The Prohibitin-Binding Compound Fluorizoline Activates the Integrated Stress Response through the eIF2α Kinase HRI
Abstract
1. Introduction
2. Results
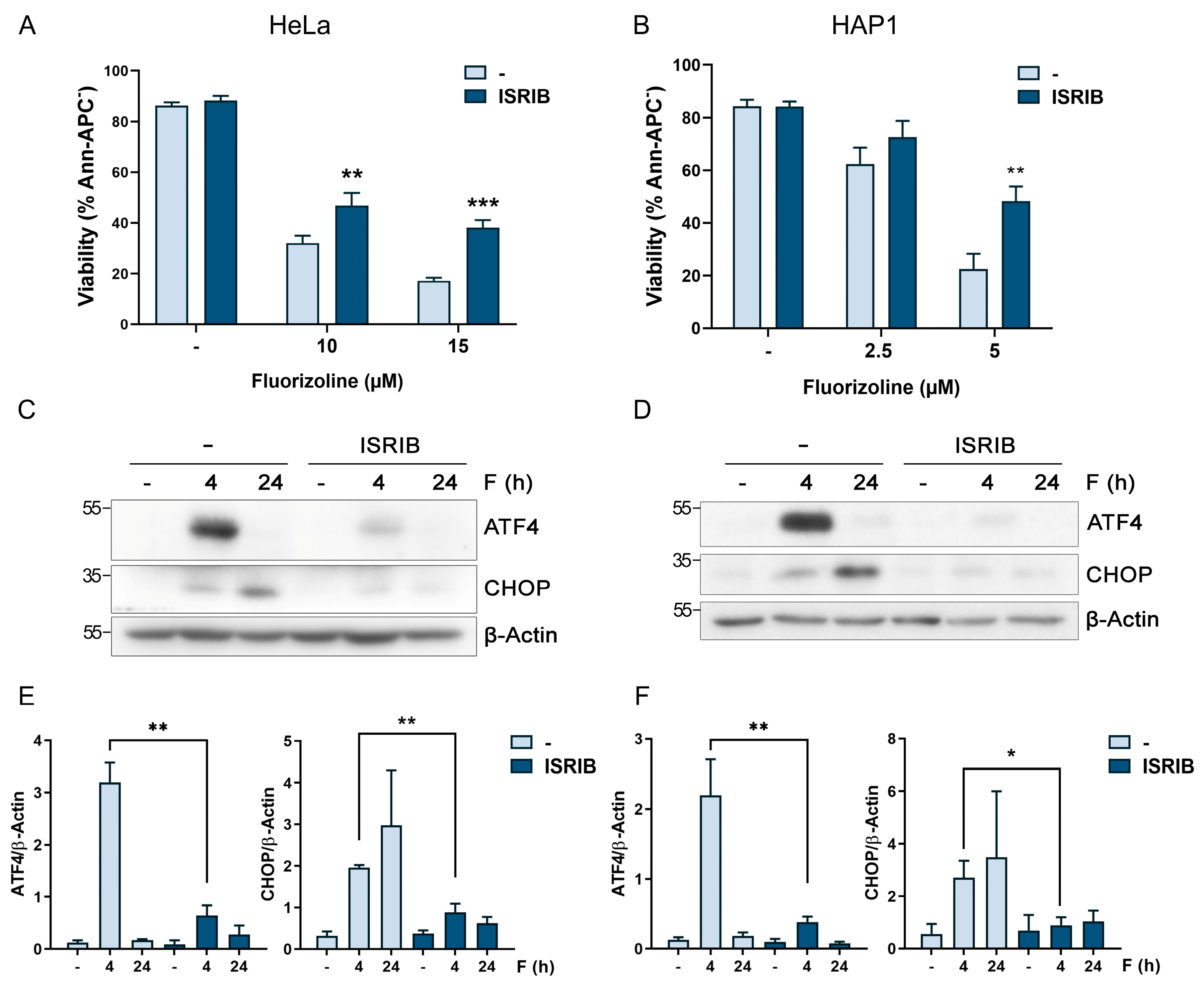
2.1. ISR Inhibition Increases Cell Resistance to Apoptosis Induction by Fluorizoline
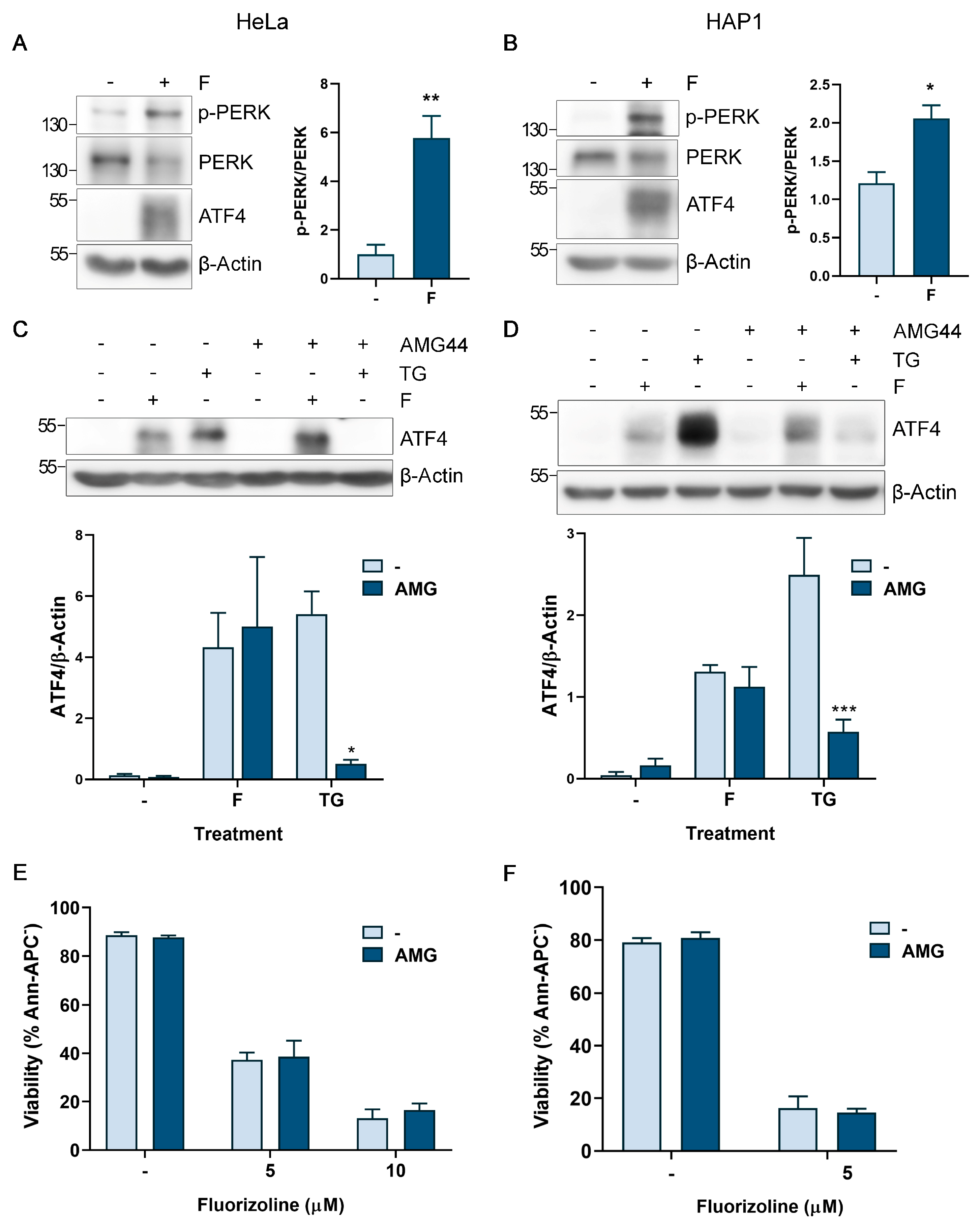
2.2. PERK Activation by Fluorizoline-Induced Stress Is Not Responsible for the Activation of the ISR
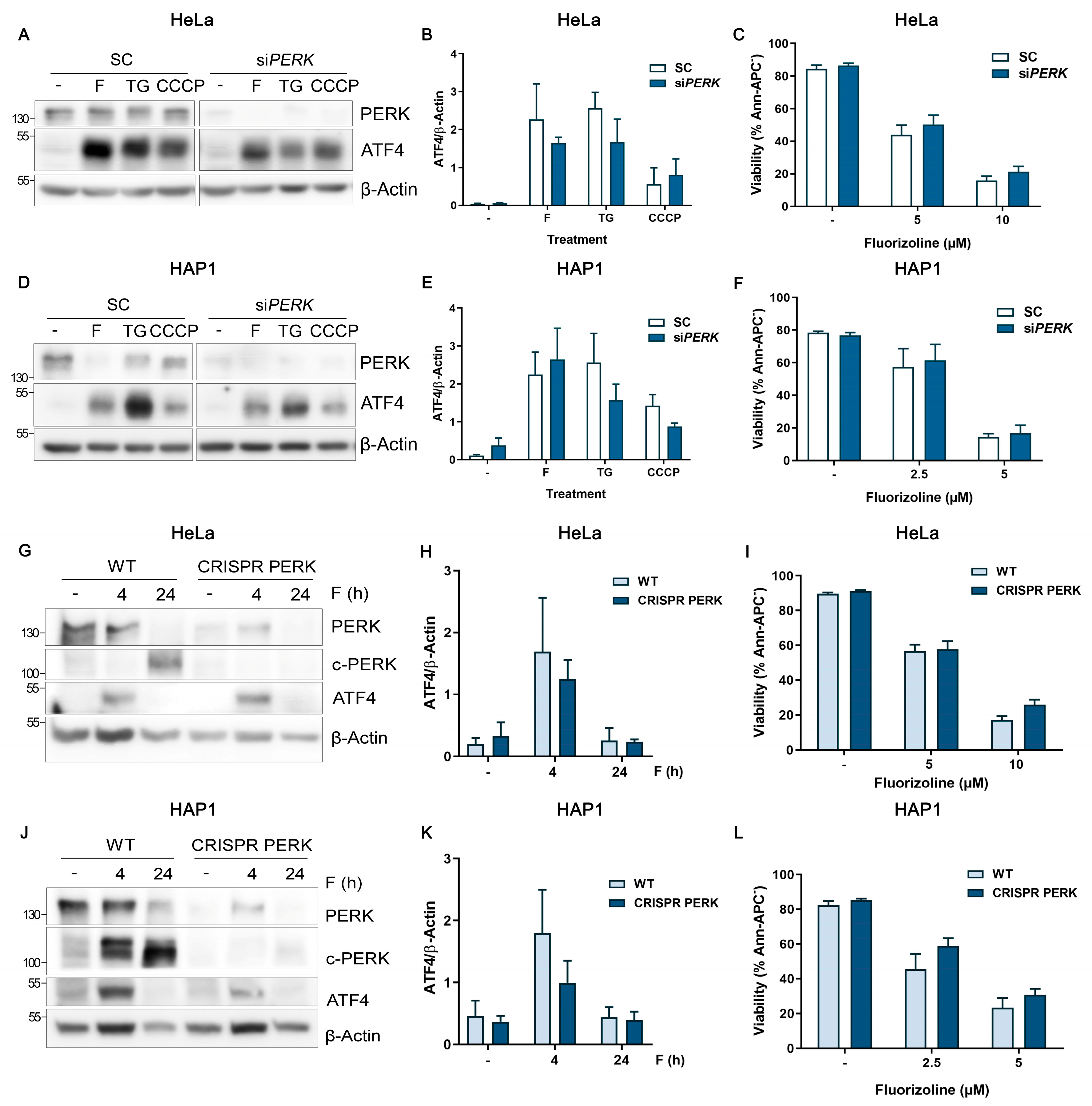
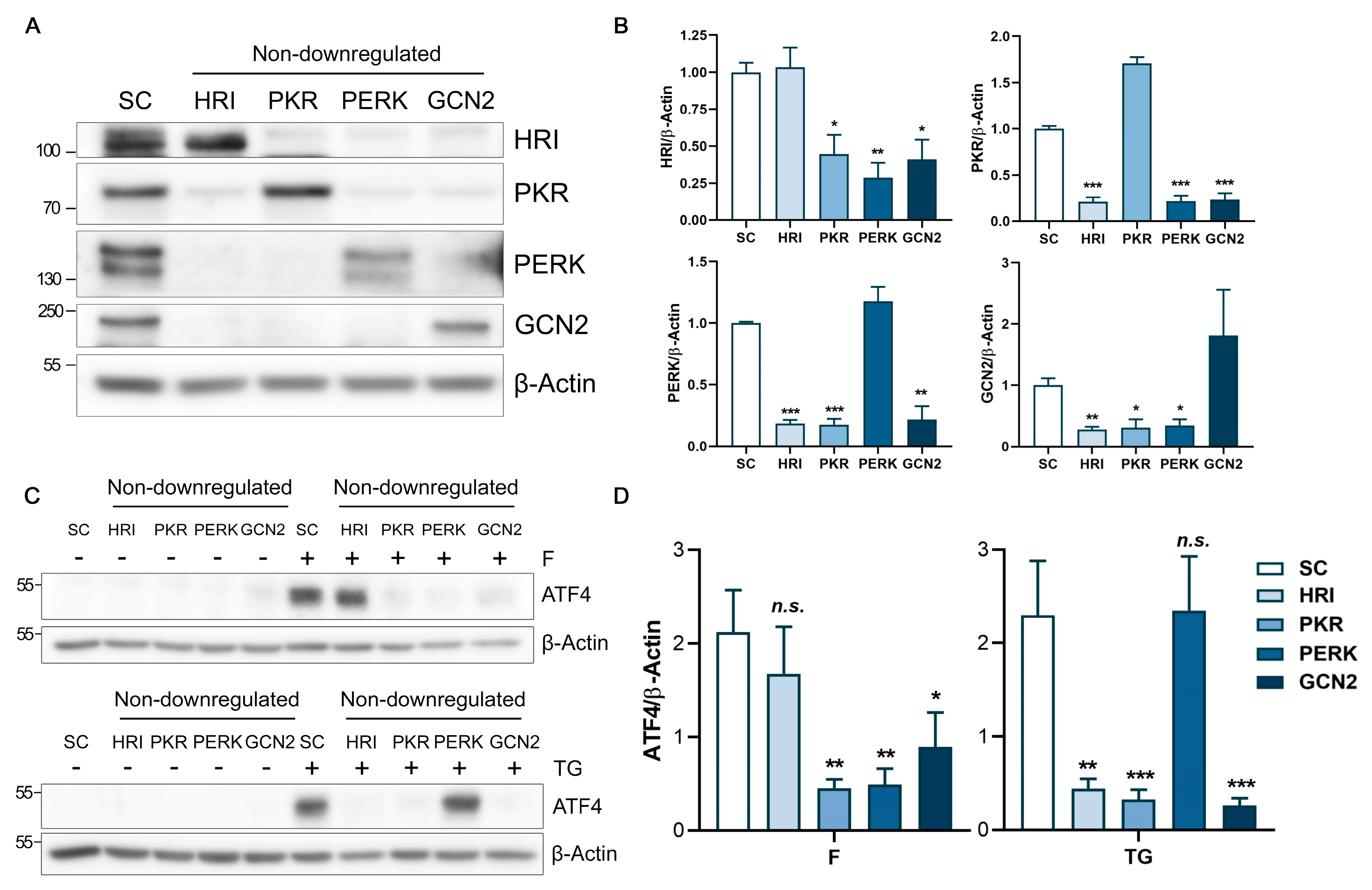
2.3. Analysis of the eIF2α Kinases in Fluorizoline-Induced Apoptosis
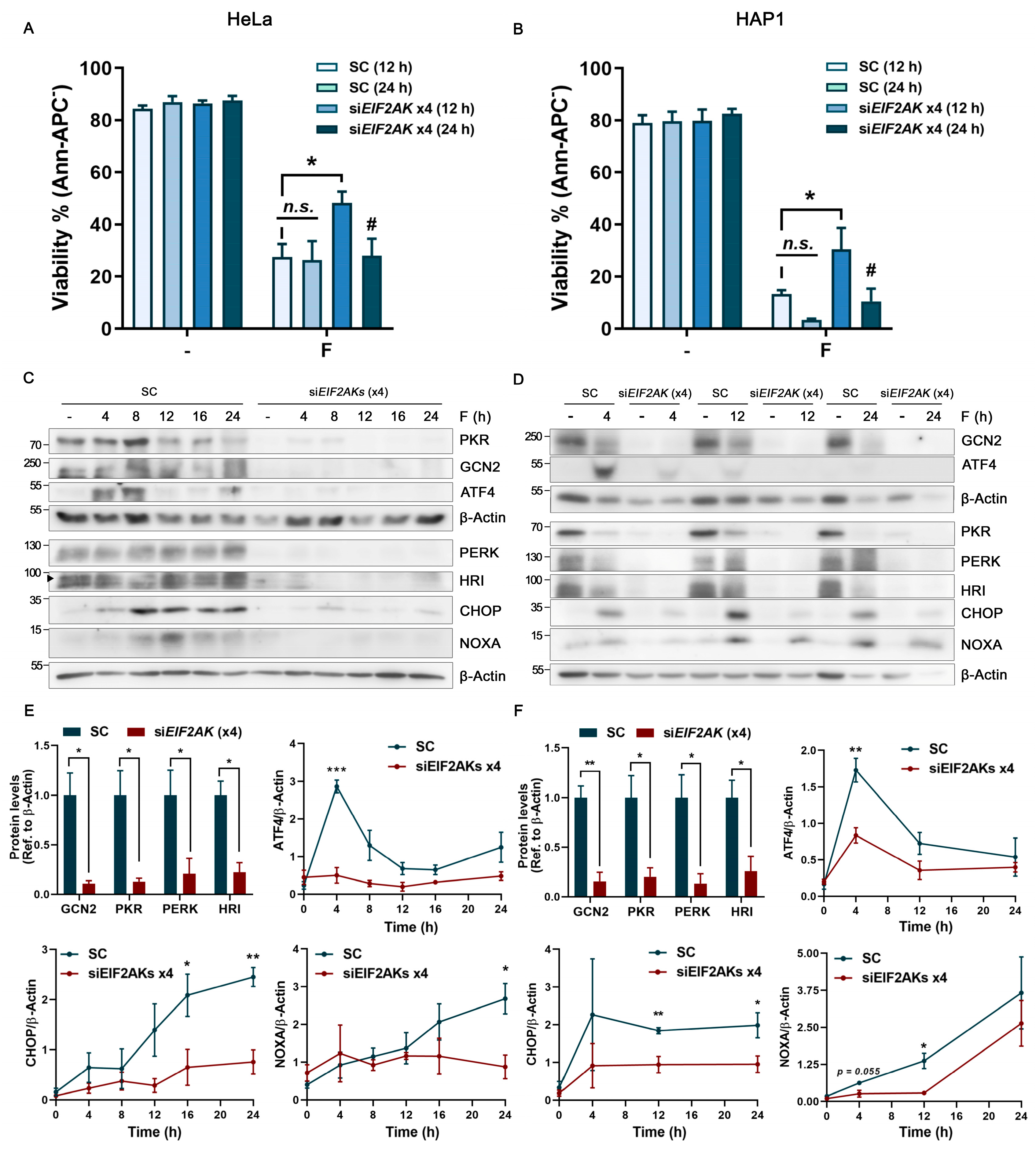
2.4. Fluorizoline Treatment Activates the eIF2α Kinase HRI to Induce the ISR
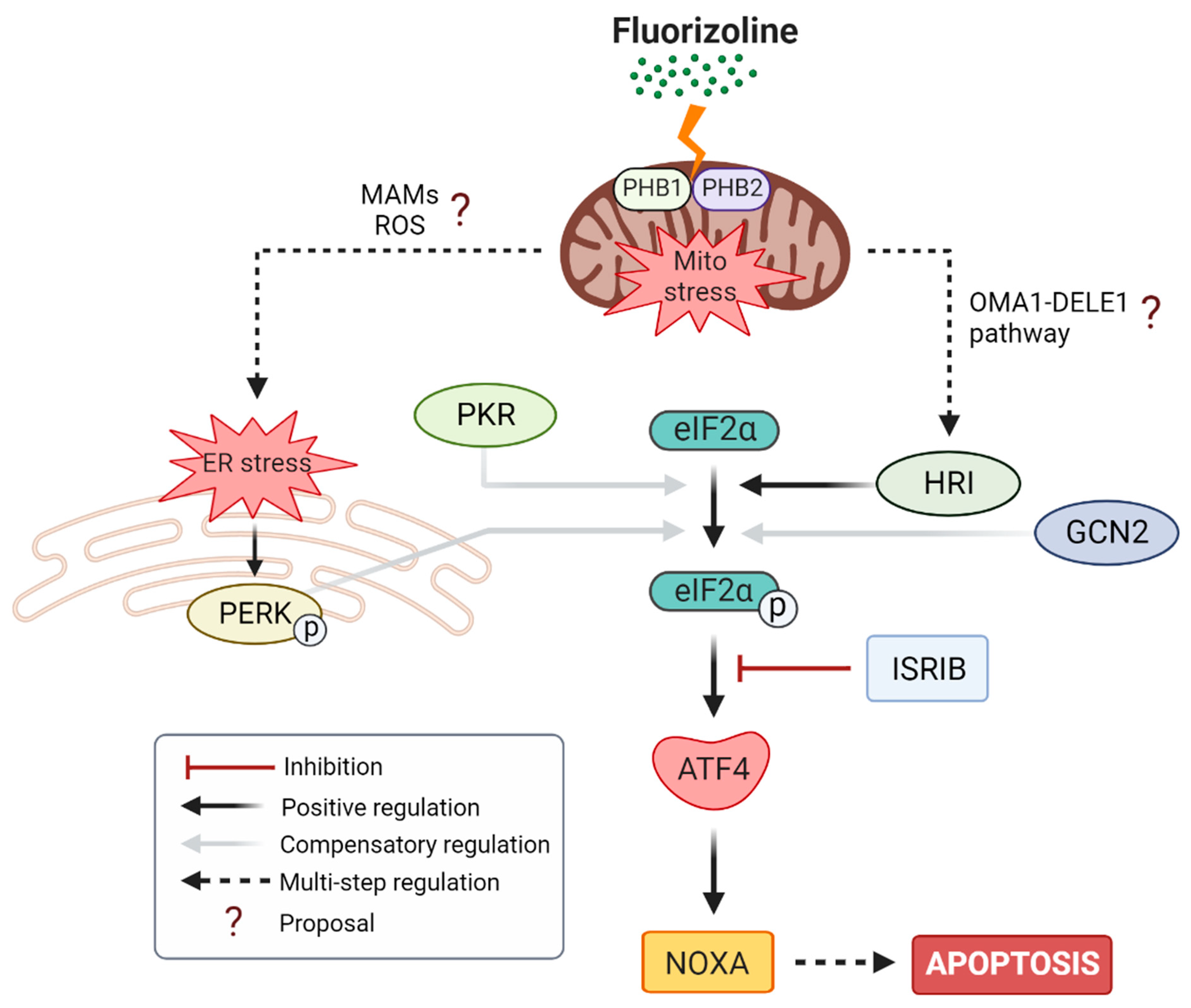
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Reagents
4.3. CRISPR/Cas9
4.4. Cell Viability
4.5. Small Interfering RNA Transfection
4.6. Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Perarnau, A.; Preciado, S.; Palmeri, C.M.; Moncunill-Massaguer, C.; Iglesias-Serret, D.; González-Gironès, D.M.; Miguel, M.; Karasawa, S.; Sakamoto, S.; Cosialls, A.M.; et al. A Trifluorinated Thiazoline Scaffold Leading to Pro-Apoptotic Agents Targeting Prohibitins. Angew. Chem. Int. Ed. 2014, 53, 10150–10154. [Google Scholar] [CrossRef]
- Moncunill-Massaguer, C.; Saura-Esteller, J.; Pérez-Perarnau, A.; Palmeri, C.M.; Núñez-Vázquez, S.; Cosialls, A.M.; González-Gironès, D.M.; Pomares, H.; Korwitz, A.; Preciado, S.; et al. A Novel Prohibitin-Binding Compound Induces the Mitochondrial Apoptotic Pathway through NOXA and BIM Upregulation. Oncotarget 2015, 6, 41750–41765. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; He, Q.Y. Significance of Prohibitin Domain Family in Tumorigenesis and Its Implication in Cancer Diagnosis and Treatment. Cell Death Dis. 2018, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, T.; Langer, T. Prohibitins. Curr. Biol. 2017, 27, R629–R631. [Google Scholar] [CrossRef]
- Thuaud, F.; Ribeiro, N.; Nebigil, C.G.; Désaubry, L. Prohibitin Ligands in Cell Death and Survival: Mode of Action and Therapeutic Potential. Chem. Biol. 2013, 20, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yu, C.; Souza, R.F.; Theiss, A.L. Prohibitin 1 Modulates Mitochondrial Function of Stat3. Cell. Signal. 2014, 26, 2086–2095. [Google Scholar] [CrossRef]
- Ma, L.L.; Shen, L.; Tong, G.H.; Tang, N.; Luo, Y.; Guo, L.L.; Hu, C.T.; Huang, Y.X.; Huang, G.; Jing, F.Y.; et al. Prohibitin, Relocated to the Front Ends, Can Control the Migration Directionality of Colorectal Cancer Cells. Oncotarget 2017, 8, 76340. [Google Scholar] [CrossRef]
- Saura-Esteller, J.; Sánchez-Vera, I.; Núñez-Vázquez, S.; Cosialls, A.M.; Gama-Pérez, P.; Bhosale, G.; Mendive-Tapia, L.; Lavilla, R.; Pons, G.; Garcia-Roves, P.M.; et al. Activation of the Integrated Stress Response and ER Stress Protect from Fluorizoline-Induced Apoptosis in HEK293T and U2OS Cell Lines. Int. J. Mol. Sci. 2021, 22, 6117. [Google Scholar] [CrossRef]
- Núñez-Vázquez, S.; Sánchez-Vera, I.; Saura-Esteller, J.; Cosialls, A.M.; Noisier, A.F.M.; Albericio, F.; Lavilla, R.; Pons, G.; Iglesias-Serret, D.; Gil, J. NOXA Upregulation by the Prohibitin-Binding Compound Fluorizoline Is Transcriptionally Regulated by Integrated Stress Response-Induced ATF3 and ATF4. FEBS J. 2021, 288, 1271–1285. [Google Scholar] [CrossRef]
- Jin, X.; Xie, J.; Zabolocki, M.; Wang, X.; Jiang, T.; Wang, D.; Désaubry, L.; Bardy, C.; Proud, C.G. The Prohibitin-Binding Compound Fluorizoline Affects Multiple Components of the Translational Machinery and Inhibits Protein Synthesis. J. Biol. Chem. 2020, 295, 9855–9867. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Moyama, C.; Taniguchi, K.; Ando, K.; Matsuda, R.; Ando, S.; Ii, H.; Kageyama, S.; Kawauchi, A.; Chouha, N.; et al. Fluorizoline Blocks the Interaction between Prohibitin-2 and γ-Glutamylcyclotransferase and Induces P21Waf1/Cip1 Expression in MCF7 Breast Cancer Cells. Mol. Pharm. 2022, 101, 78–86. [Google Scholar] [CrossRef]
- Yurugi, H.; Marini, F.; Weber, C.; David, K.; Zhao, Q.; Binder, H.; Désaubry, L.; Rajalingam, K. Targeting Prohibitins with Chemical Ligands Inhibits KRAS-Mediated Lung Tumours. Oncogene 2017, 36, 4778–4789. [Google Scholar] [CrossRef] [PubMed]
- Cosialls, A.M.; Pomares, H.; Iglesias-Serret, D.; Saura-Esteller, J.; Núñez-Vázquez, S.; González-Gironès, D.M.; de La Banda, E.; Preciado, S.; Albericio, F.; Lavilla, R.; et al. The Prohibitin-Binding Compound Fluorizoline Induces Apoptosis in Chronic Lymphocytic Leukemia Cells through the Upregulation of NOXA and Synergizes with Ibrutinib, 5-Aminoimidazole-4-Carboxamide Riboside or Venetoclax. Haematologica 2017, 102, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Cosialls, A.M.; Sánchez-Vera, I.; Pomares, H.; Perramon-Andújar, J.; Sanchez-Esteban, S.; Palmeri, C.M.; Iglesias-Serret, D.; Saura-Esteller, J.; Núñez-Vázquez, S.; Lavilla, R.; et al. The BCL-2 Family Members NOXA and BIM Mediate Fluorizoline-Induced Apoptosis in Multiple Myeloma Cells. Biochem. Pharm. 2020, 180, 114198. [Google Scholar] [CrossRef]
- Pomares, H.; Palmeri, C.M.; Iglesias-Serret, D.; Moncunill-Massaguer, C.; Saura-Esteller, J.; Núñez-Vázquez, S.; Gamundi, E.; Arnan, M.; Preciado, S.; Albericio, F.; et al. Targeting Prohibitins Induces Apoptosis in Acute Myeloid Leukemia Cells. Oncotarget 2016, 7, 64987–65000. [Google Scholar] [CrossRef]
- Wierz, M.; Pierson, S.; Chouha, N.; Désaubry, L.; François, J.H.; Berchem, G.; Paggetti, J.; Moussay, E. The Prohibitin-Binding Compound Fluorizoline Induces Apoptosis in Chronic Lymphocytic Leukemia Cells Ex Vivo but Fails to Prevent Leukemia Development in a Murine Model. Haematologica 2018, 103, e154–e157. [Google Scholar] [CrossRef] [PubMed]
- Merkwirth, C.; Dargazanli, S.; Tatsuta, T.; Geimer, S.; Löwer, B.; Wunderlich, F.T.; Von Kleist-Retzow, J.C.; Waisman, A.; Westermann, B.; Langer, T. Prohibitins Control Cell Proliferation and Apoptosis by Regulating OPA1-Dependent Cristae Morphogenesis in Mitochondria. Genes Dev. 2008, 22, 476–488. [Google Scholar] [CrossRef]
- Anderson, N.S.; Haynes, C.M. Folding the Mitochondrial UPR into the Integrated Stress Response. Trends Cell Biol. 2020, 30, 428–439. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The Integrated Stress Response: From Mechanism to Disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Ron, D. Translational Control in the Endoplasmic Reticulum Stress Response. J. Clin. Investig. 2002, 110, 1383–1388. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The Integrated Stress Response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Kalkavan, H.; Chen, M.J.; Crawford, J.C.; Quarato, G.; Fitzgerald, P.; Tait, S.W.G.; Goding, C.R.; Green, D.R. Sublethal Cytochrome c Release Generates Drug-Tolerant Persister Cells. Cell 2022, 185, 3356–3374.e22. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell Death Induced by Endoplasmic Reticulum Stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Zyryanova, A.F.; Weis, F.; Faille, A.; Abo Alard, A.; Crespillo-Casado, A.; Sekine, Y.; Harding, H.P.; Allen, F.; Parts, L.; Fromont, C.; et al. Binding of ISRIB Reveals a Regulatory Site in the Nucleotide Exchange Factor EIF2B. Science 2018, 359, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Bennett, B.S.; Cullinan, S.B.; Diehl, J.A. PERK and GCN2 Contribute to EIF2α Phosphorylation and Cell Cycle Arrest after Activation of the Unfolded Protein Response Pathway. Mol. Biol. Cell 2005, 16, 5493–5501. [Google Scholar] [CrossRef]
- Lehman, S.L.; Ryeom, S.; Koumenis, C. Signaling through Alternative Integrated Stress Response Pathways Compensates for GCN2 Loss in a Mouse Model of Soft Tissue Sarcoma. Sci. Rep. 2015, 5, srep11781. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Andrews, K.L.; Beckmann, H.; Bellon, S.F.; Beltran, P.J.; Booker, S.; Chen, H.; Chung, Y.A.; D’Angelo, N.D.; Dao, J.; et al. Discovery of 1H-Pyrazol-3(2H)-Ones as Potent and Selective Inhibitors of Protein Kinase R-like Endoplasmic Reticulum Kinase (PERK). J. Med. Chem. 2015, 58, 1426–1441. [Google Scholar] [CrossRef]
- Prudent, J.; McBride, H.M. The Mitochondria-Endoplasmic Reticulum Contact Sites: A Signalling Platform for Cell Death. Curr. Opin. Cell Biol. 2017, 47, 52–63. [Google Scholar] [CrossRef]
- Su, Y.; Huang, X.; Huang, Z.; Huang, T.; Xu, Y.; Yi, C. STAT3 Localizes in Mitochondria-Associated ER Membranes Instead of in Mitochondria. Front. Cell Dev. Biol. 2020, 8, 274. [Google Scholar] [CrossRef]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. PERK Is Required at the ER-Mitochondrial Contact Sites to Convey Apoptosis after ROS-Based ER Stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.M.; Prado, M.A.; Zamboni, N.; D’Amico, D.; Williams, R.W.; Finley, D.; Gygi, S.P.; Auwerx, J. Multi-Omics Analysis Identifies ATF4 as a Key Regulator of the Mitochondrial Stress Response in Mammals. J. Cell Biol. 2017, 216, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. Deletion of the EIF2α Kinase GCN2 Fails to Rescue the Memory Decline Associated with Alzheimer’s Disease. PLoS ONE 2013, 8, e77335. [Google Scholar] [CrossRef] [PubMed]
- Roobol, A.; Roobol, J.; Bastide, A.; Knight, J.R.P.; Willis, A.E.; Smales, C.M. P58IPK Is an Inhibitor of the EIF2α Kinase GCN2 and Its Localization and Expression Underpin Protein Synthesis and ER Processing Capacity. Biochem. J. 2015, 465, 213–225. [Google Scholar] [CrossRef]
- Mick, E.; Titov, D.V.; Skinner, O.S.; Sharma, R.; Jourdain, A.A.; Mootha, V.K. Distinct Mitochondrial Defects Trigger the Integrated Stress Response Depending on the Metabolic State of the Cell. eLife 2020, 9, e49178. [Google Scholar] [CrossRef] [PubMed]
- Fessler, E.; Eckl, E.M.; Schmitt, S.; Mancilla, I.A.; Meyer-Bender, M.F.; Hanf, M.; Philippou-Massier, J.; Krebs, S.; Zischka, H.; Jae, L.T. A Pathway Coordinated by DELE1 Relays Mitochondrial Stress to the Cytosol. Nature 2020, 579, 433. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Aviles, G.; Liu, Y.; Tian, R.; Unger, B.A.; Lin, Y.H.T.; Wiita, A.P.; Xu, K.; Correia, M.A.; Kampmann, M. Mitochondrial Stress Is Relayed to the Cytosol by an OMA1–DELE1–HRI Pathway. Nature 2020, 579, 427–432. [Google Scholar] [CrossRef]
- Baker, B.M.; Nargund, A.M.; Sun, T.; Haynes, C.M. Protective Coupling of Mitochondrial Function and Protein Synthesis via the EIF2α Kinase GCN-2. PLoS Genet. 2012, 8, e1002760. [Google Scholar] [CrossRef] [PubMed]
- Artal-Sanz, M.; Tavernarakis, N. Prohibitin Couples Diapause Signalling to Mitochondrial Metabolism during Ageing in C. elegans. Nature 2009, 461, 793–797. [Google Scholar] [CrossRef]
- Hernando-Rodríguez, B.; Artal-Sanz, M. Mitochondrial Quality Control Mechanisms and the PHB (Prohibitin) Complex. Cells 2018, 7, 238. [Google Scholar] [CrossRef]
- Merkwirth, C.; Langer, T. Prohibitin Function within Mitochondria: Essential Roles for Cell Proliferation and Cristae Morphogenesis. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2009, 1793, 27–32. [Google Scholar] [CrossRef]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; Rasmo, D. De Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.I.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine Natural Product Aurilide Activates the OPA1-Mediated Apoptosis by Binding to Prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, H.; Song, Z. Membrane Depolarization Activates the Mitochondrial Protease OMA1 by Stimulating Self-Cleavage. EMBO Rep. 2014, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yu, H.; Kong, Q.; Wang, B.; Shen, L.; Dong, D.; Sun, L. The Mitochondrial PHB2/OMA1/DELE1 Pathway Cooperates with Endoplasmic Reticulum Stress to Facilitate the Response to Chemotherapeutics in Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 1320. [Google Scholar] [CrossRef] [PubMed]
- Hartleben, G.; Schorpp, K.; Kwon, Y.; Betz, B.; Tsokanos, F.; Dantes, Z.; Schäfer, A.; Rothenaigner, I.; Kuhn, J.M.M.; Morigny, P.; et al. Combination Therapies Induce Cancer Cell Death through the Integrated Stress Response and Disturbed Pyrimidine Metabolism. EMBO Mol. Med. 2021, 13, e12461. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Ri, M.; Masaki, A.; Mori, F.; Ito, A.; Kusumoto, S.; Ishida, T.; Komatsu, H.; Iida, S. Lower Expression of Activating Transcription Factors 3 and 4 Correlates with Shorter Progression-Free Survival in Multiple Myeloma Patients Receiving Bortezomib plus Dexamethasone Therapy. Blood Cancer J. 2015, 5, e373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Safran, H.; Borsuk, R.; Lulla, R.; Tapinos, N.; Seyhan, A.A.; El-Deiry, W.S. EZH2i EPZ-6438 and HDACi Vorinostat Synergize with ONC201/TIC10 to Activate Integrated Stress Response, DR5, Reduce H3K27 Methylation, ClpX and Promote Apoptosis of Multiple Tumor Types Including DIPG. Neoplasia 2021, 23, 792–810. [Google Scholar] [CrossRef]
- Dey, S.; Sayers, C.M.; Verginadis, I.I.; Lehman, S.L.; Cheng, Y.; Cerniglia, G.J.; Tuttle, S.W.; Feldman, M.D.; Paul, P.J.; Fuchs, S.Y.; et al. ATF4-Dependent Induction of Heme Oxygenase 1 Prevents Anoikis and Promotes Metastasis. J. Clin. Investig. 2015, 125, 2592. [Google Scholar] [CrossRef]
- Jammi, N.V.; Whitby, L.R.; Beal, P.A. Small Molecule Inhibitors of the RNA-Dependent Protein Kinase. Biochem. Biophys. Res. Commun. 2003, 308, 50–57. [Google Scholar] [CrossRef]
- Kato, Y.; Kunimasa, K.; Sugimoto, Y.; Tomida, A. BCR-ABL Tyrosine Kinase Inhibition Induces Metabolic Vulnerability by Preventing the Integrated Stress Response in K562 cells. Biochem. Biophys. Res. Commun. 2018, 504, 721–726. [Google Scholar] [CrossRef]
- Mohamed, E.; Sierra, R.A.; Trillo-Tinoco, J.; Cao, Y.; Innamarato, P.; Payne, K.K.; de Mingo Pulido, A.; Mandula, J.; Zhang, S.; Thevenot, P.; et al. The Unfolded Protein Response Mediator PERK Governs Myeloid Cell-Driven Immunosuppression in Tumors through Inhibition of STING Signaling. Immunity 2020, 52, 668–682.e7. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.D.; Woods, C.R.; Goldberg, S.D.; Hack, M.D.; Bounds, A.D.; Yang, Y.; Wagaman, P.C.; Phuong, V.K.; Ameriks, A.P.; Barrett, T.D.; et al. Discovery of the First Known Small-Molecule Inhibitors of Heme-Regulated Eukaryotic Initiation Factor 2α (HRI) Kinase. Bioorg. Med. Chem. Lett. 2009, 19, 6548–6551. [Google Scholar] [CrossRef] [PubMed]
- Sidrauski, C.; McGeachy, A.M.; Ingolia, N.T.; Walter, P. The Small Molecule ISRIB Reverses the Effects of EIF2α Phosphorylation on Translation and Stress Granule Assembly. eLife 2015, 4, e05033. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.L.; Lebon, L.; Basso, A.M.; Kohlhaas, K.L.; Nikkel, A.L.; Robb, H.M.; Donnelly-Roberts, D.L.; Prakash, J.; Swensen, A.M.; Rubinstein, N.D.; et al. EIF2B Activator Prevents Neurological Defects Caused by a Chronic Integrated Stress Response. eLife 2019, 8, e42940. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, S.; Zhou, L.; Seyhan, A.A.; Hernandez Borrero, L.; Zhang, Y.; El-Deiry, W.S. Targeting the Integrated Stress Response in Cancer Therapy. Front. Pharm. 2021, 12, 2621. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Vera, I.; Núñez-Vázquez, S.; Saura-Esteller, J.; Cosialls, A.M.; Heib, J.; Nadal Rodríguez, P.; Ghashghaei, O.; Lavilla, R.; Pons, G.; Gil, J.; et al. The Prohibitin-Binding Compound Fluorizoline Activates the Integrated Stress Response through the eIF2α Kinase HRI. Int. J. Mol. Sci. 2023, 24, 8064. https://doi.org/10.3390/ijms24098064
Sánchez-Vera I, Núñez-Vázquez S, Saura-Esteller J, Cosialls AM, Heib J, Nadal Rodríguez P, Ghashghaei O, Lavilla R, Pons G, Gil J, et al. The Prohibitin-Binding Compound Fluorizoline Activates the Integrated Stress Response through the eIF2α Kinase HRI. International Journal of Molecular Sciences. 2023; 24(9):8064. https://doi.org/10.3390/ijms24098064
Chicago/Turabian StyleSánchez-Vera, Ismael, Sonia Núñez-Vázquez, José Saura-Esteller, Ana M. Cosialls, Judith Heib, Pau Nadal Rodríguez, Ouldouz Ghashghaei, Rodolfo Lavilla, Gabriel Pons, Joan Gil, and et al. 2023. "The Prohibitin-Binding Compound Fluorizoline Activates the Integrated Stress Response through the eIF2α Kinase HRI" International Journal of Molecular Sciences 24, no. 9: 8064. https://doi.org/10.3390/ijms24098064
APA StyleSánchez-Vera, I., Núñez-Vázquez, S., Saura-Esteller, J., Cosialls, A. M., Heib, J., Nadal Rodríguez, P., Ghashghaei, O., Lavilla, R., Pons, G., Gil, J., & Iglesias-Serret, D. (2023). The Prohibitin-Binding Compound Fluorizoline Activates the Integrated Stress Response through the eIF2α Kinase HRI. International Journal of Molecular Sciences, 24(9), 8064. https://doi.org/10.3390/ijms24098064





