Sex Modulates Response to Renal-Tubule-Targeted Insulin Receptor Deletion in Mice
Abstract
1. Introduction
2. Results
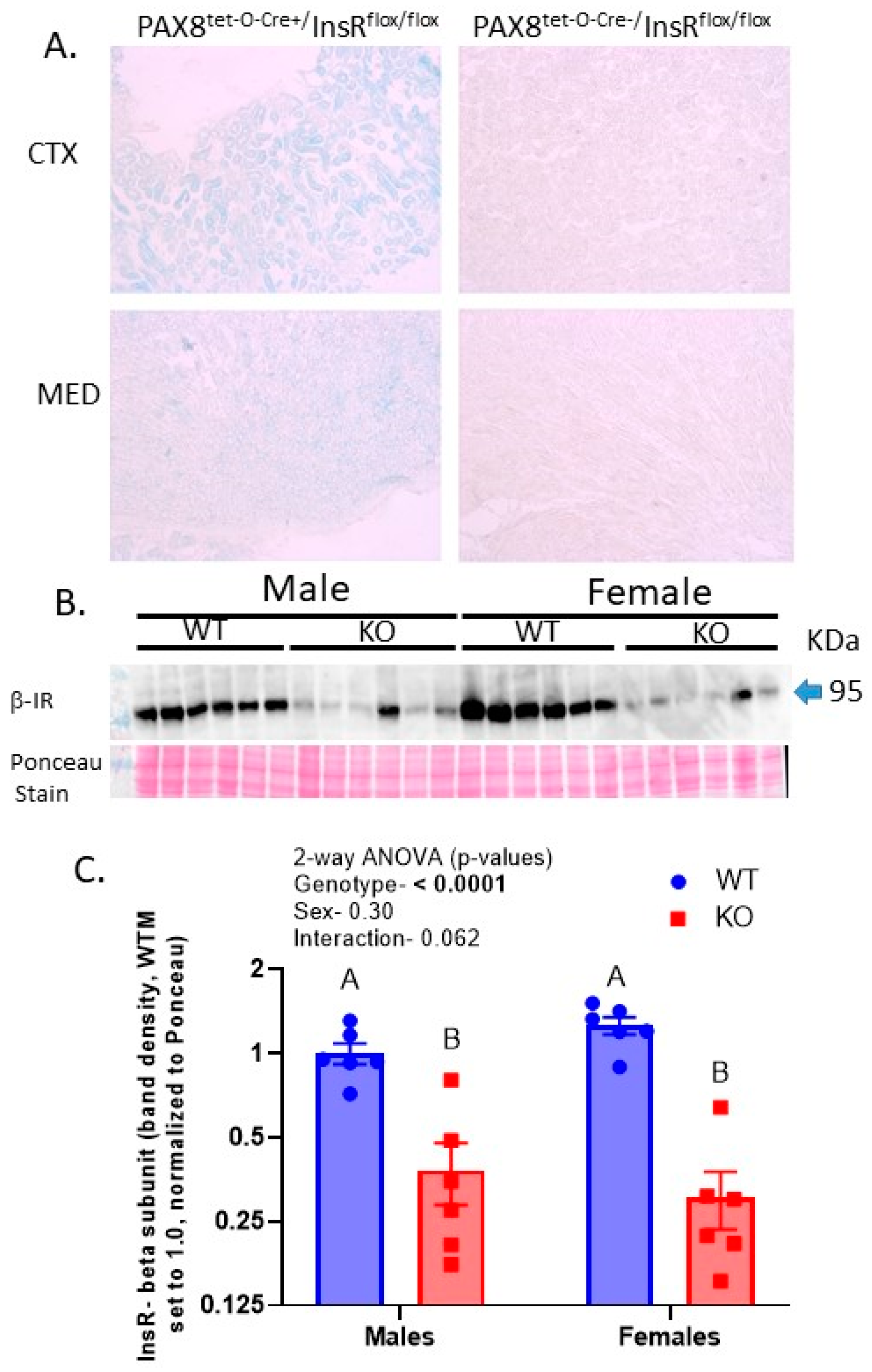
2.1. Protein Levels of the Insulin Receptor Were Reduced by over 70% in the KO Kidneys
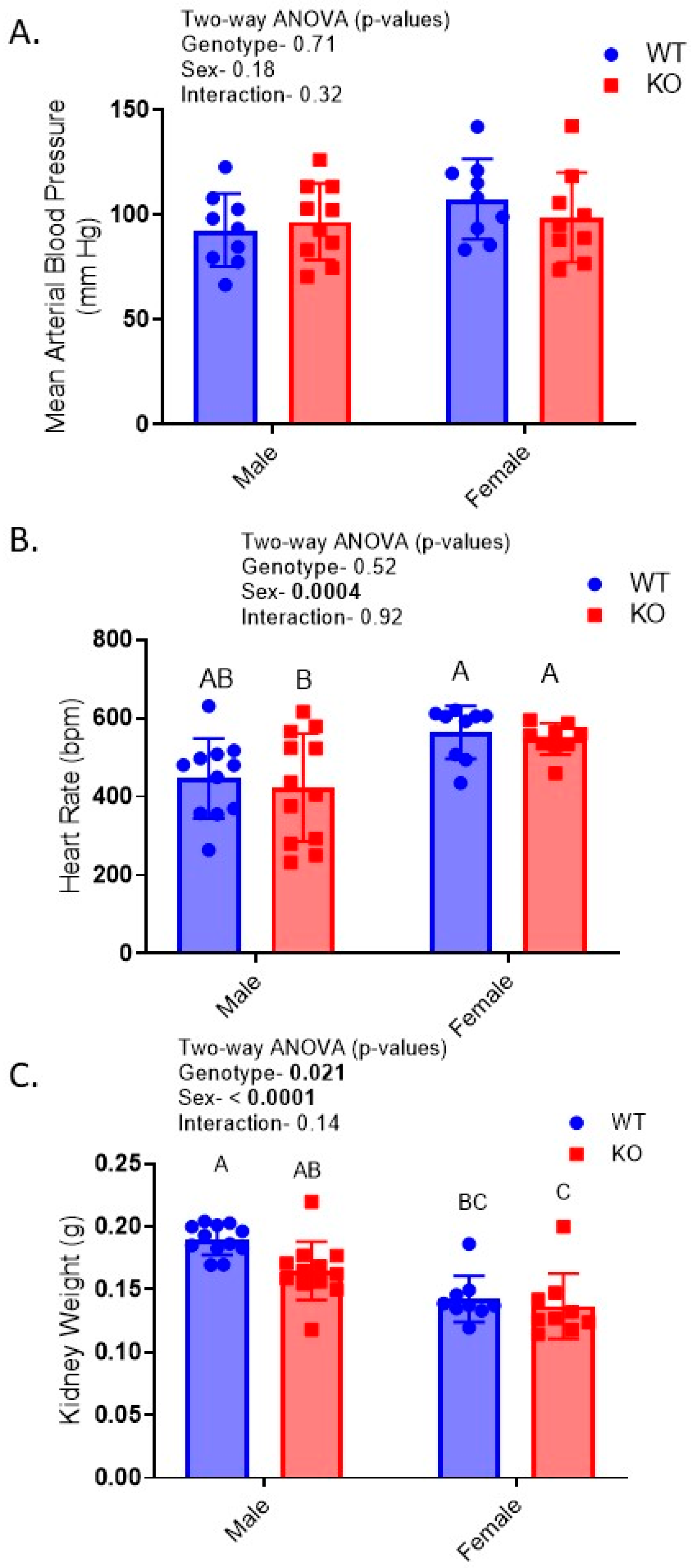
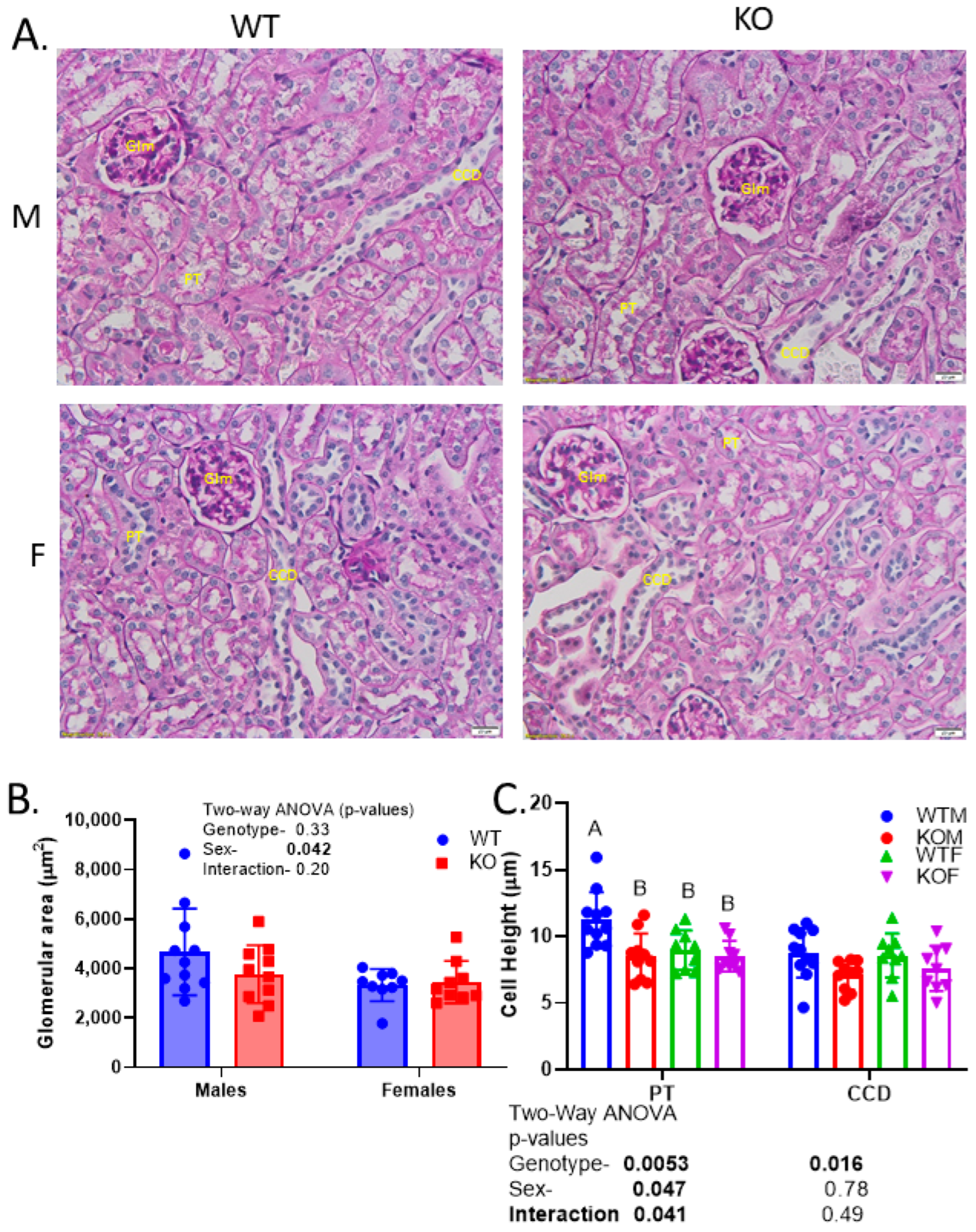
2.2. Reduced Kidney Weights and Cell Height Exacerbated in KO Males
2.3. Relative Insulin-Induced Hyperkalemia in KO
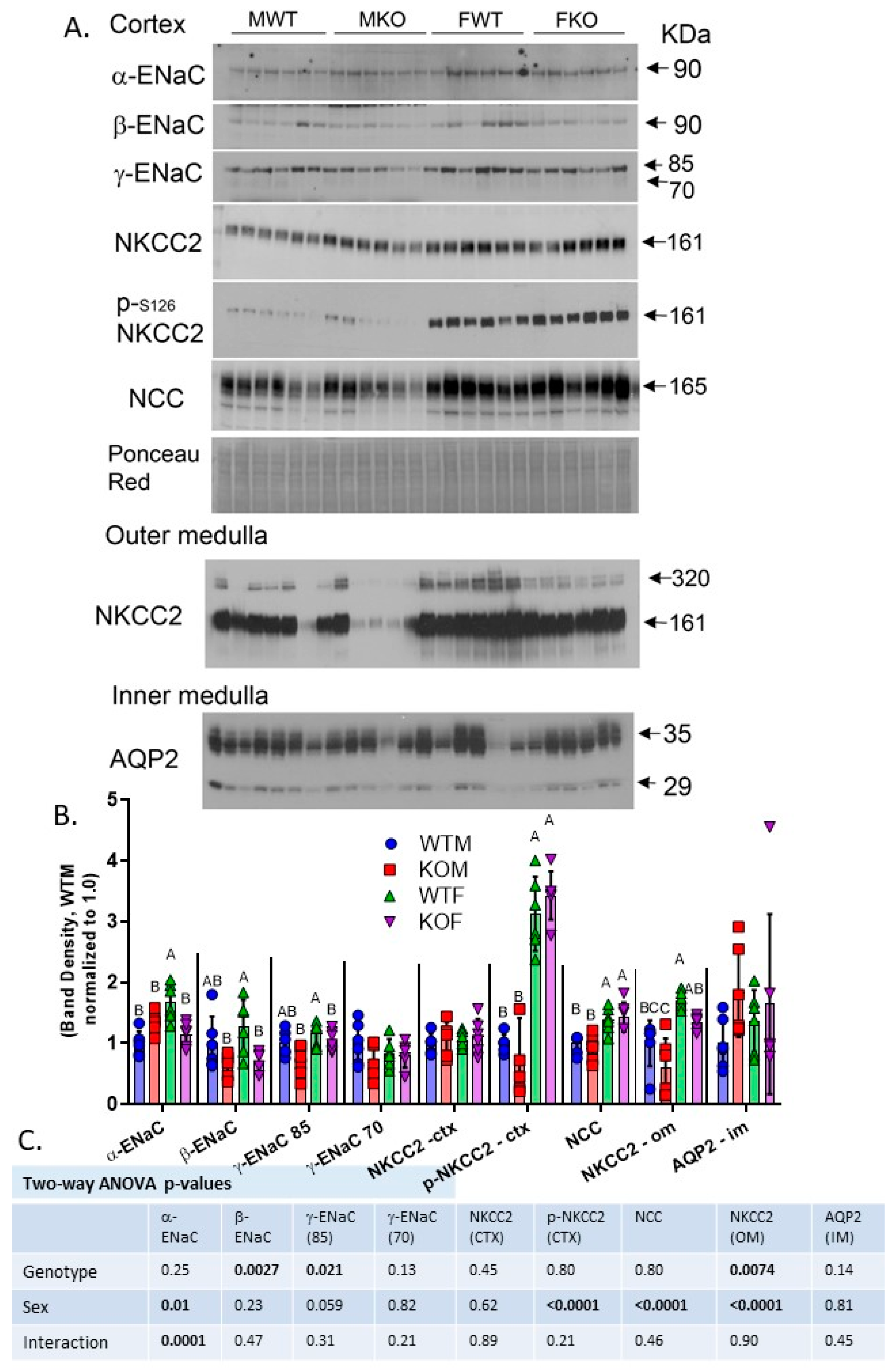
2.4. Western Blotting Revealed Reduced ENaC Subunits and NKCC2 in Kidneys of the KO Mice
2.5. Kaliuretic Response to Diuretics Relatively Blunted in Female Mice
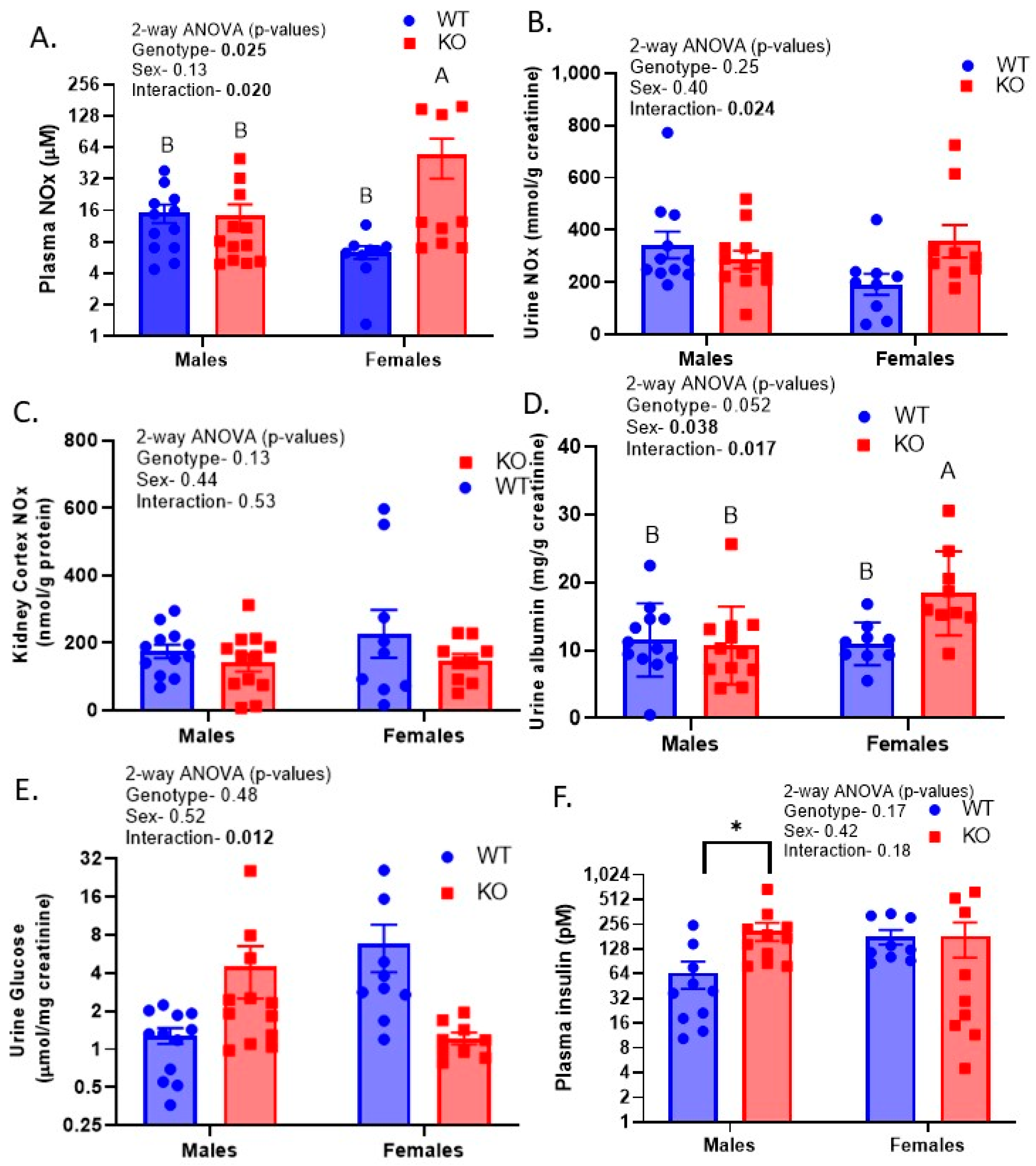
2.6. Sex-by-Genotype Interactions in Urine Nitrates plus Nitrites (NOx), Albumin, and Glucose
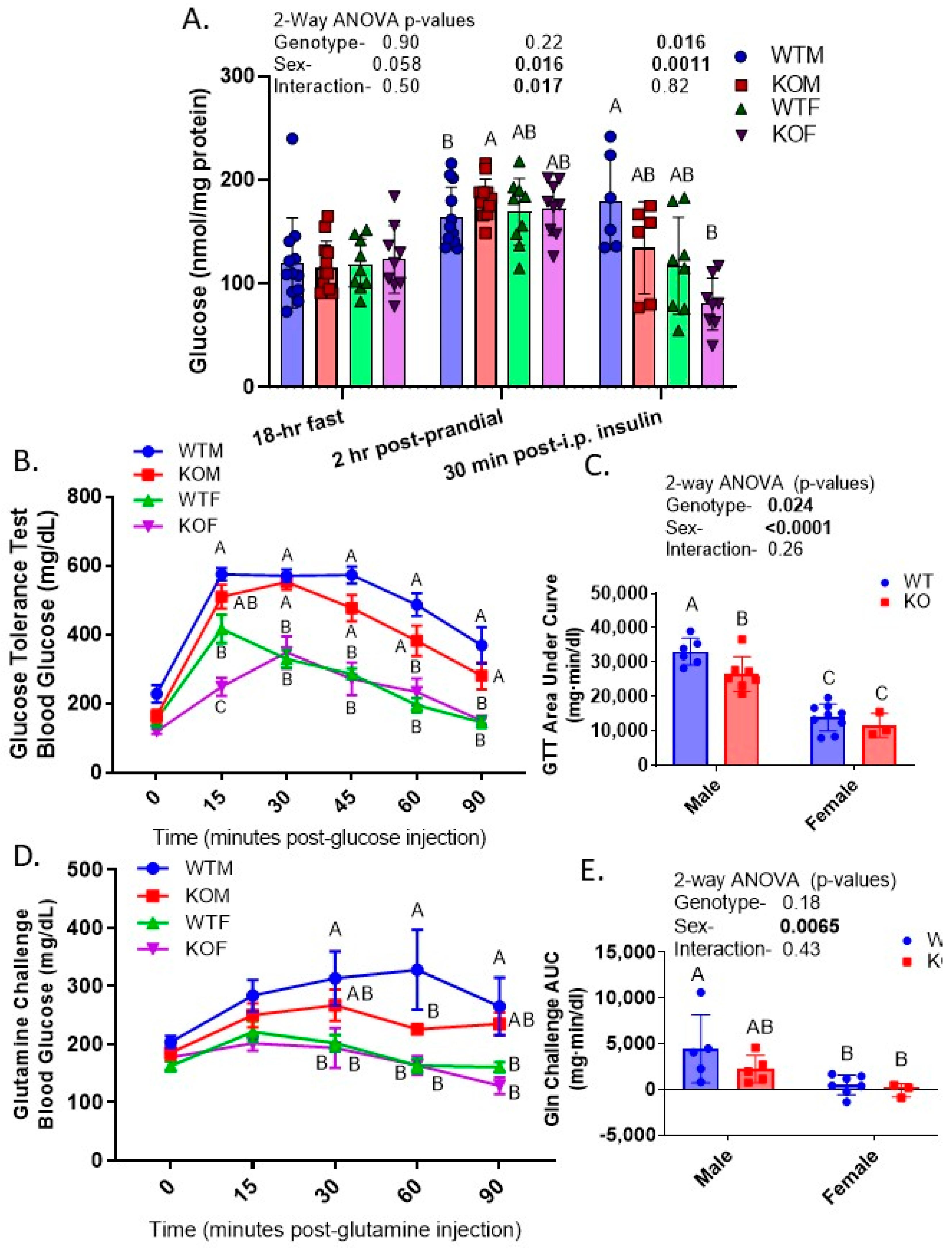
2.7. KO and Female Mice Had Lower Blood Glucose in GTT and Glutamine Challenge
2.8. Male and KO Mice Have Greater Ex Vivo Glucose-Producing Capacity of Isolated PT
2.9. Testing of Leakiness in Transgene Expression
3. Discussion
4. Materials and Methods
4.1. Generation of the Knockout
4.2. Kidney Sample Preparation and Western Blotting
4.3. Kidney Morphometry
4.4. Blood and Urine Chemistry
4.5. Blood Pressure and Insulin Administration
4.6. Natriuretic Tests
4.7. Glucose Tolerance and Glutamine Challenge
4.8. Proximal Tubule (PT) Suspensions and Gluconeogenesis
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, R.W.C. Insulin Blood Level. In The Pituitary; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. Williams Textbook of Endocrinology, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Johnson, J.L.; Duick, D.S.; Chui, M.A.; Aldasouqi, S.A. Identifying prediabetes using fasting insulin levels. Endocr. Pract. 2010, 16, 47–52. [Google Scholar] [CrossRef]
- Parks, B.W.; Sallam, T.; Mehrabian, M.; Psychogios, N.; Hui, S.T.; Norheim, F.; Castellani, L.W.; Rau, C.D.; Pan, C.; Phun, J.; et al. Genetic architecture of insulin resistance in the mouse. Cell Metab. 2015, 21, 334–347. [Google Scholar] [CrossRef]
- Tiwari, S.; Riazi, S.; Ecelbarger, C.A. Insulin’s impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am. J. Physiol. Renal. Physiol. 2007, 293, F974–F984. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt. Sinai. J. Med. 2010, 77, 511–523. [Google Scholar] [CrossRef]
- Tiwari, S.; Halagappa, V.K.; Riazi, S.; Hu, X.; Ecelbarger, C.A. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J. Am. Soc. Nephrol. 2007, 18, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Bailyes, E.M.; Nave, B.T.; Soos, M.A.; Orr, S.R.; Hayward, A.C.; Siddle, K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: Quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem. J. 1997, 327 Pt 1, 209–215. [Google Scholar] [CrossRef]
- Entingh-Pearsall, A.; Kahn, C.R. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J. Biol. Chem. 2004, 279, 38016–38024. [Google Scholar] [CrossRef]
- Slaaby, R.; Schaffer, L.; Lautrup-Larsen, I.; Andersen, A.S.; Shaw, A.C.; Mathiasen, I.S.; Brandt, J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 2006, 281, 25869–25874. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Li, L.; Ecelbarger, C.M. Sex differences in renal and metabolic responses to a high-fructose diet in mice. Am. J. Physiol. Renal. Physiol. 2015, 308, F400–F410. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Fraser, S.A.; Galic, S.; Choy, S.W.; Katerelos, M.; Gleich, K.; Kemp, B.E.; Mount, P.F.; Power, D.A. Novel mechanisms of Na+ retention in obesity: Phosphorylation of NKCC2 and regulation of SPAK/OSR1 by AMPK. Am. J. Physiol. Renal. Physiol. 2014, 307, F96–F106. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Canales, M.; Arroyo, J.P.; Ko, B.; Vazquez, N.; Bautista, R.; Castaneda-Bueno, M.; Bobadilla, N.A.; Hoover, R.S.; Gamba, G. Insulin increases the functional activity of the renal NaCl cotransporter. J. Hypertens. 2013, 31, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, T.S.; Ilatovskaya, D.V.; Levchenko, V.; Li, L.; Ecelbarger, C.M.; Staruschenko, A. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J. 2013, 27, 2723–2732. [Google Scholar] [CrossRef]
- Song, J.; Hu, X.; Riazi, S.; Tiwari, S.; Wade, J.B.; Ecelbarger, C.A. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am. J. Physiol. Renal. Physiol. 2006, 290, F1055–F1064. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Sharma, N.; Gill, P.S.; Igarashi, P.; Kahn, C.R.; Wade, J.B.; Ecelbarger, C.M. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 6469–6474. [Google Scholar] [CrossRef]
- Li, L.; Garikepati, R.M.; Tsukerman, S.; Kohan, D.; Wade, J.B.; Tiwari, S.; Ecelbarger, C.M. Reduced ENaC activity and blood pressure in mice with genetic knockout of the insulin receptor in the renal collecting duct. Am. J. Physiol. Renal. Physiol. 2013, 304, F279–F288. [Google Scholar] [CrossRef]
- Li, L.; Garikepati, R.M.; Tsukerman, S.; Tiwari, S.; Ecelbarger, C.M. Salt sensitivity of nitric oxide generation and blood pressure in mice with targeted knockout of the insulin receptor from the renal tubule. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R505–R512. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, R.S.; Li, L.; Tsukerman, S.; Godbole, M.; Pandey, G.; Ecelbarger, C.M. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J. Am. Soc. Nephrol. 2013, 24, 1209–1214. [Google Scholar] [CrossRef]
- Pandey, G.; Shankar, K.; Makhija, E.; Gaikwad, A.; Ecelbarger, C.; Mandhani, A.; Srivastava, A.; Tiwari, S. Reduced Insulin Receptor Expression Enhances Proximal Tubule Gluconeogenesis. J. Cell. Biochem. 2017, 118, 276–285. [Google Scholar] [CrossRef]
- Traykova-Brauch, M.; Schonig, K.; Greiner, O.; Miloud, T.; Jauch, A.; Bode, M.; Felsher, D.W.; Glick, A.B.; Kwiatkowski, D.J.; Bujard, H.; et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat. Med. 2008, 14, 979–984. [Google Scholar] [CrossRef]
- Kumari, M.; Sharma, R.; Pandey, G.; Ecelbarger, C.M.; Mishra, P.; Tiwari, S. Deletion of insulin receptor in the proximal tubule and fasting augment albumin excretion. J. Cell. Biochem. 2019, 120, 10688–10696. [Google Scholar] [CrossRef]
- Lewis, K.T.; Oles, L.R.; MacDougald, O.A. Tetracycline response element driven Cre causes ectopic recombinase activity independent of transactivator element. Mol. Metab. 2022, 61, 101501. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Dember, L.M.; Ingelfinger, J.R.; Vinson, A.; Neugarten, J.; Sandberg, K.L.; Sullivan, J.C.; Maric-Bilkan, C.; Rankin, T.L.; Kimmel, P.L.; et al. Sex and the kidneys: Current understanding and research opportunities. Nat. Rev. Nephrol. 2019, 15, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Nizar, J.M.; Shepard, B.D.; Vo, V.T.; Bhalla, V. Renal tubule insulin receptor modestly promotes elevated blood pressure and markedly stimulates glucose reabsorption. JCI Insight 2018, 3, e95107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Byrd, M.; Doh, K.; Dixon, P.D.; Lee, H.; Tiwari, S.; Ecelbarger, C.M. Absence of renal enlargement in fructose-fed proximal-tubule-select insulin receptor (IR), insulin-like-growth factor receptor (IGF1R) double knockout mice. Physiol. Rep. 2016, 4, e13052. [Google Scholar] [CrossRef]
- Harris, A.N.; Lee, H.W.; Osis, G.; Fang, L.; Webster, K.L.; Verlander, J.W.; Weiner, I.D. Differences in renal ammonia metabolism in male and female kidney. Am. J. Physiol. Renal. Physiol. 2018, 315, F211–F222. [Google Scholar] [CrossRef]
- Shepard, B.D.; Ecelbarger, C.M. Sodium Glucose Transporter, Type 2 (SGLT2) Inhibitors (SGLT2i) and Glucagon-Like Peptide 1-Receptor Agonists: Newer Therapies in Whole-Body Glucose Stabilization. Semin. Nephrol. 2021, 41, 331–348. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Daitoku, H.; Hatta, M.; Tanaka, K.; Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl. Acad. Sci. USA 2003, 100, 11285–11290. [Google Scholar] [CrossRef]
- Aljaylani, A.; Fluitt, M.; Piselli, A.; Shepard, B.D.; Tiwari, S.; Ecelbarger, C.M. Acid Loading Unmasks Glucose Homeostatic Instability in Proximal-Tubule-Targeted Insulin/Insulin-Like-Growth-Factor-1 Receptor Dual Knockout Mice. Cell. Physiol. Biochem. 2020, 54, 682–695. [Google Scholar] [CrossRef]
- Sharma, R.; Tiwari, S. Renal gluconeogenesis in insulin resistance: A culprit for hyperglycemia in diabetes. World J. Diabetes 2021, 12, 556–568. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, M.; Prakash, P.; Gupta, S.; Tiwari, S. Phosphoenolpyruvate carboxykinase in urine exosomes reflect impairment in renal gluconeogenesis in early insulin resistance and diabetes. Am. J. Physiol. Renal. Physiol. 2020, 318, F720–F731. [Google Scholar] [CrossRef]
- Chung, S.T.; Hsia, D.S.; Chacko, S.K.; Rodriguez, L.M.; Haymond, M.W. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia 2015, 58, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; McDonough, A.A.; Layton, A.T. Sex differences in solute and water handling in the human kidney: Modeling and functional implications. iScience 2021, 24, 102667. [Google Scholar] [CrossRef]
- Riazi, S.; Madala-Halagappa, V.K.; Hu, X.; Ecelbarger, C.A. Sex and body-type interactions in the regulation of renal sodium transporter levels, urinary excretion, and activity in lean and obese Zucker rats. Gend. Med. 2006, 3, 309–327. [Google Scholar] [CrossRef]
- Tiwari, S.; Li, L.; Riazi, S.; Halagappa, V.K.; Ecelbarger, C.M. Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am. J. Nephrol. 2009, 30, 554–562. [Google Scholar] [CrossRef]
- Chen, B.; Fluitt, M.B.; Brown, A.L.; Scott, S.; Gadicherla, A.; Ecelbarger, C.M. Selective Deletion of the Mechanistic Target of Rapamycin From the Renal Collecting Duct Principal Cell in Mice Down-Regulates the Epithelial Sodium Channel. Front. Physiol. 2021, 12, 787521. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, S.; Kim, G.H.; Mitchell, C.; Wade, J.B.; Knepper, M.A. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J. Clin. Investig. 1999, 104, R19–R23. [Google Scholar] [CrossRef]
- Veiras, L.C.; Girardi, A.C.C.; Curry, J.; Pei, L.; Ralph, D.L.; Tran, A.; Castelo-Branco, R.C.; Pastor-Soler, N.; Arranz, C.T.; Yu, A.S.L.; et al. Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J. Am. Soc. Nephrol. 2017, 28, 3504–3517. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Kaulbach, H.C.; Tian, R.; Hopkins, J.C.; Duffy, J.; Doetschman, T.; Minnemann, T.; Boers, M.E.; Hadro, E.; Oberste-Berghaus, C.; et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J. Clin. Investig. 1999, 104, 1703–1714. [Google Scholar] [CrossRef]
- Ecelbarger, C.A.; Kim, G.H.; Wade, J.B.; Knepper, M.A. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp. Neurol. 2001, 171, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Perriello, G.; Meyer, C.; Gerich, J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999, 55, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Doctor, R.B.; Chen, J.; Peters, L.L.; Lux, S.E.; Mandel, L.J. Distribution of epithelial ankyrin (Ank3) spliceoforms in renal proximal and distal tubules. Am. J. Physiol. 1998, 274, F129–F138. [Google Scholar] [CrossRef] [PubMed]

| Group | Body Weight (g) | Hct (% RBV) | Blood Na+ (mM) | Blood K+ (mM) | Blood Cl− (mM) | Blood HCO3− (mM) | BUN (mg/dL) |
|---|---|---|---|---|---|---|---|
| WTM | 33.5 ± 1.1 A | 29.5 ± 1.4 | 145 ± 3 | 3.5 ± 0.4 B | 117 ± 2 | 22.8 ± 0.7 | 19.2 ± 4 |
| KOM | 34.2 ± 1.2 A | 29.7 ± 1.4 | 141 ± 1 | 5.2 ± 0.5 A | 118 ± 2 | 24.8 ± 1.2 | 24.8 ± 0.3 |
| WTF | 24.4 ± 1.1 B | 29.1 ± 1.6 | 144 ± 2 | 4.6 ± 0.3 AB | 120 ± 2 | 21.6 ± 1.7 | 15.6 ± 1.4 |
| KOF | 24.4 ± 2.0 B | 29.5 ± 0.9 | 144 ± 2 | 4.7 ± 0.3 AB | 121 ± 1 | 22.5 ± 0.5 | 19.2 ± 1.3 |
| 2-way Analysis of Variance (Genotype × Sex) | |||||||
| G | 0.84 | 0.88 | 0.44 | 0.035 | 0.52 | 0.43 | 0.17 |
| Sex | <0.0001 | 0.88 | 0.76 | 0.53 | 0.28 | 0.35 | 0.17 |
| I | 0.80 | 0.95 | 0.41 | 0.051 | 0.99 | 0.78 | 0.76 |
| Furosemide | Thiazide | Benzamil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Na+ † | K+ † | Ratio (Na/K) | Na+ † | K+ † | Ratio (Na/K) | Na+ † | K+ † | Ratio (Na/K) |
| WTM | 115 ± 23 | 103 ± 31 | 1.37 ± 0.12 | 59 ± 19 | 98 ± 39 | 0.74 ± 0.08 | 68 ± 11 | 36 ± 14 | 2.8 ± 0.4 |
| KOM | 119 ± 18 | 72 ± 10 | 1.88 ± 0.29 | 85 ± 19 | 112 ± 37 | 0.86 ± 0.07 | 48 ± 9 | 15 ± 7 | 5.6 ± 1.3 |
| WTF | 105 ± 12 | 53 ± 8 | 2.16 ± 0.20 | 51 ± 8 | 50 ± 9 | 1.24 ± 0.16 | 69 ± 9 | 19 ± 2 | 3.7 ± 0.6 |
| KOF | 95 ± 15 | 52 ± 13 | 2.02 ± 0.26 | 69 ± 17 | 67 ± 21 | 1.17 ± 0.23 | 69 ± 12 | 22 ± 4 | 3.4 ± 0.6 |
| 2-way Analysis of Variance (Genotype × Sex) | |||||||||
| G | 0.69 | 0.28 | 0.11 | 0.11 | 0.30 | 0.84 | 0.57 | 0.40 | 0.12 |
| Sex | 0.26 | 0.034 | 0.0073 | 0.78 | 0.17 | 0.019 | 0.19 | 0.67 | 0.46 |
| I | 0.55 | 0.50 | 0.64 | 0.36 | 0.51 | 0.65 | 0.23 | 0.066 | 0.055 |
| Group | Body Weight (g) | MAP (mm Hg) | GTT (g·min/dL) | Fasting Glucose (mg/dL) | Furosemide Test Na+ † | Thiazide Test Na+ † | Benzamil Test Na+ † |
|---|---|---|---|---|---|---|---|
| WTM | 30.2 ± 1.6 A | 104 ± 8 | 24.5 ± 3 A | 108 ± 16 A | 55± 14 | 23 ± 7 B | 50 ± 15 |
| KOM | 28.7 ± 1.5 A | 104 ± 5 | 25.1 ± 1 A | 102 ± 8 A | 87± 15 | 17 ± 6 B | 46 ± 10 |
| WTF | 22.6 ± 1.6 B | 102 ± 13 | 18.4 ± 1 A | 83 ± 5 B | 71 ± 12 | 45 ± 8 A | 67 ± 13 |
| KOF | 24.7 ± 2.0 B | 104 ± 6 | 17.5 ± 2 A | 81 ± 8 B | 101 ± 12 | 68 ± 16 A | 71 ± 19 |
| 2-way Analysis of Variance (Genotype × Sex) | |||||||
| G | 0.76 | 0.11 | 0.93 | 0.51 | 0.030 | 0.40 | 0.82 |
| Sex | <0.0001 | 0.19 | 0.0013 | 0.0043 | 0.27 | 0.0011 | 0.097 |
| I | 0.37 | 0.23 | 0.70 | 0.67 | 0.93 | 0.17 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, S.; Akkawi, G.; Rechter, T.; Fluitt, M.B.; Ecelbarger, C.M. Sex Modulates Response to Renal-Tubule-Targeted Insulin Receptor Deletion in Mice. Int. J. Mol. Sci. 2023, 24, 8056. https://doi.org/10.3390/ijms24098056
Sohail S, Akkawi G, Rechter T, Fluitt MB, Ecelbarger CM. Sex Modulates Response to Renal-Tubule-Targeted Insulin Receptor Deletion in Mice. International Journal of Molecular Sciences. 2023; 24(9):8056. https://doi.org/10.3390/ijms24098056
Chicago/Turabian StyleSohail, Soha, Gabriella Akkawi, Taylor Rechter, Maurice B. Fluitt, and Carolyn M. Ecelbarger. 2023. "Sex Modulates Response to Renal-Tubule-Targeted Insulin Receptor Deletion in Mice" International Journal of Molecular Sciences 24, no. 9: 8056. https://doi.org/10.3390/ijms24098056
APA StyleSohail, S., Akkawi, G., Rechter, T., Fluitt, M. B., & Ecelbarger, C. M. (2023). Sex Modulates Response to Renal-Tubule-Targeted Insulin Receptor Deletion in Mice. International Journal of Molecular Sciences, 24(9), 8056. https://doi.org/10.3390/ijms24098056







