In Vivo Efficacy and Toxicity of an Antimicrobial Peptide in a Model of Endotoxin-Induced Pulmonary Inflammation
Abstract
1. Introduction
2. Results and Discussion
2.1. Toxicity Study by Inhalation Administration to CD-1 Mice for a Week
2.1.1. Atmosphere Analysis and Estimation of Achieved Dose
2.1.2. Clinical Observations
2.1.3. Necroscopy
2.1.4. Bioanalysis
2.2. Efficacy of SET-M33 on Endotoxin (LPS)-Induced Lung Inflammation
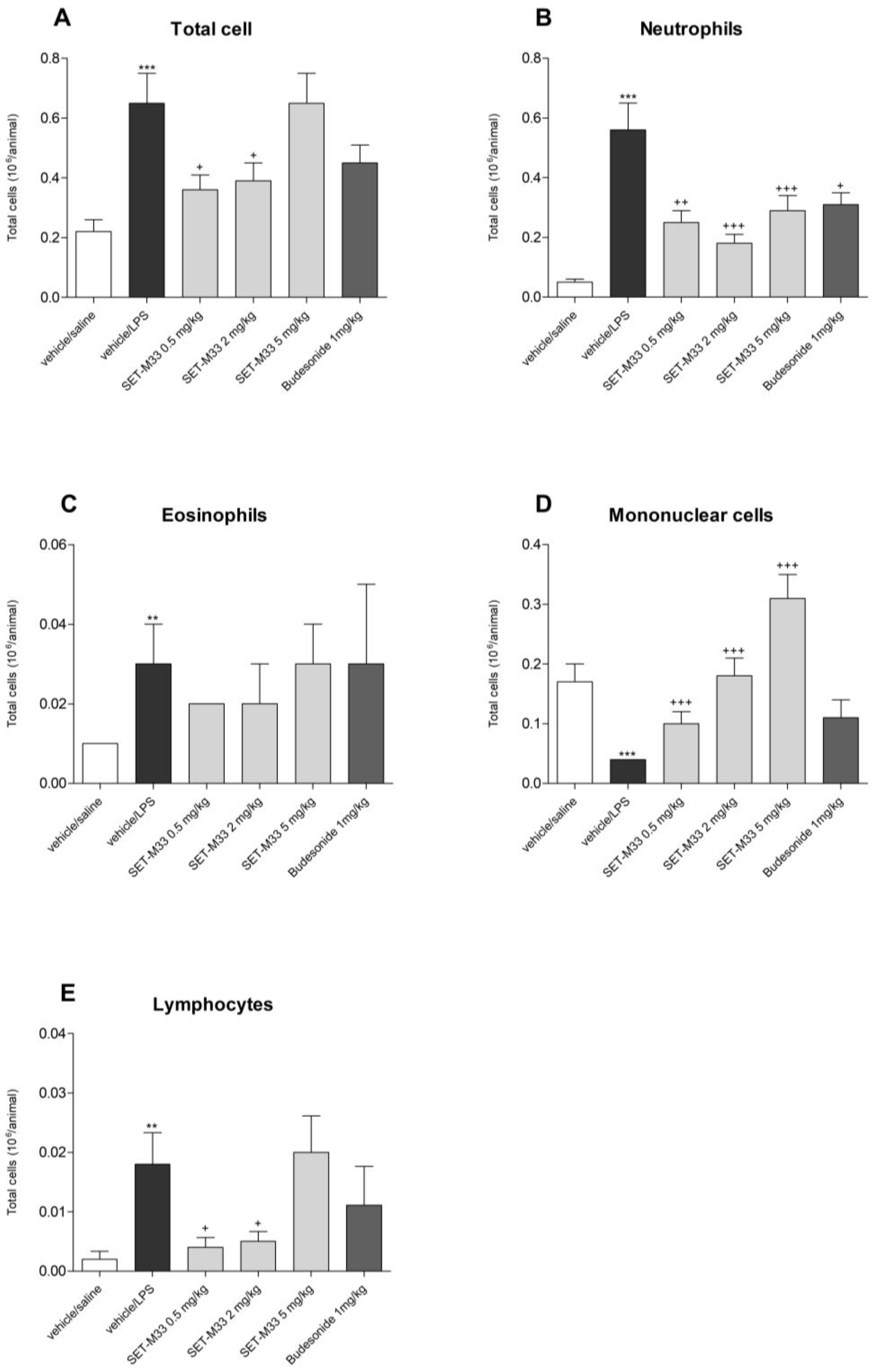
2.2.1. Total and Differential Cell Counts in BAL
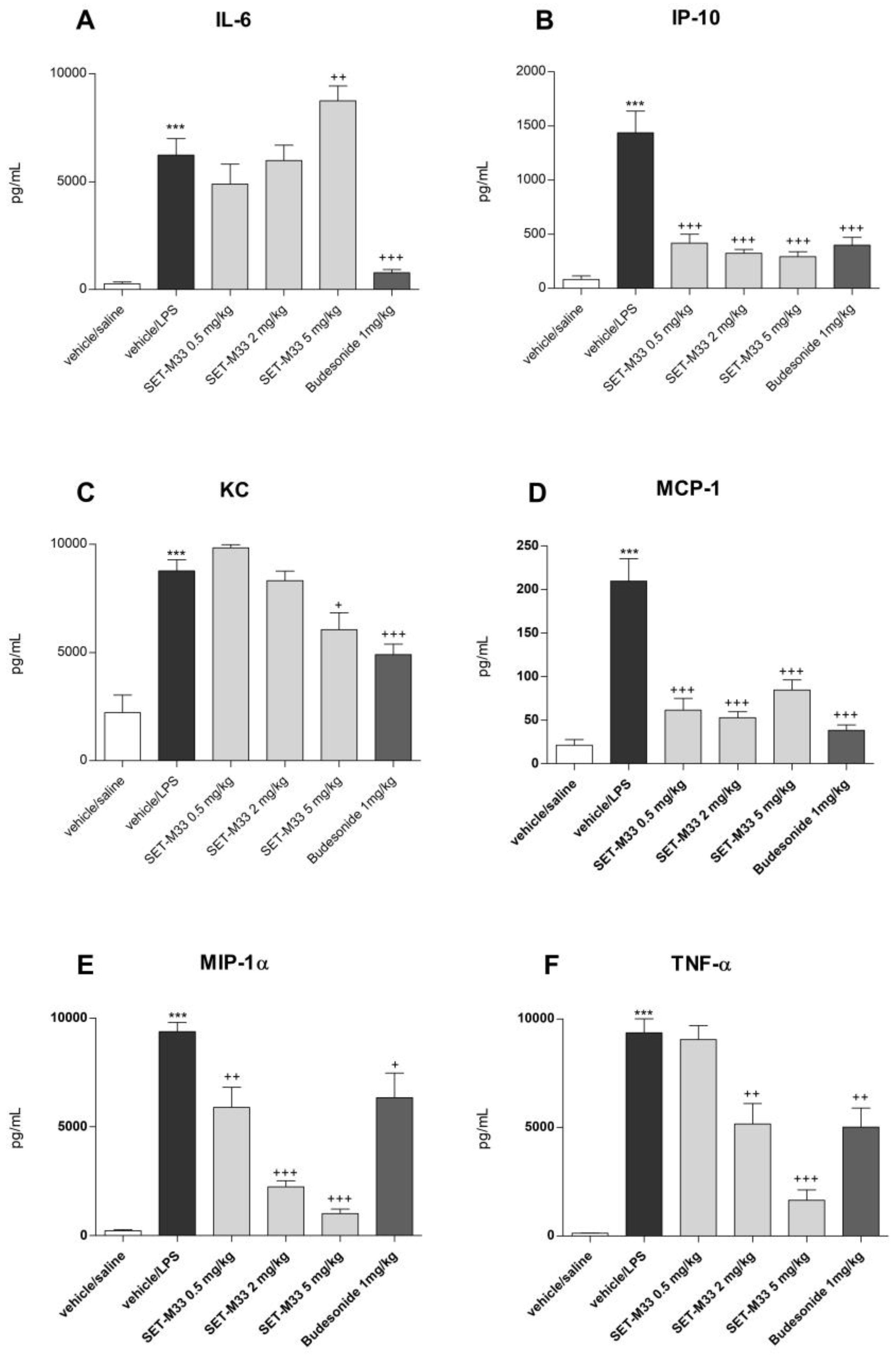
2.2.2. BAL Cytokine Levels
3. Materials and Methods
3.1. Peptide SET-M33
3.2. Animals and Experimental Procedures
3.3. Toxicity Study by Inhalation Administration to CD-1 Mice for 1 Week
3.3.1. Atmosphere Analysis and Estimation of Achieved Dose
- where C = aerosol concentration (μg/L)
- RMV = respiratory minute volume = 0.608 × BW (Kg)0.852
- D = duration of exposure (60 min)
- BW = body weight (kg).
3.3.2. Necroscopy
3.3.3. Bioanalysis
3.4. Efficacy of SET-M33 in a Murine Model of Endotoxin (LPS)-Induced Pulmonary Inflammation
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Serna, C.; Gonzalez-Zorn, B. Antimicrobial resistance and One Health. Rev. Esp. Quimioter. 2022, 35, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T. Antibiotics special issue: Challenges and opportunities in antibiotic discovery and development. ACS Infect. Dis. 2020, 6, 1286–1288. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Beyer, P.; Paulin, S. The antibacterial research and development pipeline needs urgent solutions. ACS Infect. Dis. 2020, 6, 1289–1291. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which Now Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 20 January 2022).
- World Health Organization. 2019 Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kim, W.; Prosen, K.R.; Lepore, C.J.; Coukell, A. On the road to discovering urgently needed antibiotics: So close yet so far away. ACS Infect. Dis. 2020, 6, 1292–1294. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, B.; Wang, Z. The revitalization of antimicrobial peptides in the resistance era. Pharmacol. Res. 2021, 163, 105276. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug. Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Bellotti, D.; Remelli, M. Lights and shadows on the therapeutic use of antimicrobial peptides. Molecules 2022, 27, 4584. [Google Scholar] [CrossRef]
- ErdemBüyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Bjorn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.; Middelkoop, E.; Boekema, B.K. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef]
- FDA Approved Drugs (FDA (2022) Drugs@FDA: FDA-Approved Drugs. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products (accessed on 30 November 2022).
- US National Library of Medicine. Clinical Trials. 2022. Available online: www.clinicaltrials.gov/ct2/results?recrs5d&age_v5&gndr5&type5Intr&rslt5&phase54&phase50&phase51&phase52&phase53&Search5Apply (accessed on 30 November 2022).
- Sharma, K.; Sharma, K.K.; Sharma, A.; Jain, R. Peptide-based drug discovery: Current status and recent advances. Drug. Discov. Today 2023, 28, 103464. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug. Discov. 2021, 4, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Bracci, L.; Falciani, C.; Lelli, B.; Lozzi, L.; Runci, Y.; Pini, A.; De Montis, M.G.; Tagliamonte, A.; Neri, P. Synthetic peptides in the form of dendrimers become resistant to protease activity. J. Biol. Chem. 2003, 278, 46590–46595. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Lozzi, L.; Pini, A.; Corti, F.; Fabbrini, M. Molecular basis of branched peptide resistance to enzyme proteolysis. Chem. Biol. Drug. Des. 2007, 69, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Falciani, C.; Bracci, L. Branched peptides as therapeutics. Curr. Protein Pept. Sci. 2008, 9, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Falciani, C.; Mantengoli, E.; Bindi, S.; Brunetti, J.; Iozzi, S.; Rossolini, G.M.; Bracci, L. A novel tetrabranched antimicrobial peptide that neutralizes bacterial lipopolysaccharide and prevents septic shock in vivo. FASEB J. 2010, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, J.; Falciani, C.; Roscia, G.; Pollini, S.; Bindi, S.; Scali, S.; Arrieta, U.C.; Gómez-Vallejo, V.; Quercini, L.; Ibba, E.; et al. In vitro and in vivo efficacy, toxicity, bio-distribution and resistance selection of a novel antibacterial drug candidate. Sci. Rep. 2016, 6, 26077. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, J.; Roscia, G.; Lampronti, I.; Gambari, R.; Quercini, L.; Falciani, C.; Bracci, L.; Pini, A. Immunomodulatory and Anti-inflammatory Activity in Vitro and in Vivo of a Novel Antimicrobial Candidate. J. Biol. Chem. 2016, 291, 25742–25748. [Google Scholar]
- Van der Weide, H.; Brunetti, J.; Pini, A.; Bracci, L.; Ambrosini, C.; Lupetti, P.; Paccagnini, E.; Gentile, M.; Bernini, A.; Niccolai, N.; et al. Investigations into the killing activity of an antimicrobial peptide active against extensively antibiotic-resistant K. pneumoniae and P. aeruginosa. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1796–1804. [Google Scholar] [CrossRef]
- van der Weide, H.; Vermeulen-de Jongh, D.M.C.; van der Meijden, A.; Boers, S.A.; Kreft, D.; Ten Kate, M.T.; Falciani, C.; Pini, A.; Strandh, M.; Bakker-Woudenberg, I.A.J.M.; et al. Antimicrobial activity of two novel antimicrobial peptides AA139 and SET-M33 against clinically and genotypically diverse Klebsiella pneumoniae isolates with differing antibiotic resistance profiles. Int. J. Antimicrob. Agents 2019, 54, 159–166. [Google Scholar] [CrossRef]
- Brunetti, J.; Carnicelli, V.; Ponzi, A.; Di Giulio, A.; Lizzi, A.R.; Cristiano, L.; Cresti, L.; Cappello, G.; Pollini, S.; Mosconi, L.; et al. Antibacterial and anti-inflammatory activity of an antimicrobial peptide aynthesized with D amino acids. Antibiotics 2020, 9, 840. [Google Scholar] [CrossRef]
- Quercini, L.; Brunetti, J.; Riolo, G.; Bindi, S.; Scali, S.; Lampronti, I.; D’Aversa, E.; Wronski, S.; Pollini, S.; Gentile, M.; et al. An antimicrobial molecule mitigates signs of sepsis in vivo and eradicates infections from lung tissue. FASEB J. 2020, 34, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Lozzi, L.; Scali, S.; Brunetti, J.; Bracci, L.; Pini, A. Site-specific pegylation of an antimicrobial peptide increases resistance to Pseudomonas aeruginosa elastase. Amino Acids 2014, 46, 1403–1407. [Google Scholar] [CrossRef]
- Falciani, C.; Zevolini, F.; Brunetti, J.; Riolo, G.; Gracia, R.; Marradi, M.; Loinaz, I.; Ziemann, C.; Cossío, U.; Llop, J.; et al. Antimicrobial peptide-loaded nanoparticles as inhalation therapy for Pseudomonas aeruginosa infections. Int. J. Nanomed. 2020, 15, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Cresti, L.; Conte, G.; Cappello, G.; Brunetti, J.; Falciani, C.; Bracci, L.; Quaglia, F.; Ungaro, F.; d’Angelo, I.; Pini, A. Inhalable Polymeric Nanoparticles for Pulmonary Delivery of Antimicrobial Peptide SET-M33: Antibacterial Activity and Toxicity In Vitro and In Vivo. Pharmaceutics 2023, 15, 3. [Google Scholar] [CrossRef]
- Cresti, L.; Falciani, C.; Cappello, G.; Brunetti, J.; Vailati, S.; Melloni, E.; Bracci, L.; Pini, A. Safety evaluations of a synthetic antimicrobial peptide administered intravenously in rats and dogs. Sci. Rep. 2022, 12, 19294. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Keating, G.M. Budesonide/formoterol: A review of its use in asthma. Drugs 2004, 64, 1597–1618. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Alexander, D.J.; Collins, C.J.; Coombs, D.W.; Gilkison, I.S.; Hardy, C.J.; Healey, G.; Karantabias, G.; Johnson, N.; Karlsson, A.; Kilgour, J.D.; et al. Association of Inhalation Toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhal. Toxicol. 2008, 20, 1179–1189. [Google Scholar] [CrossRef]
- Timmerman, P.; White, S.; Stuart McDougall, S.; Kall, M.A.; John Smeraglia, J.; Fjording, M.S.; Knutsson, M. Tiered approach into practice: Scientific validation for chromatography-based assays in early development—A recommendation from the European Bioanalysis Forum. Bioanalysis 2015, 7, 2387–2398. [Google Scholar] [CrossRef]
- Angervall, L.; Carlström, E. Theoretical criteria for the use of relative organ weights and similar ratios in biology. J. Theoret. Biol. 1963, 4, 254–259. [Google Scholar] [CrossRef]
- Niederman, M.S.; Torres, A. Severe community-acquired pneumonia. Eur. Respir. Rev. 2022, 31, 220123. [Google Scholar] [CrossRef]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Niederman, M.S. Aspiration pneumonia. N. Engl. J. Med. 2019, 380, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Sender, V.; Stamme, C. Lung cell-specific modulation of LPS-induced TLR4 receptor and adaptor localization. Commun. Integr. Biol. 2014, 7, e29053. [Google Scholar] [CrossRef] [PubMed]
- Palazzi, X.; Burkhardt, J.E.; Caplain, H.; Dellarco, V.; Fant, P.; Foster, J.R.; Francke, S.; Germann, P.; Gröters, S.; Harada, T.; et al. Characterizing “Adversity” of Pathology Findings in Nonclinical Toxicity Studies: Results from the 4th ESTP International Expert Workshop. Toxicol. Pathol. 2016, 44, 810–824. [Google Scholar] [CrossRef] [PubMed]
| Concentration (µg/L) | Particle Size | Dose (mg/kg/Day) | ||||
|---|---|---|---|---|---|---|
| Target | Achieved | MMAD (µm) | σg | Target | Estimated Achieved Inhaled | |
| Mean | SD | |||||
| 79 | 68.5 | 17.8 | 0.8 | 2.3 | 5 | 4.34 |
| 318 | 371 | 25.4 | 1.4 | 2.5 | 20 | 23.1 |
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| 0 mg/kg/Day | 4.34 mg/kg/Day | 23.1 mg/kg/Day | 0 mg/kg/Day | 4.34 mg/kg/Day | 23.1 mg/kg/Day | ||
| SET-M33-related findings in the lungs | |||||||
| Inflammation, interstitial | Minimal | 0 | 0 | 1 | 1 | 2 | 4 |
| Slight | 0 | 0 | 4 | 0 | 0 | 2 | |
| Total | 0 | 0 | 5 | 1 | 2 | 6 | |
| Inflammation, granulomatous | Minimal | 0 | 0 | 2 | 0 | 0 | 3 |
| Slight | 0 | 0 | 3 | 0 | 0 | 0 | |
| Total | 0 | 0 | 5 | 0 | 0 | 3 | |
| Infiltrate, inflammatory cell, perivascular | Minimal | 0 | 0 | 2 | 0 | 1 | 1 |
| Slight | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 0 | 0 | 2 | 0 | 1 | 2 | |
| Inflammation, alveolar ducts | Minimal | 0 | 0 | 2 | 1 | 0 | 2 |
| Total | 0 | 0 | 2 | 1 | 0 | 2 | |
| Fibrosis, alveolar ducts | Minimal | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 0 | 0 | 0 | 0 | 0 | 1 | |
| SET-M33-related findings in the nose/turbinates | |||||||
| Atrophy/degeneration, olfactory epithelium | Minimal | 0 | 2 | 1 | 1 | 4 | 0 |
| Slight | 0 | 4 | 5 | 0 | 2 | 3 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 3 | |
| Total | 0 | 6 | 6 | 1 | 6 | 6 | |
| Inflammatory exudate | Minimal | 0 | 4 | 0 | 0 | 0 | 2 |
| Slight | 0 | 0 | 2 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 3 | 0 | 0 | 3 | |
| Marked | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 0 | 4 | 5 | 0 | 0 | 6 | |
| Eosinophilic globules, olfactory epithelium | Minimal | 0 | 6 | 3 | 0 | 6 | 3 |
| Total | 0 | 6 | 3 | 0 | 6 | 3 | |
| Eosinophilic globules, respiratory epithelium | Minimal | 0 | 3 | 3 | 0 | 6 | 2 |
| Total | 0 | 3 | 3 | 0 | 6 | 2 | |
| Infiltrate, inflammatory cell, lamina propria | Minimal | 0 | 1 | 2 | 0 | 3 | 2 |
| Slight | 0 | 1 | 0 | 0 | 2 | 2 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 0 | 2 | 2 | 0 | 5 | 5 | |
| Haemorrhage | Moderate | 0 | 0 | 1 | 0 | 0 | 0 |
| Total | 0 | 0 | 1 | 0 | 0 | 0 | |
| SET-M33-related findings in the larynx | |||||||
| Squamous metaplasia | Minimal | 0 | 4 | 6 | 0 | 6 | 6 |
| Total | 0 | 4 | 6 | 0 | 6 | 6 | |
| Infiltrate, inflammatory cell | Minimal | 0 | 3 | 4 | 0 | 0 | 5 |
| Total | 0 | 3 | 4 | 0 | 0 | 5 | |
| SET-M33-related findings at the tracheal bifurcation | |||||||
| Loss of cilia, point of bifurcation | Minimal | 0 | 0 | 2 | 1 | 0 | 3 |
| Total | 0 | 0 | 2 | 1 | 0 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cresti, L.; Cappello, G.; Vailati, S.; Melloni, E.; Brunetti, J.; Falciani, C.; Bracci, L.; Pini, A. In Vivo Efficacy and Toxicity of an Antimicrobial Peptide in a Model of Endotoxin-Induced Pulmonary Inflammation. Int. J. Mol. Sci. 2023, 24, 7967. https://doi.org/10.3390/ijms24097967
Cresti L, Cappello G, Vailati S, Melloni E, Brunetti J, Falciani C, Bracci L, Pini A. In Vivo Efficacy and Toxicity of an Antimicrobial Peptide in a Model of Endotoxin-Induced Pulmonary Inflammation. International Journal of Molecular Sciences. 2023; 24(9):7967. https://doi.org/10.3390/ijms24097967
Chicago/Turabian StyleCresti, Laura, Giovanni Cappello, Silvia Vailati, Elsa Melloni, Jlenia Brunetti, Chiara Falciani, Luisa Bracci, and Alessandro Pini. 2023. "In Vivo Efficacy and Toxicity of an Antimicrobial Peptide in a Model of Endotoxin-Induced Pulmonary Inflammation" International Journal of Molecular Sciences 24, no. 9: 7967. https://doi.org/10.3390/ijms24097967
APA StyleCresti, L., Cappello, G., Vailati, S., Melloni, E., Brunetti, J., Falciani, C., Bracci, L., & Pini, A. (2023). In Vivo Efficacy and Toxicity of an Antimicrobial Peptide in a Model of Endotoxin-Induced Pulmonary Inflammation. International Journal of Molecular Sciences, 24(9), 7967. https://doi.org/10.3390/ijms24097967








