Synthesis of 1-(2-Hydroxyphenyl)- and (3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as Promising Scaffolds for the Development of Novel Antimicrobial and Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
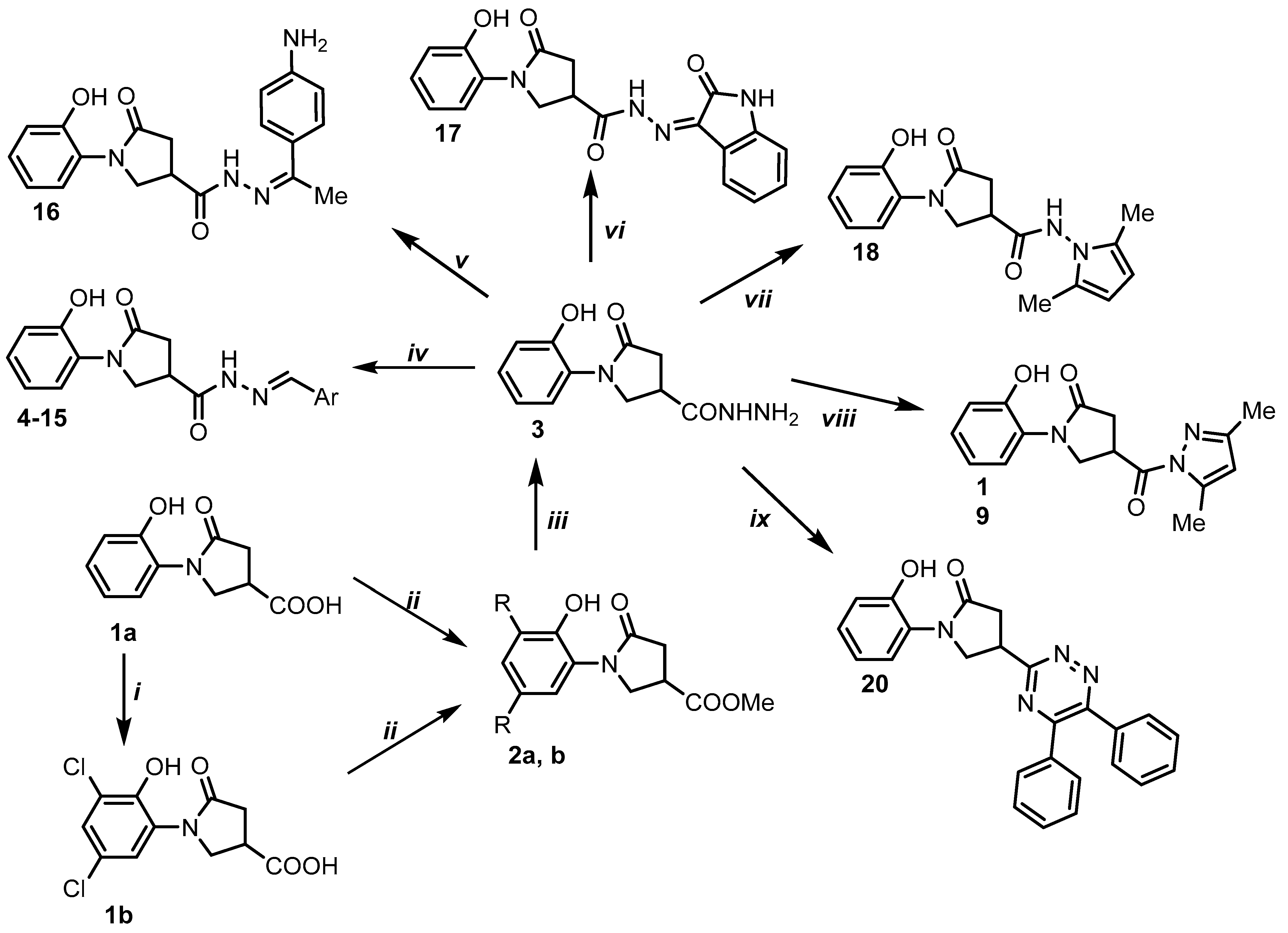
2.1. Chemistry
2.2. Novel 1-(2-Hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives Show Gram-Positive Bacteria-Directed Antimicrobial Activity
2.3. Compounds 14 and 24b Demonstrate Antibacterial Activity against Vancomycin-Intermediate Staphylococcus aureus Strains
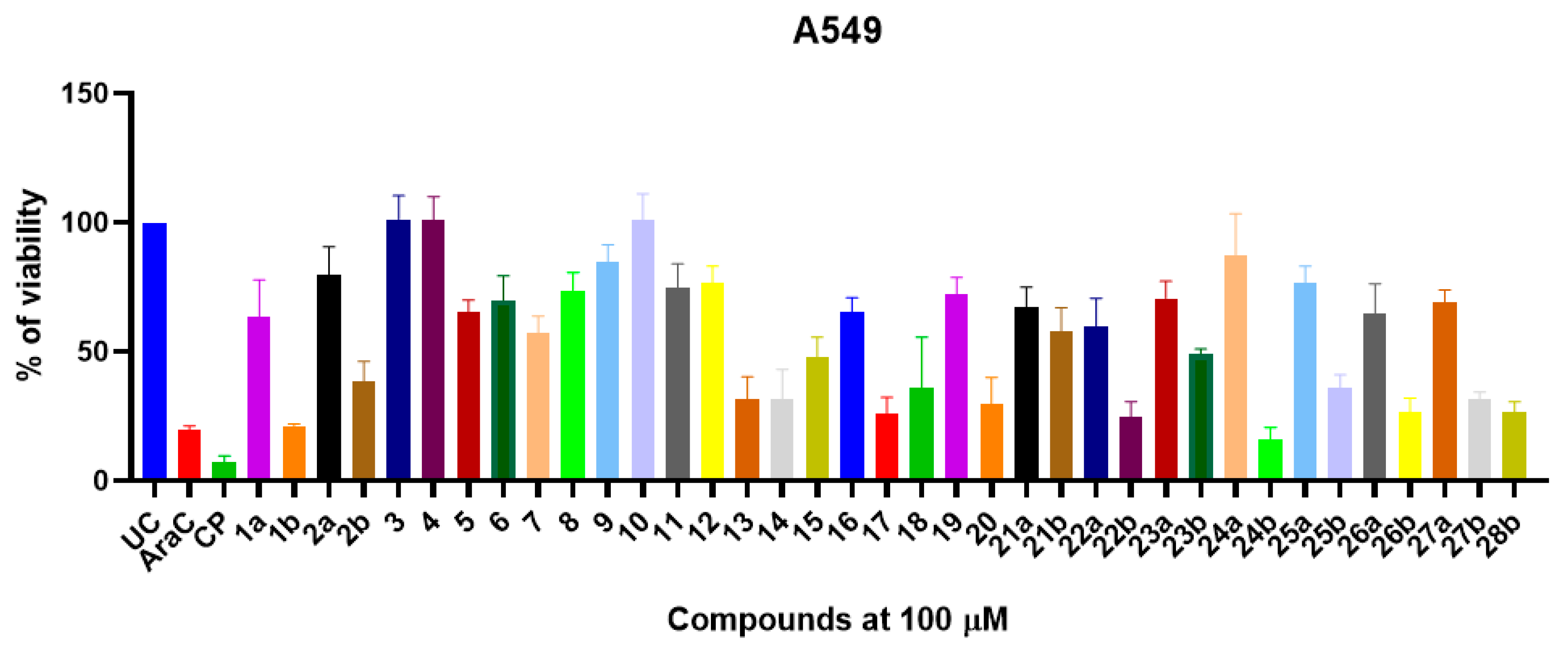
2.4. Novel 1-(2-Hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives Demonstrate Structure-Dependent Anticancer Activity
3. Materials and Methods
3.1. Synthesis
- General procedure of the preparation of esters 2a, b
- Methyl 1-(2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylate (2a). Light grey solid, yield 78.8%, m.p. 128–129 °C (from MeOH).
- Methyl 1-(3,5-dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylate (2b).
- 1-(2-Hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (3).
- General procedure for the preparation of hydrazones 4–15
- N′-benzylidene-1-(2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (4).
- N′-(4-chlorobenzylidene)-1-(2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (5).
- N′-(4-bromobenzylidene)-1-(2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (6).
- 1-(2-Hydroxyphenyl)-N′-(4-nitrobenzylidene)-5-oxopyrrolidine-3-carbohydrazide (7).
- N′-(4-(dimethylamino)benzylidene)-1-(2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (8).
- 1-(2-Hydroxyphenyl)-N′-(4-methoxybenzylidene)-5-oxopyrrolidine-3-carbohydrazide (9).
- 1-(2-Hydroxyphenyl)-N′-(2,4-dimethoxybenzylidene)-5-oxopyrrolidine-3-carbohydrazide (10).
- 1-(2-Hydroxyphenyl)-N′-(2,3,4-trimethoxybenzylidene)-5-oxopyrrolidine-3-carbohydrazide (11).
- 1-(2-Hydroxyphenyl)-N′-(3,4,5-trimethoxybenzylidene)-5-oxopyrrolidine-3-carbohydrazide (12).
- 1-(2-Hydroxyphenyl)-N′-(naphth-1-ylmethylene)-5-oxopyrrolidine-3-carbohydrazide (13).
- 1-(2-Hydroxyphenyl)-5-oxo-N′-(thien-2-ylmethylene)pyrrolidine-3-carbohydrazide (14).
- 1-(2-Hydroxyphenyl)-5-oxo-N′-(5-nitrothien-2-ylmethylene)pyrrolidine-3-carbohydrazide (15).
- N′-(1-(4-aminophenyl)ethylidene)-1-(2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (16)
- 1-(2-Hydroxyphenyl)-5-oxo-N′-(2-oxoindolin-3-ylidene)pyrrolidine-3-carbohydrazide (17).
- 1-(2-Hydroxyphenyl)-N-(2,5-dimethyl-1H-pyrrol-1-yl)-5-oxopyrrolidine-3-carboxamide (18).
- 1-(2-Hydroxyphenyl)-4-(3,5-dimethyl-1H-pyrazol-1-carbonyl)pyrrolidin-2-one (19).
- 1-(2-Hydroxyphenyl)-4-(5,6-diphenyl-1,2,4-triazin-3-yl)pyrrolidine-2-one (20)
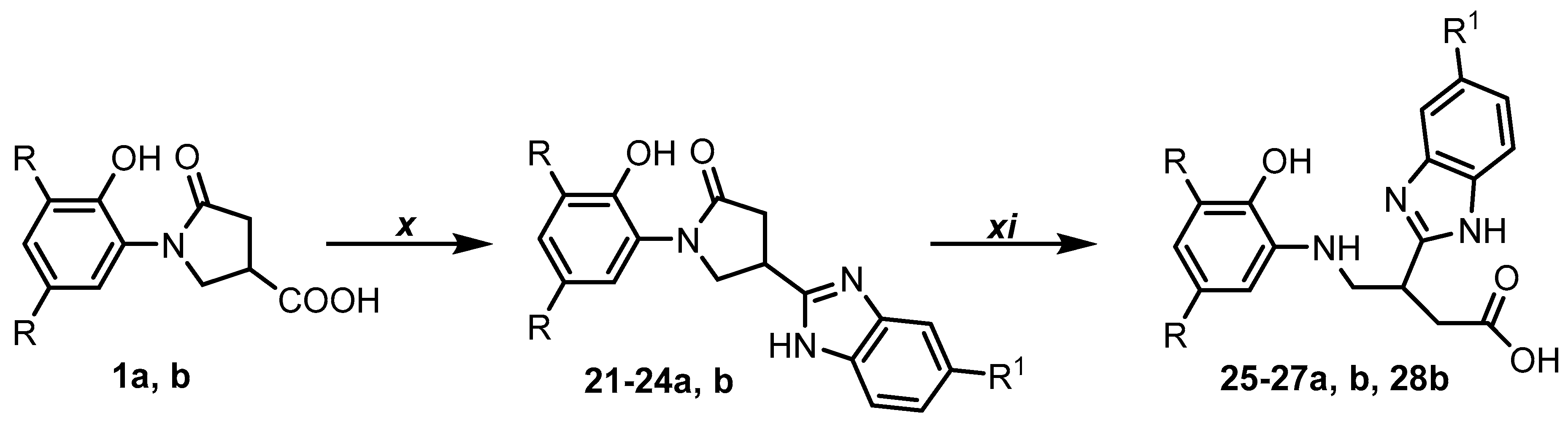
- General procedure for the preparation of benzimidazoles 21–24a, b.
- 4-(1H-benzo[d]imidazol-2-yl)-1-(2-hydroxyphenyl)pyrrolidine-2-one (21a).
- 4-(1H-benzo[d]imidazol-2-yl)-1-(3,5-dichloro-2-hydroxyphenyl)pyrrolidin-2-one (21b).
- 4-(5-Methyl-1H-benzo[d]imidazol-2-yl)-1-(2-hydroxyphenyl)pyrrolidine-2-one (22a).
- 1-(3,5-Dichloro-2-hydroxyphenyl)-4-(5-methyl-1H-benzo[d]imidazol-2-yl)pyrrolidin-2-one (22b).
- 4-(5-Chloro-1H-benzo[d]imidazol-2-yl)-1-(2-hydroxyphenyl)pyrrolidine-2-one (23a).
- 4-(5-Chloro-1H-benzo[d]imidazol-2-yl)-1-(3,5-dichloro-2-hydroxyphenyl)pyrrolidin-2-one (23b).
- 4-(5-Fluoro-1H-benzo[d]imidazol-2-yl)-1-(2-hydroxyphenyl)pyrrolidine-2-one (24a).
- 1-(3,5-Dichloro-2-hydroxyphenyl)-4-(5-fluoro-1H-benzo[d]imidazol-2-yl)pyrrolidin-2-one (24b).
- General method for the preparation of butanoic acids 25–27a, b and 28b.
- 3-(1H-benzo[d]imidazol-2-yl)-4-((2-hydroxyphenyl)amino)butanoic acid (25a).
- 3-(1H-benzo[d]imidazol-2-yl)-4-((3,5-dichloro-2-hydroxyphenyl)amino)butanoic acid (25b).
- 4-((2-Hydroxyphenyl)amino)-3-(5-methyl-1H-benzo[d]imidazol-2-yl)butanoic acid (26a).
- 4-((3,5-Dichloro-2-hydroxyphenyl)amino)-3-(5-methyl-1H-benzo[d]imidazol-2-yl)butanoic acid (26b).
- 3-(5-Chloro-1H-benzo[d]imidazol-2-yl)-4-((2-hydroxyphenyl)amino)butanoic acid (27a).
- 3-(5-Chloro-1H-benzo[d]imidazol-2-yl)-4-((3,5-dichloro-2-hydroxyphenyl)amino)butanoic acid (27b).
- 4-((3,5-Dichloro-2-hydroxyphenyl)amino)-3-(5-fluoro-1H-benzo[d]imidazol-2-yl)butanoic acid (28b).
3.2. Bacterial Strains and Culture Conditions
3.3. Minimal Inhibitory Concentration Determination
3.4. Cell Lines and Culture Conditions
3.5. In Vitro Cytotoxic Activity Determination
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vazquez-Guillamet, C.; Kollef, M.H. Treatment of gram—Positive infections in critically ill patients. BMC Infect. Dis. 2014, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Derlot, E.; Duval, J.; Courvalin, P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 1988, 319, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Ealy, V.L.; Lessard, I.A.; Roper, D.I.; Knox, J.R.; Walsh, C.T. Vancomycin resistance in enterococci: Reprogramming of the D-ala-D-ala ligases in bacterial peptidoglycan biosynthesis. Chem. Biol. 2000, 7, R109–R119. [Google Scholar]
- Périchon, B.; Courvalin, P. Glycopeptide Resistance Antibiotic Discovery and Development; Springer: Boston, MA, USA, 2011; Volume 48, pp. 515–542. [Google Scholar]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2013, 4, 165rv13. [Google Scholar] [CrossRef]
- Jeanvoine, A.; Rocchi, S.; Bellanger, A.P.; Reboux, G.; Millon, L. Azole-resistant Aspergillus fumigatus: A global phenomenon originating in the environment? Med. Mal. Infect. 2020, 50, 389–395. [Google Scholar] [CrossRef]
- Mohammadi, F.; Hashemi, S.J.; Zoll, J.; Melchers, W.J.; Rafati, H.; Dehghan, P.; Rezaie, S.; Tolooe, A.; Tamadon, Y.; van der Lee, H.A.; et al. Quantitative Analysis of Single-Nucleotide Polymorphism for Rapid Detection of TR34/L98H- and TR46/Y121F/T289A-Positive Aspergillus fumigatus Isolates Obtained from Patients in Iran from 2010 to 2014. Antimicrob. Agents Chemother. 2015, 60, 387–392. [Google Scholar] [CrossRef]
- Benhamou, R.I.; Bibi, M.; Steinbuch, K.B.; Engel, H.; Levin, M.; Roichman, Y.; Berman, J.; Fridman, M. Real-Time Imaging of the Azole-Class of Antifungal Drugs in Live Candida Cells ACS Chemical Biology. ACS Chem. Biol. 2017, 12, 1769–1777. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharm. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Wang, Y. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Z.; Gan, L.-L.; Wang, H.; Zhou, C.-H. New Progress in Azole Compounds as Antimicrobial Agents. Mini Rev. Med. Chem. 2017, 17, 122–166. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Akhtar, T.; Khalid, M.; Ali, S.; Zahra, S.; Haq, I.U.; Alhujaily, M.; de B Dias, M.C.H.; Leite, A.C.L.; Muhammad, S. Synthesis, antioxidant, antimicrobial and antiviral docking studies of ethyl 2-(2-(arylidene)hydrazinyl)thiazole-4-carboxylates. Z. Naturforsch. C J. Biosci. 2021, 76, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Tiperciuc, B.; Pârvu, A.; Tamaian, R.; Nastasă, C.; Ionuţ, I.; Oniga, O. New anti-inflammatory thiazolyl-carbonyl-thiosemicarbazides and thiazolyl-azoles with antioxidant properties as potential iNOS inhibitors. Arch. Pharm. Res. 2013, 36, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Aisha; Raza, M.A.; Farwa, U.; Rashid, U.; Maurin, J.K.; Budzianowski, A. Synthesis, Single Crystal, In-Silico and In-Vitro Assessment of the Thiazolidinones. J. Mol. Struct. 2022, 1255, 132384. [Google Scholar] [CrossRef]
- Eze, C.C.; Ezeokonkwo, A.M.; Ugwu, I.D.; Eze, U.F.; Onyeyilim, E.L.; Attah, I.S.; Okonkwo, I.V. Azole-Pyrimidine Hybrid Anticancer Agents: A Review of Molecular Structure, Structure Activity Relationship, and Molecular Docking. Anticancer Agents Med. Chem. 2022, 22, 2822–2851. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Khan, M.K.A.; Baig, M.H.; Imran, M.; Gupta, G.K. Role of azoles in cancer prevention and treatment: Present and future perspectives. Anti-Cancer Agents Med. Chem. 2018, 18, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.; Sabuncuoğlu, S.; Koçak Aslan, E.; Avci, A.; Kart, D.; Özdemir, Z.; Acar, M.F.; Sayoğlu, B.; Alagöz, M.A.; Karakurt, A.; et al. Azoles containing naphthalene with activity against Gram-positive bacteria: In vitro studies and in silico predictions for flavohemoglobin inhibition. J. Biomol. Struct. Dyn. 2022, 40, 10220–10229. [Google Scholar] [CrossRef]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial Activities of Azole Complexes Combined with Silver Nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef] [PubMed]
- Vasava, M.S.; Bhoi, M.N.; Rathwa, S.K.; Jethava, D.J.; Acharya, P.T.; Patel, D.B.; Patel, H.D. Benzimidazole: A Milestone in the Field of Medicinal Chemistry, Mini Rev. Med. Chem. 2020, 20, 532–565. [Google Scholar] [CrossRef]
- Khalifa, M.; Gobouri, A.; Kabli, F.; Altalhi, T.; Almalki, A.; Mohamed, M. Synthesis, antibacterial, and anti HepG2 cell line human hepatocyte carcinoma activity of some new potentially benzimidazole-5-(aryldiazenyl)thiazole derivatives. Molecules 2018, 23, 3285. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.E.; Zaffagni, M.; Chung, I.; Xu, Y.; Wang, Y.; Jernigan, F.E.; Zetter, B.R.; Sun, L. Synthesis and anticancer activity of novel water soluble benzimidazole carbamates. Eur. J. Med. Chem. 2018, 144, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Vertuani, S.; Manfredini, S.; Onnis, V. In-Vitro Evaluation of Antioxidant, Antiproliferative and Photo-Protective Activities of Benzimidazolehydrazone Derivatives. Pharmaceuticals 2020, 13, 68. [Google Scholar] [CrossRef]
- Law, C.S.W.; Yeong, K.Y. Benzimidazoles in Drug Discovery: A Patent Review. ChemMedChem 2021, 16, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Sapijanskaitė-Banevič, B.; Palskys, V.; Vaickelionienė, R.; Šiugždaitė, J.; Kavaliauskas, P.; Grybaitė, B.; Mickevičius, V. Synthesis and Antibacterial Activity of New Azole, Diazole and Triazole Derivatives Based on p-Aminobenzoic Acid. Molecules 2021, 26, 2597. [Google Scholar] [CrossRef] [PubMed]
- Strelčiūnaitė, V.; Jonuškienė, I.; Anusevičius, K.; Tumosienė, I.; Šiugždaitė, J.; Ramanauskaitė, I.; Mickevičius, V. N-Synthesis of Novel Benzimidazoles 2-Functionalized with Pyrrolidinone and γ-Amino Acid with a High Antibacterial Activity. Heterocycles 2016, 92, 235–251. [Google Scholar] [CrossRef]
- Malūkaitė, D.; Grybaitė, B.; Vaickelionienė, R.; Vaickelionis, G.; Sapijanskaitė-Banevič, B.; Kavaliauskas, P.; Mickevičius, V. Synthesis of Novel Thiazole Derivatives Bearing β-Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens. Molecules 2022, 27, 74. [Google Scholar] [CrossRef]
- Sapijanskaitė-Banevič, B.; Šovkovaja, B.; Vaickelionienė, R.; Šiugždaitė, J.; Mickevičiūtė, E. Synthesis, Characterization and Bioassay of Novel Substituted 1-(3-(1,3-Thiazol-2-yl)phenyl)-5-oxopyrrolidines. Molecules 2020, 25, 2433. [Google Scholar] [CrossRef]
- Voskienė, A.; Sapijanskaitė, B.; Mickevičius, V.; Jonuškienė, I.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Musyanovych, R.; Novikov, V. Synthesis and Microbiological Evaluation of New 2- and 2,3-Diphenoxysubstituted Naphthalene-1,4-diones with 5-Oxopyrrolidine Moieties. Molecules 2012, 17, 14434. [Google Scholar] [CrossRef]
- Kairytė, K.; Grybaitė, B.; Vaickelionienė, R.; Sapijanskaitė-Banevič, B.; Kavaliauskas, P.; Mickevičius, V. Synthesis and Biological Activity Characterization of Novel 5-Oxopyrrolidine Derivatives with Promising Anticancer and Antimicrobial Activity. Pharmaceuticals 2022, 15, 970. [Google Scholar] [CrossRef] [PubMed]
- Tumosienė, I.; Kantminienė, K.; Jonuškienė, I.; Peleckis, A.; Belyakov, S.; Mickevičius, V. Synthesis of 1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives and Their Antioxidant Activity. Molecules 2019, 24, 971. [Google Scholar] [CrossRef] [PubMed]
- Brokaitė, K.; Mickevičius, V.; Mikulskienė, G. Synthesis and structural investigation of some 1,4-disubstituted-2-pyrrolidinones. Arkivoc 2006, 2, 61–67. [Google Scholar] [CrossRef]
- Nath, R.; Pathania, S.; Grover, G.; Akhtar, M.J. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020, 1222, 128900. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Amawi, H.; Ashby, C.R., Jr.; Khare, V.M.; Jones, V.; Moorthy, N.S.H.N.; Trivedi, P.; Tiwari, A.K. Novel 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives produce anticancer efficacy in ovarian cancer in vitro. Heliyon 2019, 5, e01603. [Google Scholar] [CrossRef]
- Mickevicius, M.; Beresnevicius, Z.J.; Mickevicius, V.; Mikulskiene, G. Condensation products of 1-aryl-4-carboxy-2-pyrrolidinones with o-diaminoarenes, o-aminophenol, and their structural studies. Heteroat. Chem. 2006, 17, 47–56. [Google Scholar] [CrossRef]
- Marturano, J.E.; Lowery, T.J. ESKAPE Pathogens in Bloodstream Infections Are Associated with Higher Cost and Mortality but Can Be Predicted Using Diagnoses Upon Admission. Open Forum Infect. Dis. 2019, 6, ofz503. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Kavaliauskas, P.; Naing, E.; Garcia, A.; Sutherland, C.; Kau, A.Y.; Goldner, N.; Bulow, C.; Nicolau, D.P.; et al. Pharmacokinetics, Tissue Distribution, and Efficacy of VIO-001 (Meropenem/Piperacillin/Tazobactam) for Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia in Immunocompetent Rabbits with Chronic Indwelling Vascular Catheters. Antimicrob. Agents Chemother. 2021, 65, e0116821. [Google Scholar] [CrossRef]
- Kavaliauskas, P.; Grybaite, B.; Mickevicius, V.; Petraitiene, R.; Grigaleviciute, R.; Planciuniene, R.; Gialanella, P.; Pockevicius, A.; Petraitis, V. Synthesis, ADMET Properties, and In Vitro Antimicrobial and Antibiofilm Activity of 5-Nitro-2-thiophenecarbaldehyde N-((E)-(5-Nitrothienyl)methylidene)hydrazone (KTU-286) against Staphylococcus aureus with Defined Resistance Mechanisms. Antibiotics 2020, 9, 612. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; CLSI document M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Hastey, C.J.; Dale, S.E.; Nary, J.; Citron, D.; Law, J.H.; Roe-Carpenter, D.E.; Chesnel, L. Comparison of Clostridium difficile minimum inhibitory concentrations obtained using agar dilution vs broth microdilution methods. Anaerobe 2017, 44, 73–77. [Google Scholar] [CrossRef]
| Compound | Minimal Inhibitory Concentration (MIC, µg/mL) | ||||
|---|---|---|---|---|---|
| S. aureus TCH 1516 | A. baumannii AR-0033 | K. pneumoniae AR-0049 | P. aeruginosa AR-0064 | C. difficile AR-1067 | |
| 1a | >128 | >128 | >128 | >128 | >128 |
| 1b | >128 | >128 | >128 | >128 | >128 |
| 2a | >128 | >128 | >128 | >128 | >128 |
| 2b | 64 | 128 | >128 | >128 | >128 |
| 3 | >128 | >128 | >128 | >128 | >128 |
| 4 | >128 | >128 | >128 | >128 | >128 |
| 5 | >128 | >128 | >128 | >128 | >128 |
| 6 | >128 | >128 | >128 | >128 | >128 |
| 7 | 64 | >128 | >128 | >128 | 16 |
| 8 | 64 | >128 | >128 | >128 | >128 |
| 9 | 128 | >128 | >128 | >128 | >128 |
| 10 | >128 | >128 | >128 | >128 | >128 |
| 11 | >128 | >128 | >128 | >128 | >128 |
| 12 | >128 | >128 | >128 | >128 | >128 |
| 13 | >128 | >128 | >128 | >128 | >128 |
| 14 | 16 | >128 | >128 | >128 | 32 |
| 15 | >128 | >128 | >128 | >128 | >128 |
| 16 | >128 | >128 | >128 | >128 | >128 |
| 17 | >128 | >128 | >128 | >128 | >128 |
| 18 | >128 | >128 | >128 | >128 | >128 |
| 19 | >128 | >128 | >128 | >128 | >128 |
| 20 | 32 | >128 | >128 | 128 | 128 |
| 21a | >128 | >128 | >128 | >128 | >128 |
| 21b | 32 | >128 | >128 | >128 | 64 |
| 22a | >128 | >128 | >128 | >128 | >128 |
| 22b | 64 | >128 | >128 | >128 | >128 |
| 23a | 128 | >128 | >128 | >128 | >128 |
| 23b | 64 | >128 | >128 | >128 | >128 |
| 24a | >128 | >128 | >128 | >128 | >128 |
| 24b | 8 | >128 | 128 | 64 | 128 |
| 25a | >128 | >128 | >128 | >128 | >128 |
| 25b | 128 | >128 | >128 | >128 | 128 |
| 26a | >128 | >128 | >128 | >128 | >128 |
| 26b | >128 | >128 | >128 | >128 | >128 |
| 27a | >128 | >128 | >128 | >128 | >128 |
| 27b | >128 | >128 | >128 | >128 | >128 |
| 28b | >128 | >128 | >128 | >128 | >128 |
| Clindamycin | 32 | N/A | N/A | N/A | 32 |
| Metronidazole | N/A | N/A | N/A | N/A | 2 |
| Vancomycin | 2 | N/A | N/A | N/A | 2 |
| Meropenem | N/A | 32 | 16 | 16 | N/A |
| Compounds | Minimal Inhibitory Concentration (MIC, µg/mL) | |||||
|---|---|---|---|---|---|---|
| C. auris AR-381 | C. auris AR-382 | C. auris AR-383 | A. fumigatus AR-731 | A. fumigatus AR-732 | A. fumigatus AR-733 | |
| 1a | >128 | >128 | >128 | >128 | >128 | >128 |
| 1b | >128 | >128 | >128 | >128 | >128 | >128 |
| 2a | >128 | >128 | >128 | >128 | >128 | >128 |
| 2b | >128 | >128 | >128 | >128 | >128 | >128 |
| 3 | >128 | >128 | >128 | >128 | >128 | >128 |
| 4 | >128 | >128 | >128 | >128 | >128 | >128 |
| 5 | >128 | >128 | >128 | >128 | >128 | >128 |
| 6 | >128 | >128 | >128 | >128 | >128 | >128 |
| 7 | >128 | >128 | >128 | >128 | >128 | >128 |
| 8 | >128 | >128 | >128 | >128 | >128 | >128 |
| 9 | >128 | >128 | >128 | >128 | >128 | >128 |
| 10 | >128 | >128 | >128 | >128 | >128 | >128 |
| 11 | >128 | >128 | >128 | >128 | >128 | >128 |
| 12 | >128 | >128 | >128 | >128 | >128 | >128 |
| 13 | >128 | >128 | >128 | >128 | >128 | >128 |
| 14 | >128 | >128 | >128 | >128 | >128 | >128 |
| 15 | 16 | 16 | 16 | 32 | 64 | 32 |
| 16 | >128 | >128 | >128 | >128 | >128 | >128 |
| 17 | >128 | >128 | >128 | >128 | >128 | >128 |
| 18 | >128 | >128 | >128 | >128 | >128 | >128 |
| 19 | >128 | >128 | >128 | >128 | >128 | >128 |
| 20 | >128 | >128 | >128 | >128 | >128 | >128 |
| 21a | >128 | >128 | >128 | >128 | >128 | >128 |
| 21b | >128 | >128 | >128 | >128 | >128 | >128 |
| 22a | >128 | 128 | 128 | >128 | >128 | >128 |
| 22b | >128 | >128 | >128 | >128 | >128 | >128 |
| 23a | >128 | >128 | >128 | >128 | >128 | >128 |
| 23b | >128 | >128 | >128 | >128 | >128 | >128 |
| 24a | >128 | >128 | >128 | >128 | >128 | >128 |
| 24b | >128 | >128 | >128 | >128 | >128 | >128 |
| 25a | >128 | >128 | >128 | >128 | >128 | >128 |
| 25b | >128 | >128 | >128 | >128 | >128 | >128 |
| 26a | >128 | >128 | >128 | >128 | >128 | >128 |
| 26b | 128 | >128 | >128 | >128 | >128 | >128 |
| 27a | >128 | >128 | >128 | >128 | >128 | >128 |
| 27b | 128 | >128 | >128 | >128 | >128 | >128 |
| 28b | >128 | >128 | >128 | >128 | >128 | >128 |
| Fluconazole | 8 | 16 | 128 | N/A | N/A | N/A |
| Flucytosine | 2 | <0.5 | <0.5 | N/A | N/A | N/A |
| Voriconazole | <0.5 | <0.5 | <0.5 | 2 | 1 | 2 |
| Bacterial Strain | Resistance Mechanisms | Antimicrobial Activity (MIC, µg/mL) | |||
|---|---|---|---|---|---|
| 14 | 24b | VAN | DAP | ||
| S. aureus AR-215 | aph-STPH, DHA1, erm(A), mecA, spc, tet(38) | 16 | 8 | 4 | 2 |
| S. aureus AR-216 | aph(3′)-III, aph-STPH, fosB, mecA, mph(C), sat-4A | 8 | 8 | 8 | 4 |
| S. aureus AR-217 | aph-STPH, blaI, dfrG, fosB, mecA, Z | 16 | 4 | 4 | 2 |
| S. aureus AR-218 | aph(3′)-III, aph-STPH, DHA1, erm(A), mecA, norA, spc, tet(38), tet(K) | 8 | 4 | 4 | 2 |
| S. aureus AR-219 | aac(6′)-aph(2″), aadD, aph-STPH, DHA1, erm(A), mecA, norA, spc, tet(38), tet(K) | 4 | 2 | 8 | 4 |
| S. aureus AR-220 | aph-STPH, DHA1, erm(A), mecA, norA, spc, tet(38) | 16 | 8 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertašiūtė, M.; Kavaliauskas, P.; Vaickelionienė, R.; Grybaitė, B.; Petraitis, V.; Petraitienė, R.; Naing, E.; Garcia, A.; Šiugždaitė, J.; Lelešius, R.; et al. Synthesis of 1-(2-Hydroxyphenyl)- and (3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as Promising Scaffolds for the Development of Novel Antimicrobial and Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 7966. https://doi.org/10.3390/ijms24097966
Bertašiūtė M, Kavaliauskas P, Vaickelionienė R, Grybaitė B, Petraitis V, Petraitienė R, Naing E, Garcia A, Šiugždaitė J, Lelešius R, et al. Synthesis of 1-(2-Hydroxyphenyl)- and (3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as Promising Scaffolds for the Development of Novel Antimicrobial and Anticancer Agents. International Journal of Molecular Sciences. 2023; 24(9):7966. https://doi.org/10.3390/ijms24097966
Chicago/Turabian StyleBertašiūtė, Monika, Povilas Kavaliauskas, Rita Vaickelionienė, Birutė Grybaitė, Vidmantas Petraitis, Rūta Petraitienė, Ethan Naing, Andrew Garcia, Jūratė Šiugždaitė, Raimundas Lelešius, and et al. 2023. "Synthesis of 1-(2-Hydroxyphenyl)- and (3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as Promising Scaffolds for the Development of Novel Antimicrobial and Anticancer Agents" International Journal of Molecular Sciences 24, no. 9: 7966. https://doi.org/10.3390/ijms24097966
APA StyleBertašiūtė, M., Kavaliauskas, P., Vaickelionienė, R., Grybaitė, B., Petraitis, V., Petraitienė, R., Naing, E., Garcia, A., Šiugždaitė, J., Lelešius, R., & Mickevičius, V. (2023). Synthesis of 1-(2-Hydroxyphenyl)- and (3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic Acid Derivatives as Promising Scaffolds for the Development of Novel Antimicrobial and Anticancer Agents. International Journal of Molecular Sciences, 24(9), 7966. https://doi.org/10.3390/ijms24097966







