Improving Solubility and Permeability of Hesperidin through Electrospun Orange-Peel-Extract-Loaded Nanofibers
Abstract
1. Introduction
2. Results and Discussion
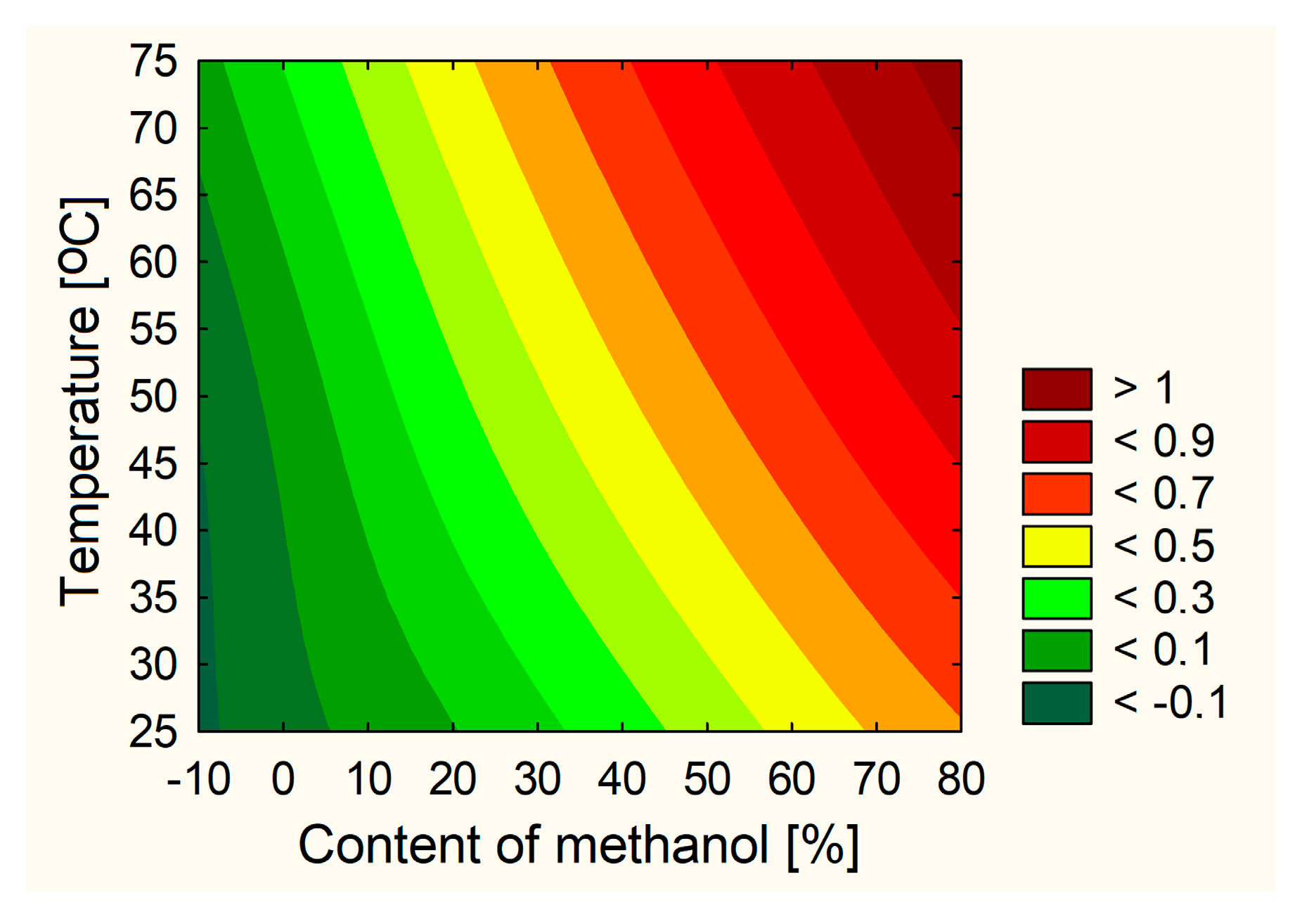
2.1. Optimization of the Orange Peel Extraction Process and Characterization of Biological Activity
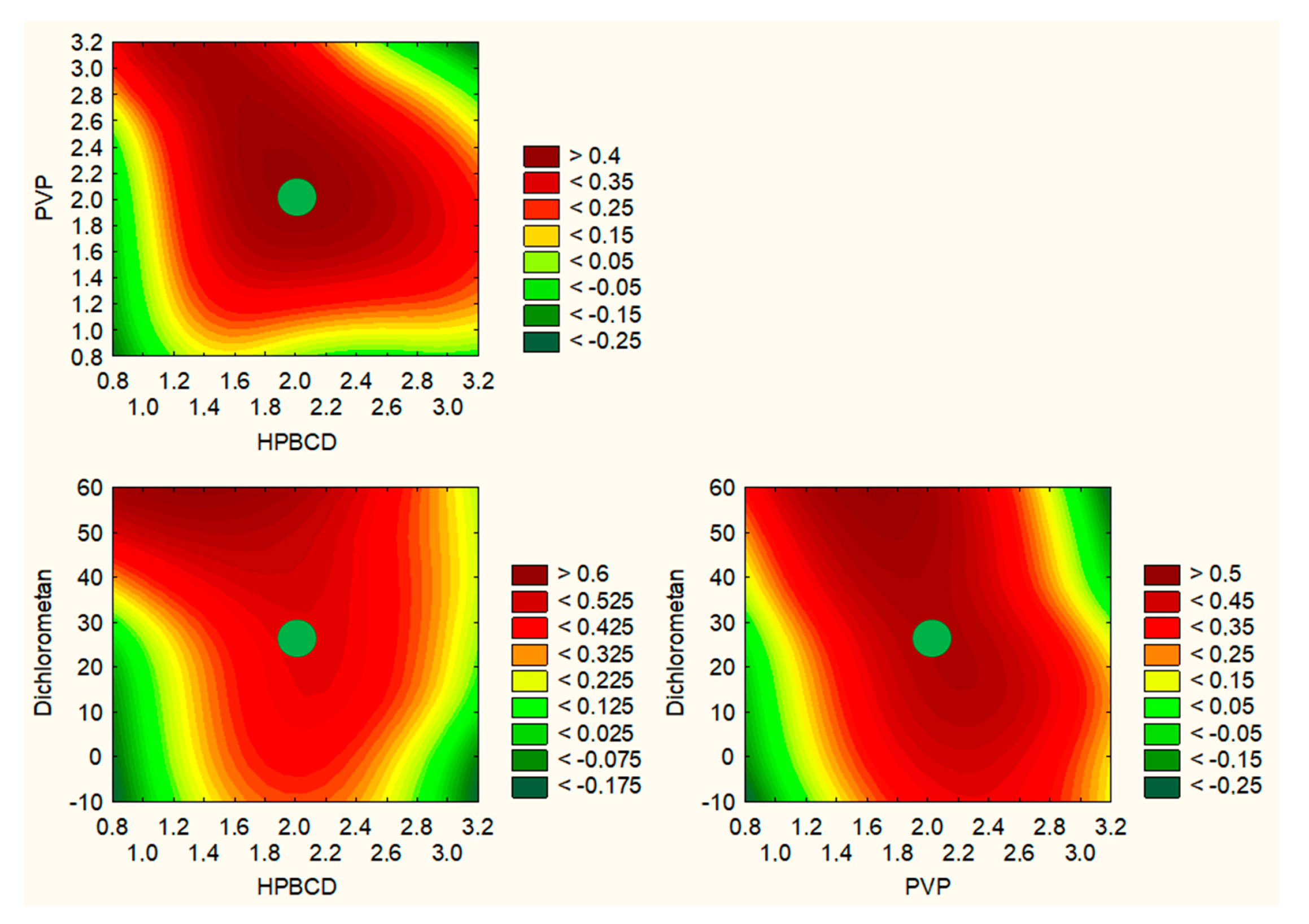
2.2. Optimization of the Electrospinning Process of Nanofibers Involving Orange Peel Extract
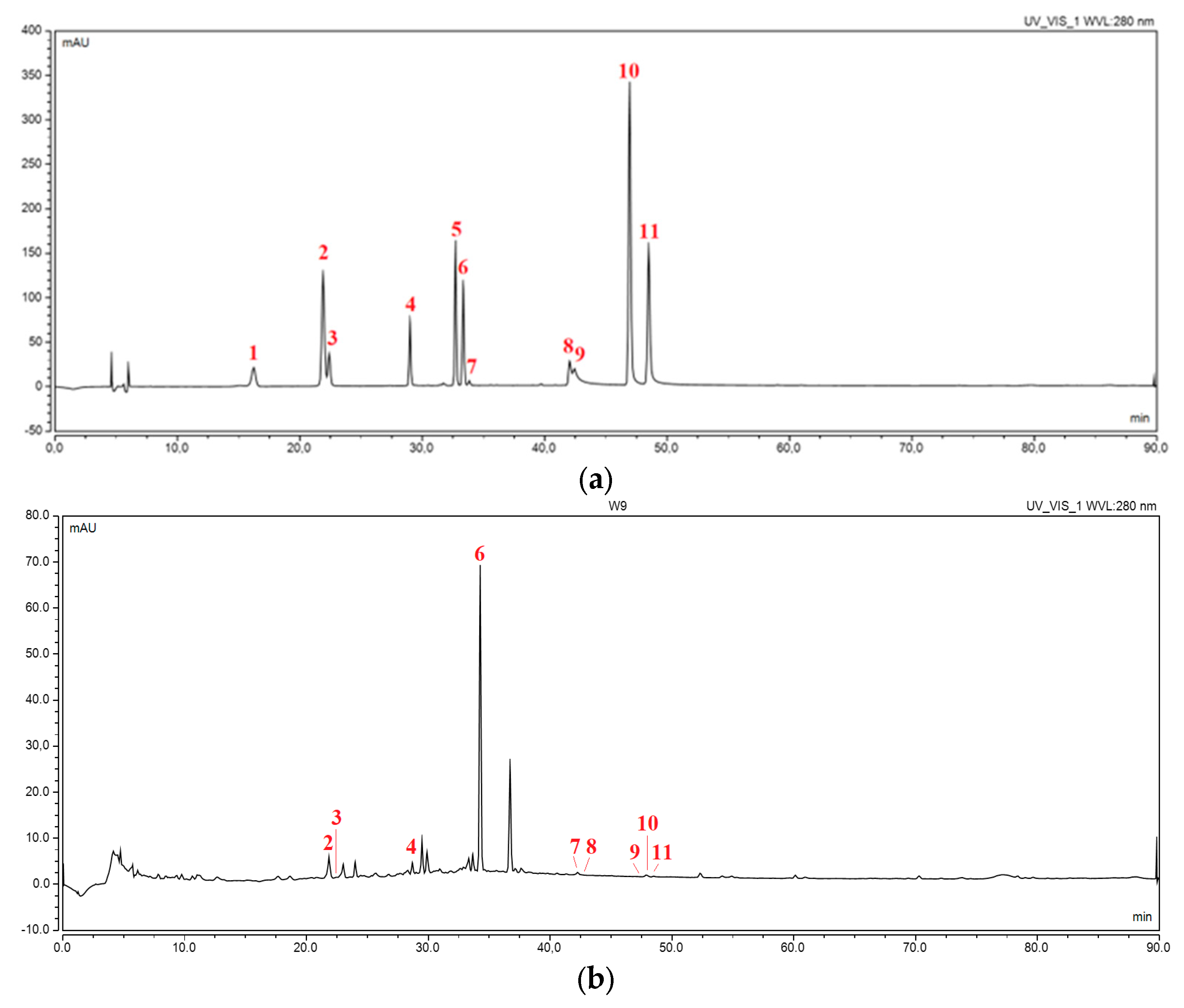
2.2.1. Preparation and Identification of Electrospun Nanofibers
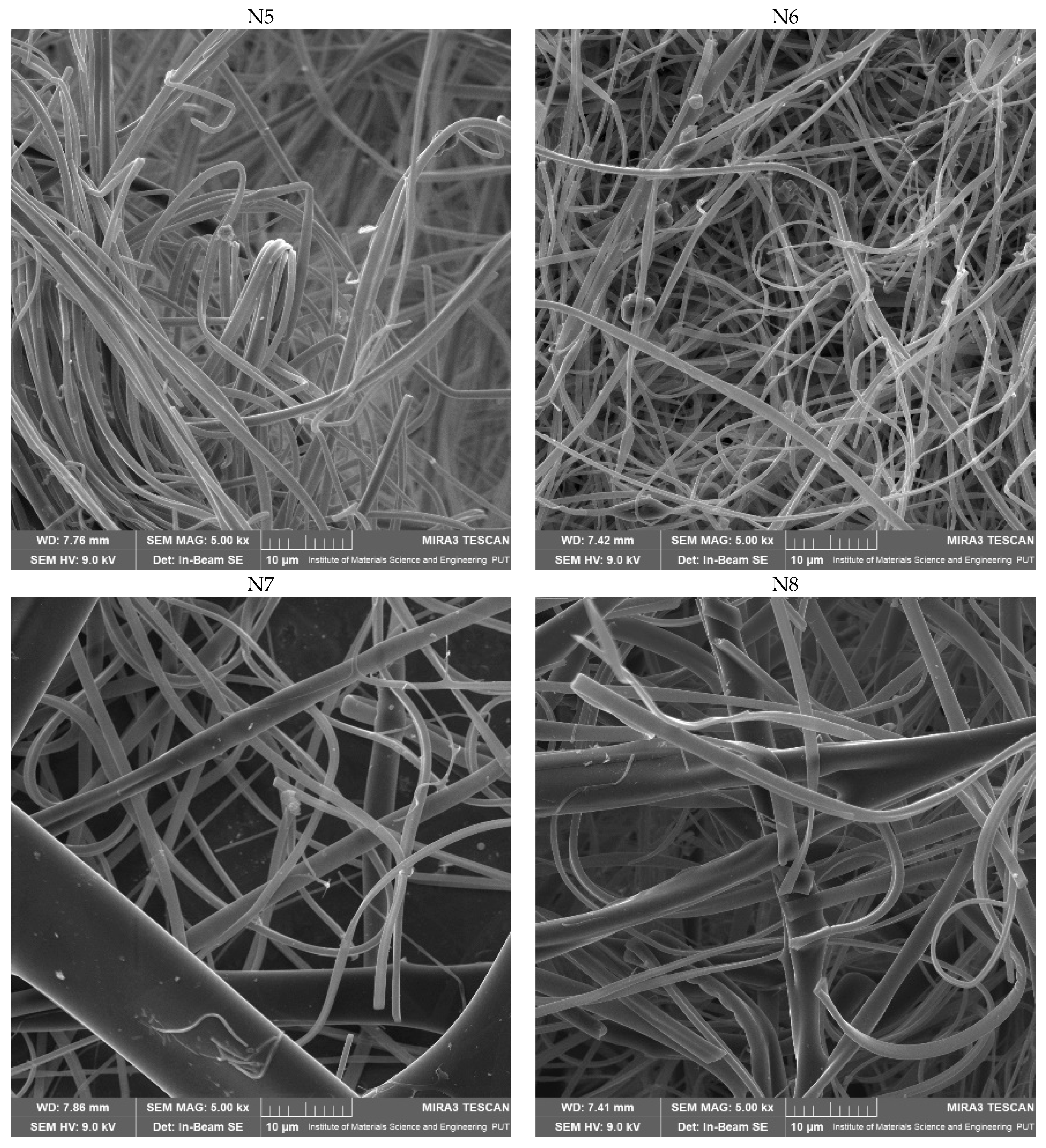
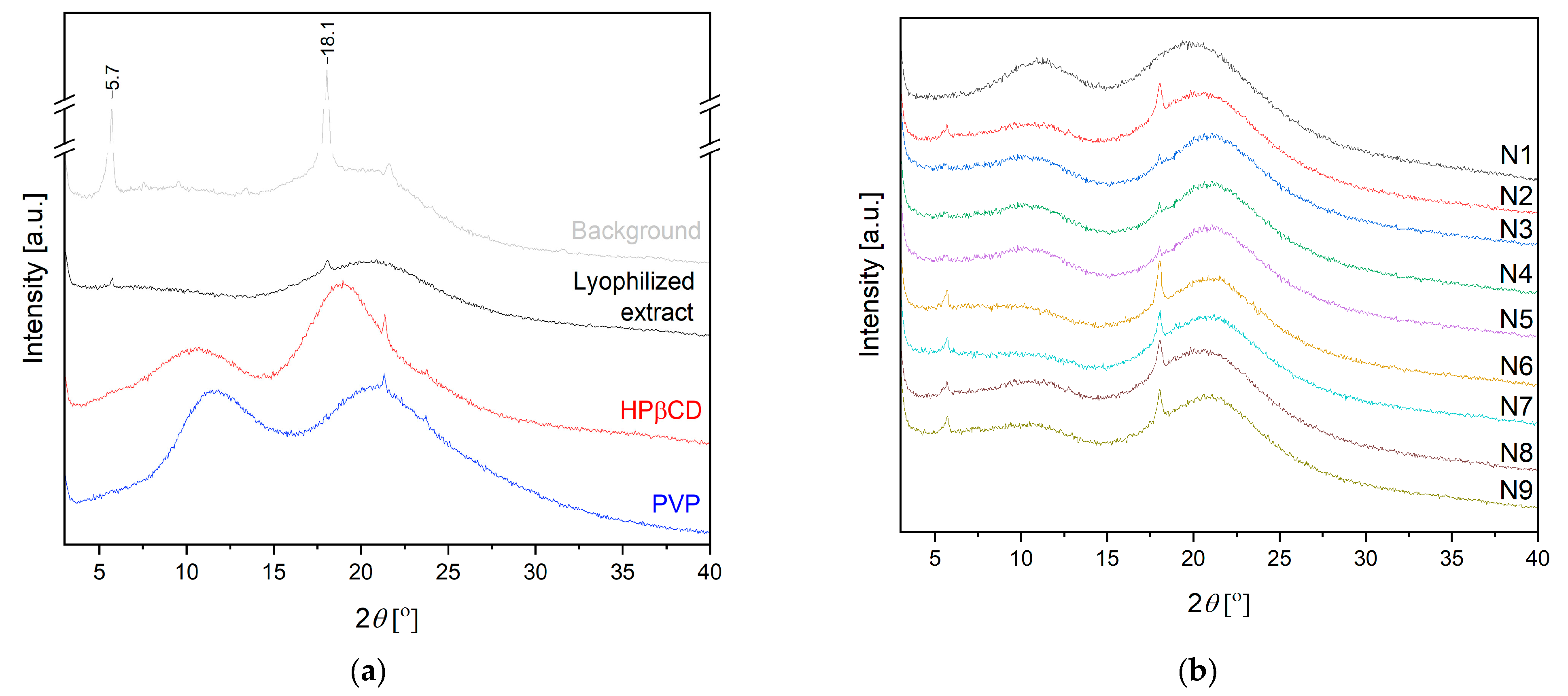
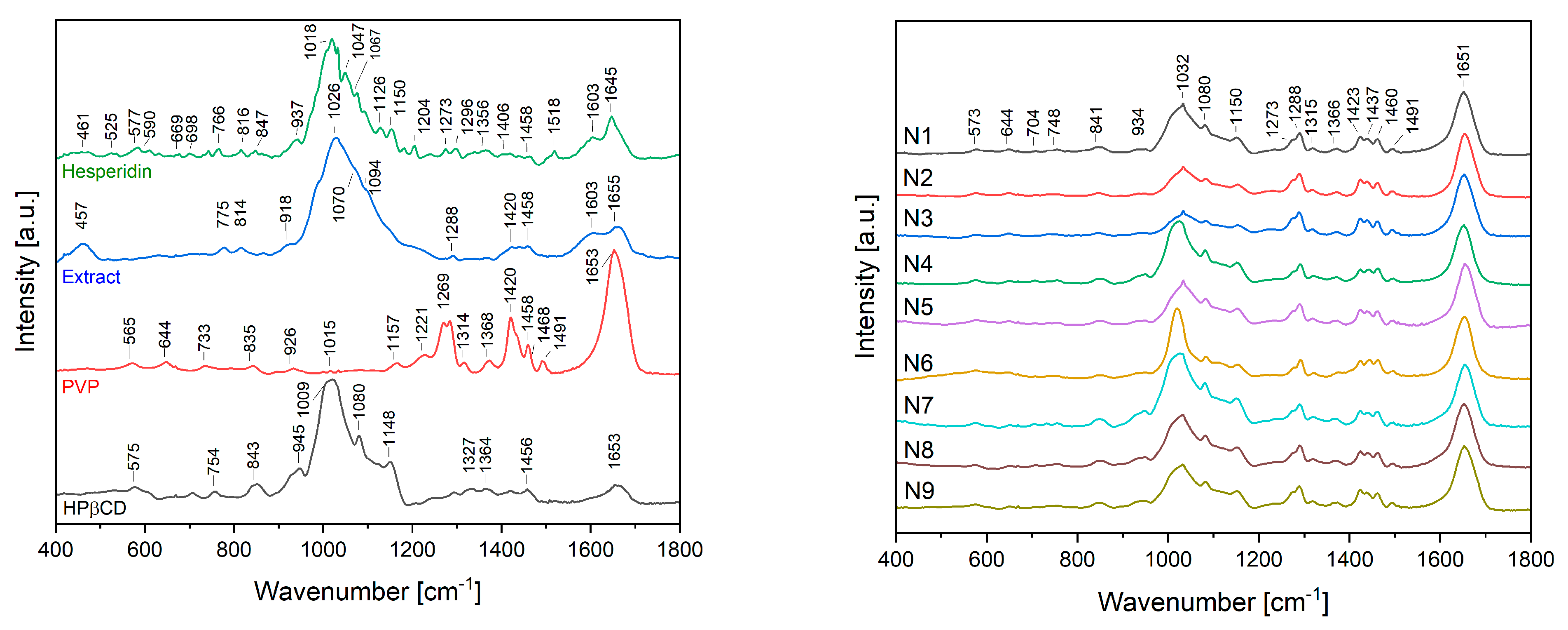
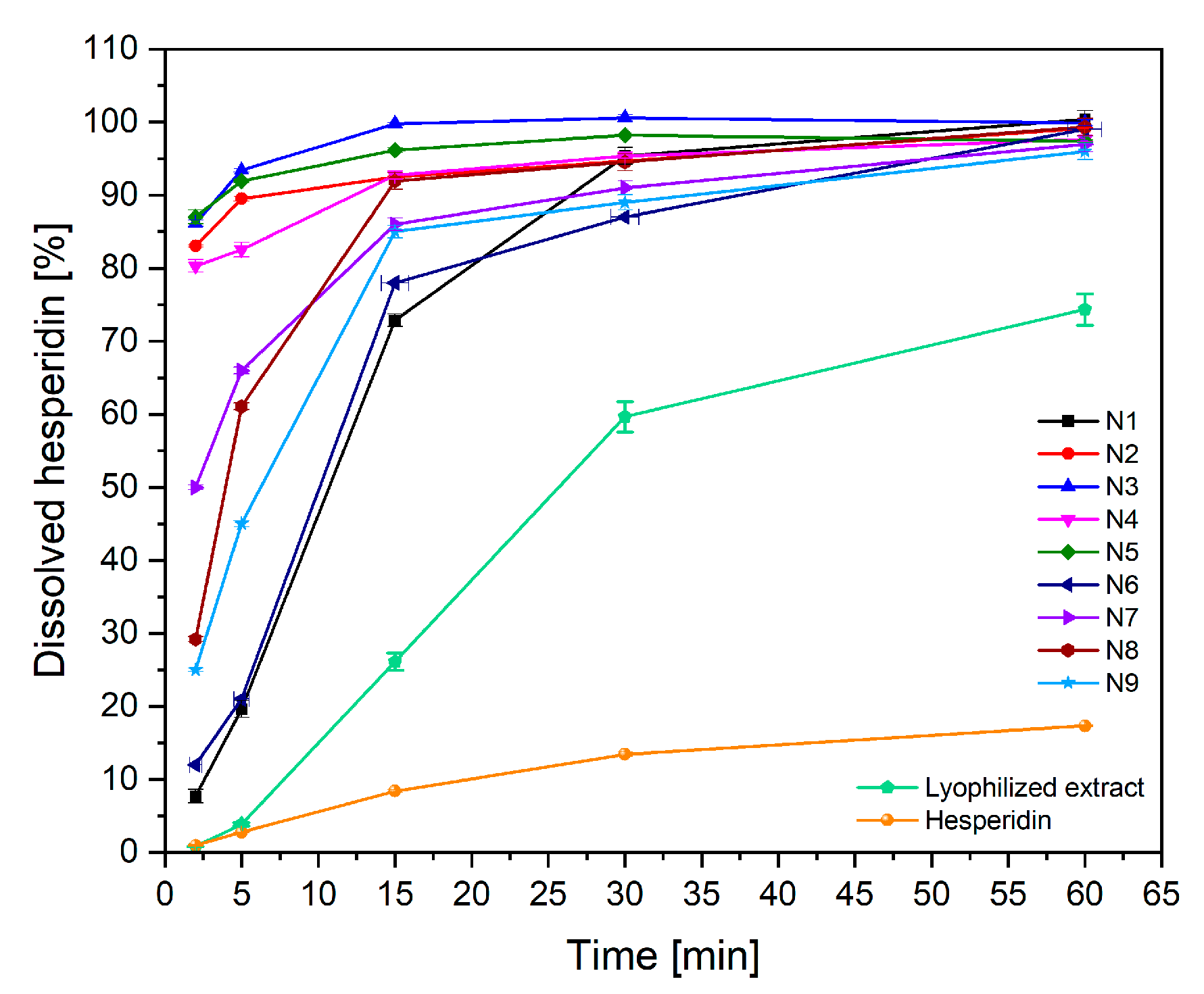
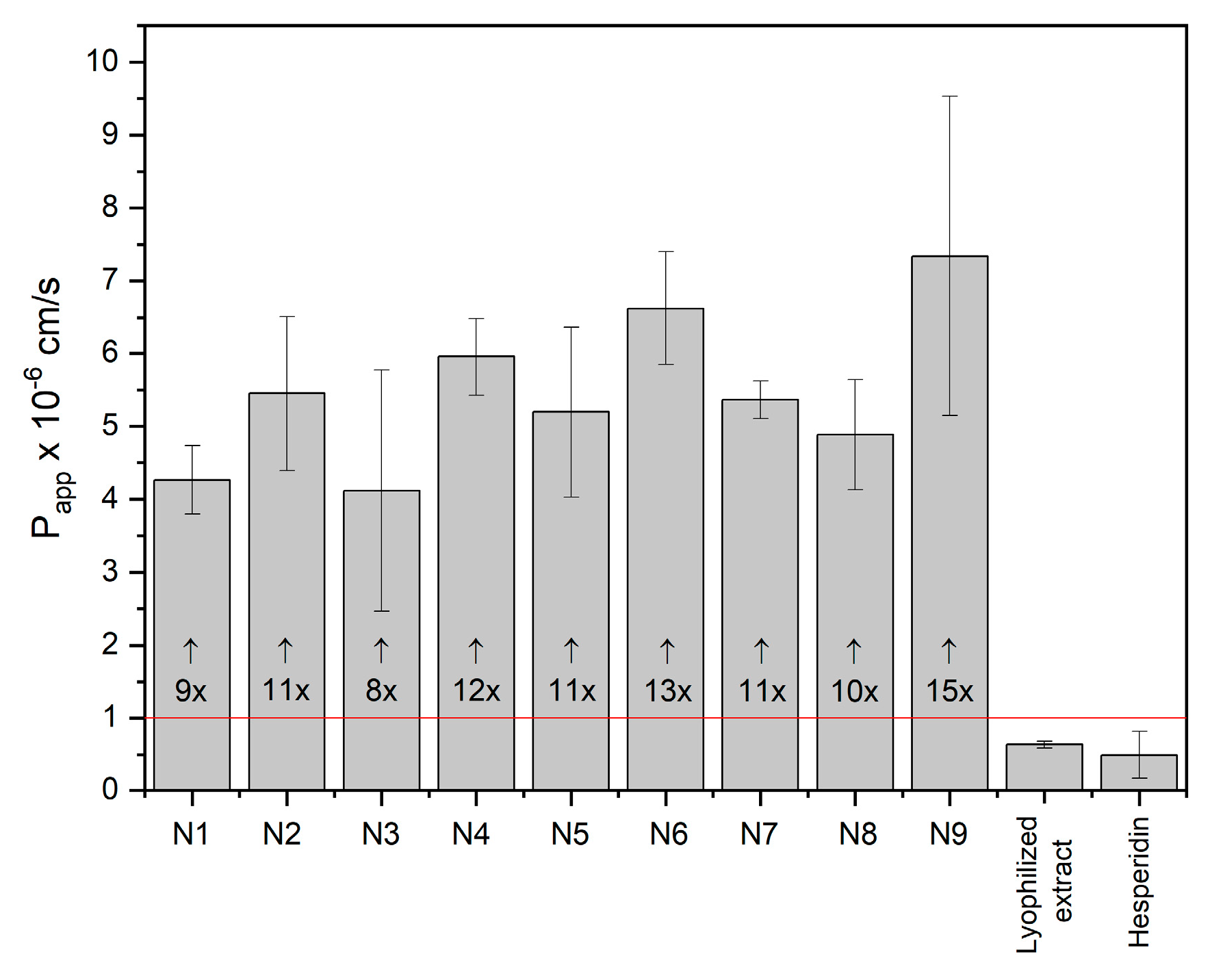
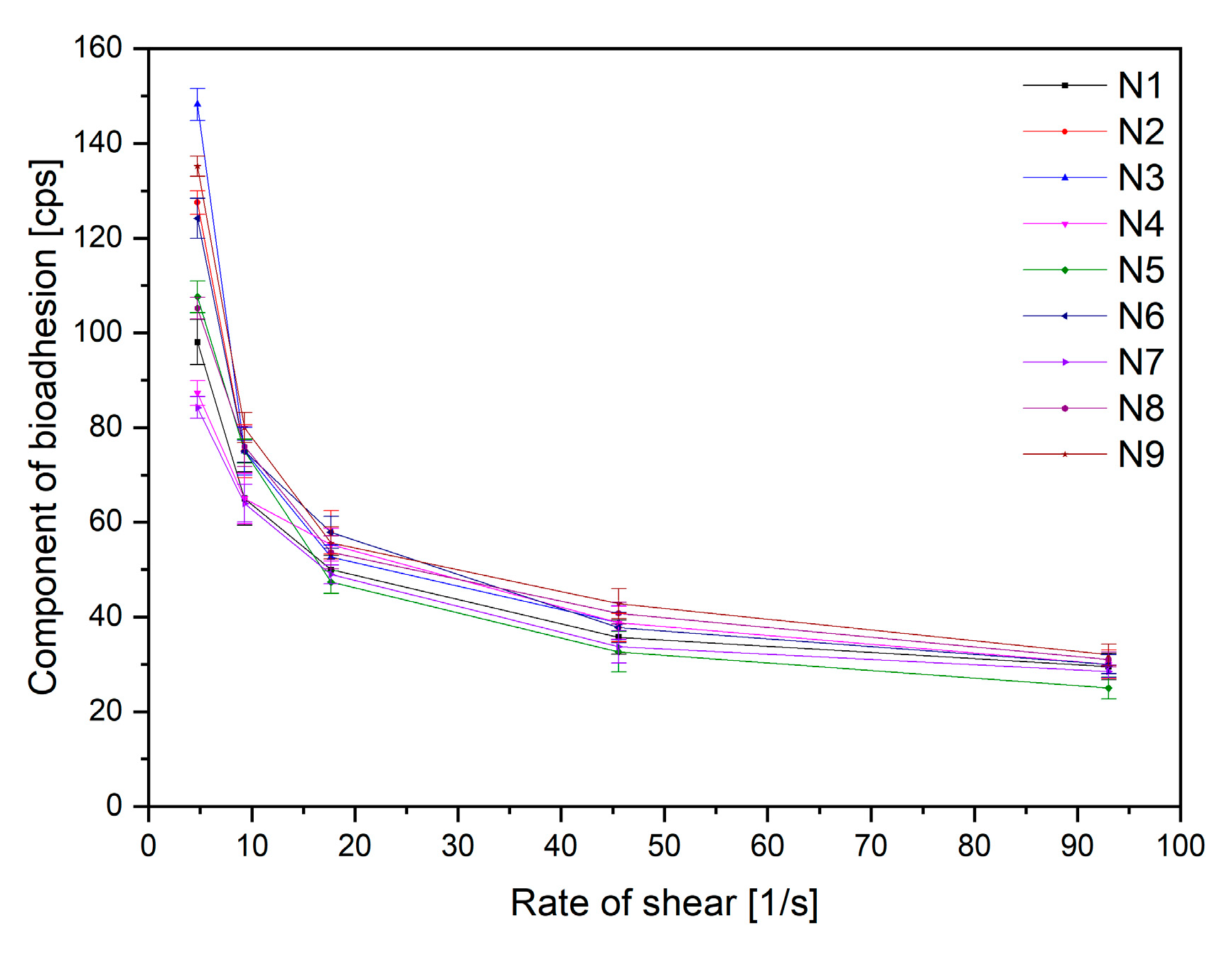
2.2.2. Characterization of Electrospun Nanofibers
3. Materials and Methods
3.1. Plant Raw Material
3.2. Chemicals and Reagents
3.3. Optimization of the Orange Peel Extraction Process and Characterization of Biological Activity of Extracts
3.3.1. Plant Extraction Using Design of Experiment (DoE)
3.3.2. Determination of Selected Active Component Content and Total Phenolic Content (TPC)
3.3.3. Determination of Biological Activity
Antioxidant Activity
Anti-Hyaluronidase Activity
3.4. Optimization of the Electrospinning Process of Nanofibers with Orange Peel Extract
Electrospun Nanofibers Preparation Using Design of Experiment (DoE)
3.5. The Identification of Optimized Electrospun Nanofibers
3.5.1. Dynamic Viscosity
3.5.2. Scanning Electron Microscopy (SEM)
3.5.3. XRPD
3.5.4. Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR)
3.6. Characterization of Electrospun Nanofibers
3.6.1. Determination of Active Component Content
3.6.2. Dissolution Studies
3.6.3. Permeability Studies
3.6.4. Determination of Biological Activity
3.6.5. In Vitro Assessment of Mucin–Biopolymer Bioadhesive Bond Strength
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oikeh, E.I.; Oviasogie, F.E.; Omoregie, E.S. Quantitative Phytochemical Analysis and Antimicrobial Activities of Fresh and Dry Ethanol Extracts of Citrus Sinensis (L.) Osbeck (Sweet Orange) Peels. Clin. Phytosci. 2020, 6, 46. [Google Scholar] [CrossRef]
- Karoui, I.J.; Marzouk, B. Characterization of Bioactive Compounds in Tunisian Bitter Orange (Citrus aurantium L.) Peel and Juice and Determination of Their Antioxidant Activities. BioMed Res. Int. 2013, 2013, 345415. [Google Scholar]
- Shawky, E. Determination of Synephrine and Octopamine in Bitter Orange Peel by HPTLC with Densitometry. J. Chromatogr. Sci. 2014, 52, 899–904. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Qiong, P. Process for Extracting Hesperidin and Tangerine Peel Yellow Pigment from Orange Peel. China Patent CN104447912A, 25 March 2015. Available online: https://patents.google.com/patent/CN104447912A/en (accessed on 8 March 2023).
- Victor, M.M.; David, J.M.; Cortez, M.V.M.; Leite, J.L.; da Silva, G.S.B. A High-Yield Process for Extraction of Hesperidin from Orange (Citrus Sinensis L. Osbeck) Peels Waste, and Its Transformation to Diosmetin, A Valuable and Bioactive Flavonoid. Waste Biomass Valor. 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in Humans of the Flavanones Hesperidin and Narirutin after the Ingestion of Two Doses of Orange Juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef]
- Pla-Pagà, L.; Companys, J.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Valls, R.M.; Pedret, A. Effects of Hesperidin Consumption on Cardiovascular Risk Biomarkers: A Systematic Review of Animal Studies and Human Randomized Clinical Trials. Nutr. Rev. 2019, 77, 845–864. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the Water Solubility and Antioxidant Activity of Hesperidin by Chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef]
- Kao, H.-H.; Kuo, C.-Y.; Tagadur Govindaraju, D.; Chen, K.-S.; Chen, J.-P. Polycaprolactone/Chitosan Composite Nanofiber Membrane as a Preferred Scaffold for the Culture of Mesothelial Cells and the Repair of Damaged Mesothelium. Int. J. Mol. Sci. 2022, 23, 9517. [Google Scholar] [CrossRef]
- Tommasini, S.; Calabrò, M.L.; Stancanelli, R.; Donato, P.; Costa, C.; Catania, S.; Villari, V.; Ficarra, P.; Ficarra, R. The Inclusion Complexes of Hesperetin and Its 7-Rhamnoglucoside with (2-Hydroxypropyl)-Beta-Cyclodextrin. J. Pharm. Biomed. Anal. 2005, 39, 572–580. [Google Scholar] [CrossRef]
- Tomás-Navarro, M.; Vallejo, F.; Borrego, F.; Tomás-Barberán, F.A. Encapsulation and Micronization Effectively Improve Orange Beverage Flavanone Bioavailability in Humans. J. Agric. Food Chem. 2014, 62, 9458–9462. [Google Scholar] [CrossRef]
- Salehi, H.; Karimi, M.; Raofie, F. Micronization and Coating of Bioflavonoids Extracted from Citrus Sinensis L. Peels to Preparation of Sustained Release Pellets Using Supercritical Technique. J. Iran. Chem. Soc. 2021, 18, 3235–3248. [Google Scholar] [CrossRef]
- Wei, Q.; Keck, C.M.; Müller, R.H. Oral Hesperidin-Amorphization and Improved Dissolution Properties by Controlled Loading onto Porous silica. Int. J. Pharm. 2017, 518, 253–263. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-Β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, R.G. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Farhaj, S.; Conway, B.R.; Ghori, M.U. Nanofibres in Drug Delivery Applications. Fibers 2023, 11, 21. [Google Scholar] [CrossRef]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the Design and Development of “Fast-Dissolving” Electrospun Nanofibers Based Drug Delivery Systems—A Systematic Review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef]
- Fenyvesi, F.; Nguyen, T.L.P.; Haimhoffer, Á.; Rusznyák, Á.; Vasvári, G.; Bácskay, I.; Vecsernyés, M.; Ignat, S.-R.; Dinescu, S.; Costache, M.; et al. Cyclodextrin Complexation Improves the Solubility and Caco-2 Permeability of Chrysin. Materials 2020, 13, 3618. [Google Scholar] [CrossRef]
- Taymouri, S.; Hashemi, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A. Fabrication and Evaluation of Hesperidin Loaded Polyacrylonitrile/Polyethylene Oxide Nanofibers for Wound Dressing Application. J. Biomater. Sci. Polym. Ed. 2021, 32, 1944–1965. [Google Scholar] [CrossRef]
- Jangde, R.; Elhassan, G.O.; Khute, S.; Singh, D.; Singh, M.; Sahu, R.K.; Khan, J. Hesperidin-Loaded Lipid Polymer Hybrid Nanoparticles for Topical Delivery of Bioactive Drugs. Pharmaceuticals 2022, 15, 211. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, L.; Lu, H.; Kang, T. Effect of Different Solvent Systems on PHBV/PEO Electrospun Fibers. RSC Adv. 2017, 7, 4000–4010. [Google Scholar] [CrossRef]
- Kim, W.-T.; Park, D.-C.; Yang, W.-H.; Cho, C.-H.; Choi, W.-Y. Effects of Electrospinning Parameters on the Microstructure of PVP/TiO2 Nanofibers. Nanomaterials 2021, 11, 1616. [Google Scholar] [CrossRef]
- Häusler, O.; Müller-Goymann, C.C. Properties and Structure of Aqueous Solutions of Hydroxypropyl-Beta-Cyclodextrin. Starch Stärke 1993, 45, 183–187. [Google Scholar] [CrossRef]
- Higashi, S.; Hirai, T.; Matsubara, M.; Yoshida, H.; Beniya, A. Dynamic Viscosity Recovery of Electrospinning Solution for Stabilizing Elongated Ultrafine Polymer Nanofiber by TEMPO-CNF. Sci. Rep. 2020, 10, 13427. [Google Scholar] [CrossRef]
- Jalalah, M.; Ahmad, A.; Saleem, A.; Qadir, M.B.; Khaliq, Z.; Khan, M.Q.; Nazir, A.; Faisal, M.; Alsaiari, M.; Irfan, M.; et al. Electrospun Nanofiber/Textile Supported Composite Membranes with Improved Mechanical Performance for Biomedical Applications. Membranes 2022, 12, 1158. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Venkatraman, S.S. Importance of Viscosity Parameters in Electrospinning: Of Monolithic and Core–Shell Fibers. Mater. Sci. Eng. C 2012, 32, 1037–1042. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012092. [Google Scholar] [CrossRef]
- Korycka, P.; Mirek, A.; Kramek-Romanowska, K.; Grzeczkowicz, M.; Lewińska, D. Effect of Electrospinning Process Variables on the Size of Polymer Fibers and Bead-on-String Structures Established with a 23 Factorial Design. Beilstein J. Nanotechnol. 2018, 9, 2466–2478. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Abdelghany, A.M.; Badr, S.I.; Morsi, M.A. Structural, Optical, Morphological and Thermal Properties of PEO/PVP Blend Containing Different Concentrations of Biosynthesized Au Nanoparticles. J. Mater. Res. Technol. 2018, 7, 419–431. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Cielecka-Piontek, J. Is It Possible to Improve the Bioavailability of Resveratrol and Polydatin Derived from Polygoni Cuspidati Radix as a Result of Preparing Electrospun Nanofibers Based on Polyvinylpyrrolidone/Cyclodextrin? Nutrients 2022, 14, 3897. [Google Scholar] [CrossRef]
- Ali, S.H.; Sulaiman, G.M.; Al-Halbosiy, M.M.F.; Jabir, M.S.; Hameed, A.H. Fabrication of Hesperidin Nanoparticles Loaded by Poly Lactic Co-Glycolic Acid for Improved Therapeutic Efficiency and Cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 378–394. [Google Scholar] [CrossRef]
- Kamaruddin; Edikresnha, D.; Sriyanti, I.; Munir, M.M.; Khairurrijal. Synthesis of Polyvinylpyrrolidone (PVP)-Green Tea Extract Composite Nanostructures Using Electrohydrodynamic Spraying Technique. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012043. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Rosiak, N.; Tykarska, E.; Michalska, K.; Płazińska, A.; Płaziński, W.; Szymanowska, D.; Cielecka-Piontek, J. Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. Int. J. Mol. Sci. 2020, 22, 115. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. Dissolution Improvement of Electrospun Nanofiber-Based Solid Dispersions for Acetaminophen. AAPS PharmSciTech 2010, 11, 809–817. [Google Scholar] [CrossRef]
- Suknuntha, K.; Tantishaiyakul, V.; Worakul, N.; Taweepreda, W. Characterization of Muco- and Bioadhesive Properties of Chitosan, PVP, and Chitosan/PVP Blends and Release of Amoxicillin from Alginate Beads Coated with Chitosan/PVP. Drug Dev. Ind. Pharm. 2011, 37, 408–418. [Google Scholar] [CrossRef]
- Asim, M.H.; Ijaz, M.; Mahmood, A.; Knoll, P.; Jalil, A.; Arshad, S.; Bernkop-Schnürch, A. Thiolated Cyclodextrins: Mucoadhesive and Permeation Enhancing Excipients for Ocular Drug Delivery. Int. J. Pharm. 2021, 599, 120451. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Szymańska, E.; Winnicka, K.; Szwajgier, D.; Baranowska-Wójcik, E.; Ruchała, M.A.; Simon, M.; Cielecka-Piontek, J. Cyclodextrin as Functional Carrier in Development of Mucoadhesive Tablets Containing Polygoni Cuspidati Extract with Potential for Dental Applications. Pharmaceutics 2021, 13, 1916. [Google Scholar] [CrossRef]


| Content of Active Compounds | ||
|---|---|---|
| No. | Content of Hesperidin (mg/g Plant Material) | TPC (mg GAE/g Plant Material) |
| E1 | 10.17 ± 0.92 | 5.34 ± 1.33 |
| E2 | 10.33 ± 0.52 | 5.85 ± 1.34 |
| E3 | 27.80 ± 0.13 | 5.89 ± 1.31 |
| E4 | 12.14 ± 0.75 | 6.64 ± 0.62 |
| E5 | 24.40 ± 0.09 | 6.77 ± 0.51 |
| E6 | 27.74 ± 0.01 | 6.73 ± 0.75 |
| E7 | 23.15 ± 0.96 | 9.58 ± 2.25 |
| E8 | 28.45 ± 0.54 | 9.92 ± 2.79 |
| E9 | 33.73 ± 0.54 | 11.01 ± 1.17 |
| Antioxidant Activity | Anti-Inflammatory Activity | ||||
|---|---|---|---|---|---|
| No. | DPPH IC50 (mg/mL) | ABTS IC50 (µg/mL) | CUPRAC IC0.5 (µg/mL) | FRAP IC0.5 (µg/mL) | IC0.5 (µg/mL) |
| E1 | 2.82 ± 0.07 | 760.90 ± 23.65 | 1670.37 ± 34.84 | 500.18 ± 48.94 | 21.87 ± 6.28 |
| E2 | 2.41 ± 0.20 | 632.99 ± 8.56 | 1673.53 ± 13.09 | 498.09 ± 79.76 | 32.38 ± 4.45 |
| E3 | 2.18 ± 0.34 | 567.31 ± 16.76 | 1560.13 ± 74.88 | 487.65 ± 82.75 | 43.41 ± 4.11 |
| E4 | 2.35 ± 0.36 | 546.32 ± 44.24 | 696.91 ± 109.96 | 482.12 ± 9.80 | 54.78 ± 12.07 |
| E5 | 2.22 ± 0.00 | 535.17 ± 30.63 | 797.48 ± 117.39 | 469.29 ± 38.55 | 81.12 ± 1.56 |
| E6 | 2.11 ± 0.04 | 479.29 ± 14.77 | 860.33 ± 76.27 | 456.33 ± 61.61 | 84.80 ± 0.00 |
| E7 | 2.27 ± 0.07 | 603.92 ± 45.75 | 682.87 ± 57.92 | 485.52 ± 18.56 | >1000 |
| E8 | 2.13 ± 0.04 | 471.58 ± 13.84 | 764.48 ± 104.93 | 449.87 ± 54.57 | >1000 |
| E9 | 1.93 ± 0.22 | 338.32 ± 5.36 | 705.62 ± 145.90 | 436.02 ± 13.51 | >1000 |
| N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 |
| Diameter of nanofibers (µm) | ||||||||
| - | 0.67 ± 0.19 | 1.14 ± 0.55 | 0.75 ± 0.33 | 0.94 ± 0.30 | 1.33 ± 0.39 | 4.53 ± 3.74 | 2.73 ± 1.12 | 1.18 ± 0.46 |
| N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 |
| % | ||||||||
| 20.79 | 53.71 | 41.49 | 42.25 | 50.52 | 1.12 | 1.17 | 4.40 | 0.54 |
| N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 |
| Content µg in 100 mg of nanofibers | ||||||||
| 522.90 ± 13.19 | 182.86 ± 0.28 | 115.91 ± 1.59 | 199.50 ± 0.44 | 233.46 ± 0.80 | 158.70 ± 1.88 | 212.80 ± 0.43 | 164.03 ± 0.46 | 65.74 ± 0.03 |
| No. | % of Methanol in the Extraction Mixture | Temperature | Number of Cycles |
|---|---|---|---|
| E1 | 0 | 30 | 3 |
| E2 | 0 | 50 | 5 |
| E3 | 0 | 70 | 4 |
| E4 | 35 | 30 | 5 |
| E5 | 35 | 50 | 4 |
| E6 | 35 | 70 | 3 |
| E7 | 70 | 30 | 4 |
| E8 | 70 | 50 | 3 |
| E9 | 70 | 70 | 5 |
| No. | HPBCD (g) | PVP (g) | Solvent Composition (Percentage of Methanol; Supplemented with Dichloromethane) |
|---|---|---|---|
| N1 | 1 | 1 | 100 |
| N2 | 1 | 2 | 50 |
| N3 | 1 | 3 | 75 |
| N4 | 2 | 1 | 50 |
| N5 | 2 | 2 | 75 |
| N6 | 2 | 3 | 100 |
| N7 | 3 | 1 | 75 |
| N8 | 3 | 2 | 100 |
| N9 | 3 | 3 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Miklaszewski, A.; Cielecka-Piontek, J. Improving Solubility and Permeability of Hesperidin through Electrospun Orange-Peel-Extract-Loaded Nanofibers. Int. J. Mol. Sci. 2023, 24, 7963. https://doi.org/10.3390/ijms24097963
Paczkowska-Walendowska M, Miklaszewski A, Cielecka-Piontek J. Improving Solubility and Permeability of Hesperidin through Electrospun Orange-Peel-Extract-Loaded Nanofibers. International Journal of Molecular Sciences. 2023; 24(9):7963. https://doi.org/10.3390/ijms24097963
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Andrzej Miklaszewski, and Judyta Cielecka-Piontek. 2023. "Improving Solubility and Permeability of Hesperidin through Electrospun Orange-Peel-Extract-Loaded Nanofibers" International Journal of Molecular Sciences 24, no. 9: 7963. https://doi.org/10.3390/ijms24097963
APA StylePaczkowska-Walendowska, M., Miklaszewski, A., & Cielecka-Piontek, J. (2023). Improving Solubility and Permeability of Hesperidin through Electrospun Orange-Peel-Extract-Loaded Nanofibers. International Journal of Molecular Sciences, 24(9), 7963. https://doi.org/10.3390/ijms24097963








