Exploring the Genetic Predisposition to Epigenetic Changes in Alzheimer’s Disease
Abstract
1. Introduction
2. Results
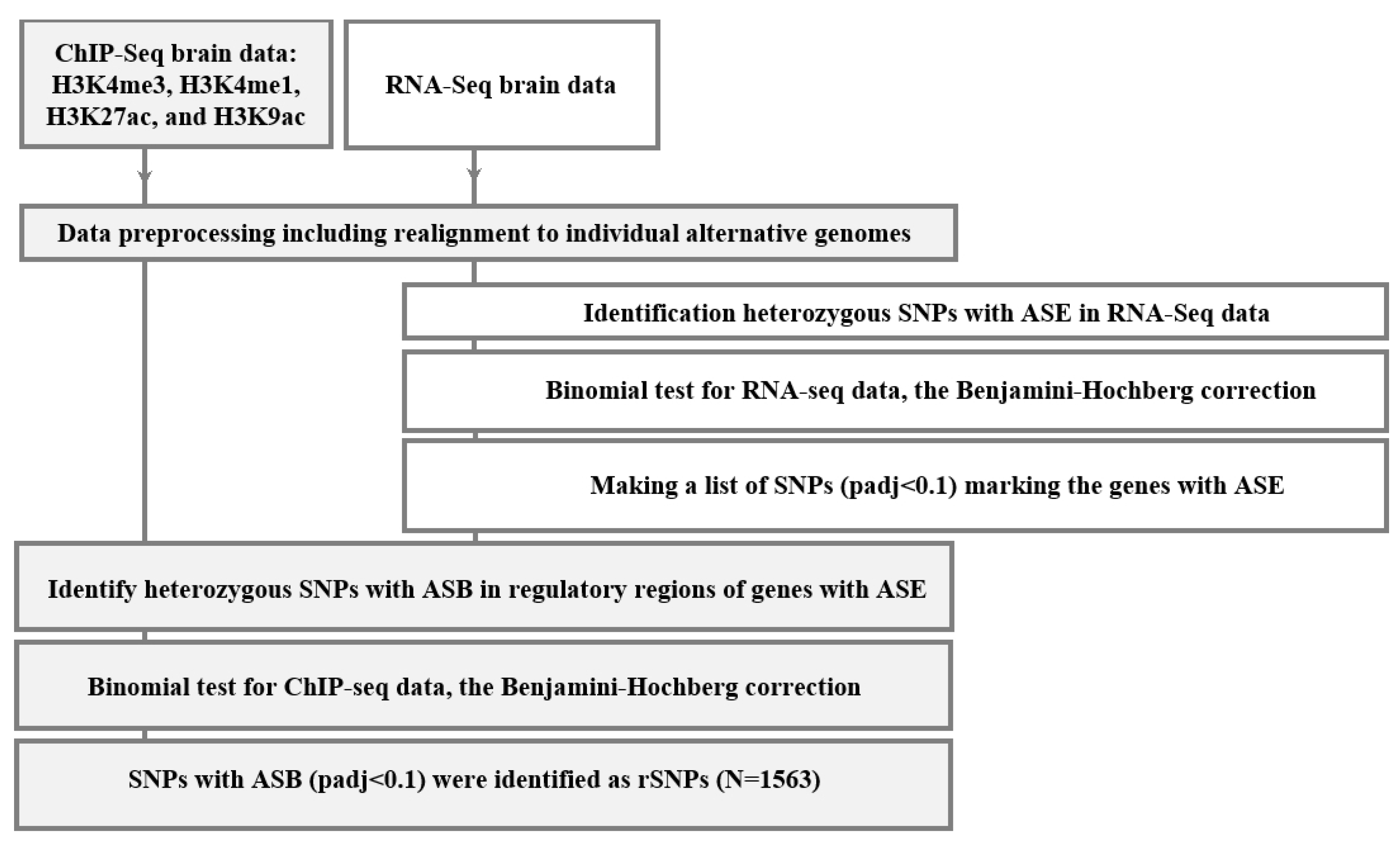
2.1. Discovering the rSNPs Associated with Allele-Specific Events
2.2. Identifying the rSNPs Affecting the Expression of DEGs (Differentially Expressed Genes) between the Temporal Lobe of AD Patients and Controls
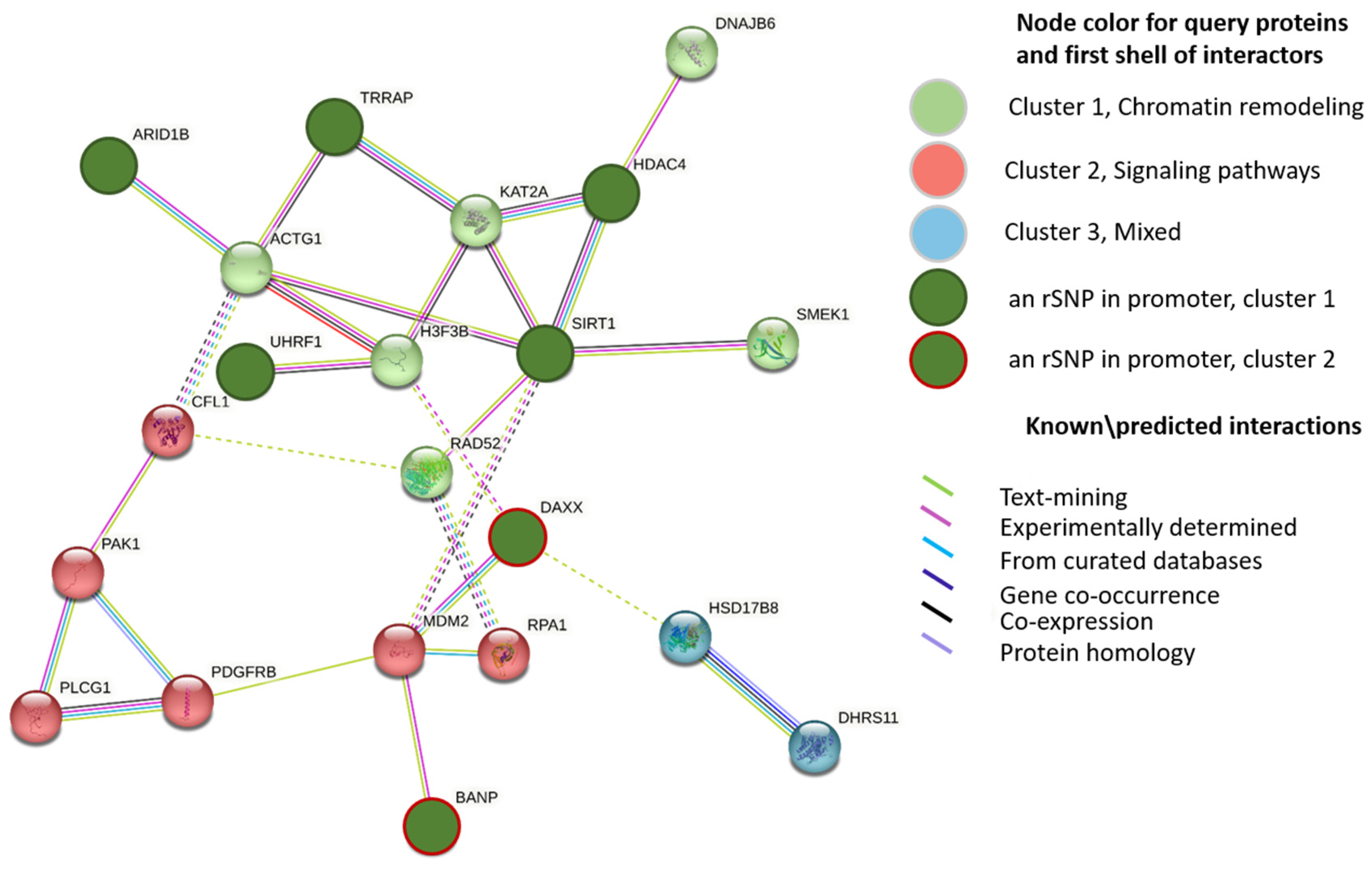
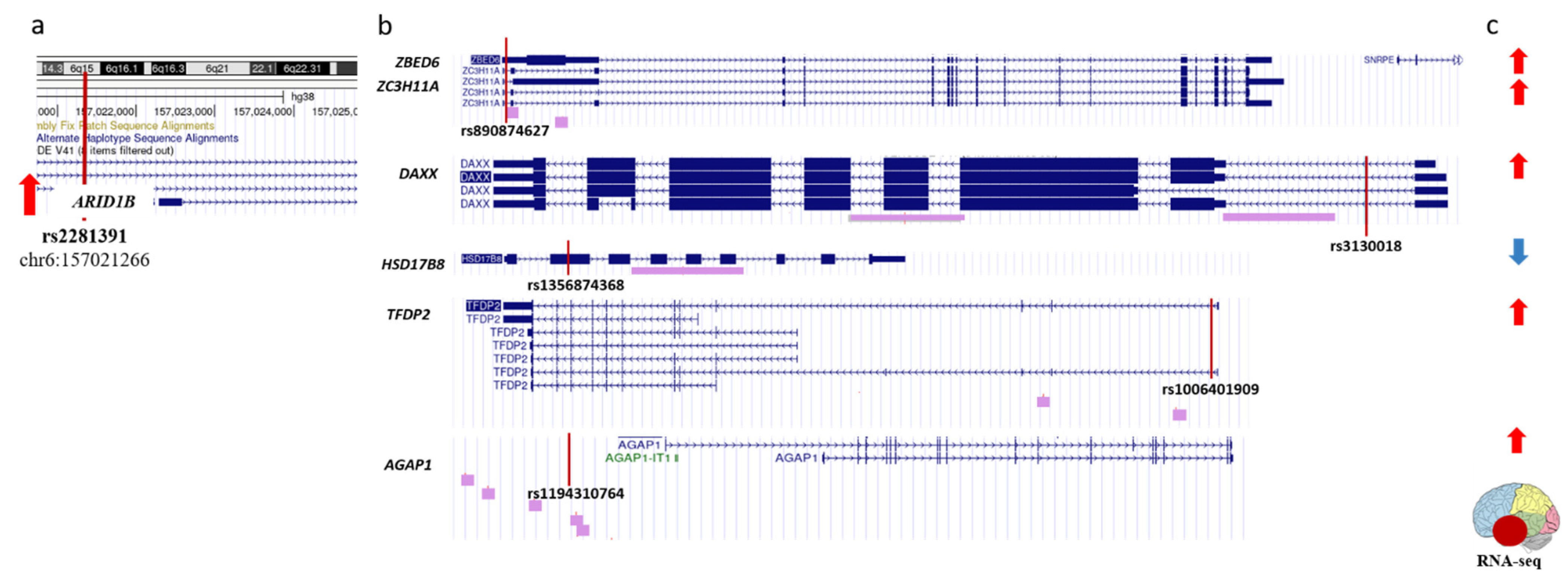
2.3. Identifying the rSNPs That Affect the Expression of Known Epigenetic Regulators
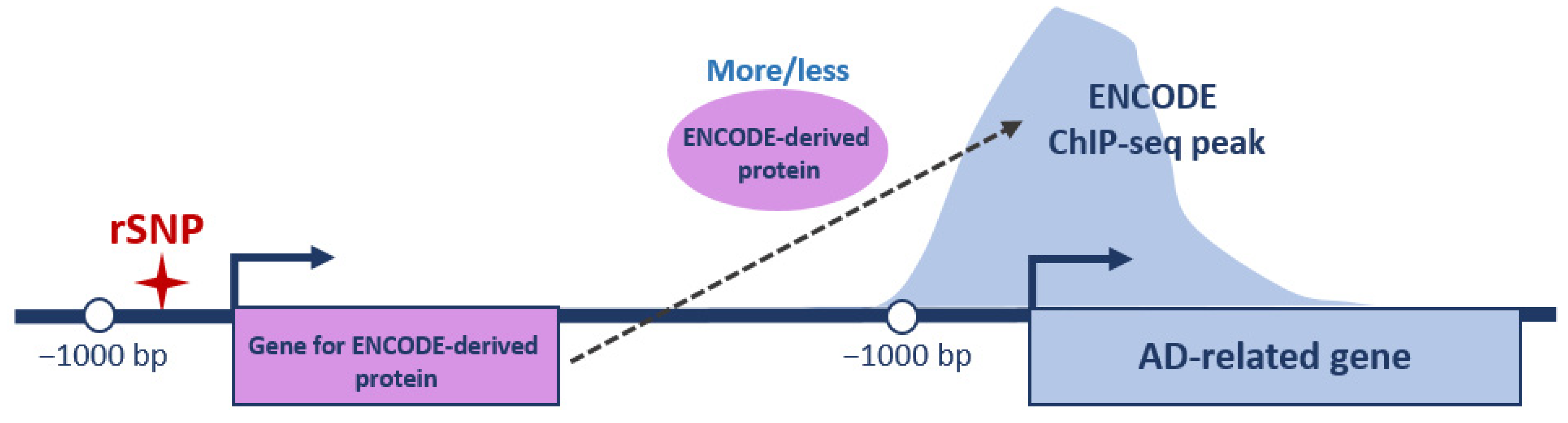
2.4. Identifying the rSNPs in the Promoters of Transcription Factors Involved in the Regulation of AD-Related Genes
3. Discussion
4. Materials and Methods
4.1. Brain Epigenomic and Transcriptomic Data
4.2. Human Genome Data
4.3. Sequencing Data Preprocessing and Alignment
4.4. Assessing Allele-Specific Events
4.5. Assignment of Gene Promoter Regions
4.6. Deposited Data
4.6.1. The Encyclopedia of DNA Elements (ENCODE)
4.6.2. GWAS Catalog
4.6.3. The EpiFactors Database
4.6.4. PubMed Resource
4.7. p-Value Correction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; King, A.; Wu, F.; Simpson-Yap, S.; Woodhouse, A.; Phipps, A.; Vickers, J.C. The potential roles of genetic factors in predicting ageing-related cognitive change and Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101402. [Google Scholar] [CrossRef] [PubMed]
- Leo, L.; Colonna Romano, N. Emerging Single-Cell Technological Approaches to Investigate Chromatin Dynamics and Centromere Regulation in Human Health and Disease. Int. J. Mol. Sci. 2021, 22, 8809. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Pivac, N. Genetic Markers of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1192, 27–52. [Google Scholar]
- Husain, M.A.; Laurent, B.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef]
- Seripa, D.; D’Onofrio, G.; Panza, F.; Cascavilla, L.; Masullo, C.; Pilotto, A. The Genetics of the Human APOE Polymorphism. Rejuvenation Res. 2011, 14, 491–500. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s Disease: The Challenge of the Second Century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef]
- Qiao, S.-Y.; Shang, K.; Chu, Y.-H.; Yu, H.-H.; Chen, X.; Qin, C.; Pan, D.-J.; Tian, D.-S. Apolipoprotein E ε4 Polymorphism as a Risk Factor for Ischemic Stroke: A Systematic Review and Meta-Analysis. Dis. Markers 2022, 2022, 1407183. [Google Scholar] [CrossRef]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, Q.; Yu, Z.; Wu, H.; Zhong, Z. The SNPs rs429358 and rs7412 of APOE gene are association with cerebral infarction but not SNPs rs2306283 and rs4149056 of SLCO1B1 gene in southern Chinese Hakka population. Lipids Health Dis. 2020, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Escott-Price, V.; Hardy, J. Genome-wide association studies for Alzheimer’s disease: Bigger is not always better. Brain Commun. 2022, 4, fcac125. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.K.; Piers, T.M.; Collier, D.; Cousins, O.; Pocock, J.M. Pathways linking Alzheimer’s disease risk genes expressed highly in microglia. Neuroimmunol. Neuroinflamm. 2021, 8, 245. [Google Scholar] [CrossRef]
- Katsumata, Y.; Nelson, P.T.; Estus, S.; Fardo, D.W. Translating Alzheimer’s disease–associated polymorphisms into functional candidates: A survey of IGAP genes and SNPs. Neurobiol. Aging 2019, 74, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Cheng, B.; Li, Y.; Li, X.; Chen, X.; Zhang, Y. TREM2 in Alzheimer’s Disease: Microglial Survival and Energy Metabolism. Front. Aging Neurosci. 2018, 10, 395. [Google Scholar] [CrossRef]
- Patel, D.; Zhang, X.; Farrell, J.J.; Chung, J.; Stein, T.D.; Lunetta, K.L.; Farrer, L.A. Cell-type-specific expression quantitative trait loci associated with Alzheimer disease in blood and brain tissue. Transl. Psychiatry 2021, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Li, L. Cell type-specific potential pathogenic genes and functional pathways in Alzheimer’s Disease. BMC Neurol. 2021, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Kuo, H.-C. Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J. Biomed. Sci. 2020, 27, 49. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Dabrowski, K.; Praticò, D. The neurobiology of non-coding RNAs and Alzheimer’s disease pathogenesis: Pathways, mechanisms and translational opportunities. Ageing Res. Rev. 2021, 71, 101425. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; Pivac, N. Epigenetics of Alzheimer’s Disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Xu, Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int. J. Environ. Res. Public Health 2020, 17, 4878. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, X.; Jiao, B. Epigenetics: Recent Advances and Its Role in the Treatment of Alzheimer’s Disease. Front. Neurol. 2020, 11, 538301. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mehta, V.; Singh, T.G. Alzheimer’s Disorder: Epigenetic Connection and Associated Risk Factors. Curr. Neuropharmacol. 2020, 18, 740–753. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Kim, K.; Yi, S.-J. The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Yu, C.; Yu, D.; Yu, G. Histone Acetylation Modifiers in the Pathogenesis of Alzheimer’s Disease. Front. Cell. Neurosci. 2015, 9, 226. [Google Scholar] [CrossRef]
- Berson, A.; Nativio, R.; Berger, S.L.; Bonini, N.M. Epigenetic Regulation in Neurodegenerative Diseases. Trends Neurosci. 2018, 41, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Nativio, R.; Lan, Y.; Donahue, G.; Sidoli, S.; Berson, A.; Srinivasan, A.R.; Shcherbakova, O.; Amlie-Wolf, A.; Nie, J.; Cui, X.; et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat. Genet. 2020, 52, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiao, B.; Shen, L. The Epigenetics of Alzheimer’s Disease: Factors and Therapeutic Implications. Front. Genet. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.; Shulha, H.P.; Jiang, Y.; Matevossian, A.; Wang, J.; Weng, Z.; Akbarian, S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 8824–8829. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Q.; Yao, H.; Tan, J.; Liu, Z.; Zhou, Y.; Zou, Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 911635. [Google Scholar] [CrossRef]
- Gupta, S.; Kim, S.Y.; Artis, S.; Molfese, D.L.; Schumacher, A.; Sweatt, J.D.; Paylor, R.E.; Lubin, F.D. Histone Methylation Regulates Memory Formation. J. Neurosci. 2010, 30, 3589–3599. [Google Scholar] [CrossRef]
- Ryu, H.; Barrup, M.; Kowall, N.W.; McKee, A.C. P3-260: Epigenetic modification in a monozygotic twin with Alzheimer’s disease. Alzheimer’s Dement. 2008, 4, T598. [Google Scholar] [CrossRef]
- Coneys, R.; Wood, I.C. Alzheimer’s disease: The potential of epigenetic treatments and current clinical candidates. Neurodegener. Dis. Manag. 2020, 10, 543–558. [Google Scholar] [CrossRef]
- Maity, S.; Farrell, K.; Navabpour, S.; Narayanan, S.N.; Jarome, T.J. Epigenetic Mechanisms in Memory and Cognitive Decline Associated with Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12280. [Google Scholar] [CrossRef]
- Surguchov, A. α-Synuclein and Mechanisms of Epigenetic Regulation. Brain Sci. 2023, 13, 150. [Google Scholar] [CrossRef]
- Stefanelli, G.; Walters, B.J.; Ramzan, F.; Narkaj, K.; Tao, C.; Zovkic, I.B. Epigenetic Mechanisms of Learning and Memory. In Molecular-Genetic and Statistical Techniques for Behavioral and Neural Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–382. [Google Scholar]
- López, A.J.; Hecking, J.K.; White, A.O. The Emerging Role of ATP-Dependent Chromatin Remodeling in Memory and Substance Use Disorders. Int. J. Mol. Sci. 2020, 21, 6816. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Spengler, D. Chromatin Remodeling Complex NuRD in Neurodevelopment and Neurodevelopmental Disorders. Front. Genet. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Iwasa, J.; Cairns, B.R.; Peterson, C.L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 2017, 18, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Alfert, A.; Moreno, N.; Kerl, K. The BAF complex in development and disease. Epigenetics Chromatin 2019, 12, 19. [Google Scholar] [CrossRef]
- Romanowska, J.; Joshi, A. From Genotype to Phenotype: Through Chromatin. Genes 2019, 10, 76. [Google Scholar] [CrossRef]
- Marakulina, D.; Vorontsov, I.E.; Kulakovskiy, I.V.; Lennartsson, A.; Drabløs, F.; Medvedeva, Y.A. EpiFactors 2022: Expansion and enhancement of a curated database of human epigenetic factors and complexes. Nucleic Acids Res. 2023, 51, D564–D570. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
- van der Sluijs, P.J.; Alders, M.; Dingemans, A.J.M.; Parbhoo, K.; van Bon, B.W.; Dempsey, J.C.; Doherty, D.; den Dunnen, J.T.; Gerkes, E.H.; Milller, I.M.; et al. A Case Series of Familial ARID1B Variants Illustrating Variable Expression and Suggestions to Update the ACMG Criteria. Genes 2021, 12, 1275. [Google Scholar] [CrossRef]
- Wang, X.; Nagl, N.G.; Wilsker, D.; van Scoy, M.; Pacchione, S.; Yaciuk, P.; Dallas, P.B.; Moran, E. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem. J. 2004, 383, 319–325. [Google Scholar] [CrossRef]
- Azad, P.; Caldwell, A.B.; Ramachandran, S.; Spann, N.J.; Akbari, A.; Villafuerte, F.C.; Bermudez, D.; Zhao, H.; Poulsen, O.; Zhou, D.; et al. ARID1B, a molecular suppressor of erythropoiesis, is essential for the prevention of Monge’s disease. Exp. Mol. Med. 2022, 54, 777–787. [Google Scholar] [CrossRef]
- Nishiyama, A.; Yamaguchi, L.; Sharif, J.; Johmura, Y.; Kawamura, T.; Nakanishi, K.; Shimamura, S.; Arita, K.; Kodama, T.; Ishikawa, F.; et al. Uhrf1-dependent H3K23 ubiquitylation couples maintenance DNA methylation and replication. Nature 2013, 502, 249–253. [Google Scholar] [CrossRef]
- Xie, S.; Qian, C. The Growing Complexity of UHRF1-Mediated Maintenance DNA Methylation. Genes 2018, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Bashtrykov, P.; Jankevicius, G.; Jurkowska, R.Z.; Ragozin, S.; Jeltsch, A. The UHRF1 Protein Stimulates the Activity and Specificity of the Maintenance DNA Methyltransferase DNMT1 by an Allosteric Mechanism. J. Biol. Chem. 2014, 289, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.V.C. Get Out and Stay Out: New Insights Into DNA Methylation Reprogramming in Mammals. Front. Cell Dev. Biol. 2021, 8, 629068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef]
- Nemeth, Z.; Kiss, E.; Takacs, I. The Role of Epigenetic Regulator SIRT1 in Balancing the Homeostasis and Preventing the Formation of Specific “Soil” of Metabolic Disorders and Related Cancers. Front. Biosci. 2022, 27, 253. [Google Scholar] [CrossRef]
- Adamkova, K.; Yi, Y.-J.; Petr, J.; Zalmanova, T.; Hoskova, K.; Jelinkova, P.; Moravec, J.; Kralickova, M.; Sutovsky, M.; Sutovsky, P.; et al. SIRT1-dependent modulation of methylation and acetylation of histone H3 on lysine 9 (H3K9) in the zygotic pronuclei improves porcine embryo development. J. Anim. Sci. Biotechnol. 2017, 8, 83. [Google Scholar] [CrossRef]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11, 585821. [Google Scholar] [CrossRef]
- Dobbin, M.M.; Madabhushi, R.; Pan, L.; Chen, Y.; Kim, D.; Gao, J.; Ahanonu, B.; Pao, P.-C.; Qiu, Y.; Zhao, Y.; et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 2013, 16, 1008–1015. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lu, C.-H.; Ke, C.-C.; Chang, C.-W.; Yang, B.-H.; Gelovani, J.G.; Liu, R.-S. Monitoring HDAC4 Expression in Alzheimer’s Disease Using [18F]TFAHA-PET. Springer Proc. Phys. 2022, 272, 61–70. [Google Scholar]
- Mielcarek, M.; Zielonka, D.; Carnemolla, A.; Marcinkowski, J.T.; Guidez, F. HDAC4 as a potential therapeutic target in neurodegenerative diseases: A summary of recent achievements. Front. Cell. Neurosci. 2015, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Colussi, C.; Aceto, G.; Ripoli, C.; Bertozzi, A.; Li Puma, D.D.; Paccosi, E.; D’Ascenzo, M.; Grassi, C. Cytoplasmic HDAC4 recovers synaptic function in the 3×Tg mouse model of Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2023, 49, 12861. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Tekwani, B.L. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front. Pharmacol. 2020, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.M.; Farinelli-Scharly, M.; Hugues-Ascery, S.; Sanchez-Mut, J.V.; Santoni, G.; Gräff, J. The HDAC inhibitor CI-994 acts as a molecular memory aid by facilitating synaptic and intracellular communication after learning. Proc. Natl. Acad. Sci. USA 2022, 119, e2116797119. [Google Scholar] [CrossRef]
- Janczura, K.J.; Volmar, C.-H.; Sartor, G.C.; Rao, S.J.; Ricciardi, N.R.; Lambert, G.; Brothers, S.P.; Wahlestedt, C. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E11148–E11157. [Google Scholar] [CrossRef]
- Yin, B.-K.; Wang, Z.-Q. Beyond HAT Adaptor: TRRAP Liaisons with Sp1-Mediated Transcription. Int. J. Mol. Sci. 2021, 22, 12445. [Google Scholar] [CrossRef]
- Grand, R.S.; Burger, L.; Gräwe, C.; Michael, A.K.; Isbel, L.; Hess, D.; Hoerner, L.; Iesmantavicius, V.; Durdu, S.; Pregnolato, M.; et al. BANP opens chromatin and activates CpG-island-regulated genes. Nature 2021, 596, 133–137. [Google Scholar] [CrossRef]
- Elsässer, S.J.; Huang, H.; Lewis, P.W.; Chin, J.W.; Allis, C.D.; Patel, D.J. DAXX envelops a histone H3.3–H4 dimer for H3.3-specific recognition. Nature 2012, 491, 560–565. [Google Scholar] [CrossRef]
- Voon, H.P.J.; Wong, L.H. New players in heterochromatin silencing: Histone variant H3.3 and the ATRX/DAXX chaperone. Nucleic Acids Res. 2016, 44, 1496–1501. [Google Scholar] [CrossRef]
- Huang, L.; Agrawal, T.; Zhu, G.; Yu, S.; Tao, L.; Lin, J.; Marmorstein, R.; Shorter, J.; Yang, X. DAXX represents a new type of protein-folding enabler. Nature 2021, 597, 132–137. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Grotzinger, A.D.; Marioni, R.E.; Nivard, M.G.; Tucker-Drob, E.M. Integrated analysis of direct and proxy genome wide association studies highlights polygenicity of Alzheimer’s disease outside of the APOE region. PLoS Genet. 2022, 18, e1010208. [Google Scholar] [CrossRef] [PubMed]
- Schriml, L.M.; Arze, C.; Nadendla, S.; Chang, Y.-W.W.; Mazaitis, M.; Felix, V.; Feng, G.; Kibbe, W.A. Disease Ontology: A backbone for disease semantic integration. Nucleic Acids Res. 2012, 40, D940–D946. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Motoki, K.; Tagawa, K.; Chen, X.; Hama, H.; Nakajima, K.; Homma, H.; Tamura, T.; Watanabe, H.; Katsuno, M.; et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci. Rep. 2016, 6, 31895. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Koolen, D.A.; Kramer, J.M.; Neveling, K.; Nillesen, W.M.; Moore-Barton, H.L.; Elmslie, F.V.; Toutain, A.; Amiel, J.; Malan, V.; Tsai, A.C.-H.; et al. Mutations in the chromatin modifier gene KANSL1 cause the 17q21.31 microdeletion syndrome. Nat. Genet. 2012, 44, 639–641. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, I.; Youm, E.M.; Lee, S.; Park, J.-H.; Lee, J.; Lee, D.Y.; Byun, M.S.; Lee, J.H.; Yi, D.; et al. Novel Alzheimer’s disease risk variants identified based on whole-genome sequencing of APOE ε4 carriers. Transl. Psychiatry 2021, 11, 296. [Google Scholar] [CrossRef]
- Yuan, J.; Chang, S.-Y.; Yin, S.-G.; Liu, Z.-Y.; Cheng, X.; Liu, X.-J.; Jiang, Q.; Gao, G.; Lin, D.-Y.; Kang, X.-L.; et al. Two conserved epigenetic regulators prevent healthy ageing. Nature 2020, 579, 118–122. [Google Scholar] [CrossRef]
- Hu, K.; Li, Y.; Yu, H.; Hu, Y. CTBP1 Confers Protection for Hippocampal and Cortical Neurons in Rat Models of Alzheimer’s Disease. Neuroimmunomodulation 2019, 26, 139–152. [Google Scholar] [CrossRef]
- Byun, J.S.; Gardner, K. C-Terminal Binding Protein: A Molecular Link between Metabolic Imbalance and Epigenetic Regulation in Breast Cancer. Int. J. Cell Biol. 2013, 2013, 647975. [Google Scholar] [CrossRef]
- Chen, Z. The transrepression and transactivation roles of CtBPs in the pathogenesis of different diseases. J. Mol. Med. 2021, 99, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Donmez, G. The Effects of SIRT1 on Alzheimer’s Disease Models. Int. J. Alzheimers Dis. 2012, 2012, 509529. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuźnicka, M.; Kuźnicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.A.; Hitz, B.C.; Sloan, C.A.; Chan, E.T.; Davidson, J.M.; Gabdank, I.; Hilton, J.A.; Jain, K.; Baymuradov, U.K.; Narayanan, A.K.; et al. The Encyclopedia of DNA elements (ENCODE): Data portal update. Nucleic Acids Res. 2018, 46, D794–D801. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, F.; Guo, X.; Jiang, Y. Decreased MEF2A Expression Regulated by Its Enhancer Methylation Inhibits Autophagy and May Play an Important Role in the Progression of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 682247. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Roussos, P.; McKenzie, A.; Zhou, X.; Kajiwara, Y.; Brennand, K.J.; De Luca, G.C.; Crary, J.F.; Casaccia, P.; Buxbaum, J.D.; et al. Integrative network analysis of nineteen brain regions identifies molecular signatures and networks underlying selective regional vulnerability to Alzheimer’s disease. Genome Med. 2016, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, M.; Wu, Y.; Jiang, D.; Wu, T.; Zhao, Y.; Wu, D.; Cui, J.; Li, G. Regulation of the Late Onset alzheimer’s Disease Associated HLA-DQA1/DRB1 Expression. Am. J. Alzheimer’s Dis. Other Dement. 2022, 37, 153331752210850. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hoekzema, K.; Vecchio, D.; Wu, H.; Sulovari, A.; Coe, B.P.; Gillentine, M.A.; Wilfert, A.B.; Perez-Jurado, L.A.; Kvarnung, M.; et al. Large-scale targeted sequencing identifies risk genes for neurodevelopmental disorders. Nat. Commun. 2020, 11, 4932. [Google Scholar] [CrossRef]
- Rikin, A.; Evans, T. The tbx/bHLH transcription factor mga regulates gata4 and organogenesis. Dev. Dyn. 2010, 239, 535–547. [Google Scholar] [CrossRef]
- Zhang, B.; Chambers, K.J.; Faller, D.V.; Wang, S. Reprogramming of the SWI/SNF complex for co-activation or co-repression in prohibitin-mediated estrogen receptor regulation. Oncogene 2007, 26, 7153–7157. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Makaremi, S.; Rahimi, A.; Asghariazar, V.; Taghadosi, M.; Safarzadeh, E. DNA methylation changes and inflammaging in aging-associated diseases. Epigenomics 2022, 14, 965–986. [Google Scholar] [CrossRef] [PubMed]
- Mitchener, M.M.; Muir, T.W. Oncohistones: Exposing the nuances and vulnerabilities of epigenetic regulation. Mol. Cell 2022, 82, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Occean, J.R.; Sen, P. Epigenetic dysregulation in cardiovascular aging and disease. J. Cardiovasc. Aging 2021, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Gu, X.; Jia, R.; Ge, S.; Chai, P.; Zhuang, A.; Fan, X. Crosstalk between metabolic reprogramming and epigenetics in cancer: Updates on mechanisms and therapeutic opportunities. Cancer Commun. 2022, 42, 1049–1082. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A.; Mehler, M.F. Understanding Neurological Disease Mechanisms in the Era of Epigenetics. JAMA Neurol. 2013, 70, 703. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, B.; Montecino, M. Epigenetic Changes and Chromatin Reorganization in Brain Function: Lessons from Fear Memory Ensemble and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12081. [Google Scholar] [CrossRef]
- Giallongo, S.; Longhitano, L.; Denaro, S.; D’Aprile, S.; Torrisi, F.; La Spina, E.; Giallongo, C.; Mannino, G.; Lo Furno, D.; Zappalà, A.; et al. The Role of Epigenetics in Neuroinflammatory-Driven Diseases. Int. J. Mol. Sci. 2022, 23, 15218. [Google Scholar] [CrossRef]
- Yu, M.; Gilbert, S.; Li, Y.; Zhang, H.; Qiao, Y.; Lu, Y.; Tang, Y.; Zhen, Q.; Cheng, Y.; Liu, Y. Association of NCOA3 polymorphisms with Dyslipidemia in the Chinese Han population. Lipids Health Dis. 2015, 14, 124. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Liao, W.-L.; Lin, Y.-J.; Chen, S.-Y.; Tsai, F.-J. Association Between SRC-1 Gene Polymorphisms and Coronary Artery Aneurysms Formation in Taiwanese Children With Kawasaki Disease. J. Clin. Lab. Anal. 2014, 28, 435–439. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Li, L.; Yang, Y.; Yang, J.; Wang, Y.; Wu, J.; Wu, X.; Shan, L.; Pei, F.; et al. SNP rs4971059 predisposes to breast carcinogenesis and chemoresistance via TRIM46-mediated HDAC1 degradation. EMBO J. 2021, 40, e107974. [Google Scholar] [CrossRef]
- Liu, L.-C.; Chien, Y.-C.; Wu, G.-W.; Hua, C.-H.; Tsai, I.-C.; Hung, C.-C.; Wu, T.-K.; Pan, Y.-R.; Yang, S.-F.; Yu, Y.-L. Analysis of EZH2 Genetic Variants on Triple-Negative Breast Cancer Susceptibility and Pathology. Int. J. Med. Sci. 2022, 19, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, G.; Liu, P.; Lu, K.; Han, Z.; Liu, H.; Du, M.; Wang, M.; Chu, H.; Zhang, Z. Evaluation of genetic variants in nucleosome remodeling and deacetylase (NuRD) complex subunits encoding genes and gastric cancer susceptibility. Arch. Toxicol. 2022, 96, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Huet, S.; Xerri, L.; Tesson, B.; Mareschal, S.; Taix, S.; Mescam-Mancini, L.; Sohier, E.; Carrère, M.; Lazarovici, J.; Casasnovas, O.; et al. EZH2 alterations in follicular lymphoma: Biological and clinical correlations. Blood Cancer J. 2017, 7, e555. [Google Scholar] [CrossRef]
- Li, J.; You, Y.; Yue, W.; Yu, H.; Lu, T.; Wu, Z.; Jia, M.; Ruan, Y.; Liu, J.; Zhang, D.; et al. Chromatin remodeling gene EZH2 involved in the genetic etiology of autism in Chinese Han population. Neurosci. Lett. 2016, 610, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Colli, L.M.; Jessop, L.; Myers, T.A.; Camp, S.Y.; Machiela, M.J.; Choi, J.; Cunha, R.; Onabajo, O.; Mills, G.C.; Schmid, V.; et al. Altered regulation of DPF3, a member of the SWI/SNF complexes, underlies the 14q24 renal cancer susceptibility locus. Am. J. Hum. Genet. 2021, 108, 1590–1610. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Verma, H.; Choudhary, S.; Kaur, S.; Silakari, O. Functional relationship of SNP (Ala490Thr) of an epigenetic gene EZH2 results in the progression and poor survival of ER+/tamoxifen treated breast cancer patients. J. Genet. 2021, 100, 86. [Google Scholar] [CrossRef]
- Zhao, J.; Huai, J. Role of primary aging hallmarks in Alzheimer’s disease. Theranostics 2023, 13, 197–230. [Google Scholar] [CrossRef]
- Maurano, M.T.; Humbert, R.; Rynes, E.; Thurman, R.E.; Haugen, E.; Wang, H.; Reynolds, A.P.; Sandstrom, R.; Qu, H.; Brody, J.; et al. Systematic Localization of Common Disease-Associated Variation in Regulatory DNA. Science 2012, 337, 1190–1195. [Google Scholar] [CrossRef]
- Farh, K.K.-H.; Marson, A.; Zhu, J.; Kleinewietfeld, M.; Housley, W.J.; Beik, S.; Shoresh, N.; Whitton, H.; Ryan, R.J.H.; Shishkin, A.A.; et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015, 518, 337–343. [Google Scholar] [CrossRef]
- Degtyareva, A.O.; Antontseva, E.V.; Merkulova, T.I. Regulatory SNPs: Altered Transcription Factor Binding Sites Implicated in Complex Traits and Diseases. Int. J. Mol. Sci. 2021, 22, 6454. [Google Scholar] [CrossRef]
- Korbolina, E.E.; Bryzgalov, L.O.; Ustrokhanova, D.Z.; Postovalov, S.N.; Poverin, D.V.; Damarov, I.S.; Merkulova, T.I. A Panel of rSNPs Demonstrating Allelic Asymmetry in Both ChIP-seq and RNA-seq Data and the Search for Their Phenotypic Outcomes through Analysis of DEGs. Int. J. Mol. Sci. 2021, 22, 7240. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kim, H.-R.; Chun, M.Y.; Jang, H.; Cho, M.; Kim, B.; Kim, S.; Jeong, J.H.; Yoon, S.J.; Park, K.W.; et al. Transferability of Alzheimer Disease Polygenic Risk Score Across Populations and Its Association With Alzheimer Disease-Related Phenotypes. JAMA Netw. Open 2022, 5, e2247162. [Google Scholar] [CrossRef]
- Harvey, C.T.; Moyerbrailean, G.A.; Davis, G.O.; Wen, X.; Luca, F.; Pique-Regi, R. QuASAR: Quantitative allele-specific analysis of reads. Bioinformatics 2015, 31, 1235–1242. [Google Scholar] [CrossRef]
- Cavalli, M.; Pan, G.; Nord, H.; Wallerman, O.; Wallén Arzt, E.; Berggren, O.; Elvers, I.; Eloranta, M.-L.; Rönnblom, L.; Lindblad Toh, K.; et al. Allele-specific transcription factor binding to common and rare variants associated with disease and gene expression. Hum. Genet. 2016, 135, 485–497. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Gu, Z.; Chen, L.; He, B. Zinc-dependent deacetylases (HDACs) as potential targets for treating Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2022, 76, 129015. [Google Scholar] [CrossRef]
- Kumar, V.; Kundu, S.; Singh, A.; Singh, S. Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr. Neuropharmacol. 2022, 20, 158–178. [Google Scholar] [CrossRef]
- Harris, L.D.; Jasem, S.; Licchesi, J.D.F. The Ubiquitin System in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020, 1233, 195–221. [Google Scholar]
- Watanabe, Y.; Taguchi, K.; Tanaka, M. Ubiquitin, Autophagy and Neurodegenerative Diseases. Cells 2020, 9, 2022. [Google Scholar] [CrossRef]
- Genome Reference Consortium Human Build 38. Available online: https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.26/ (accessed on 14 March 2023).
- Korbolina, E.E.; Brusentsov, I.I.; Bryzgalov, L.O.; Leberfarb, E.Y.; Degtyareva, A.O.; Merkulova, T.I. Novel approach to functional SNPs discovery from genome-wide data reveals promising variants for colon cancer risk. Hum. Mutat. 2018, 39, 851–859. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Adwan, L.; Zawia, N.H. Epigenetics: A novel therapeutic approach for the treatment of Alzheimer’s disease. Pharmacol. Ther. 2013, 139, 41–50. [Google Scholar] [CrossRef]
| rSNP_ID | Gene Symbol | Function | Modification | PMID | Complex Name | Target | Specific Target |
|---|---|---|---|---|---|---|---|
| rs2281391 | ARID1B | HMW | Histone ubiquitination | 20086098 | BAF, nBAF, npBAF, PBAF, SWI/SNF-like_EPAFa, SWI/SNF-like EPAFB, SWI/SNF BRM-BRG1 | Histone, DNA | H2BK120, DNA motif |
| rs536224963 | BANP | HMW | Histone acetylation | 16166625 | # | Histone | H3K9, H4K8 |
| rs3130018 | DAXX | # | # | 23075851 | # | Histone | H3.3 |
| rs946554101 | HDAC4 | HME | Histone acetylation | 10220385 | # | Histone | H2AKac, H2BKac, H3Kac, H4Kac |
| rs1053224730 | SIRT1 | HME, HMW cofactor | Histone acetylation, Histone methylation | 15469825 | eNoSc | Histone | H1K26ac, H3K9ac, H4K16ac |
| rs1249650489 | TRRAP | HMW cofactor | Histone acetylation | 14966270 | SWR, PCAF, TFTC-HAT, NuA4, SAGA, NuA4-related complex, STAGA | Histone | # |
| rs958341678 | UHRF1 | HMR, HMW cofactor | Histone ubiquitination | 17967883 | # | Histone, DNA | H3K9me3, H3R2, H3, mCG |
| rSNP ID | TF | Target Genes | N |
|---|---|---|---|
| rs1022095596 | CLOCK | ZBED6, HSD17B8, ZC3H11A, SMG5, DAXX, GPC1, HDAC4 | 7 |
| rs977886453 rs1279727503 rs924734233 | DPF2 | AGAP1, HERC6, DAXX, ZBED6, ZC3H11A, HSD17B8, HDAC4, TFDP2, SMG5 | 9 |
| rs1370216229 | CUX1 | TFDP2, ZBED6, ZC3H11A | 3 |
| rs1255551090 | BCL11A | HERC6, AGAP1 | 2 |
| rs995147107 | FOSL2 | TFDP2, HDAC4, DAXX, AGAP1, HSD17B8, SMG5 | 6 |
| rs992579579 | FOXK2 | DAXX, HSD17B8, HDAC4, TFDP2, AGAP1, ZC3H11A, SMG5, ZBED6 | 8 |
| rs1465639308 rs975045833 | IRF1 | DAXX, ZC3H11A, HDAC4, TFDP2, HERC6, GPC1, AGAP1, SMG5, ZBED6, HSD17B8 | 10 |
| rs2570800 rs1402353341 | MEF2A | SMG5, HERC6, ZC3H11A, AGAP1, ZBED6, SMG5, HERC6, ZC3H11A, AGAP1, ZBED6 | 10 |
| rs1286079777 | MGA | HDAC4, TFDP2, AGAP1, ZC3H11A, ZBED6, DAXX | 6 |
| rs954995579 rs1043408625 rs954995579 | NFIB | HERC6, ZBED6, DAXX, HDAC4 | 4 |
| rs983776002 | NFIC | SMG5, DAXX, ZBED6, ZC3H11A, HSD17B8, HERC6, AGAP1, TFDP2, HDAC4 | 9 |
| rs1484805397 | SREBF1 | HDAC4, ZBED6, ZC3H11A | 3 |
| rs1044184380 | TCF12 | AGAP1, ZBED6, ZC3H11A, HSD17B8, HDAC4, HERC6, GPC1, SMG5, TFDP2, DAXX | 10 |
| rSNP ID | ENCODE-Derived Protein | Target Genes | Function | Modification | PMID | Complex Name | Specific Target |
|---|---|---|---|---|---|---|---|
| rs2281391 | ARID1B | AGAP1 DAXX HSD17B8 TFDP2 ZBED6 ZC3H11A | HMW | Histone ubiquitination | 20086098 | BAF, nBAF, npBAF, PBAF, SWI/SNF-like_EPAFa, SWI/SNF-like EPAFB, SWI/SNF BRM-BRG1 | H2BK120DNA motif |
| rs1363175143 | CHD4 | DAXX HSD17B8 | CR | # | 12592387 | NuRD | # |
| rs1022095596 | CLOCK | DAXX GPC1 HDAC4 HSD17B8 SMG5 ZBED6 ZC3H11A | HMW | Histone acetylation | # | # | H3, H4 |
| rs1013929495 | CTBP1 | AGAP1 DAXX GPC1 HDAC4 HERC6 HSD17B8 SMG5 TFDP2 ZBED6 ZC3H11A | CR | # | 21102443 | LSD-CoREST | # |
| rs977886453 rs1279727503 rs924734233 | DPF2 | AGAP1 DAXX HDAC4 HERC6 HSD17B8 SMG5 TFDP2 ZBED6 ZC3H11A | CR | # | 21888896 | SWI/SNF BRM-BRG1 | # |
| rs1465945079 | EHMT2 | AGAP1 DAXX HDAC4 ZBED6 | HMW | Histone methylation | 18264113 | # | H3K9 |
| rs777573795 | KAT8 | ZBED6 ZC3H11A | HMW | Histone acetylation | 10786633 | NSL, CHD8, MLL2/3, COMPASS-like MLL1,2, MLL4/WBP7 | H2A, H3, H4 |
| rs1286079777 | MGA | AGAP1 DAXX HDAC4 TFDP2 ZBED6 ZC3H11A | HMW cofactor, TF | Histone methylation, histone acetylation, TF activator, TF repressor | # | RING2-L3MBTL2, CHD8, MLL2/3, MLL4/WBP7 | DNA motif |
| rs563166047 | NCOA2 | ZC3H11A | CR cofactor | # | 9590696 | # | # |
| rs1320061320 rs1395087048 | RCOR1 | AGAP1 DAXX GPC1 HDAC4 HERC6 HSD17B8 SMG5 TFDP2 | HME cofactor | Histone acetylation, | 10449787 | BHC, SCL, LSD-CoREST | # |
| rs1346876773 | SAP30 | DAXX GPC1 HSD17B8 SMG5 TFDP2 ZBED6 ZC3H11A | HME cofactor | Histone acetylation | 9651585 | mSin3A, mSin3A-like complex | # |
| rs930121077 rs907151175 | SMARCE1 | AGAP1 DAXX HSD17B8 ZBED6 ZC3H11A ZC3H11A | CR cofactor | # | 12672490 | BAF, nBAF, npBAF, PBAF, SWI/SNF_Brg1(I), SWI/SNF_Brg1(II), SWI/SNF_Brm, SWI/SNF-like_EPAFa, WINAC, SWI/SNF-like EPAFB, bBAF | # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryzgalov, L.O.; Korbolina, E.E.; Merkulova, T.I. Exploring the Genetic Predisposition to Epigenetic Changes in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 7955. https://doi.org/10.3390/ijms24097955
Bryzgalov LO, Korbolina EE, Merkulova TI. Exploring the Genetic Predisposition to Epigenetic Changes in Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(9):7955. https://doi.org/10.3390/ijms24097955
Chicago/Turabian StyleBryzgalov, Leonid O., Elena E. Korbolina, and Tatiana I. Merkulova. 2023. "Exploring the Genetic Predisposition to Epigenetic Changes in Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 9: 7955. https://doi.org/10.3390/ijms24097955
APA StyleBryzgalov, L. O., Korbolina, E. E., & Merkulova, T. I. (2023). Exploring the Genetic Predisposition to Epigenetic Changes in Alzheimer’s Disease. International Journal of Molecular Sciences, 24(9), 7955. https://doi.org/10.3390/ijms24097955





