Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury
Abstract
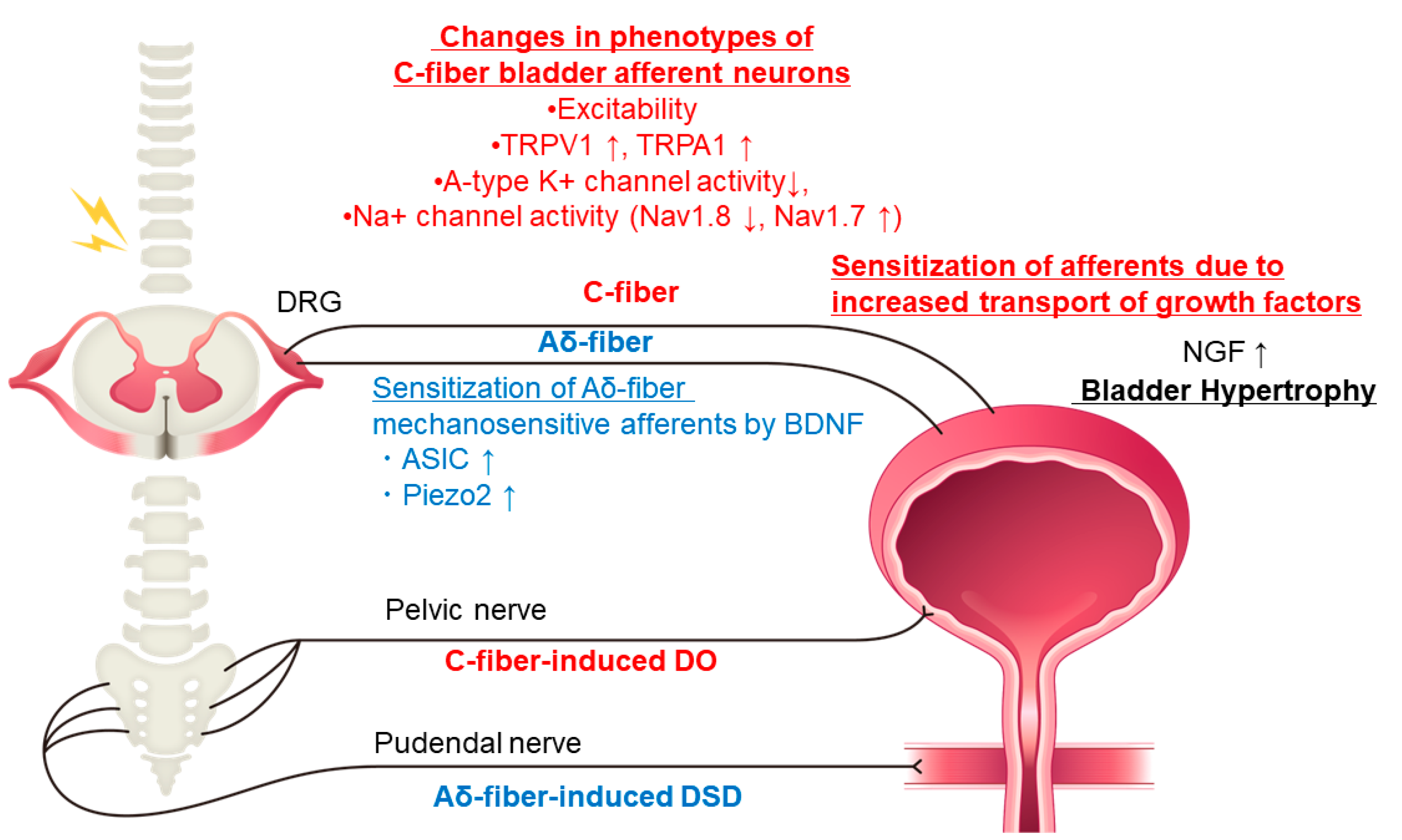
1. Introduction
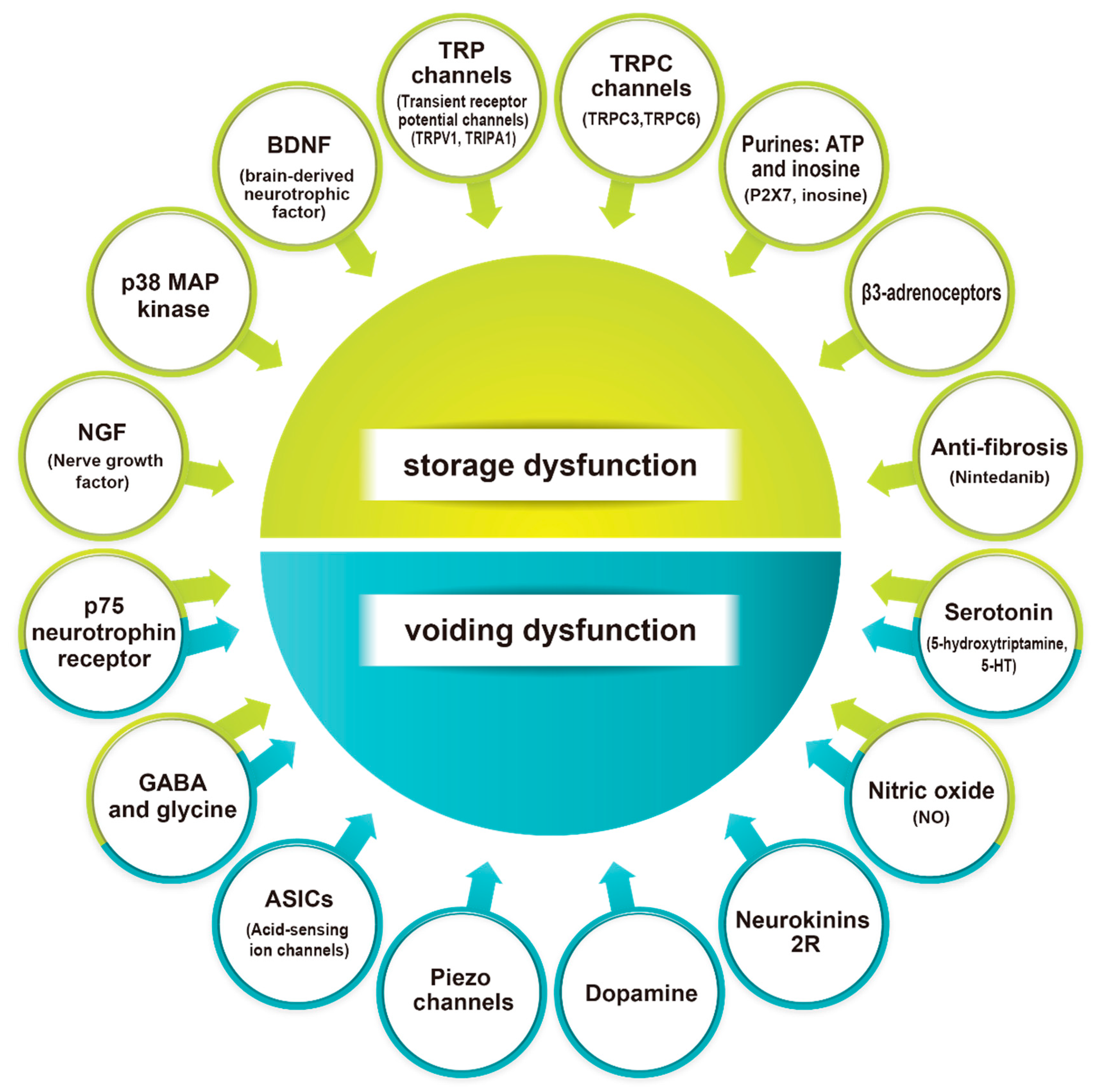
2. Neurotrophic Factors
2.1. NGF (Nerve Growth Factor)
2.2. p38 MAP Kinase
2.3. BDNF (Brain-Derived Neurotrophic Factor)
2.4. p75 Neurotrophin Receptor
3. Transient Receptor Potential (TRP) Channels
3.1. TRPV1 and TRPA1 Receptors
3.2. TRPC Channels (TRPC1, TRPC3, and TRPC6)
4. Mechanosensitive Channels
4.1. ASICs (Acid-Sensing Ion Channels)
4.2. Piezo Channels
5. Neurotransmitters and Their Receptors
5.1. Purines: ATP and Inosine
5.2. Nitric Oxide (NO)
5.3. Inhibitory Neurotransmitters; GABA and Glycine
5.4. Serotonin (5-Hydroxytriptamine, 5-HT)
5.5. Dopamine
5.6. Neurokinins
5.7. β3-Adrenoceptors
6. Tissue Fibrosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Yoshimura, N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2006, 152, 59–84. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Afferent nerve regulation of bladder function in health and disease. In Sensory Nerves; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 91–138. [Google Scholar] [CrossRef]
- Panicker, J.N.; Fowler, C.J.; Kessler, T.M. Lower urinary tract dysfunction in the neurological patient: Clinical assessment and management. Lancet Neurol. 2015, 14, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Lo, S.-H.; Meng, E.; Shen, J.-D.; Chou, E.; Chen, S.-F.; Lee, M.-H.; Hsu, C.-Y.; Ong, H.-L.; Chen, J.-T.; et al. Clinical guidelines of patient-centered bladder management of neurogenic lower urinary tract dysfunction due to chronic spinal cord injury-part 1: Pathophysiology, treatment strategy, and priority. Urol. Sci. 2023, 34, 3–9. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef]
- David, S.; Zarruk, J.G.; Ghasemlou, N. Inflammatory pathways in spinal cord injury. Int. Rev. Neurobiol. 2012, 106, 127–152. [Google Scholar] [CrossRef]
- Shimizu, N.; Doyal, M.F.; Goins, W.F.; Kadekawa, K.; Wada, N.; Kanai, A.J.; de Groat, W.C.; Hirayama, A.; Uemura, H.; Glorioso, J.C.; et al. Morphological changes in different populations of bladder afferent neurons detected by herpes simplex virus (HSV) vectors with cell-type-specific promoters in mice with spinal cord injury. Neuroscience 2017, 364, 190–201. [Google Scholar] [CrossRef]
- Sartori, A.M.; Hofer, A.S.; Scheuber, M.I.; Rust, R.; Kessler, T.M.; Schwab, M.E. Slow development of bladder malfunction parallels spinal cord fiber sprouting and interneurons’ loss after spinal cord transection. Exp. Neurol. 2022, 348, 113937. [Google Scholar] [CrossRef]
- Ochodnicky, P.; Cruz, C.D.; Yoshimura, N.; Cruz, F. Neurotrophins as regulators of urinary bladder function. Nat. Rev. Urol. 2012, 9, 628–637. [Google Scholar] [CrossRef]
- Vizzard, M.A. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog. Brain Res. 2006, 152, 97–115. [Google Scholar] [CrossRef]
- Yoshimura, N.; Bennett, N.E.; Hayashi, Y.; Ogawa, T.; Nishizawa, O.; Chancellor, M.B.; de Groat, W.C.; Seki, S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10847–10855. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Seki, S.; Sasaki, K.; Fraser, M.O.; Igawa, Y.; Nishizawa, O.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J. Urol. 2002, 168, 2269–2274. [Google Scholar] [CrossRef]
- Seki, S.; Sasaki, K.; Igawa, Y.; Nishizawa, O.; Chancellor, M.B.; De Groat, W.C.; Yoshimura, N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J. Urol. 2004, 171, 478–482. [Google Scholar] [CrossRef]
- Wada, N.; Shimizu, T.; Shimizu, N.; de Groat, W.C.; Kanai, A.J.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. The effect of neutralization of nerve growth factor (NGF) on bladder and urethral dysfunction in mice with spinal cord injury. Neurourol. Urodyn. 2018, 37, 1889–1896. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp. Neurol. 2012, 235, 123–132. [Google Scholar] [CrossRef]
- Kadekawa, K.; Majima, T.; Shimizu, T.; Wada, N.; de Groat, W.C.; Kanai, A.J.; Goto, M.; Yoshiyama, M.; Sugaya, K.; Yoshimura, N. The role of capsaicin-sensitive C-fiber afferent pathways in the control of micturition in spinal-intact and spinal cord-injured mice. Am. J. Physiol. Ren. Physiol. 2017, 313, F796–F804. [Google Scholar] [CrossRef]
- Seki, S.; Sasaki, K.; Igawa, Y.; Nishizawa, O.; Chancellor, M.; de Groat, W.; Yoshimura, N. Detrusor overactivity induced by increased levels of nerve growth factor in bladder afferent pathways in rats. Neurourol. Urodyn. 2003, 22, 8. [Google Scholar]
- Steers, W.D.; Tuttle, J.B. Mechanisms of Disease: The role of nerve growth factor in the pathophysiology of bladder disorders. Nat. Clin. Pract. Urol. 2006, 3, 101–110. [Google Scholar] [CrossRef]
- Wada, N.; Matsumoto, S.; Kita, M.; Hashizume, K.; Kakizaki, H. Decreased urinary nerve growth factor reflects prostatic volume reduction and relief of outlet obstruction in patients with benign prostatic enlargement treated with dutasteride. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2014, 21, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Fraser, M.O.; Yu, Y.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J. Urol. 2001, 165, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.; Gebhart, G.F.; Bielefeldt, K. Increased nerve growth factor expression triggers bladder overactivity. J. Pain 2004, 5, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.Y.; Vizzard, M.A. Spinal cord injury-induced expression of TrkA, TrkB, phosphorylated CREB, and c-Jun in rat lumbosacral dorsal root ganglia. J. Comp. Neurol. 2005, 482, 142–154. [Google Scholar] [CrossRef]
- Vizzard, M.A. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2000, 279, R295–R305. [Google Scholar] [CrossRef]
- Shimizu, T.; Majima, T.; Suzuki, T.; Shimizu, N.; Wada, N.; Kadekawa, K.; Takai, S.; Takaoka, E.; Kwon, J.; Kanai, A.J.; et al. Nerve growth factor-dependent hyperexcitability of capsaicin-sensitive bladder afferent neurones in mice with spinal cord injury. Exp. Physiol. 2018, 103, 896–904. [Google Scholar] [CrossRef]
- Takahashi, R.; Yoshizawa, T.; Yunoki, T.; Tyagi, P.; Naito, S.; de Groat, W.C.; Yoshimura, N. Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 α-subunit in rats with spinal cord injury. J. Urol. 2013, 190, 2296–2304. [Google Scholar] [CrossRef]
- Ni, J.; Suzuki, T.; Karnup, S.V.; Gu, B.; Yoshimura, N. Nerve growth factor-mediated Na(+) channel plasticity of bladder afferent neurons in mice with spinal cord injury. Life Sci. 2022, 298, 120524. [Google Scholar] [CrossRef]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Et Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Cheng, H.T.; Dauch, J.R.; Oh, S.S.; Hayes, J.M.; Hong, Y.; Feldman, E.L. p38 mediates mechanical allodynia in a mouse model of type 2 diabetes. Mol. Pain 2010, 6, 28. [Google Scholar] [CrossRef]
- Shimizu, N.; Wada, N.; Shimizu, T.; Suzuki, T.; Kurobe, M.; Kanai, A.J.; de Groat, W.C.; Hashimoto, M.; Hirayama, A.; Uemura, H.; et al. Role of p38 MAP kinase signaling pathways in storage and voiding dysfunction in mice with spinal cord injury. Neurourol. Urodyn. 2020, 39, 108–115. [Google Scholar] [CrossRef]
- Vizzard, M.A. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 2000, 161, 273–284. [Google Scholar] [CrossRef]
- Wada, N.; Shimizu, T.; Shimizu, N.; Kurobe, M.; de Groat, W.C.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. Therapeutic effects of inhibition of brain-derived neurotrophic factor on voiding dysfunction in mice with spinal cord injury. Am. J. Physiology. Ren. Physiol. 2019, 317, F1305–F1310. [Google Scholar] [CrossRef]
- Wada, N.; Yoshimura, N.; Kurobe, M.; Saito, T.; Tyagi, P.; Kakizaki, H. The early, long-term inhibition of brain-derived neurotrophic factor improves voiding, and storage dysfunctions in mice with spinal cord injury. Neurourol. Urodyn. 2020, 39, 1345–1354. [Google Scholar] [CrossRef]
- Garraway, S.M.; Huie, J.R. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast. 2016, 2016, 9857201. [Google Scholar] [CrossRef]
- Paddock, N.; Sheppard, P.; Gardiner, P. Chronic Increases in Daily Neuromuscular Activity Promote Changes in Gene Expression in Small and Large Dorsal Root Ganglion Neurons in Rat. Neuroscience 2018, 388, 171–180. [Google Scholar] [CrossRef]
- Qiao, L.; Vizzard, M.A. Up-regulation of tyrosine kinase (Trka, Trkb) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8-T10) injury. J. Comp. Neurol. 2002, 449, 217–230. [Google Scholar] [CrossRef]
- Frias, B.; Santos, J.; Morgado, M.; Sousa, M.M.; Gray, S.M.; McCloskey, K.D.; Allen, S.; Cruz, F.; Cruz, C.D. The role of brain-derived neurotrophic factor (BDNF) in the development of neurogenic detrusor overactivity (NDO). J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 2146–2160. [Google Scholar] [CrossRef]
- Kadekawa, K.; Yoshimura, N.; Majima, T.; Wada, N.; Shimizu, T.; Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Sugaya, K.; Yoshiyama, M. Characterization of bladder and external urethral activity in mice with or without spinal cord injury—A comparison study with rats. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2016, 310, R752–R758. [Google Scholar] [CrossRef]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Karnup, S.; Kadekawa, K.; Shimizu, N.; Kwon, J.; Shimizu, T.; Gotoh, D.; Kakizaki, H.; de Groat, W.C.; Yoshimura, N. Current Knowledge and Novel Frontiers in Lower Urinary Tract Dysfunction after Spinal Cord Injury: Basic Research Perspectives. Urol. Sci. 2022, 33, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.C.; Tooke, K.; Malley, S.E.; Soulas, A.; Weiss, T.; Ganesh, N.; Saidi, N.; Daugherty, S.; Saragovi, U.; Ikeda, Y.; et al. Role of proNGF/p75 signaling in bladder dysfunction after spinal cord injury. J. Clin. Investig. 2018, 128, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Zabbarova, I.V.; Ikeda, Y.; Carder, E.J.; Wipf, P.; Wolf-Johnston, A.S.; Birder, L.A.; Yoshimura, N.; Getchell, S.E.; Almansoori, K.; Tyagi, P.; et al. Targeting p75 neurotrophin receptors ameliorates spinal cord injury-induced detrusor sphincter dyssynergia in mice. Neurourol. Urodyn. 2018, 37, 2452–2461. [Google Scholar] [CrossRef]
- Zhang, X.; Douglas, K.L.; Jin, H.; Eldaif, B.M.; Nassar, R.; Fraser, M.O.; Dolber, P.C. Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2008, 295, R2084–R2096. [Google Scholar] [CrossRef]
- Andrade, E.L.; Forner, S.; Bento, A.F.; Leite, D.F.; Dias, M.A.; Leal, P.C.; Koepp, J.; Calixto, J.B. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am. J. Physiol. Ren. Physiol. 2011, 300, F1223–F1234. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Charrua, A.; Cruz, C.D.; Gharat, L.; Avelino, A.; Cruz, F. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Auton. Neurosci. 2012, 166, 35–38. [Google Scholar] [CrossRef]
- Shimizu, N.; Wada, N.; Shimizu, T.; Suzuki, T.; Takaoka, E.I.; Kanai, A.J.; de Groat, W.C.; Hirayama, A.; Hashimoto, M.; Uemura, H.; et al. Effects of nerve growth factor neutralization on TRP channel expression in laser-captured bladder afferent neurons in mice with spinal cord injury. Neurosci. Lett. 2018, 683, 100–103. [Google Scholar] [CrossRef]
- Srinivasan, R.; Huang, S.; Chaudhry, S.; Sculptoreanu, A.; Krisky, D.; Cascio, M.; Friedman, P.A.; de Groat, W.C.; Wolfe, D.; Glorioso, J.C. An HSV vector system for selection of ligand-gated ion channel modulators. Nat. Methods 2007, 4, 733–739. [Google Scholar] [CrossRef]
- Shimizu, N.; Saito, T.; Igarashi, N.; Gotoh, D.; Majima, T.; Takai, S.; Hirayama, A.; William, F.G.; Joseph, C.G.; Uemura, H.; et al. Gene therapy with replication-deficient herpes simplex virus vectors encoding poreless TRPV1 or protein phosphatase 1α (PP1α) reduces detrusor overactivity in mice with spinal cord injury. Neurourol. Urodyn. 2019, 38, S145–S146. [Google Scholar]
- Elg, S.; Marmigere, F.; Mattsson, J.P.; Ernfors, P. Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J. Comp. Neurol. 2007, 503, 35–46. [Google Scholar] [CrossRef]
- Mazzone, G.L.; Veeraraghavan, P.; Gonzalez-Inchauspe, C.; Nistri, A.; Uchitel, O.D. ASIC channel inhibition enhances excitotoxic neuronal death in an in vitro model of spinal cord injury. Neuroscience 2017, 343, 398–410. [Google Scholar] [CrossRef]
- McIlwrath, S.L.; Hu, J.; Anirudhan, G.; Shin, J.B.; Lewin, G.R. The sensory mechanotransduction ion channel ASIC2 (acid sensitive ion channel 2) is regulated by neurotrophin availability. Neuroscience 2005, 131, 499–511. [Google Scholar] [CrossRef]
- Lewin, G.R.; Moshourab, R. Mechanosensation and pain. J. Neurobiol. 2004, 61, 30–44. [Google Scholar] [CrossRef]
- Dang, K.; Bielefeldt, K.; Gebhart, G.F. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 3973–3984. [Google Scholar] [CrossRef]
- Lin, S.H.; Cheng, Y.R.; Banks, R.W.; Min, M.Y.; Bewick, G.S.; Chen, C.C. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat. Commun. 2016, 7, 11460. [Google Scholar] [CrossRef]
- Romero, L.O.; Caires, R.; Nickolls, A.R.; Chesler, A.T.; Cordero-Morales, J.F.; Vásquez, V. A dietary fatty acid counteracts neuronal mechanical sensitization. Nat. Commun. 2020, 11, 2997. [Google Scholar] [CrossRef]
- Szczot, M.; Pogorzala, L.A.; Solinski, H.J.; Young, L.; Yee, P.; Le Pichon, C.E.; Chesler, A.T.; Hoon, M.A. Cell-Type-Specific Splicing of Piezo2 Regulates Mechanotransduction. Cell Rep. 2017, 21, 2760–2771. [Google Scholar] [CrossRef]
- Rutlin, M.; Ho, C.Y.; Abraira, V.E.; Cassidy, C.; Bai, L.; Woodbury, C.J.; Ginty, D.D. The cellular and molecular basis of direction selectivity of Aδ-LTMRs. Cell 2014, 159, 1640–1651. [Google Scholar] [CrossRef]
- Schrenk-Siemens, K.; Wende, H.; Prato, V.; Song, K.; Rostock, C.; Loewer, A.; Utikal, J.; Lewin, G.R.; Lechner, S.G.; Siemens, J. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat. Neurosci. 2015, 18, 10–16. [Google Scholar] [CrossRef]
- Dalghi, M.G.; Ruiz, W.G.; Clayton, D.R.; Montalbetti, N.; Daugherty, S.L.; Beckel, J.M.; Carattino, M.D.; Apodaca, G. Functional roles for PIEZO1 and PIEZO2 in urothelial mechanotransduction and lower urinary tract interoception. JCI Insight 2021, 6, e152984. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.; Saade, D.; Ghitani, N.; Coombs, A.M.; Szczot, M.; Keller, J.; Ogata, T.; Daou, I.; Stowers, L.T.; Bönnemann, C.G.; et al. PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 2020, 588, 290–295. [Google Scholar] [CrossRef]
- Saito, T.; Gotoh, D.; Wada, N.; Tyagi, P.; Minagawa, T.; Ogawa, T.; Ishizuka, O.; Yoshimura, N. Time-dependent progression of neurogenic lower urinary tract dysfunction after spinal cord injury in the mouse model. Am. J. Physiol. Ren. Physiol. 2021, 321, F26–F32. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Somogyi, G.T.; Boone, T.B.; Ford, A.P.; Smith, C.P. Modulation of bladder afferent signals in normal and spinal cord-injured rats by purinergic P2X3 and P2X2/3 receptors. BJU Int. 2012, 110, E409–E414. [Google Scholar] [CrossRef]
- Smith, C.P.; Gangitano, D.A.; Munoz, A.; Salas, N.A.; Boone, T.B.; Aoki, K.R.; Francis, J.; Somogyi, G.T. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 2008, 52, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Yazdi, I.K.; Tang, X.; Rivera, C.; Taghipour, N.; Grossman, R.G.; Boone, T.B.; Tasciotti, E. Localized inhibition of P2X7R at the spinal cord injury site improves neurogenic bladder dysfunction by decreasing urothelial P2X3R expression in rats. Life Sci. 2017, 171, 60–67. [Google Scholar] [CrossRef]
- Salas, N.A.; Somogyi, G.T.; Gangitano, D.A.; Boone, T.B.; Smith, C.P. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem. Int. 2007, 50, 345–350. [Google Scholar] [CrossRef]
- Chung, Y.G.; Seth, A.; Doyle, C.; Franck, D.; Kim, D.; Cristofaro, V.; Benowitz, L.I.; Tu, D.D.; Estrada, C.R.; Mauney, J.R.; et al. Inosine Improves Neurogenic Detrusor Overactivity following Spinal Cord Injury. PLoS ONE 2015, 10, e0141492. [Google Scholar] [CrossRef]
- Doyle, C.; Cristofaro, V.; Sullivan, M.P.; Adam, R.M. Inosine—A Multifunctional Treatment for Complications of Neurologic Injury. Cell Physiol. Biochem. 2018, 49, 2293–2303. [Google Scholar] [CrossRef]
- Doyle, C.; Cristofaro, V.; Sack, B.S.; Lukianov, S.N.; Schäfer, M.; Chung, Y.G.; Sullivan, M.P.; Adam, R.M. Inosine attenuates spontaneous activity in the rat neurogenic bladder through an A(2B) pathway. Sci. Rep. 2017, 7, 44416. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kanai, A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: Role of mucosal muscarinic receptors. Am. J. Physiol. Ren. Physiol. 2008, 295, F454–F461. [Google Scholar] [CrossRef]
- McCarthy, C.J.; Zabbarova, I.V.; Brumovsky, P.R.; Roppolo, J.R.; Gebhart, G.F.; Kanai, A.J. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J. Urol. 2009, 181, 1459–1466. [Google Scholar] [CrossRef]
- Gotoh, D.; Saito, T.; Karnup, S.; Morizawa, Y.; Hori, S.; Nakai, Y.; Miyake, M.; Torimoto, K.; Fujimoto, K.; Yoshimura, N. Therapeutic effects of a soluble guanylate cyclase activator, BAY 60-2770, on lower urinary tract dysfunction in mice with spinal cord injury. Am. J. Physiol. Ren. Physiol. 2022, 323, F447–F454. [Google Scholar] [CrossRef]
- Zhang, F.; Liao, L.; Ju, Y.; Song, A.; Liu, Y. Neurochemical plasticity of nitric oxide synthase isoforms in neurogenic detrusor overactivity after spinal cord injury. Neurochem. Res. 2011, 36, 1903–1909. [Google Scholar] [CrossRef]
- Lin, G.; Huang, Y.C.; Wang, G.; Lue, T.F.; Lin, C.S. Prominent expression of phosphodiesterase 5 in striated muscle of the rat urethra and levator ani. J. Urol. 2010, 184, 769–774. [Google Scholar] [CrossRef]
- Reitz, A.; Knapp, P.A.; Müntener, M.; Schurch, B. Oral nitric oxide donors: A new pharmacological approach to detrusor-sphincter dyssynergia in spinal cord injured patients? Eur. Urol. 2004, 45, 516–520. [Google Scholar] [CrossRef]
- Kadekawa, K.; Majima, T.; Kawamorita, N.; Okada, H.; Yoshizawa, T.; Mori, K.; Tyagi, P.; Sugaya, K.; Yoshimura, N. Effects of an alpha1A/D-adrenoceptor antagonist, naftopidil, and a phosphodiesterase type 5 inhibitor, tadalafil, on urinary bladder remodeling in rats with spinal cord injury. Neurourol. Urodyn. 2017, 36, 1488–1495. [Google Scholar] [CrossRef]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Ashitomi, K.; Ohyama, C.; Ogawa, Y. Rectal distention inhibits bladder activity via glycinergic and GABAergic mechanisms in rats. J. Urol. 2004, 171, 1353–1356. [Google Scholar] [CrossRef]
- Miyazato, M.; Sasatomi, K.; Hiragata, S.; Sugaya, K.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R336–R342. [Google Scholar] [CrossRef]
- Miyazato, M.; Sasatomi, K.; Hiragata, S.; Sugaya, K.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J. Urol. 2008, 179, 1178–1183. [Google Scholar] [CrossRef]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Ashitomi, K.; Hatano, T.; Ogawa, Y. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp. Neurol. 2003, 183, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Ashitomi, K.; Morozumi, M.; Ogawa, Y. Dietary glycine inhibits bladder activity in normal rats and rats with spinal cord injury. J. Urol. 2005, 173, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sugaya, K.; Goins, W.F.; Wolfe, D.; Goss, J.R.; Chancellor, M.B.; de Groat, W.C.; Glorioso, J.C.; Yoshimura, N. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther. 2009, 16, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sugaya, K.; Saito, S.; Chancellor, M.B.; Goins, W.F.; Goss, J.R.; de Groat, W.C.; Glorioso, J.C.; Yoshimura, N. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J. Urol. 2010, 184, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Ni, J.; Wang, X.; Tu, H.; Gu, B.; Si, J.; Wu, G.; Andersson, K.E. Chronic spinal cord injury causes upregulation of serotonin (5-HT)(2A) and 5-HT(2C) receptors in lumbosacral cord motoneurons. BJU Int. 2018, 121, 145–154. [Google Scholar] [CrossRef]
- Lin, C.Y.; Sparks, A.; Lee, Y.S. Improvement of lower urinary tract function by a selective serotonin 5-HT(1A) receptor agonist, NLX-112, after chronic spinal cord injury. Exp. Neurol. 2020, 332, 113395. [Google Scholar] [CrossRef]
- Ni, J.; Cao, N.; Wang, X.; Zhan, C.; Si, J.; Gu, B.; Andersson, K.E. The serotonin (5-hydroxytryptamine) 5-HT(7) receptor is up-regulated in Onuf’s nucleus in rats with chronic spinal cord injury. BJU Int. 2019, 123, 718–725. [Google Scholar] [CrossRef]
- Hou, S.; Carson, D.M.; Wu, D.; Klaw, M.C.; Houlé, J.D.; Tom, V.J. Dopamine is produced in the rat spinal cord and regulates micturition reflex after spinal cord injury. Exp. Neurol. 2016, 285, 136–146. [Google Scholar] [CrossRef]
- Hou, S.; DeFinis, J.H.; Daugherty, S.L.; Tang, C.; Weinberger, J.; de Groat, W.C. Deciphering Spinal Endogenous Dopaminergic Mechanisms That Modulate Micturition Reflexes in Rats with Spinal Cord Injury. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Qiao, Y.; Brodnik, Z.D.; Zhao, S.; Trueblood, C.T.; Li, Z.; Tom, V.J.; España, R.A.; Hou, S. Spinal Dopaminergic Mechanisms Regulating the Micturition Reflex in Male Rats with Complete Spinal Cord Injury. J. Neurotrauma 2021, 38, 803–817. [Google Scholar] [CrossRef]
- Seki, S.; Erickson, K.A.; Seki, M.; Nishizawa, O.; Igawa, Y.; Ogawa, T.; de Groat, W.C.; Chancellor, M.B.; Yoshimura, N. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am. J. Physiol. Ren. Physiol. 2005, 288, F466–F473. [Google Scholar] [CrossRef]
- Burcher, E.; Shang, F.; Warner, F.J.; Du, Q.; Lubowski, D.Z.; King, D.W.; Liu, L. Tachykinin NK2 receptor and functional mechanisms in human colon: Changes with indomethacin and in diverticular disease and ulcerative colitis. J. Pharmacol. Exp. Ther. 2008, 324, 170–178. [Google Scholar] [CrossRef]
- Warner, F.J.; Miller, R.C.; Burcher, E. Human tachykinin NK2 receptor: A comparative study of the colon and urinary bladder. Clin. Exp. Pharmacol. Physiol. 2003, 30, 632–639. [Google Scholar] [CrossRef]
- Kullmann, F.A.; Katofiasc, M.; Thor, K.B.; Marson, L. Pharmacodynamic evaluation of Lys(5), MeLeu(9), Nle(10)-NKA((4-10)) prokinetic effects on bladder and colon activity in acute spinal cord transected and spinally intact rats. Naunyn. Schmiedebergs Arch. Pharm. 2017, 390, 163–173. [Google Scholar] [CrossRef]
- Marson, L.; Thor, K.B.; Katofiasc, M.; Burgard, E.C.; Rupniak, N.M.J. Prokinetic effects of neurokinin-2 receptor agonists on the bladder and rectum of rats with acute spinal cord transection. Eur. J. Pharmacol. 2018, 819, 261–269. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Katofiasc, M.; Walz, A.; Thor, K.B.; Burgard, E.C. [Lys(5),MeLeu(9),Nle(10)]-NKA((4-10)) Elicits NK2 Receptor-Mediated Micturition and Defecation, and NK1 Receptor-Mediated Emesis and Hypotension, in Conscious Dogs. J. Pharmacol. Exp. Ther. 2018, 366, 136–144. [Google Scholar] [CrossRef]
- Marson, L.; Piatt, R.K., 2nd; Katofiasc, M.A.; Bobbitt, C.; Thor, K.B. Chronic, Twice-Daily Dosing of an NK2 Receptor Agonist [Lys(5),MeLeu(9),Nle(10)]-NKA(4-10), Produces Consistent Drug-Induced Micturition and Defecation in Chronic Spinal Rats. J. Neurotrauma 2020, 37, 868–876. [Google Scholar] [CrossRef]
- Yamaguchi, O. Beta3-adrenoceptors in human detrusor muscle. Urology 2002, 59, 25–29. [Google Scholar] [CrossRef]
- Birder, L.A.; Nealen, M.L.; Kiss, S.; de Groat, W.C.; Caterina, M.J.; Wang, E.; Apodaca, G.; Kanai, A.J. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 8063–8070. [Google Scholar] [CrossRef]
- Aizawa, N.; Igawa, Y.; Nishizawa, O.; Wyndaele, J.J. Effects of CL316,243, a beta 3-adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol. Urodyn. 2010, 29, 771–776. [Google Scholar] [CrossRef]
- Kanai, A.; Andersson, K.E. Bladder afferent signaling: Recent findings. J. Urol. 2010, 183, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Igawa, Y.; Nishizawa, O.; Wyndaele, J.J. Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesical acrolein treatment. Eur. Urol. 2011, 59, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Igawa, Y.; Aizawa, N.; Michel, M.C. β3-Adrenoceptors in the normal and diseased urinary bladder-What are the open questions? Br. J. Pharmacol. 2019, 176, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Takasu, T.; Ukai, M.; Sato, S.; Matsui, T.; Nagase, I.; Maruyama, T.; Sasamata, M.; Miyata, K.; Uchida, H.; Yamaguchi, O. Effect of (R)-2-(2-aminothiazol-4-yl)-4’-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J. Pharmacol. Exp. Ther. 2007, 321, 642–647. [Google Scholar] [CrossRef]
- Alexandre, E.C.; Kiguti, L.R.; Calmasini, F.B.; Silva, F.H.; da Silva, K.P.; Ferreira, R.; Ribeiro, C.A.; Mónica, F.Z.; Pupo, A.S.; Antunes, E. Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3 -adrenoceptor activation and α1 -adrenoceptor blockade. Br. J. Pharmacol. 2016, 173, 415–428. [Google Scholar] [CrossRef]
- Sugaya, K.; Nishijima, S.; Kadekawa, K.; Noguchi, K.; Ueda, T.; Yamamoto, H. Mirabegron causes vesical and urethral relaxation in rats with spinal cord injury. Low. Urin. Tract Symptoms 2020, 12, 92–98. [Google Scholar] [CrossRef]
- Krhut, J.; Borovička, V.; Bílková, K.; Sýkora, R.; Míka, D.; Mokriš, J.; Zachoval, R. Efficacy and safety of mirabegron for the treatment of neurogenic detrusor overactivity-Prospective, randomized, double-blind, placebo-controlled study. Neurourol. Urodyn. 2018, 37, 2226–2233. [Google Scholar] [CrossRef]
- Shimizu, N.; Gotoh, D.; Nishimoto, M.; Hashimoto, M.; Saito, T.; Fujita, K.; Hirayama, A.; Yoshimura, N.; Uemura, H. Efficacy of vibegron, a novel β3-adrenoreceptor agonist, for lower urinary tract dysfunction in mice with spinal cord injury. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2021, 28, 1068–1072. [Google Scholar] [CrossRef]
- Gotoh, D.; Shimizu, N.; Wada, N.; Kadekawa, K.; Saito, T.; Mizoguchi, S.; Morizawa, Y.; Hori, S.; Miyake, M.; Torimoto, K.; et al. Effects of a new β3-adrenoceptor agonist, vibegron, on neurogenic bladder dysfunction and remodeling in mice with spinal cord injury. Neurourol. Urodyn. 2020, 4, 100516. [Google Scholar] [CrossRef]
- Lee, K.; Takahashi, R.; Imada, K.; Okabe, A.; Kajioka, S.; Kashiwagi, E.; Shiota, M.; Inokuchi, J.; Eto, M. Initial experience with vibegron for the treatment of neurogenic lower urinary tract storage dysfunction in patients with spinal cord injury. Continence 2022, 4, 100516. [Google Scholar] [CrossRef]
- Yamada, S.; Chimoto, J.; Shiho, M.; Okura, T.; Morikawa, K.; Kagota, S.; Shinozuka, K. Muscarinic receptor binding activity in rat tissues by vibegron and prediction of its receptor occupancy levels in the human bladder. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2021, 28, 1298–1303. [Google Scholar] [CrossRef]
- Iguchi, N.; Dönmez, M.; Malykhina, A.P.; Carrasco, A., Jr.; Wilcox, D.T. Preventative effects of a HIF inhibitor, 17-DMAG, on partial bladder outlet obstruction-induced bladder dysfunction. Am. J. Physiol. Ren. Physiol. 2017, 313, F1149–F1160. [Google Scholar] [CrossRef]
- Lee, H.J.; An, J.; Doo, S.W.; Kim, J.H.; Choi, S.S.; Lee, S.R.; Park, S.W.; Song, Y.S.; Kim, S.U. Improvement in Spinal Cord Injury-Induced Bladder Fibrosis Using Mesenchymal Stem Cell Transplantation Into the Bladder Wall. Cell Transpl. 2015, 24, 1253–1263. [Google Scholar] [CrossRef]
- Carlson, B.M. The Biology of Long-Term Denervated Skeletal Muscle. Eur. J. Transl. Myol. 2014, 24, 3293. [Google Scholar] [CrossRef]
- Fry, C.H.; Kitney, D.G.; Paniker, J.; Drake, M.J.; Kanai, A.; Andersson, K.E. Fibrosis and the bladder, implications for function ICI-RS 2017. Neurourol. Urodyn. 2018, 37, S7–S12. [Google Scholar] [CrossRef]
- Wada, N.; Shimizu, T.; Takai, S.; Shimizu, N.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. Combinational effects of muscarinic receptor inhibition and β3-adrenoceptor stimulation on neurogenic bladder dysfunction in rats with spinal cord injury. Neurourol. Urodyn. 2017, 36, 1039–1045. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, E.J.; Cho, H.J.; Jang, J.A.; Han, M.S.; Kwak, E.; Kim, H.; An, J.; Park, D.; Han, S.; et al. Antifibrosis treatment by inhibition of VEGF, FGF, and PDGF receptors improves bladder wall remodeling and detrusor overactivity in association with modulation of C-fiber afferent activity in mice with spinal cord injury. Neurourol. Urodyn. 2021, 40, 1460–1469. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, N.; Saito, T.; Wada, N.; Hashimoto, M.; Shimizu, T.; Kwon, J.; Cho, K.J.; Saito, M.; Karnup, S.; de Groat, W.C.; et al. Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 7885. https://doi.org/10.3390/ijms24097885
Shimizu N, Saito T, Wada N, Hashimoto M, Shimizu T, Kwon J, Cho KJ, Saito M, Karnup S, de Groat WC, et al. Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury. International Journal of Molecular Sciences. 2023; 24(9):7885. https://doi.org/10.3390/ijms24097885
Chicago/Turabian StyleShimizu, Nobutaka, Tetsuichi Saito, Naoki Wada, Mamoru Hashimoto, Takahiro Shimizu, Joonbeom Kwon, Kang Jun Cho, Motoaki Saito, Sergei Karnup, William C. de Groat, and et al. 2023. "Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury" International Journal of Molecular Sciences 24, no. 9: 7885. https://doi.org/10.3390/ijms24097885
APA StyleShimizu, N., Saito, T., Wada, N., Hashimoto, M., Shimizu, T., Kwon, J., Cho, K. J., Saito, M., Karnup, S., de Groat, W. C., & Yoshimura, N. (2023). Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury. International Journal of Molecular Sciences, 24(9), 7885. https://doi.org/10.3390/ijms24097885





