Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methodology
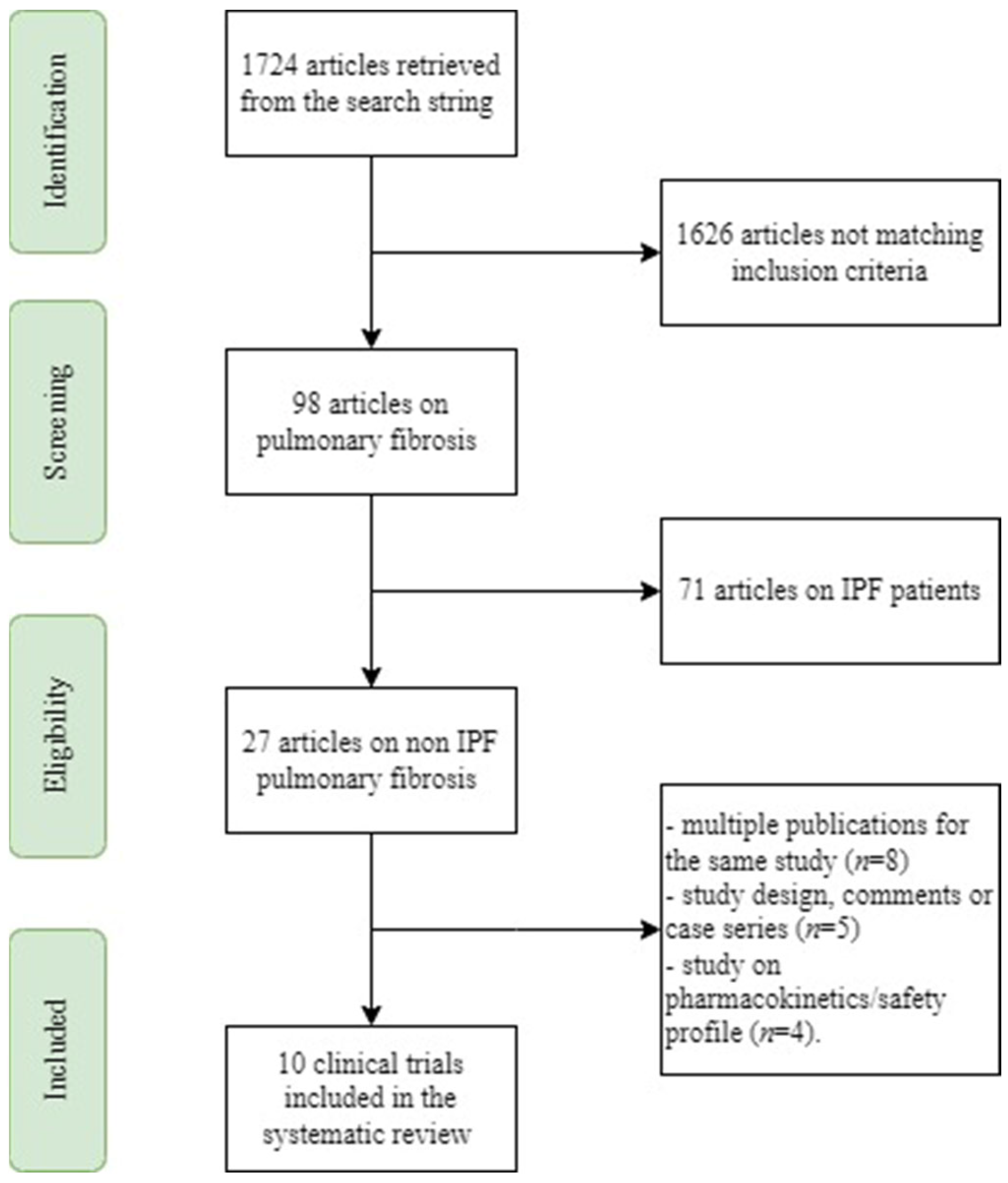
2.2. Study Selection
2.3. Data Extraction
2.4. Data Analysis
2.5. Critical Assessment of Evidence Quality
3. Results
3.1. Summary of the Main Results
3.2. Efficacy of Ninitedanib
3.3. Efficacy of Pirfenidone
4. Discussion
5. Ongoing Clinical Trials
5.1. Ongoing Clinical Trials on Nintedanib
5.2. Ongoing Clinical Trials on Pirfenidone
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Cuello-Garcia, C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19, Erratum in Am. J. Respir. Crit. Care Med. 2015, 192, 644. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082, Erratum in N. Engl. J. Med. 2015, 373, 782. [Google Scholar] [CrossRef] [PubMed]
- Petnak, T.; Lertjitbanjong, P.; Thongprayoon, C.; Moua, T. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Chest 2021, 160, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Amati, F.; Spagnolo, P.; Oldham, J.M.; Ryerson, C.J.; Stainer, A.; Gramegna, A.; Mantero, M.; Lacedonia, D.; Sverzellati, N.; Richeldi, L.; et al. Treatable traits in interstitial lung diseases: A call to action. Lancet Respir. Med. 2023. ahead of print. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Weigt, S.S.; Saggar, R.; Palchevskiy, V.; Volkmann, E.R.; Liang, L.L.; Ross, D.; Ardehali, A.; Lynch, J.P.; Belperio, J.A. Endotype-phenotyping may predict a treatment response in progressive fibrosing interstitial lung disease. Ebiomedicine 2019, 50, 379–386. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in progressive Fibrosing interstitial lung diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Brozek, J.L.; Akl, E.A.; Alonso-Coello, P.; Lang, D.; Jaeschke, R.; Williams, J.W.; Phillips, B.; Lelgemann, M.; Lethaby, A.; Bousquet, J.; et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 2009, 64, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.J.; Danoff, S.K.; Woodhead, F.A.; Hurwitz, S.; Maurer, R.; Glaspole, I.; Dellaripa, P.F.; Gooptu, B.; Vassallo, R.; Cox, P.G.; et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir. Med. 2022. ahead of print. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Qi, X.; Sun, Z.; Zhang, T.; Cui, Y.; Shu, Q. The Efficacy and Safety of Pirfenidone Combined with Immunosuppressant Therapy in Connective Tissue Disease-Associated Interstitial Lung Disease: A 24-Week Prospective Controlled Cohort Study. Front. Med. 2022, 9, 871861. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Maher, T.M.; Corte, T.J.; Fischer, A.; Kreuter, M.; Lederer, D.J.; Molina-Molina, M.; Axmann, J.; Kirchgaessler, K.-U.; Samara, K.; Gilberg, F.; et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2020, 8, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Sharma, S.K.; Mishra, D.; Dhooria, S.; Dhir, V.; Jain, S. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease-a randomised controlled trial. Rheumatol. Int. 2020, 40, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Toledo, H.; Mejía-Ávila, M.; Rodríguez-Barreto, Ó.; Mejía-Hurtado, J.G.; Rojas-Serrano, J.; Estrada, A.; Castillo-Pedroza, J.; Castillo-Castillo, K.; Gaxiola, M.; Buendia-Roldan, I.; et al. An Open-label Study with Pirfenidone on Chronic Hypersensitivity Pneumonitis. Arch. Bronconeumol. 2020, 56, 163–169. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Troendle, J.; Gochuico, B.R.; Markello, T.C.; Salas, J.; Cardona, H.; Yao, J.; Bernardini, I.; Hess, R.; Gahl, W.A. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol. Genet. Metab. 2011, 103, 128–134. [Google Scholar] [CrossRef]
- Gahl, W.A.; Brantly, M.; Troendle, J.; Avila, N.; Padua, A.; Montalvo, C.; Cardona, H.; Calis, K.A.; Gochuico, B. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol. Genet. Metab. 2002, 76, 234–242. [Google Scholar] [CrossRef]
- Cottin, V.; Richeldi, L.; Rosas, I.; Otaola, M.; Song, J.W.; Tomassetti, S.; Wijsenbeek, M.; Schmitz, M.; Coeck, C.; Stowasser, S.; et al. Nintedanib and immunomodulatory therapies in progressive fibrosing interstitial lung diseases. Respir. Res. 2021, 22, 84. [Google Scholar] [CrossRef]
- Wells, A.U.; Flaherty, K.R.; Brown, K.K.; Inoue, Y.; Devaraj, A.; Richeldi, L.; Moua, T.; Crestani, B.; Wuyts, W.A.; Stowasser, S.; et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir. Med. 2020, 8, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Maher, T.M.; Corte, T.J.; Molina-Molina, M.; Axmann, J.; Gilberg, F.; Kirchgaessler, K.-U.; Cottin, V. Pirfenidone in Unclassifiable Interstitial Lung Disease: A Subgroup Analysis by Concomitant Mycophenolate Mofetil and/or Previous Corticosteroid Use. Adv. Ther. 2022, 39, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, M.; Kreuter, M.; Cottin, V.; Corte, T.J.; Gilberg, F.; Kirchgaessler, K.-U.; Axmann, J.; Maher, T.M. Efficacy of Pirfenidone vs. Placebo in Unclassifiable Interstitial Lung Disease, by Surgical Lung Biopsy Status: Data From a post-hoc Analysis. Front. Med. 2022, 9, 897102. [Google Scholar] [CrossRef]
- Velázquez-Díaz, P.; Nakajima, E.; Sorkhdini, P.; Hernandez-Gutierrez, A.; Eberle, A.; Yang, D.; Zhou, Y. Hermansky-Pudlak Syndrome and Lung Disease: Pathogenesis and Therapeutics. Front. Pharmacol. 2021, 12, 644671. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Identifier: NCT05065190. Available online: https://clinicaltrials.gov/ct2/show/NCT05065190 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT05067517. Available online: https://clinicaltrials.gov/ct2/show/NCT05067517 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT05335278. Available online: https://clinicaltrials.gov/ct2/show/NCT05335278 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04856111. Available online: https://clinicaltrials.gov/ct2/show/NCT04856111 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04619680. Available online: https://clinicaltrials.gov/ct2/show/NCT04619680 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04541680. Available online: https://clinicaltrials.gov/ct2/show/NCT04541680 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04338802. Available online: https://clinicaltrials.gov/ct2/show/NCT04338802 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04161014. Available online: https://clinicaltrials.gov/ct2/show/NCT04161014 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03805477. Available online: https://clinicaltrials.gov/ct2/show/NCT03805477 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03283007. Available online: https://clinicaltrials.gov/ct2/show/NCT03283007 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03062943. Available online: https://clinicaltrials.gov/ct2/show/NCT03062943 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT02496585. Available online: https://clinicaltrials.gov/ct2/show/NCT02496585 (accessed on 22 December 2022).
- Crinò, L.; Metro, G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2014, 23, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, N.; Liu, Y.; Yang, X.; He, Y.; Li, Q.; Shen, X.; Zhu, Y.; Yang, Y. The Interaction Between Pulmonary Fibrosis and COVID-19 and the Application of Related Anti-Fibrotic Drugs. Front. Pharmacol. 2022, 12, 805535. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Identifier: NCT05505409. Available online: https://clinicaltrials.gov/ct2/show/NCT05505409 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT05288179. Available online: https://clinicaltrials.gov/ct2/show/NCT05288179 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT05133453. Available online: https://clinicaltrials.gov/ct2/show/NCT05133453 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT05118256. Available online: https://clinicaltrials.gov/ct2/show/NCT05118256 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT05075161. Available online: https://clinicaltrials.gov/ct2/show/NCT05075161 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04928586. Available online: https://clinicaltrials.gov/ct2/show/NCT04928586 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04675619. Available online: https://clinicaltrials.gov/ct2/show/NCT04675619 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04607928. Available online: https://clinicaltrials.gov/ct2/show/NCT04607928 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04461587. Available online: https://clinicaltrials.gov/ct2/show/NCT04461587 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT04193592. Available online: https://clinicaltrials.gov/ct2/show/NCT04193592 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03902509. Available online: https://clinicaltrials.gov/ct2/show/NCT03902509 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03857854. Available online: https://clinicaltrials.gov/ct2/show/NCT03857854 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03856853. Available online: https://clinicaltrials.gov/ct2/show/NCT03856853 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03385668. Available online: https://clinicaltrials.gov/ct2/show/NCT03385668 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03315741. Available online: https://clinicaltrials.gov/ct2/show/NCT03315741 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT03221257. Available online: https://clinicaltrials.gov/ct2/show/NCT03221257 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT02958917. Available online: https://clinicaltrials.gov/ct2/show/NCT02958917 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT02496182. Available online: https://clinicaltrials.gov/ct2/show/NCT02496182 (accessed on 22 December 2022).
- ClinicalTrials.gov. Identifier: NCT02262299. Available online: https://clinicaltrials.gov/ct2/show/NCT02262299 (accessed on 22 December 2022).
| Article | Study Design | Sample Size | Type of ILD | Inclusion Criteria | Intervention | Primary Outcome | Results | Side Effects | GRADE |
|---|---|---|---|---|---|---|---|---|---|
| Solomon JJ; Lancet Resp Med 2022 [13] | Randomized, double-blind, placebo-controlled, 1:1, phase 2 trial | 123 | RA-ILD | ILD diagnosis on a HRCT scan and, when available, a lung biopsy. | Pirfenidone, 2403 mg oral daily, or placebo | Composite endpoint of a decline from baseline FVC% of 10% or more or death during the 52-week treatment period | 11% of patients in the pirfenidone group vs. 15% of patients in the placebo group; (p = 0.48). | There was no significant difference in the rate of serious AEs between the two groups. | Low |
| Wang J; Front Med (Lausanne) 2022 [14] | Non-randomized, single-center, 1:1, controlled cohort study | 136 | CTD-ILD | FVC < 80%; DLCO < 80% No pulmonary fibrosis improvement with glucocorticoid and/or immunosuppressant treatment. | Pirfenidone, 1800 mg/day, or placebo | Change in FVC and DLCO after 24 weeks of treatment according to 4 groups (SSc; RA; inflammatory myopathy; other CTD) | FVC% in the SSc-pirfenidone group was improved by 6.60%, while this value was 0.55% in the SSc-non-pirfenidone group (p = 0.042). The elevation in FVC% was also different between the pirfenidone and control groups of patients with inflammatory myopathy: 7.50% vs. 1.00% (p = 0.016). The DLCO% of RA-pirfenidone was enhanced by 7.40% compared with RA-non-pirfenidone which decreased by 5.50% from baseline (p = 0.002). | No differences were found in terms of total AEs between the two groups. Gastrointestinal events were more often found in the pirfenidone group than the control group. | Very Low |
| Behr; Lancet Respir Med 2021 [15] | Randomized double-blind, placebo-controlled, 1:1, 2b trial | 127 | CTD-ILD, NSIP, CHP, and asbestosis-ILD | FVC 40–90%; DLCO 10–90%; Annual decline of FVC of at least 5% despite conventional therapy. | Pirfenidone, 2403 mg daily, or placebo | Absolute change in percentage of FVC % predicted from baseline to week 48 in the intention-to-treat population | There was significantly lower decline in FVC% predicted in the pirfenidone group compared with placebo group (p = 0.043). | Severe AEs (grades 3–4) of nausea (two patients on pirfenidone, two on placebo), dyspnea (one patient on pirfenidone, one on placebo), and diarrhea (one patient on pirfenidone) were occasionally observed. | Low |
| Maher; Lancet Resp Med 2020 [16] | Randomized double-blind, placebo-controlled, 1:1, phase 2 | 253 | Progressive fibrosing uILD * | FVC > 45%; DLCO > 30%; Fibrosis affecting at least 10% of the lung volume on HRCT. | Pirfenidone, 2403 mg daily, or placebo | The mean predicted change in FVC from baseline over 24 weeks | −87.7 mL in the pirfenidone group versus−157.1 mL in the placebo group (p = 0.002). | The most common TEAEs were gastrointestinal disorders (47% in the pirfenidone group vs. 26% in the placebo group), fatigue (13% vs. 10%), and rash (10% vs. 7%). | Low |
| Acharya; Rheumatol Int. 2020 [17] | Randomized double-blind, placebo-controlled, 1:1, phases 2–3 | 34 | SSc-ILD | FVC 50–80%. | Pirfenidone, 2403 mg daily, or placebo | Proportion of subjects with either stabilization or improvement in FVC at 6 months | Stabilization/improvement in FVC was seen in 94.1% and 76.5% of subjects in the pirfenidone and placebo groups, respectively (p = 0.33). | Common AEs were gastrointestinal disturbances and skin rashes. | Very low |
| Mateos-Toledo; Arch Bronconeumol 2020 [18] | Open-label, proof-of-concept study | 22 | CHP | More than 12 months of symptoms before diagnosis; The presence of fibrosis and architectural distortion in the histopathological evaluation of the lung biopsy affected more than 10% of the parenchima. | Pirfenidone 1800 mg/day plus prednisone (to reach a maintenance dose of 0.125 mg/kg) plus azathioprine (1–2 mg/kg per day) or prednisone plus azathioprine for 1 year. | Change of predicted value of the FVC in % and ml at 12 months | No significant changes were observed in the predicted value of FVC (% and mL) from baseline to 12 months in any of the groups. | Nausea (27% vs. 0%), diarrhea (27% vs. 11%), and dyspepsia (100% vs. 67%) were more frequent in the pirfenidone group. | Very low |
| Flaherty; NEJM 2019 [9] | Randomized double blind, placebo controlled, 1:1, phase 3 | 663 | CHP, CTD-ILD, NSIP, uILD, and others | Fibrosing ILDs affecting at least 10% of lung volume on HRCT; ILD Progression **; FVC > 45%; DLCO 30–80%. | Nintedanib, 150 mg twice daily, or placebo | Annual rate of decline in FVC | −80.8 mL per year with nintedanib versus−187.8 per year with placebo (p < 0.001) | Diarrhea 66.9% in nintedanib group and 23.9% in placebo group. Abnormalities on liver-function testing were more common in the nintedanib group. | High |
| Distler; NEJM 2019 [10] | Randomized double blind, placebo controlled, 1:1, phase 3 | 576 | SSc-ILD | Onset of the first non-Raynaud’s symptom within the past 7 years; Fibrosis affecting at least 10% of the lung volume on HRCT. | Nintedanib 150 mg twice daily or placebo | Annual rate of decline in FVC | −52.4 mL per year in the nintedanib group versus −93.3 mL per year in the placebo group (p = 0.04) | Diarrhea was reported in 75.7% of the patients in the nintedanib group and in 31.6% of those in the placebo group. AEs led to the permanent discontinuation of the trial drug in 16.0% of the nintedanib group and 8.7% in the placebo group. | High |
| O’Brien; Mol Genet Metab 2011 [19] | Randomized, double-blind, placebo-controlled trial, 2:1 | 35 | Hermansky-Pudlak syndrome ILD | FVC 51–85%. | Pirfenidone, 1602 mg daily, or placebo | Rate of change in post-bronchodilator FVC at 12 months | There was no statistical difference between the placebo and pirfenidone groups. | Nausea (17% vs. 8%) and photosensitivity rash (9% vs. 0%) were more frequent in the pirfenidone group. | Very low |
| Gahl; Mol Genet Metab. 2002 [20] | Randomized, placebo-controlled, and 1:1 trial | 21 | Hermansky-Pudlak syndrome ILD | FVC 40–75%. | Pirfenidone, 800 mg t.i.d., or placebo | Rate of change in FVC at 21, 32, 36, and 44 months | 11 pirfenidone-treated patients lost FVC at a rate of 5% of predicted (∼400 mL) per year, slower than 10 placebo-treated patients (p = 0.001). | There was no statistical difference between the placebo and pirfenidone groups in terms of AEs. | Very low |
| NCT Number | Disease | Phase | Enrollment | Study Design | Inclusion Criteria | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|---|
| NCT05065190 [26] | PF-ILD | 3 | 90 | Interventional Randomized Quadruple blind | Progressive fibrosis * Fibrosing lung disease on HRCT FVC ≥ 45% | Change in FVC [Time frame 52 weeks] | N/A |
| NCT05067517 [27] | Progressive Fibrosing Coal Mine Dust-Induced ILD | 3 | 160 | Interventional Randomized Triple blind | 30% ≤ DLCO < 80%. FVC ≥ 45% | Change in FVC [Time frame 12–24–36–52 weeks] | Change in pulmonary function Absolute change from baseline in the L-PF Symptoms (cough and dyspnea) domain score Absolute change from baseline in the K- BILD total score Progression on HRCT 6MWT Time to all-cause and respiratory mortality Time for progression |
| NCT05335278 [28] | Myositis Associated ILD | N/A | 25 | Interventional Open label | Extent of ILD disease ≥ 10% on HRCT done within 12 months of enrolment Progressive disease within 24 months of the screening visit Current and ongoing treatment with immunosuppressive medications, on a stable medication regimen and dosage for at least 6 weeks (considered standard of care medical therapy) | Tolerability AE [Time frame 24 weeks] | Change in FVC Change in DLCO Change in 6MWD |
| NCT04856111 [29] | COVID-19 | 4 | 48 | Interventional Randomized Single blind | Post-COVID parenchymal involvement >10% of the lung parenchyma or having persistent reticulation or persistent consolidation despite a trial of glucocorticoids (minimum prednisolone dose of 10 mg/day, or equivalent) for a minimum period of 4 weeks after discharge for the acute COVID-19 illness | Change in the FVC [Time frame 24 weeks] | Proportion of subjects with FVC improvement or stabilization Change in dyspnoea Change in resting oxygen saturation Proportion of subjects with oxygen desaturation on exercise testing Change in the 6MWD Change the SF-36 and K-BILD questionnaires Changes in HRCT scores AE |
| NCT04619680 [30] | COVID-19 | 4 | 170 | Interventional Randomized Triple blind | Required one of the following after diagnosis with SARS-CoV-2:
| Change in FVC [Time frame 180 days] | Chest CT visual score Change in the SGRQ, K-BILD, LCQ, and SF-36 questionnaires Change in 6MWT Functional Assessment of Chronic Illness Number of deaths due to any or respiratory cause AE |
| NCT04541680 [31] | COVID-19 | 3 | 250 | Interventional Randomized Triple blind | 1. History of hospitalization for COVID-19 infection documented with positive PCR or positive serology in the previous 2 to 12 months 2. Lung opacities on HRCT involving > 10% of the lung volume with fibrotic features 3. DLCO ≤ 70% | Change in FVC [Time frame 12 months] | Change in DLCO Change in 6MWT HRCT lung opacities extension Change in health-related quality of life Evolution of dyspnoea over time AE |
| NCT04338802 [32] | COVID-19 | 2 | 96 | Interventional Open Label | 18–70 years old. CT examination of patients with multiple fibrotic shadows in both lungs. | Change in FVC [Time frame 8 weeks] | Changes in DLCO Changes in the 6MWT Changes in the HRCT score |
| NCT04161014 [33] | Pneumoconiosis | 2 | 100 | Interventional Open Label | 1. Pneumoconiosis diagnosis confirmed at the Occupational MDT 2. Diffuse fibrosing lung disease >10% on HRCT with protocol criteria for progression 3. Asbestosis, silicosis, coal worker’s pneumoconiosis, and diffuse dust fibrosis 3. FVC ≥ 45% and DLCO > 30% | Change in FVC [Time frame 36 months] | K-BILD score Time to acute exacerbation Time to referral for lung transplantation Time to death |
| NCT03805477 [34] | BOS | 2 | 20 | Interventional Open Label | Time interval from transplant ≤ 5 years at the time of inclusion Absolute decline of FEV1 ≥ 10% within the past 12 months | AE rate leading to interruption/ discontinuation of study treatment [Time frame 12 months] | Changes in pulmonary function parameters Change in eNO Nitrogen-washout Changes in in 6MWD Cumulative steroid doses Occurrence of GvHD in other organs Disease-free survival of underlying hematologic disease Overall survival |
| NCT03283007 [35] | BOS | 3 | 80 | Interventional Randomized Quadruple Blind | At least 6 months post-lung transplant Progressive BOS ** | Change in FEV1 [Time frame 1, 2, 3, 6, 9, 12, and after 13 months] | Exercise tolerance Quality of life improvement Efficacy to hamper FEV1 decrease Efficacy to hamper the progression of BOS Change in oxygen saturation nintedanib tolerance Explanatory parameters of fibrotic pathways |
| NCT03062943 [36] | LAM | 2 | 30 | Interventional Open Label | LAM patients with proven side effects and/or toxicities/contraindications to sirolimus therapy will be eligible for this study. | Change in FEV1 [Time frame 12 months] | AE |
| NCT02496585 [37] | Radiation induced lung injury | 2 | 33 | Interventional Randomized Double Blind | Prior treatment with thoracic radiotherapy completed >4 weeks and ≤9 months prior to enrolment | Number of patients who are free from pulmonary exacerbations [Time frame 12 months] | N/A |
| NCT Number | Disease | Phase | Enrollment | Study Design | Inclusion Criteria | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|---|
| NCT05505409 [41] | CTD-ILD | 4 | 120 | Interventional Open Label | Patients with clinical deterioration more than 1 month after diagnosis of ILD history, or poor response or intolerance to glucocorticoids or immunosuppressants treatment, or poor response or intolerance to other antifibrotic drugs. | Change in FVC% [Time Frame: 6 months] | Changes in pulmonary function parameters Progression-free survival Change in 6MWTD Radiological changes BORG dyspnea index score Changes in inflammatory biomarkers Changes in primary disease activity AE |
| NCT05288179 [42] | Pneumoconiosis | 3 | 272 | Interventional Randomized Quadruple Blind | 40% ≥ FVC > 80% 30% ≥ DLCO > 80% | Change in FVC% [Time Frame: 52 weeks] | Change in FVC (L) Change in DLCO% |
| NCT05133453 [43] | Asbestosis | N/A | 40 | Interventional Randomized Open Label | FVC ≥ 50% DLCO ≥ 30% Duration since diagnosis at least one year before the study. | FVC % DLCO Radiological findings change [Time frame: 6–12 months] | N/A |
| NCT05118256 [44] | Silicosis | 2 | 18 | Interventional Randomized Single Blind | Age range: 18–65 years. Progressive massive fibrosis due to silicosis. | Metabolic pulmonary activity assessed by PET-CT scan (18 FFDG) [Time Frame: baseline (day 1), 6–12 months] | Cell biomarkers in peripheral blood AE EQ-5D-5L test Respiratory function parameters |
| NCT05075161 [45] | Post ARDS fibrosis | 3 | 130 | Interventional Randomized Quadruple Blind | The inflammatory ARDS phenotype is defined by at least one of the following: - High plasma levels of inflammatory biomarkers; - Vasopressor dependence; - Lower serum bicarbonate or increased serum lactate. | The number of ventilator free days (VFD) [Time frame: 28 days] | ICU-free days at day 28 Cumulative SOFA-free point at day 28 Hospital length of stay Fibroproliferative changes on HRCT Mortality at ICU/hospital discharge Quality of life assessment at follow-up (6–12 months) with SF-36 and EQ-5D scores Percentage change in pulmonary function parameters Proportion of subjects who develop heart dysfunction AE Use of rescue therapies for severe hypoxemia |
| NCT04928586 [46] | CTD-ILD | 4 | 200 | Interventional Randomized Open Label | DMARDs treatment. | Change in FVC Change in DLCO [Time frame: 12 months] | Changes in dyspnea score Imaging changes Changes in 6MWD Changes in CRP and ESR Changes in VAS score AE |
| NCT04675619 [47] | PF-CHP | 2 | 40 | Interventional Randomized Open Label | Progressive fibrosis * | Change in FVC Change in 6MWD [Time frame: 12 months] | N/A |
| NCT04607928 [48] | COVID-19 | 2 | 148 | Interventional Randomized Triple Blind | Fibrotic radiological changes ≥ 5% after recovery from the acute process. | FVC% change HRCT fibrosis % change [Time frame: 24 weeks] | FVC stability or improvement Decreased oxygen requirement for physical activity Improved exercise capacity Visits to the Emergency or DH for respiratory causes Lung transplantation Death |
| NCT04461587 [49] | Pneumoconiosis | 2 | 50 | Interventional Open Label | Progressive fibrosis ** FEV1 ≤ 75%, or FVC ≤ 80%, or DLCO ≤ 70%, or abnormal 6MWT (oxygen desaturation ≥ 4% of resting at screening or within the past 6 months) | Change in FVC [Time frame: 12 months] | Changes in FEV1, DLCO, and 6MWT Changes in HRCT Inflammatory biomarkers SGRQ |
| NCT04193592 [50] | HPS-ILD | 2 | 50 | Interventional Open Label | Fibrotic abnormality affecting more than 5% of the lung parenchyma. Stable dose of corticosteroids. No cytotoxic, immunosuppressive, cytokine-modulating, or receptor antagonist agents were used in treatment. | Change in FVC [Time Frame: baseline, 6–12 months] | Changes in DLCO, FVC, and AE SAE |
| NCT03902509 [51] | Radiation-induced lung injury | 2 | 126 | Interventional Open Label | 18–75 years old. Course of radiation-induced lung injury < 2 months. ECOG 0–2 Capable of eating solid food upon enrolment. | Change in DLCO% [Time frame: 8–24 weeks] | Changes in radiation-induced lung injury Score-change in HRCT Increase in effective lung volume Change in grade of cough, dyspnea, and fever |
| NCT03857854 [52] | Dm-ILD | 3 | 152 | Interventional Quadruple Blind | 40% < FVC < 80% predicted. 30% < DLCO < 89% predicted Glucocorticoid and immunosuppressive therapy for more than 3 months | Change in FVC % [Time frame 52 weeks] | N/A |
| NCT03856853 [53] | SSc-ILD | 3 | 144 | Interventional Quadruple Blind | 18–75 years old. SSc disease onset within 5 years. 40% < FVC 70% predicted. | Change in FVC % [Time frame 52 weeks] | N/A |
| NCT03385668 [54] | Pulmonary Fibrosis with MPO | 2 | 7 | Interventional Open Label | Possible UIP or NSIP based on HRCT. Pulmonary fibrosis refractory (according to the investigator’s judgment) to a conventional regimen used for anti-MPO associated vasculitis | Change in FVC % [Time frame 52 weeks] | AE Change in FVC% Change in DLCO% Change in 6MWT distance Progression-free survival Changes in dyspnea Changes in HRCT HAQ SF-36 |
| NCT03315741 [55] | BOS | 1 | 30 | Interventional Open Label | Presence of cGVHD in an organ other than the lung Decrease in %FVC and/or %FEV1 ≥ 20% at screening compared with the pre-transplant baseline. Bronchodilator response on PFT testing those results in an FEV1 < 75% Life expectancy > 6 months | Number of participants that do not require a reduction in drug dose for more than 21 days due to AEs [Time frame 52 weeks] | AE The number of patients who experience treatment-emergent deaths during the study period and for 28 days after the last dose of study treatment. All-cause mortality BMI |
| NCT03221257 [56] | SSc-ILD | 2 | 51 | Interventional Triple blind | Grade ≥ 2 on the Magnitude of Task component of the MMDI FVC% ≤ 85% Onset of the first non-Raynaud manifestation of SSc within the prior 84 months. The presence of any GGO on HRCT | Change in FVC % [Baseline to 18 months, measured at 3-month intervals] | mRSS Changes in FVC and DLCO TDI SHAQ SGRQ HRCT measurements of quantitative lung fibrosis AEs |
| NCT02958917 [57] | PF-CHP | 2 | 40 | Interventional Double Blind | FVC ≥ 40%, DLCO ≥ 30%. Progressive fibrosis *** | Change in FVC % [Time frame 52 weeks] | Progression-free survival Change in DLCO% Proportion of patients with all-cause mortality, all-cause hospitalization, hospitalization for a respiratory cause, respiratory exacerbations requiring hospitalizations, evidence of progression in fibrosis on HRCT |
| NCT02496182 [58] | CHP | 3 | 60 | Interventional Quadruple Blind | CHP with a recent diagnosis confirmed by HRCT with or without biopsy | Change in FVC [Time frame 52 weeks] | 6MWD San George Qty Score, SOBQ, and EQ5D Quality Scores Pulmonary artery systolic pressure with an echocardiogram Oxygen desaturation in exercise |
| NCT02262299 [59] | BOS | 2–3 | 90 | Interventional Triple Blind | Azithromycin therapy for ≥4 weeks prior to study start At least 6 months after transplantation BOS grades 1–3 Progressive disease **** | Change in FEV1 [Time frame 6 months] | Number of patients with treatment failure Change in BOS grade Change in pulmonary function parameters Change in 6MWTD All-cause hospital admission Death or re-transplantation rates Changes in EQ5D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amati, F.; Stainer, A.; Polelli, V.; Mantero, M.; Gramegna, A.; Blasi, F.; Aliberti, S. Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7849. https://doi.org/10.3390/ijms24097849
Amati F, Stainer A, Polelli V, Mantero M, Gramegna A, Blasi F, Aliberti S. Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(9):7849. https://doi.org/10.3390/ijms24097849
Chicago/Turabian StyleAmati, Francesco, Anna Stainer, Veronica Polelli, Marco Mantero, Andrea Gramegna, Francesco Blasi, and Stefano Aliberti. 2023. "Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review" International Journal of Molecular Sciences 24, no. 9: 7849. https://doi.org/10.3390/ijms24097849
APA StyleAmati, F., Stainer, A., Polelli, V., Mantero, M., Gramegna, A., Blasi, F., & Aliberti, S. (2023). Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. International Journal of Molecular Sciences, 24(9), 7849. https://doi.org/10.3390/ijms24097849








