Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis
Abstract
1. Introduction
2. Results
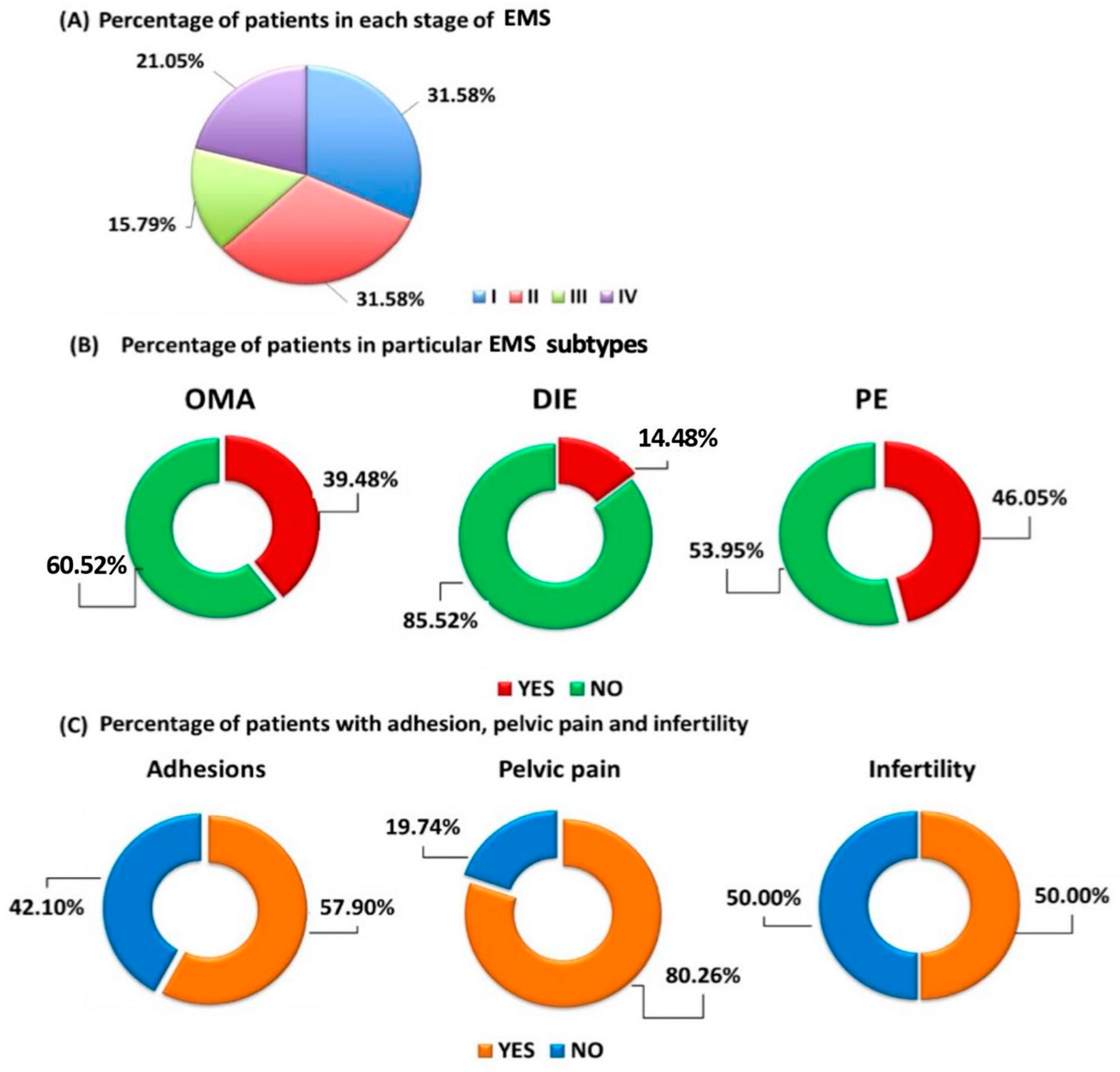
2.1. Basic Characteristics of Patients Diagnosed with Endometriosis and Healthy Volunteers Included in the Study
2.2. Evaluation of Ucn1 Concentration in the Serum and Peritoneal Fluid of Patients with EMS and the Control Group and Its Possible Use as a Biomarker Molecule in the Diagnosis of EMS
2.3. Evaluation of the Effectiveness of Serum Ucn1, CA 125, HE4 and Peritoneal Ucn1 as a Potential Biomarker Molecules of Particular EMS Types
2.4. Assessment of the Relationship between Ucn1 Concentrations in a Group of Patients with EMS and the Stage of the Disease
2.5. Evaluation of Selected Parameters of the Functioning of the Immune System in the Course of EMS in Relation to Healthy Volunteers
2.6. Assessment of the Relationship between Urocortin Concentration and Selected Parameters of the Functioning of the Immune System of Patients with Endometriosis
3. Discussion
3.1. Ucn1 as a Potential EMS Biomarker
3.2. Evaluation of Ucn1 as a Beneficial Tool Able to Distinguishing Different EMS Types
3.3. The Role of Ucn1 in Distinguishing of Different Stages of EMS
3.4. Ucn1 and the Immune System in the Pathogenesis of EMS
4. Materials and Methods
4.1. Patients and Controls
4.2. Material Collection
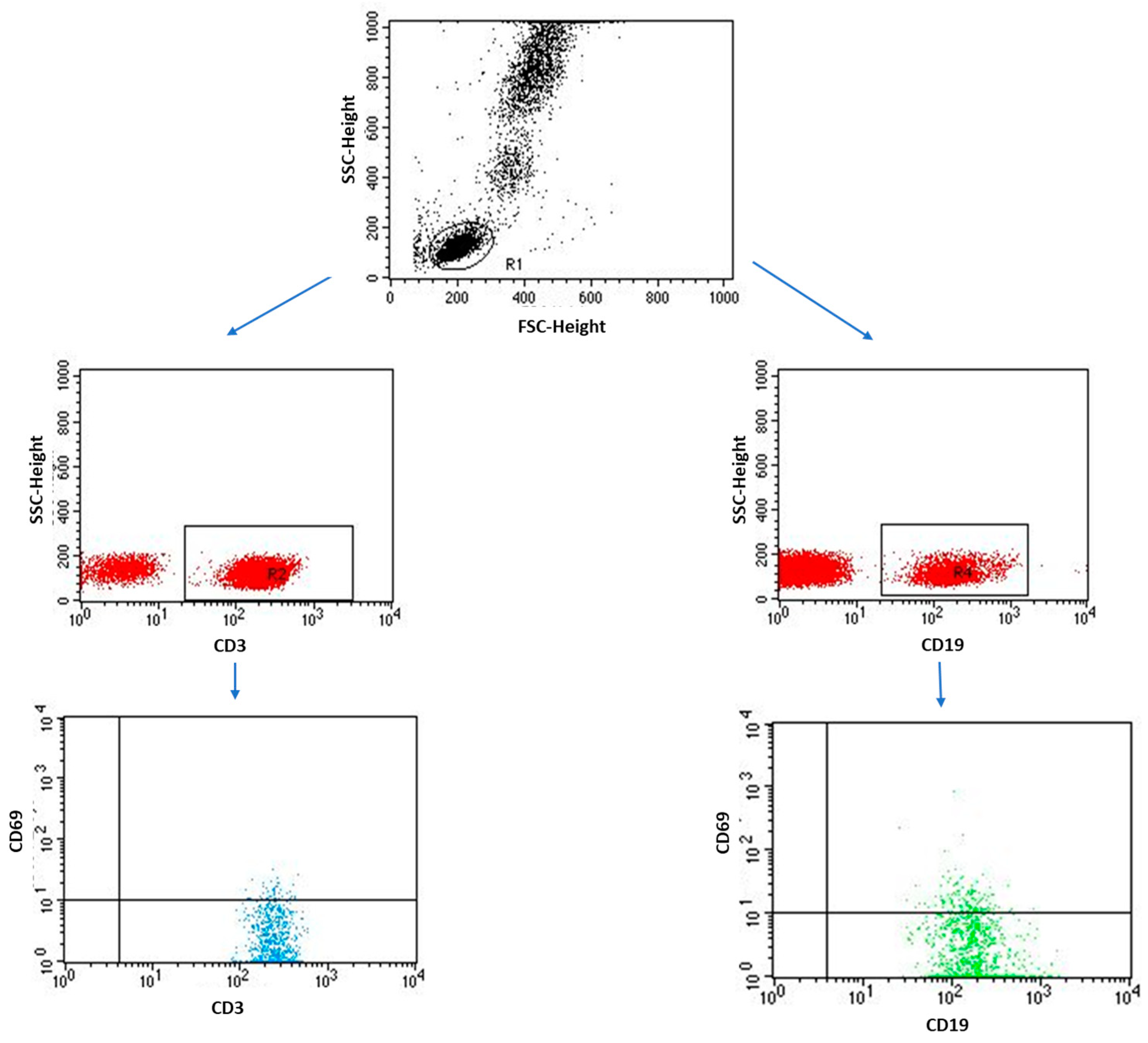
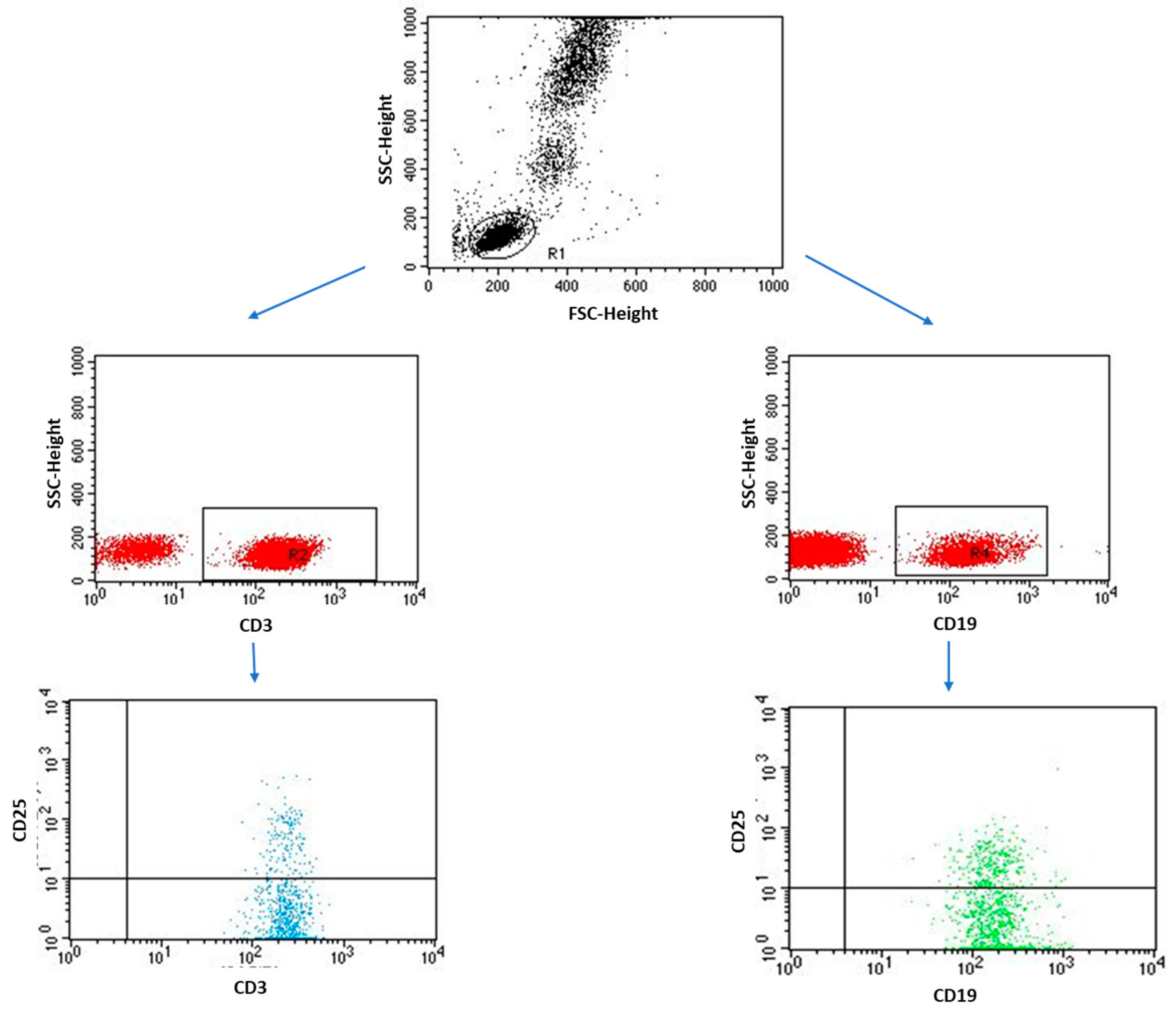
4.3. Immunophenotyping
4.4. Measurements of Serum Cytokines (IL-2, IL-4, IL-6, IL-10, IFN-gamma) Concentrations
4.5. Measurement of Urocortin 1 (Ucn1) Concentration
4.6. Measurement of Cancer Antigen 125 (CA 125) and Human Epididymis Protein 4 (HE-4) Levels
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti, C.; Johnson, N.P.; Petrozza, J.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Lee, T.T.M.; Missmer, S.; Vermeulen, N.; et al. An International Terminology for Endometriosis, 2021. Hum. Reprod. Open 2021, 2021, hoab029. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.A.; Markham, S.M. Pathogenesis of Endometriosis. Lancet 1992, 340, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Clement, P.B. The Pathology of Endometriosis: A Survey of the Many Faces of a Common Disease Emphasizing Diagnostic Pitfalls and Unusual and Newly Appreciated Aspects. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Kho, R.M.; Andres, M.P.; Borrelli, G.M.; Neto, J.S.; Zanluchi, A.; Abrão, M.S. Surgical Treatment of Different Types of Endometriosis: Comparison of Major Society Guidelines and Preferred Clinical Algorithms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking Mechanisms, Diagnosis and Management of Endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of Endometriosis on Quality of Life and Work Productivity: A Multicenter Study across Ten Countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of Endometriosis and Its Comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- de Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and Infertility: Pathophysiology and Management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and Treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and Pathophysiology of Endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; De Carolis, C.; Man, G.C.W.; Wang, C.C. The Link between Immunity, Autoimmunity and Endometriosis: A Literature Update. Autoimmun. Rev. 2018, 17, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Moein Mahini, S.; Younesi, M.; Mortazavi, G.; Samare-Najaf, M.; Karim Azadbakht, M.; Jamali, N. Non-Invasive Diagnosis of Endometriosis: Immunologic and Genetic Markers. Clin. Chim. Acta 2023, 538, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.; Donaldson, C.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C.; et al. Urocortin, a Mammalian Neuropeptide Related to Fish Urotensin I and to Corticotropin-Releasing Factor. Nature 1995, 378, 287–292. [Google Scholar] [CrossRef]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF Family of Neuropeptides and Their Receptors—Mediators of the Central Stress Response. Curr. Mol. Pharmacol. 2018, 11, 4–31. [Google Scholar] [CrossRef]
- Liapakis, G.; Venihaki, M.; Margioris, A.; Grigoriadis, D.; Gkountelias, K. Members of CRF Family and Their Receptors: From Past to Future. Curr. Med. Chem. 2011, 18, 2583–2600. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A. Urocortin and the Brain. Prog. Neurobiol. 2008, 84, 148–156. [Google Scholar] [CrossRef]
- Oki, Y.; Sasano, H. Localization and Physiological Roles of Urocortin. Peptides 2004, 25, 1745–1749. [Google Scholar] [CrossRef]
- Emeto, T.I.; Moxon, J.V.; Rush, C.; Woodward, L.; Golledge, J. Relevance of Urocortins to Cardiovascular Disease. J. Mol. Cell. Cardiol. 2011, 51, 299–307. [Google Scholar] [CrossRef]
- Souza-Moreira, L.; Campos-Salinas, J.; Caro, M.; Gonzalez-Rey, E. Neuropeptides as Pleiotropic Modulators of the Immune Response. Neuroendocrinology 2011, 94, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Baka, S.; Malamitsi-Puchner, A. The Role of Urocortin in Gynecological and Obstetrical Conditions. Arch. Gynecol. Obstet. 2009, 279, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Laganà, A.S.; Rapisarda, A.M.C.; Scarale, M.G.; Corrado, F.; Cignini, P.; Butticè, S.; Rossetti, D. Role of Urocortin in Pregnancy: An Update and Future Perspectives. World J. Clin. Cases 2016, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Arcuri, F.; Ciarmela, P.; Runci, Y.; Romagnoli, R.; Cintorino, M.; Di Blasio, A.; Petraglia, F. Identification of Urocortin MRNA and Peptide in the Human Endometrium. J. Endocrinol. 2002, 173, R9–R14. [Google Scholar] [CrossRef]
- Florio, P.; De Falco, G.; Leucci, E.; Torricelli, M.; Torres, P.B.; Toti, P.; Dell’Anna, A.; Tiso, E.; Santopietro, R.; Leoncini, L.; et al. Urocortin Expression Is Downregulated in Human Endometrial Carcinoma. J. Endocrinol. 2006, 190, 99–105. [Google Scholar] [CrossRef]
- Torricelli, M.; De Falco, G.; Florio, P.; Rossi, M.; Leucci, E.; Viganò, P.; Leoncini, L.; Petraglia, F. Secretory Endometrium Highly Expresses Urocortin Messenger RNA and Peptide: Possible Role in the Decidualization Process. Hum. Reprod. 2007, 22, 92–96. [Google Scholar] [CrossRef]
- Florio, P.; Vale, W.; Petraglia, F. Urocortins in Human Reproduction. Peptides 2004, 25, 1751–1757. [Google Scholar] [CrossRef]
- Dolińska, W.; Draper, H.; Othman, L.; Thompson, C.; Girvan, S.; Cunningham, K.; Allen, J.; Rigby, A.; Phillips, K.; Guinn, B. Accuracy and Utility of Blood and Urine Biomarkers for the Non-Invasive Diagnosis of Endometriosis: A Systematic Literature Review and Meta-Analysis. FS Rev. 2022, S2666571922000214. [Google Scholar] [CrossRef]
- Maia, L.M.; Rocha, A.L.; Del Puerto, H.L.; Petraglia, F.; Reis, F.M. Plasma Urocortin-1 as a Preoperative Marker of Endometriosis in Symptomatic Women. Gynecol. Endocrinol. 2018, 34, 202–205. [Google Scholar] [CrossRef]
- Kempuraj, D.; Papadopoulou, N.; Stanford, E.J.; Christodoulou, S.; Madhappan, B.; Sant, G.R.; Solage, K.; Adams, T.; Theoharides, T.C. Increased Numbers of Activated Mast Cells in Endometriosis Lesions Positive for Corticotropin-Releasing Hormone and Urocortin. Am. J. Reprod. Immunol. 2004, 52, 267–275. [Google Scholar] [CrossRef]
- Florio, P.; Reis, F.M.; Torres, P.B.; Calonaci, F.; Toti, P.; Bocchi, C.; Linton, E.A.; Petraglia, F. Plasma Urocortin Levels in the Diagnosis of Ovarian Endometriosis. Obstet. Gynecol. 2007, 110, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chmaj-Wierzchowska, K.; Kampioni, M.; Wilczak, M.; Sajdak, S.; Opala, T. Novel Markers in the Diagnostics of Endometriomas: Urocortin, Ghrelin, and Leptin or Leukocytes, Fibrinogen, and CA-125? Taiwan. J. Obstet. Gynecol. 2015, 54, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, A.; Ugur, M.; Tonguc, E.; Var, T.; Moraloğlu, O.; Ozaksit, G. The Value of Urocortin and Ca-125 in the Diagnosis of Endometrioma. Arch. Gynecol. Obstet. 2011, 283, 1075–1079. [Google Scholar] [CrossRef]
- Eris Yalcin, S.; Ocal, I.; Yalcin, Y.; Sen Selim, H.; Demir Caltekin, M.; Aydogmus, H.; Kelekci, S. Evaluation of the Ki-67 Proliferation Index and Urocortin Expression in Women with Ovarian Endometriomas. Eurasian J. Med. 2017, 49, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Carrarelli, P.; Luddi, A.; Funghi, L.; Arcuri, F.; Batteux, F.; Dela Cruz, C.; Tosti, C.; Reis, F.M.; Chapron, C.; Petraglia, F. Urocortin and Corticotrophin-Releasing Hormone Receptor Type 2 MRNA Are Highly Expressed in Deep Infiltrating Endometriotic Lesions. Reprod. BioMedicine Online 2016, 33, 476–483. [Google Scholar] [CrossRef]
- Scioscia, M.; Virgilio, B.A.; Laganà, A.S.; Bernardini, T.; Fattizzi, N.; Neri, M.; Guerriero, S. Differential Diagnosis of Endometriosis by Ultrasound: A Rising Challenge. Diagnostics 2020, 10, 848. [Google Scholar] [CrossRef]
- Rousset, P.; Florin, M.; Bharwani, N.; Touboul, C.; Monroc, M.; Golfier, F.; Nougaret, S.; Thomassin-Naggara, I. Deep Pelvic Infiltrating Endometriosis: MRI Consensus Lexicon and Compartment-Based Approach from the ENDOVALIRM Group. Diagn. Interv. Imaging 2023, 104, 95–112. [Google Scholar] [CrossRef]
- Cosma, S.; Salgarello, M.; Ceccaroni, M.; Gorgoni, G.; Riboni, F.; La Paglia, E.; Danese, S.; Benedetto, C. Accuracy of a New Diagnostic Tool in Deep Infiltrating Endometriosis: Positron Emission Tomography-Computed Tomography with 16α-[18F]Fluoro-17β-Estradiol: Endometriosis: Radiotracer Diagnosis. J. Obstet. Gynaecol. Res. 2016, 42, 1724–1733. [Google Scholar] [CrossRef]
- Li, W.; Challis, J.R.G. Corticotropin-Releasing Hormone and Urocortin Induce Secretion of Matrix Metalloproteinase-9 (MMP-9) without Change in Tissue Inhibitors of MMP-1 by Cultured Cells from Human Placenta and Fetal Membranes. J. Clin. Endocrinol. Metab. 2005, 90, 6569–6574. [Google Scholar] [CrossRef]
- Barbe, A.M.; Berbets, A.M.; Davydenko, I.S.; Koval, H.D.; Yuzko, V.O.; Yuzko, O.M. Expression and Significance of Matrix Metalloproteinase-2 and Matrix Metalloproteinas-9 in Endometriosis. J. Med. Life 2020, 13, 314–320. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, H.; Tang, N.; Li, Y.; Zhang, Y.; Hao, T. Correlation between Matrix Metalloproteinase-9 and Endometriosis. Int. J. Clin. Exp. Pathol. 2015, 8, 13399–13404. [Google Scholar] [PubMed]
- Kageyama, K.; Hanada, K.; Nigawara, T.; Moriyama, T.; Terui, K.; Sakihara, S.; Suda, T. Urocortin Induces Interleukin-6 Gene Expression via Cyclooxygenase-2 Activity in Aortic Smooth Muscle Cells. Endocrinology 2006, 147, 4454–4462. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, V.T.; Nácul, A.P.; Dos Santos, B.R.; Lecke, S.B.; Spritzer, P.M.; Morsch, D.M. Circulating and Peritoneal Fluid Interleukin-6 Levels and Gene Expression in Pelvic Endometriosis. Exp. Ther. Med. 2017, 14, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Mamillapalli, R.; Habata, S.; Taylor, H.S. Endometriosis Cell Proliferation Induced by Bone Marrow Mesenchymal Stem Cells. Reprod. Sci. 2021, 28, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.W.; Nawy, S.S.; Wei, E.T.; Sadée, W.; Filov, V.A.; Rezsova, V.V.; Slominski, A.; Quillan, J.M. Inhibition of Mouse Melanoma Cell Proliferation by Corticotropin-Releasing Hormone and Its Analogs. Anticancer Res. 2001, 21, 1173–1179. [Google Scholar] [PubMed]
- Slominski, A.T.; Roloff, B.; Zbytek, B.; Wei, E.T.; Fechner, K.; Curry, J.; Wortsman, J. Corticotropin Releasing Hormone and Related Peptides Can Act as Bioregulatory Factors in Human Keratinocytes. Vitr. Cell. Dev. Biol. Anim. 2000, 36, 211–216. [Google Scholar] [CrossRef]
- Moghaddam, M.Z.; Ansariniya, H.; Seifati, S.M.; Zare, F.; Fesahat, F. Immunopathogenesis of Endometriosis: An Overview of the Role of Innate and Adaptive Immune Cells and Their Mediators. Am. J. Rep. Immunol. 2022, 87, e13537. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Bamberger, C.M.; Wald, M.; Bamberger, A.M.; Ergün, S.; Beil, F.U.; Schulte, H.M. Human Lymphocytes Produce Urocortin, But Not Corticotropin-Releasing Hormone. J. Clin. Endocrinol. Metab. 1998, 83, 708–711. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Borges, L.E.; Bloise, E.; Dela Cruz, C.; Galleri, L.; Apa, R.; Petraglia, F.; Reis, F.M. Urocortin 1 Expression and Secretion by Human Umbilical Vein Endothelial Cells: In Vitro Effects of Interleukin 8, Interferon γ, Lipopolysaccharide, Endothelin 1, Prostaglandin F-2α, Estradiol, Progesterone and Dexamethasone. Peptides 2015, 74, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Honjo, T.; Inoue, N.; Shiraki, R.; Kobayashi, S.; Otsui, K.; Takahashi, M.; Hirata, K.; Kawashima, S.; Yokozaki, H.; Yokoyama, M. Endothelial Urocortin Has Potent Antioxidative Properties and Is Upregulated by Inflammatory Cytokines and Pitavastatin. J. Vasc. Res. 2006, 43, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Novembri, R.; Carrarelli, P.; Toti, P.; Rocha, A.L.L.; Borges, L.E.; Reis, F.M.; Piomboni, P.; Florio, P.; Petraglia, F. Urocortin 2 and Urocortin 3 in Endometriosis: Evidence for a Possible Role in Inflammatory Response. Mol. Hum. Reprod. 2011, 17, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Takahashi, K.; Suzuki, T.; Saruta, M.; Watanabe, M.; Nakata, T.; Sasano, H. Urocortin 1, Urocortin 3/Stresscopin, and Corticotropin-Releasing Factor Receptors in Human Adrenal and Its Disorders. J. Clin. Endocrinol. Metab. 2005, 90, 4671–4678. [Google Scholar] [CrossRef]



| Parameter | Patients with EMS (n = 76) | Control Group (n = 20) | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Age | 35.00 ± 6.14 | 35.00 (22.00–48.00) | 36.65 ± 6.69 | 36.00 (26.00–53.00) | 0.428 |
| White blood cells (103 /mm3) | 8.39 ± 1.59 | 8.31 (5.51–11.33) | 7.42 ± 0.75 | 7.31 (6.37–8.66) | 0.014 * |
| Neutrophils (103 /mm3) | 5.04 ± 1.38 | 5.50 (2.08–7.91) | 4.32 ± 1.01 | 3.93 (2.71–6.03) | 0.0138 * |
| Monocytes (103 /mm3) | 0.54 ± 0.16 | 0.56 (0.24–0.86) | 0.46 ± 0.09 | 0.48 (0.28–0.59) | 0.053 |
| Lymphocytes (103 /mm3) | 2.24 ± 0.72 | 2.05 (1.20–3.83) | 2.43 ± 0.44 | 2.53 (1.53–3.07) | 0.188 |
| TSH (thyrotrophic hormone) (μIU/mL) | 1.38 ± 0.62 | 1.40 (0.33–2.58) | 1.42 ± 0.67 | 1.49 (0.44–2.55) | 0.813 |
| fT3 (free triiodothyronine) (pg/mL) | 3.15 ± 0.56 | 3.15 (2.01–4.34) | 3.13 ± 0.56 | 3.15 (2.02–4.12) | 0.870 |
| fT4 (free thyroxin) (pg/mL) | 1.31 ± 0.24 | 1.32 (0.92–1.73) | 1.29 ± 0.21 | 1.30 (0.93–1.67) | 0.758 |
| Estradiol (pg/mL) | 57.93 ± 26.18 | 51.00 (19.30–114.80) | 56.23 ± 26.74 | 44.95 (25.6–112.30) | 0.659 |
| FSH (follicle-stimulating hormone) (mIU/mL) | 5.36 ± 1.79 | 4.90 (2.20–9.50) | 6.16 ± 1.54 | 6.00 (3.50–8.50) | 0.068 |
| LH (luteinizing hormone) (mIU/mL) | 6.64 ± 2.73 | 6.00 (2.20–14.60) | 5.96 ± 1.78 | 5.75 (3.20–10.50) | 0.525 |
| Parameter | Patients with EMS (n = 76) | Control Group (n = 20) | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Level of Ucn1 in serum (ng/mL) | 12.38 ± 6.18 | 11.78 (1.75–26.85) | 4.52 ± 2.71 | 3.50 (1.30–10.55) | 0.0000 * |
| Level Ucn1 in peritoneal fluid (ng/mL) | 1.30 ± 0.68 | 1.22 (0.15–2.52) | N/A | N/A | N/A |
| Level of CA 125 (U/mL) | 37.78 ± 28.68 | 30.84 (7.67–178.12) | 9.23 ± 5.23 | 9.02 (2.50–21.73) | 0.0000 * |
| Level of HE4 (pmol/L) | 39.96 ± 9.60 | 39.15 (26.00–67.00) | 37.05 ± 8.90 | 37.63 (16.92–53.18) | 0.414 |
| Parameter | Optimal Cutpoint | AUC | N | N Positive | N Negative | TP | FN | FP | TN | Se | Sp | Sp + Se | Acc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum Ucn1 (ng/mL) | 5.94 | 0.85 | 96 | 76 | 20 | 58 | 11 | 5 | 21 | 0.83 | 0.80 | 1.63 | 0.82 |
| CA 125 (U/mL) | 16.87 | 0.94 | 96 | 76 | 20 | 58 | 11 | 2 | 25 | 0.83 | 0.95 | 1.78 | 0.87 |
| HE4 (pmol/L) | 40.4 | 0.56 | 96 | 76 | 20 | 30 | 40 | 7 | 19 | 0.42 | 0.75 | 1.18 | 0.51 |
| Parameter | OMA (n = 30) (Group 1) | PE (n = 35) (Group 2) | DIE (n = 11) (Group 3) | Control (n = 20) (Group 4) | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ±SD | Median (Range) | Mean ±SD | Median (Range) | Mean ±SD | Median (Range) | Mean ±SD | Median (Range) | p-Value | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| Level of Ucn1 in serum | 12.37 ± 6.49 | 11.87 (2.43–26.85) | 12.83 ± 7.20 | 11.86 (1.75–26.85) | 16.70 ± 6.86 | 16.20 (6.32–26.85) | 4.52 ± 2.71 | 3.50 (1.30–10.55) | 0.000 * | 0.911 | 0.103 | 0.000 * | 0.144 | 0.000 * | 0.000 * |
| Level of CA 125 | 44.12 ± 32.64 | 33.47 (9.98–178.12) | 33.98 ± 23.43 | 29.26 (7.67–101.00) | 54.85 ± 45.11 | 32.56 (17.11–178.12) | 9.23 ± 5.23 | 9.02 (2.50–21.73) | 0.000 * | 0.129 | 0.551 | 0.000 * | 0.089 | 0.000 * | 0.000 * |
| Level of HE4 | 41.81 ± 10.85 | 40.25 (26.00–69.00) | 38.61 ± 8.99 | 38.20 (26.00–67.00) | 43.65 ± 7.34 | 42.30 (31.00–59.30) | 37.05 ± 8.90 | 37.63 (16.92–53.18) | 0.136 | 0.268 | 0.374 | 0.000 * | 0.033 * | 0.775 | 0.014 * |
| Parameter | Patients with EMS (n = 76) | Control Group (n = 20) | p-Value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | |||
| Frequency of occurrence of individual subpopulations of immune cells in peripheral blood (%) | CD3+ T lymphocytes | 70.83 ± 4.59 | 71.86 (61.31–78.77) | 68.26 ± 3.74 | 68.08 (60.63–74.49) | 0.020 * |
| CD3+/CD4+ T lymphocytes | 43.09 ± 7.56 | 44.01 (26.13–65.45) | 44.46 ± 2.44 | 44.16 (40.71–48.84) | 0.731 | |
| CD3+/CD8+ T lymphocytes | 28.07 ± 6.79 | 27.99 (16.25–42.50) | 34.36 ± 3.20 | 34.74 (29.33–39.60) | 0.0000 * | |
| CD4+/CD25/Foxp3+ T lymphocytes | 6.30 ± 3.13 | 5.46 (0.39–13.55) | 6.20 ± 1.69 | 6.25 (3.13–9.68) | 0.956 | |
| CD19+ B lymphocytes | 10.55 ± 3.08 | 9.76 (6.12–16.84) | 11.25 ± 2.44 | 11.40 (6.04–16.90) | 0.176 | |
| CD3+/CD25+ T lymphocytes | 28.58 ± 8.32 | 26.96 (10.86–56.29) | 7.60 ± 2.69 | 8.03 (1.08–11.13) | 0.0000 * | |
| CD4+/CD25+ T lymphocytes | 13.90 ± 6.03 | 14.36 (0.82–29.48) | 5.66 ± 2.40 | 6.35 (0.95–8.83) | 0.0000 * | |
| CD8+/CD25+ T lymphocytes | 14.69 ± 8.83 | 13.66 (2.33–34.98) | 1.94 ± 1.11 | 1.63 (0.13–5.11) | 0.0000 * | |
| CD19+/CD25+ B lymphocytes | 3.50 ± 1.74 | 3.10 (0.55–8.14) | 1.77 ± 1.29 | 1.81 (0.06–5.12) | 0.0001 * | |
| CD3+/CD69+ T lymphocytes | 14.80 ± 8.69 | 14.65 (1.21–33.66) | 3.38 ± 1.66 | 3.36 (0.52–6.89) | 0.0000 * | |
| CD4+/CD69+ T lymphocytes | 9.52 ± 5.58 | 9.42 (0.78–21.64) | 2.20 ± 1.00 | 2.30 (0.18–3.48) | 0.0000 * | |
| CD8+/CD69+ T lymphocytes | 5.29 ± 3.10 | 5.23 (0.43–12.02) | 1.18 ± 1.19 | 0.70 (0.02–3.87) | 0.0000 * | |
| CD19+/CD69+ B lymphocytes | 2.17 ± 0.95 | 2.02 (0.06–6.39) | 0.12 ± 0.06 | 0.09 (0.06–0.25) | 0.0000 * | |
| Frequency of Occurrence of Individual Subpopulations of Immune Cells in Peripheral Blood | OMA (n = 30) (Group 1) | PE (n = 35) (Group 2) | DIE (n = 11) (Group 3) | Control (n = 20) (Group 4) | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | p-Value | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| CD3+ T lymphocytes | 70.32 ± 4.29 | 69.94 (61.88–76.55) | 70.76 ± 4.93 | 72.08 (61.31–78.77) | 72.33 ± 5.60 | 74.40 (61.31–78.77) | 68.26 ± 3.74 | 68.08 (60.63–74.49) | 0.074 | 0.615 | 0.183 | 0.087 * | 0.372 | 0.042 * | 0.054 |
| CD3+/CD4+ T lymphocytes | 41.97 ± 8.00 | 43.78 (26.13–54.97) | 41.81 ± 6.20 | 43.20 (26.62–53.10) | 45.27 ± 9.60 | 46.59 (27.34–65.45) | 44.46 ± 2.44 | 44.16 (40.71–48.84) | 0.525 | 0.605 | 0.441 | 0.536 | 0.320 | 0.182 | 0.982 |
| CD3+/CD8+ T lymphocytes | 26.85 ± 6.78 | 25.35 (16.37–42.90) | 29.31 ± 6.45 | 29.06 (18.13–42.90) | 23.88 ± 7.93 | 18.73 (16.25–40.97) | 34.36 ± 3.20 | 34.74 (29.33–39.60) | 0.000 * | 0.086 | 0.164 | 0.000 * | 0.027 * | 0.000 * | 0.001 * |
| CD4+/CD25/Foxp3+ T lymphocytes | 5.74 ± 3.10 | 4.57 (1.09–13.55) | 6.39 ± 2.97 | 6.46 (0.39–13.55) | 9.88 ± 2.66 | 10.21 (3.58–13.55) | 6.20 ± 1.69 | 6.25 (3.13–9.68) | 0.003 * | 0.303 | 0.000 * | 0.350 | 0.001 * | 0.896 | 0.001 * |
| CD19+ B lymphocytes | 10.41 ± 3.19 | 9.76 (6.12–16.83) | 10.80 ± 3.04 | 10.64 (6.12–16.84) | 11.53 ± 3.09 | 11.40 (7.69–16.82) | 11.25 ± 2.44 | 11.40 (6.04–16.90) | 0.509 | 0.569 | 0.329 | 0.164 | 0.558 | 0.380 | 0.812 |
| CD3+/CD25+ T lymphocytes | 26.93 ± 7.52 | 25.96 (10.86–41.20) | 29.03 ± 8.63 | 27.03 (10.86–56.29) | 34.63 ± 9.84 | 29.99 (23.37–56.29) | 7.60 ± 2.69 | 8.03 (1.08–11.13) | 0.000 * | 0.406 | 0.036 * | 0.000 * | 0.117 | 0.000 * | 0.000 * |
| CD4+/CD25+ T lymphocytes | 13.05 ± 6.03 | 13.5 (0.82–29.48) | 13.95 ± 5.46 | 14.40 (0.82–22.10) | 18.96 ± 3.53 | 18.20 (11.08–25.90) | 5.66 ± 2.40 | 6.35 (0.95–8.83) | 0.000 * | 0.362 | 0.001 * | 0.000 * | 0.004 * | 0.000 * | 0.000 * |
| CD8+/CD25+ T lymphocytes | 13.88 ± 8.19 | 13.68 (2.75– 34.05) | 15.08 ± 9.64 | 12.04 (2.23–34.08) | 15.68 ± 8.70 | 12.29 (5.29–34.98) | 1.94 ± 1.11 | 1.63 (0.13–5.11) | 0.000 * | 0.624 | 0.532 | 0.000 * | 0.741 | 0.000 * | 0.000 * |
| CD19+/CD25+ B lymphocytes | 3.32 ± 1.77 | 2.94 (0.55–8.14) | 3.41 ± 1.43 | 3.10 (1.35–7.33) | 5.52 ± 1.89 | 5.93 (1.99–8.14) | 1.77 ± 1.29 | 1.81 (0.06–5.12) | 0.000 * | 0.596 | 0.003 * | 0.000 * | 0.003* | 0.000 * | 0.000 * |
| CD3+/CD69+ T lymphocytes | 13.82 ± 7.55 | 13.68 (1.24–27.30) | 14.09 ± 9.26 | 12.75 (1.21–33.66) | 7.14 ± 7.02 | 4.41 (1.21–21.11) | 3.38 ± 1.66 | 3.36 (0.52–6.89) | 0.000 * | 1.00 | 0.027 * | 0.000 * | 0.025 * | 0.000 * | 0.619 |
| CD4+/CD69+ T lymphocytes | 8.88 ± 4.85 | 8.80 (0.80–17.55) | 9.06 ± 5.95 | 8.20 (0.78–21.64) | 4.59 ± 4.51 | 2.84 (0.78–13.57) | 2.20 ± 1.00 | 2.30 (0.18–3.48) | 0.000 * | 1.00 | 0.027 * | 0.000 * | 0.025 * | 0.000 * | 0.619 |
| CD8+/CD69+ T lymphocytes | 4.94 ± 2.70 | 4.89 (0.44–9.75) | 5.03 ± 3.31 | 4.55 (0.43–12.02) | 2.55 ± 2.51 | 1.58 (0.43–7.54) | 1.18 ± 1.19 | 0.70 (0.02–3.87) | 0.000 * | 0.994 | 0.021 * | 0.000 * | 0.003 * | 0.000 * | 0.074 |
| CD19+/CD69+ B lymphocytes | 2.08 ± 0.91 | 2.00 (0.82–6.39) | 2.15 ± 0.88 | 1.99 (0.06–4.59) | 1.98 ± 0.86 | 2.10 (0.06–3.25) | 0.12 ± 0.06 | 0.09 (0.06–0.25) | 0.000 * | 0.680 | 0.424 | 0.000 * | 0.899 | 0.000 * | 0.000 * |
| Parameter | Patients with EMS (n = 76) | Control Group (n = 20) | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| IL-4 concentration in serum (pg/mL) | 3.14 ± 2.22 | 2.77 (0.47–12.04) | 4.80 ± 0.31 | 4.67 (4.29–5.51) | 0.000 * |
| IL-6 concentration in serum (pg/mL) | 22.00 ± 7.12 | 22.06 (5.17–41.38) | 3.11 ± 4.03 | 1.17 (0.15–17.20) | 0.000 * |
| IL-10 concentration in serum (pg/mL) | 10.22 ± 12.22 | 6.77 (0.07–65.30) | 3.96 ± 0.97 | 3.91 (2.77–6.16) | 0.013 * |
| IL-2 concentration in serum (pg/mL) | 14.20 ± 12.89 | 8.18 (0.98–50.12) | 2.76 ± 1.95 | 2.36 (0.48–7.16) | 0.000 * |
| IFN-γ concentration in serum (pg/mL) | 6.45 ± 3.75 | 5.45 (1.28–23.30) | 2.33 ± 1.16 | 2.34 (0.61–4.38) | 0.000 * |
| IL-4 concentration in peritoneal fluid (pg/mL) | 3.34 ± 2.93 | 2.32 (0.42–14.93) | N/C | N/C | N/A |
| IL-6 concentration in peritoneal fluid (pg/mL) | 141.02 ± 29.57 | 66.72 (10.24–395.58) | N/C | N/C | N/A |
| IL-10 concentration in peritoneal fluid (pg/mL) | 41.41 ± 11.30 | 33.58 (10.04–156.88) | N/C | N/C | N/A |
| IL-2 concentration in peritoneal fluid (pg/mL) | 17.15 ± 4.44 | 16.80 (1.19–27.11) | N/C | N/C | N/A |
| IFN-γ concentration in peritoneal fluid (pg/mL) | 112.34 ± 37.19 | 99.21 (13.34–496.38) | N/C | N/C | N/A |
| Parameter | OMA (n = 30) (Group 1) | PE (n = 35) (Group 2) | DIE (n = 11) (Group 3) | Control (n = 20) (Group 4) | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | p-Value | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| IL-4 concentration in serum (pg/mL) | 3.28 ± 2.52 | 2.77 (0.64–12.04) | 3.40 ± 2.48 | 2.97 (0.47–12.04) | 2.38 ± 1.41 | 2.54 (0.86–6.20) | 4.80 ± 0.31 | 4.67 (4.29–5.51) | 0.001 * | 0.683 | 0.249 | 0.000 * | 0.158 | 0.002 * | 0.003 * |
| IL-6 concentration in serum (pg/mL) | 30.59 ± 8.07 | 22.28 (0.44–41.38) | 18.93 ± 8.23 | 11.98 (0.17–38.98) | 40.57 ± 10.48 | 31.98 (0.46–37.98) | 3.11 ± 4.03 | 1.17 (0.15–17.20) | 0.001 * | 0.029 * | 0.076 | 0.180 | 0.001 * | 0.000 * | 0.000 * |
| IL-10 concentration in serum (pg/mL) | 12.64 ± 14.34 | 9.09 (0.07–65.30) | 9.66 ± 13.84 | 5.71 (0.07–65.30) | 13.69 ± 7.49 | 9.48 (1.18–65.30) | 3.96 ± 0.97 | 3.91 (2.77–6.16) | 0.027 * | 0.059 * | 0.94 | 0.002 * | 0.035 * | 0.000 * | 0.000 * |
| IL-2 concentration in serum (pg/mL) | 12.42 ± 6.01 | 7.33 (0.98–50.12) | 17.41 ± 7.16 | 9.60 (1.29–50.12) | 26.07 ± 10.84 | 19.62 (1.29–50.12) | 2.76 ± 1.95 | 2.36 (0.48–7.16) | 0.000 * | 0.042 * | 0.001 * | 0.000 * | 0.027 * | 0.000 * | 0.000 * |
| IFN-γ concentration in serum (pg/mL) | 6.43 ± 3.47 | 5.56 (3.83–23.30) | 6.48 ± 4.28 | 5.44 (1.28–23.30) | 6.65 ± 2.88 | 5.29 (4.75–13.40) | 2.33 ± 1.16 | 2.34 (0.61–4.38) | 0.000 * | 0.633 | 0.980 | 0.000 * | 0.780 | 0.000 * | 0.000 * |
| IL-4 concentration in peritoneal fluid (pg/mL) | 3.54 ± 3.27 | 2.32 (0.42–14.93) | 2.92 ± 2.28 | 2.02 (0.42–10.29) | 5.65 ± 4.06 | 5.92 (0.71–14.93) | N/C | N/C | N/A | 0.690 | 0.024 * | N/A | 0.001 * | N/A | N/A |
| IL-6 concentration in peritoneal fluid (pg/mL) | 112.21 ± 30.43 | 64.40 (10.39–395.56) | 166.52 ± 37.81 | 166.77 (50.24–387.91) | 49.84 ± 22.51 | 37.80 (10.24–44.16) | N/C | N/C | N/A | 0.000 * | 0.032 * | N/A | 0.00 * | N/A | N/A |
| IL-10 concentration in peritoneal fluid (pg/mL) | 29.89 ± 11.36 | 21.08 (10.04–99.63) | 48.26 ± 7.24 | 23.51 (10.04–56.88) | 17.73 ± 7.78 | 11.17 (3.63–65.71) | N/C | N/C | N/A | 0.480 | 0.001 * | N/A | 0.000 * | N/A | N/A |
| IL-2 concentration in peritoneal fluid (pg/mL) | 20.94 ± 7.99 | 6.60 (1.19–27.11) | 13.08 ± 7.53 | 6.31 (1.19–26.80) | 8.04 ± 3.53 | 8.58 (2.78–22.64) | N/C | N/C | N/A | 0.279 | 0.059 | N/A | 0.062 | N/A | N/A |
| IFN-γ concentration in peritoneal fluid (pg/mL) | 139.85 ± 51.75 | 111.73 (13.34–496.38) | 123.54 ± 57.77 | 106.73 (33.34–495.29) | 91.91 ± 20.04 | 85.86 (23.86–380.88) | N/C | N/C | N/A | 0.436 | 0.001 * | N/A | 0.037 * | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramiuk, M.; Frankowska, K.; Kułak, K.; Tarkowski, R.; Mertowska, P.; Mertowski, S.; Grywalska, E. Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis. Int. J. Mol. Sci. 2023, 24, 7787. https://doi.org/10.3390/ijms24097787
Abramiuk M, Frankowska K, Kułak K, Tarkowski R, Mertowska P, Mertowski S, Grywalska E. Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis. International Journal of Molecular Sciences. 2023; 24(9):7787. https://doi.org/10.3390/ijms24097787
Chicago/Turabian StyleAbramiuk, Monika, Karolina Frankowska, Krzysztof Kułak, Rafał Tarkowski, Paulina Mertowska, Sebastian Mertowski, and Ewelina Grywalska. 2023. "Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis" International Journal of Molecular Sciences 24, no. 9: 7787. https://doi.org/10.3390/ijms24097787
APA StyleAbramiuk, M., Frankowska, K., Kułak, K., Tarkowski, R., Mertowska, P., Mertowski, S., & Grywalska, E. (2023). Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis. International Journal of Molecular Sciences, 24(9), 7787. https://doi.org/10.3390/ijms24097787





