Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart
Abstract
1. Introduction
2. Adipose-Tissue-Derived Extracellular Vesicles
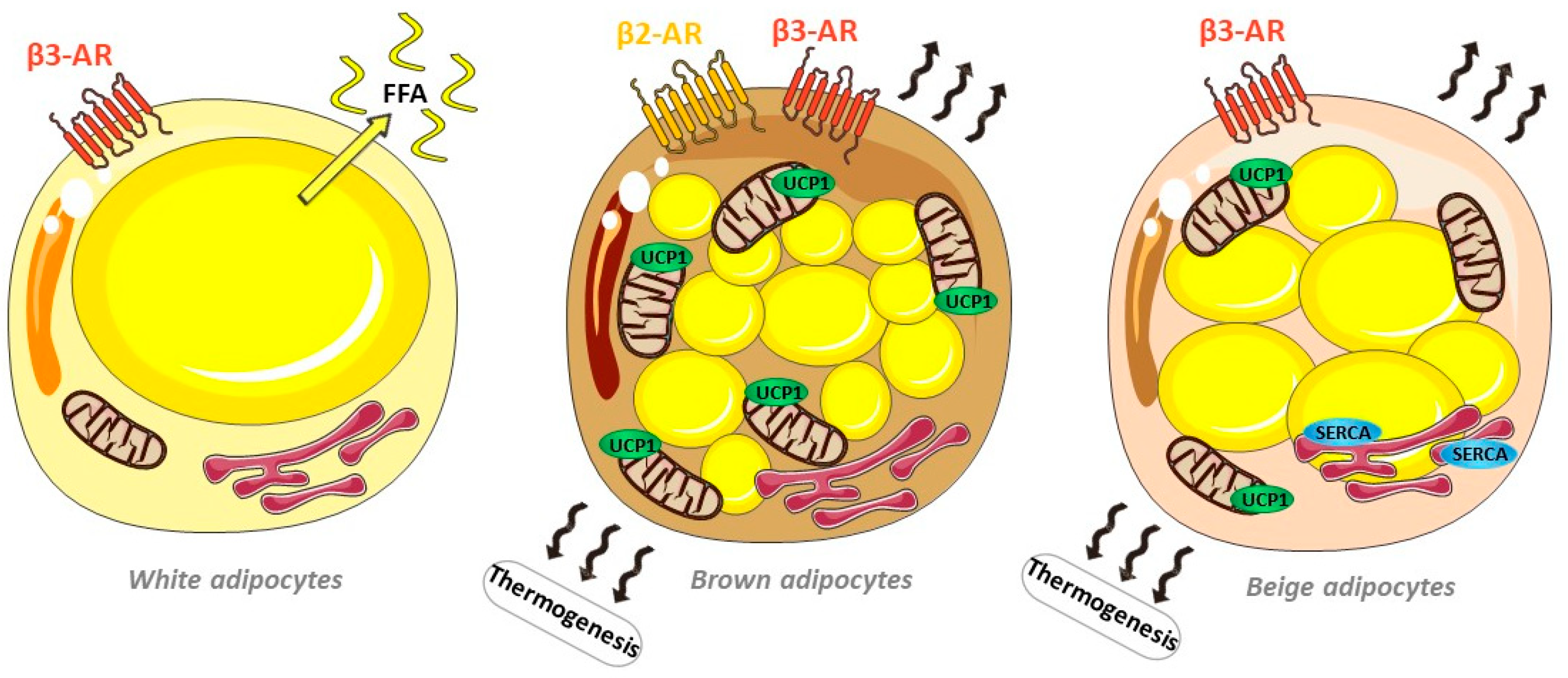
2.1. Adipose Tissue Diversity
2.1.1. Multiple and Diverse Adipose Depots
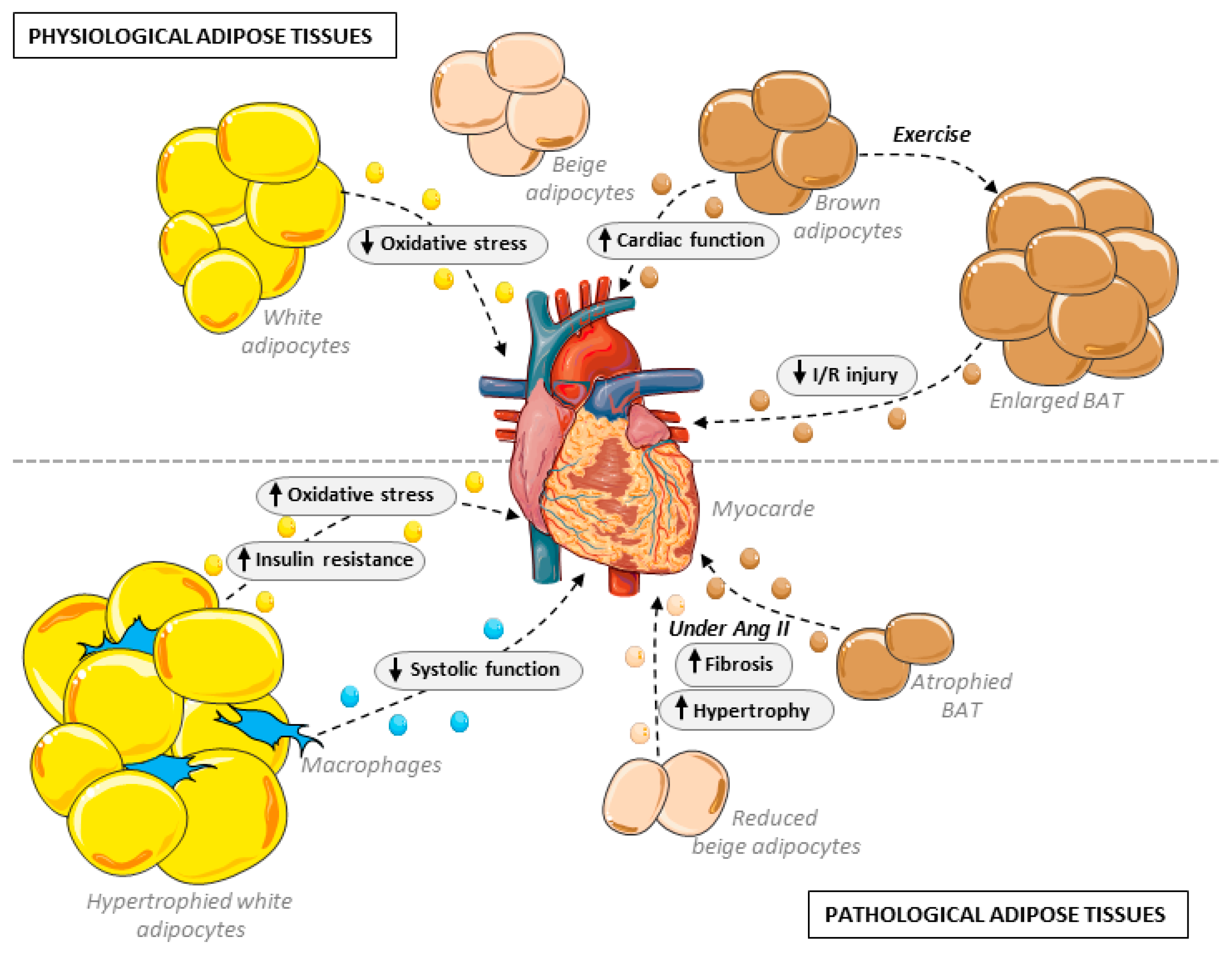
2.1.2. Endocrine Role in Cardiovascular Setting
2.2. Paracrine and Endocrine Communication by Extracellular Vesicles
2.2.1. Importance of Extracellular Vesicles in Adipose Tissue Cross-Talk
2.2.2. Type of Extracellular Vesicles and Biogenesis
3. Adipose Tissue Diversity and Remodeling: EV-Mediated Influence on the Heart
3.1. Brown Adipose Tissue and Beige Adipocytes
3.2. Cardiovascular Effects through Vascular Implications
3.3. Adipose Tissue Remodeling and Cardiac Consequences
3.3.1. Aging
3.3.2. Obesity
4. Adipose-Derived EVs in the Pathological Heart
4.1. Cardiac Remodeling
4.2. Diabetes and Diabetic Cardiomyopathy
4.3. Epicardial Adipose Tissue and Atrial Fibrosis and Fibrillation
4.4. Ischemia/Reperfusion
| Species | MI Model | Identified/Implicated Cargo | Effect on Cardiac Function and Cardiac Myocytes | Effect on Cardiac Fibroblasts | Effects on Other Cell Types | Reference |
|---|---|---|---|---|---|---|
| Rat | MI/R | miR-425-5 | ↓ mitochondrial dysfunction ↓ ROS production | - | ↑ angiogenesis ↑ M2 macrophage polarization | [106] |
| miR-196a-5p | ↓ mitochondrial dysfunction ↓ ROS production | ↓ myofibroblast activation | ↑ angiogenesis ↑ M2 macrophage polarization | [106] | ||
| miR-146a | ↓ reduced apoptosis | - | - | [107] | ||
| miR-126 | ↓ proinflammatory cytokine | ↓ fibrosis | ↑ angiogenesis | [108] | ||
| miR-93-5p | ↓ hypoxia-induced autophagy ↓ inflammatory cytokine expression by targeting Atg7 and TLR4 | - | - | [109] | ||
| Mouse | MI/R | miR-221/222 | ↓ PUMA/ETS-1 pathway ↓ H2O2-induced cell apoptosis | - | - | [110] |
| miR-214 | ↓ reduced apoptosis ↓ EGR1 and hypoxia-induced TLR4/NFκB | ↓ fibrosis | - | [111] | ||
| miR-210 | ↑ mitochondrial bioenergetics ↓ ROS production | - | - | [112] | ||
| miR-31 | - | - | ↑ angiogenesis ↑ endothelial cell migration ↑ tube formation Target FIH1 and ↑ HIF-1α activation | [113] | ||
| MI/R anoxic preconditioning of ADSCs | miRNA224-5p | ↓ reduced infarct size ↓ reduced apoptosis TXNIP downregulation | - | - | [114] | |
| Pig | MI/R | - | ↓ end-diastolic pressure–volume ↓ CM hypertrophy | - | - | [115] |
| - | ↑ contractile function | - | ↑ angiogenesis ↑ myocardial perfusion | [116] | ||
| Primary cells | H/R in NMCM | miR-671 | ↓ TGFBR2/Smad2 pathway ↓ reduced apoptosis | - | - | [117] |
| H/R in NRCM | - | ↓ reduced apoptosis ↑ VEGF, bFGF, HGF | - | - | [118] | |
| Cell lines | H/R on HL-1 | circ_0001747 | Sequestration of miRNA-199b-3p ↑ MCL1 pathway | - | - | [119] |
| H/R on H9c2 | - | ↑ S1P/SK1/S1PR1 signaling | - | ↑ M2 macrophage polarization | [120] |
5. Conclusions
Funding
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Cook, K.S.; Usher, P.; Spiegelman, B.M. Severely impaired adipsin expression in genetic and acquired obesity. Science 1987, 237, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Miyoshi, T.; Koyama, Y.; Sato, S.; Akagi, N.; Morimitsu, Y.; Kubo, M.; Sugiyama, H.; Nakamura, K.; Morita, H.; et al. Differential association of visceral adipose tissue with coronary plaque characteristics in patients with and without diabetes mellitus. Cardiovasc. Diabetol. 2014, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Lottati, M.; Kolka, C.M.; Stefanovski, D.; Kirkman, E.L.; Bergman, R.N. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obes. Silver Spring 2009, 17, 674–680. [Google Scholar] [CrossRef]
- Sawaki, D.; Czibik, G.; Pini, M.; Ternacle, J.; Suffee, N.; Mercedes, R.; Marcelin, G.; Surenaud, M.; Marcos, E.; Gual, P.; et al. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018, 138, 809–822. [Google Scholar] [CrossRef]
- Muzzin, P.; Revelli, J.P.; Kuhne, F.; Gocayne, J.D.; McCombie, W.R.; Venter, J.C.; Giacobino, J.P.; Fraser, C.M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J. Biol. Chem. 1991, 266, 24053–24058. [Google Scholar] [CrossRef]
- Lowell, B.B.; S-Susulic, V.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schoder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular characterization of the human beta 3-adrenergic receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.V.; Snedden, S.K.; Raimbault, S.; Ricquier, D.; Collins, S. Transcriptional regulation of the mouse uncoupling protein-2 gene. Double E-box motif is required for peroxisome proliferator-activated receptor-gamma-dependent activation. J. Biol. Chem. 2001, 276, 10817–10823. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by beta2-AR Stimulation. Cell Metab. 2020, 32, 287–300.e287. [Google Scholar] [CrossRef]
- Riis-Vestergaard, M.J.; Richelsen, B.; Bruun, J.M.; Li, W.; Hansen, J.B.; Pedersen, S.B. Beta-1 and Not Beta-3 Adrenergic Receptors May Be the Primary Regulator of Human Brown Adipocyte Metabolism. J. Clin. Endocrinol. Metab. 2020, 105, e994–e1005. [Google Scholar] [CrossRef]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The beta3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020, 130, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.H.; Cypess, A.M. beta3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 2021, 6, e139160. [Google Scholar] [CrossRef]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Farah, C.; Balligand, J.L. The Beta3 Adrenergic Receptor in Healthy and Pathological Cardiovascular Tissues. Cells 2020, 9, 2584. [Google Scholar] [CrossRef]
- Himms-Hagen, J.; Melnyk, A.; Zingaretti, M.C.; Ceresi, E.; Barbatelli, G.; Cinti, S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 2000, 279, C670–C681. [Google Scholar] [CrossRef]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab. 2019, 30, 174–189.e175. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; Skibicka, K.P.; Leichner, T.M.; Guarnieri, D.J.; DiLeone, R.J.; Bence, K.K.; Grill, H.J. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010, 11, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.M.; Williams, K.W.; Rossi, J.; Lee, C.E.; Elmquist, J.K. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Investig. 2011, 121, 2413–2421. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhao, X.Y.; Meng, Z.X.; Kern, M.; Dietrich, A.; Chen, Z.; Cozacov, Z.; Zhou, D.; Okunade, A.L.; Su, X.; et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 2014, 20, 1436–1443. [Google Scholar] [CrossRef]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef]
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Shibata, R.; Ouchi, N.; Ito, M.; Kihara, S.; Shiojima, I.; Pimentel, D.R.; Kumada, M.; Sato, K.; Schiekofer, S.; Ohashi, K.; et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004, 10, 1384–1389. [Google Scholar] [CrossRef]
- Ye, R.; Wang, M.; Wang, Q.A.; Scherer, P.E. Adiponectin-mediated antilipotoxic effects in regenerating pancreatic islets. Endocrinology 2015, 156, 2019–2028. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, C.; Xu, S.; Wang, J.; Guo, F.; Duan, S.; Deng, Q.; Sun, J.; Yu, F.; Zhou, Y.; et al. Metabolism regulator adiponectin prevents cardiac remodeling and ventricular arrhythmias via sympathetic modulation in a myocardial infarction model. Basic Res. Cardiol. 2022, 117, 34. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Margaritis, M.; Verheule, S.; Recalde, A.; Sanna, F.; Herdman, L.; Psarros, C.; Nasrallah, H.; Coutinho, P.; Akoumianakis, I.; et al. Mutual Regulation of Epicardial Adipose Tissue and Myocardial Redox State by PPAR-gamma/Adiponectin Signalling. Circ. Res. 2016, 118, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Dutour, A.; Achard, V.; Sell, H.; Naour, N.; Collart, F.; Gaborit, B.; Silaghi, A.; Eckel, J.; Alessi, M.C.; Henegar, C.; et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J. Clin. Endocrinol. Metab. 2010, 95, 963–967. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clement, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.J.; Akar, J.G. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.M.; Hu, W.C.; Wu, M.H.; Tai, C.T.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Wu, T.J.; et al. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am. J. Cardiol. 2011, 107, 1498–1503. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e1113. [Google Scholar] [CrossRef]
- Pinckard, K.M.; Shettigar, V.K.; Wright, K.R.; Abay, E.; Baer, L.A.; Vidal, P.; Dewal, R.S.; Das, D.; Duarte-Sanmiguel, S.; Hernandez-Saavedra, D.; et al. A Novel Endocrine Role for the BAT-Released Lipokine 12,13-diHOME to Mediate Cardiac Function. Circulation 2021, 143, 145–159. [Google Scholar] [CrossRef]
- Hartwig, S.; De Filippo, E.; Goddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 140172. [Google Scholar] [CrossRef] [PubMed]
- Gesmundo, I.; Pardini, B.; Gargantini, E.; Gamba, G.; Birolo, G.; Fanciulli, A.; Banfi, D.; Congiusta, N.; Favaro, E.; Deregibus, M.C.; et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic beta cells. JCI Insight 2021, 6, e141962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Buyel, J.J.; Hanssen, M.J.; Siegel, F.; Pan, R.; Naumann, J.; Schell, M.; van der Lans, A.; Schlein, C.; Froehlich, H.; et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat. Commun. 2016, 7, 11420. [Google Scholar] [CrossRef]
- Connolly, K.D.; Wadey, R.M.; Mathew, D.; Johnson, E.; Rees, D.A.; James, P.E. Evidence for Adipocyte-Derived Extracellular Vesicles in the Human Circulation. Endocrinology 2018, 159, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Kim, H.K.; Cho, Y.; Choi, J.S.; Woo, C.H.; Lee, K.S.; Sul, J.H.; Lee, C.M.; Han, J.; Park, J.H.; et al. Cell reprogramming using extracellular vesicles from differentiating stem cells into white/beige adipocytes. Sci. Adv. 2020, 6, eaay6721. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Joffin, N.; Rutkowski, J.M.; Kim, M.; Zhang, F.; Towler, D.A.; Gordillo, R.; Scherer, P.E. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 2018, 175, 695–708.e613. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021, 33, 1853–1868.e1811. [Google Scholar] [CrossRef]
- Lin, J.R.; Ding, L.L.; Xu, L.; Huang, J.; Zhang, Z.B.; Chen, X.H.; Cheng, Y.W.; Ruan, C.C.; Gao, P.J. Brown Adipocyte ADRB3 Mediates Cardioprotection via Suppressing Exosomal iNOS. Circ. Res. 2022, 131, 133–147. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Oberhammer, F.A.; Hochegger, K.; Froschl, G.; Tiefenbacher, R.; Pavelka, M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J. Cell Biol. 1994, 126, 827–837. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell. Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Wu, Y.; Zhang, Q.; Liu, P.; Zhang, S.; Yu, M.; Tian, W. Identification of thermogenesis-related lncRNAs in small extracellular vesicles derived from adipose tissue. BMC Genom. 2022, 23, 660. [Google Scholar] [CrossRef]
- Fang, X.; Stroud, M.J.; Ouyang, K.; Fang, L.; Zhang, J.; Dalton, N.D.; Gu, Y.; Wu, T.; Peterson, K.L.; Huang, H.D.; et al. Adipocyte-specific loss of PPARgamma attenuates cardiac hypertrophy. JCI Insight 2016, 1, e89908. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.D.; Wang, K.; Zhang, A.D.; Cheng, D.; Grossetta Nardini, H.K.; Lin, H.; Bracken, M.B.; Desai, M.; Krumholz, H.M.; Ross, J.S. Updating insights into rosiglitazone and cardiovascular risk through shared data: Individual patient and summary level meta-analyses. BMJ 2020, 368, l7078. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.M.; Nielsen, R.; Petrovic, N.; Jacobsson, A.; Mandrup, S.; Cannon, B.; Nedergaard, J. Noradrenaline represses PPAR (peroxisome-proliferator-activated receptor) gamma2 gene expression in brown adipocytes: Intracellular signalling and effects on PPARgamma2 and PPARgamma1 protein levels. Biochem. J. 2004, 382, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARgamma-ABCA1/ABCG1 pathways. Vasc. Pharm. 2022, 143, 106968. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular Adipose-Derived Exosomes Reduce Foam Cell Formation by Regulating Expression of Cholesterol Transporters. Front. Cardiovasc. Med. 2021, 8, 697510. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef] [PubMed]
- Akawi, N.; Checa, A.; Antonopoulos, A.S.; Akoumianakis, I.; Daskalaki, E.; Kotanidis, C.P.; Kondo, H.; Lee, K.; Yesilyurt, D.; Badi, I.; et al. Fat-Secreted Ceramides Regulate Vascular Redox State and Influence Outcomes in Patients With Cardiovascular Disease. J. Am. Coll. Cardiol. 2021, 77, 2494–2513. [Google Scholar] [CrossRef]
- Osikoya, O.; Cushen, S.C.; Gardner, J.J.; Raetz, M.M.; Nagarajan, B.; Raut, S.; Goulopoulou, S. Exosomes facilitate intercellular communication between uterine perivascular adipose tissue and vascular smooth muscle cells in pregnant rats. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H577–H584. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Romero-Garcia, N.; Mas-Bargues, C.; Monleon, D.; Gordevicius, J.; Brooke, R.T.; Dromant, M.; Diaz, A.; Derevyanko, A.; Guio-Carrion, A.; et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 2022, 8, eabq2226. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Kelly, J.P.; von Lueder, T.G.; Voors, A.A.; Lam, C.S.; Cowie, M.R.; Kjeldsen, K.; Jankowska, E.A.; Atar, D.; Butler, J.; et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 64, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Mancini, C.; Gohlke, S.; Garcia-Carrizo, F.; Zagoriy, V.; Stephanowitz, H.; Schulz, T.J. Identification of biomarkers of brown adipose tissue aging highlights the role of dysfunctional energy and nucleotide metabolism pathways. Sci. Rep. 2021, 11, 19928. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Lazic, M.; Armando, A.M.; Phillips, S.A.; Katebian, R.; Maraka, S.; Quehenberger, O.; Sears, D.D.; Feldstein, A.E. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J. Mol. Med. 2016, 94, 1241–1253. [Google Scholar] [CrossRef]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal 2021, 14, eabj2807. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Claggett, B.L.; Liu, J.; Jackson, A.M.; Jhund, P.S.; Kober, L.; Widimsky, J.; Boytsov, S.A.; Chopra, V.K.; Anand, I.S.; et al. Global Differences in Heart Failure With Preserved Ejection Fraction: The PARAGON-HF Trial. Circ. Heart Fail. 2021, 14, e007901. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Bi, Y.; Ajoolabady, A.; You, F.; Sowers, J.; Wang, Q.; Ceylan, A.F.; Zhang, Y.; Ren, J. Parkin Insufficiency Accentuates High-Fat Diet-Induced Cardiac Remodeling and Contractile Dysfunction Through VDAC1-Mediated Mitochondrial Ca(2+) Overload. JACC Basic Transl. Sci. 2022, 7, 779–796. [Google Scholar] [CrossRef]
- Zhao, X.; Si, L.; Bian, J.; Pan, C.; Guo, W.; Qin, P.; Zhu, W.; Xia, Y.; Zhang, Q.; Wei, K. Adipose tissue macrophage-derived exosomes induce ferroptosis via glutathione synthesis inhibition by targeting SLC7A11 in obesity-induced cardiac injury. Free Radic. Biol. Med. 2022, 182, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Pan, Y.; Zhou, Y. Exosomal microRNA-194 causes cardiac injury and mitochondrial dysfunction in obese mice. Biochem. Biophys. Res. Commun. 2018, 503, 3174–3179. [Google Scholar] [CrossRef]
- Li, F.; Zhang, K.; Xu, T.; Du, W.; Yu, B.; Liu, Y.; Nie, H. Exosomal microRNA-29a mediates cardiac dysfunction and mitochondrial inactivity in obesity-related cardiomyopathy. Endocrine 2019, 63, 480–488. [Google Scholar] [CrossRef]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic Adipocyte-Derived Exosomal miR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obes. Silver Spring 2020, 28, 1932–1940. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Attane, C.; Carrie, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef]
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trotzmuller, M.; Kofeler, H.; Mabilleau, G.; et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e312. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Li, J.; Gong, L.; Liu, S.; Zhang, Y.; Zhang, C.; Tian, M.; Lu, H.; Bu, P.; Yang, J.; Ouyang, C.; et al. Adipose HuR protects against diet-induced obesity and insulin resistance. Nat. Commun. 2019, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.R.; Anthony, S.R.; Gozdiff, A.; Green, L.C.; Fleifil, S.M.; Slone, S.; Nieman, M.L.; Alam, P.; Benoit, J.B.; Owens, A.P.; et al. Adipocyte-specific deletion of HuR induces spontaneous cardiac hypertrophy and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H228–H241. [Google Scholar] [CrossRef] [PubMed]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Qi, M.; Zhao, P.; Duan, Y.; Yang, G.; Yuan, L. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics 2020, 10, 8197–8210. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Watanabe, T.; Kadowaki, S.; Kinoshita, D.; Yokoyama, M.; Honda, Y.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; et al. Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc. Diabetol. 2014, 13, 84. [Google Scholar] [CrossRef]
- Wu, Z.J.; Cheng, Y.J.; Gu, W.J.; Aung, L.H. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: A systematic review and meta-analysis. Metabolism 2014, 63, 1157–1166. [Google Scholar] [CrossRef]
- de Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef]
- Dracheva, K.V.; Pobozheva, I.A.; Anisimova, K.A.; Balandov, S.G.; Hamid, Z.M.; Panteleeva, A.A.; Vasilevsky, D.; Pchelina, S.; Miroshnikova, V. Omentin-1 and adiponectin secretion via adipose tissue extracellular vesicles. Atherosclerosis 2021, 331, e56–e293. [Google Scholar] [CrossRef]
- Feijoo-Bandin, S.; Aragon-Herrera, A.; Morana-Fernandez, S.; Anido-Varela, L.; Tarazon, E.; Rosello-Lleti, E.; Portoles, M.; Moscoso, I.; Gualillo, O.; Gonzalez-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Su, M.; Li, W.; Yuan, Y.; Liu, S.; Liang, C.; Liu, H.E.; Zhang, R.; Liu, Y.; Sun, L.I.; Wei, Y.; et al. Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner. Transl. Res. 2022, 248, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.D.; Stewart, J.A., Jr. Rap1a Regulates Cardiac Fibroblast Contraction of 3D Diabetic Collagen Matrices by Increased Activation of the AGE/RAGE Cascade. Cells 2021, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Nishida, H.; Takahashi, Y.; Kawachi, Y.; Tsugawa-Shimizu, Y.; et al. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.; Comarita, I.K.; Alexandru, N.; Filippi, A.; Bojin, F.; Gherghiceanu, M.; Vilcu, A.; Nemecz, M.; Niculescu, L.S.; Paunescu, V.; et al. Stem cell-derived extracellular vesicles reduce the expression of molecules involved in cardiac hypertrophy-In a model of human-induced pluripotent stem cell-derived cardiomyocytes. Front. Pharm. 2022, 13, 1003684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, X.Y.; Zhao, Y.; Eirin, A.; Liu, L.; Ferguson, C.M.; Tang, H.; Lerman, A.; Lerman, L.O. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic Res. Cardiol. 2020, 115, 16. [Google Scholar] [CrossRef]
- Jiang, Y.; Hong, S.; Zhu, X.; Zhang, L.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Huang, W.; Lerman, A.; Eirin, A.; et al. IL-10 partly mediates the ability of MSC-derived extracellular vesicles to attenuate myocardial damage in experimental metabolic renovascular hypertension. Front. Immunol. 2022, 13, 940093. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Pan, J.; Ke, J.; Zhang, A.; Liu, Y.; Wang, C.; Chang, A.C.Y.; Gu, J. Secretion of miRNA-326-3p by senescent adipose exacerbates myocardial metabolism in diabetic mice. J. Transl. Med. 2022, 20, 278. [Google Scholar] [CrossRef]
- Gan, L.; Xie, D.; Liu, J.; Bond Lau, W.; Christopher, T.A.; Lopez, B.; Zhang, L.; Gao, E.; Koch, W.; Ma, X.L.; et al. Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation 2020, 141, 968–983. [Google Scholar] [CrossRef]
- Shan, Y.X.; Yang, T.L.; Mestril, R.; Wang, P.H. Hsp10 and Hsp60 suppress ubiquitination of insulin-like growth factor-1 receptor and augment insulin-like growth factor-1 receptor signaling in cardiac muscle: Implications on decreased myocardial protection in diabetic cardiomyopathy. J. Biol. Chem. 2003, 278, 45492–45498. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Gaborit, B.; Venteclef, N.; Ancel, P.; Pelloux, V.; Gariboldi, V.; Leprince, P.; Amour, J.; Hatem, S.N.; Jouve, E.; Dutour, A.; et al. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc. Res. 2015, 108, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J. Am. Coll. Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Shaihov-Teper, O.; Ram, E.; Ballan, N.; Brzezinski, R.Y.; Naftali-Shani, N.; Masoud, R.; Ziv, T.; Lewis, N.; Schary, Y.; Levin-Kotler, L.P.; et al. Extracellular Vesicles From Epicardial Fat Facilitate Atrial Fibrillation. Circulation 2021, 143, 2475–2493. [Google Scholar] [CrossRef] [PubMed]
- Kita, S.; Shimomura, I. Stimulation of exosome biogenesis by adiponectin, a circulating factor secreted from adipocytes. J. Biochem. 2021, 169, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, D.; Xie, D.; Bond Lau, W.; Liu, J.; Christopher, T.A.; Lopez, B.; Liu, L.; Hu, H.; Yao, P.; et al. Ischemic Heart-Derived Small Extracellular Vesicles Impair Adipocyte Function. Circ. Res. 2022, 130, 48–66. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Oliveira, N.C.; Neri, E.A.; Silva, C.M.; Valadao, I.C.; Fonseca-Alaniz, M.H.; Zogbi, C.; Levy, D.; Bydlowski, S.P.; Krieger, J.E. Multicellular regulation of miR-196a-5p and miR-425-5 from adipose stem cell-derived exosomes and cardiac repair. Clin. Sci. 2022, 136, 1281–1301. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J. Cell Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Deng, S.; Lu, J.; Huang, H.; Zhang, Y.; Gong, P.; Shen, X.; Ruan, H.; Jin, M.; et al. miR-93-5p-Containing Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced Myocardial Damage. Mol. Nucleic. Acids 2018, 11, 103–115. [Google Scholar] [CrossRef]
- Lai, T.C.; Lee, T.L.; Chang, Y.C.; Chen, Y.C.; Lin, S.R.; Lin, S.W.; Pu, C.M.; Tsai, J.S.; Chen, Y.L. MicroRNA-221/222 Mediates ADSC-Exosome-Induced Cardioprotection Against Ischemia/Reperfusion by Targeting PUMA and ETS-1. Front. Cell Dev. Biol. 2020, 8, 569150. [Google Scholar] [CrossRef]
- Eguchi, S.; Takefuji, M.; Sakaguchi, T.; Ishihama, S.; Mori, Y.; Tsuda, T.; Takikawa, T.; Yoshida, T.; Ohashi, K.; Shimizu, Y.; et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J. Biol. Chem. 2019, 294, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Dasgupta, C.; Mulder, C.; Zhang, L. MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction. Circulation 2022, 145, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Y.; Thomas, M.; McLaughlin, K.; Oguljahan, B.; Henderson, J.; Yang, Q.; Chen, Y.E.; Liu, D. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1alpha pathway. J. Mol. Cell. Cardiol. 2022, 162, 10–19. [Google Scholar] [CrossRef]
- Mao, C.; Li, D.; Zhou, E.; Gao, E.; Zhang, T.; Sun, S.; Gao, L.; Fan, Y.; Wang, C. Extracellular vesicles from anoxia preconditioned mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury. Aging 2021, 13, 6156–6170. [Google Scholar] [CrossRef] [PubMed]
- Aboulgheit, A.; Karbasiafshar, C.; Sabra, M.; Zhang, Z.; Sodha, N.; Abid, M.R.; Sellke, F.W. Extracellular vesicles improve diastolic function and substructure in normal and high-fat diet models of chronic myocardial ischemia. J. Thorac. Cardiovasc. Surg. 2022, 164, e371–e384. [Google Scholar] [CrossRef]
- Aboulgheit, A.; Potz, B.A.; Scrimgeour, L.A.; Karbasiafshar, C.; Shi, G.; Zhang, Z.; Machan, J.T.; Schorl, C.; Brodsky, A.S.; Braga, K.; et al. Effects of High Fat Versus Normal Diet on Extracellular Vesicle-Induced Angiogenesis in a Swine Model of Chronic Myocardial Ischemia. J. Am. Heart Assoc. 2021, 10, e017437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Wu, C.; Liu, W.; He, Y.; Yang, Q. Adipose-Derived Mesenchymal Stem Cells-Derived Exosomes Carry MicroRNA-671 to Alleviate Myocardial Infarction Through Inactivating the TGFBR2/Smad2 Axis. Inflammation 2021, 44, 1815–1830. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Liu, L.; Zhang, B.; Li, B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J. Cell Biochem. 2020, 121, 2089–2102. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, Z.; Ren, M.; Yang, M. Adipose-Derived Stem Cells-Derived Exosomes with High Amounts of Circ_0001747 Alleviate Hypoxia/Reoxygenation-Induced Injury in Myocardial Cells by Targeting MiR-199b-3p/MCL1 Axis. Int. Heart J. 2022, 63, 356–366. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X.; Zhang, D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell Biol. 2019, 114, 105564. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, L.Y.M. Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. Int. J. Mol. Sci. 2023, 24, 7745. https://doi.org/10.3390/ijms24097745
Michel LYM. Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. International Journal of Molecular Sciences. 2023; 24(9):7745. https://doi.org/10.3390/ijms24097745
Chicago/Turabian StyleMichel, Lauriane Y. M. 2023. "Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart" International Journal of Molecular Sciences 24, no. 9: 7745. https://doi.org/10.3390/ijms24097745
APA StyleMichel, L. Y. M. (2023). Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. International Journal of Molecular Sciences, 24(9), 7745. https://doi.org/10.3390/ijms24097745






