Puerarin Alleviates H2O2-Induced Oxidative Stress and Blood–Milk Barrier Impairment in Dairy Cows
Abstract
1. Introduction
2. Results
2.1. Effect of PUE on the Viability of BMECs Treated with H2O2
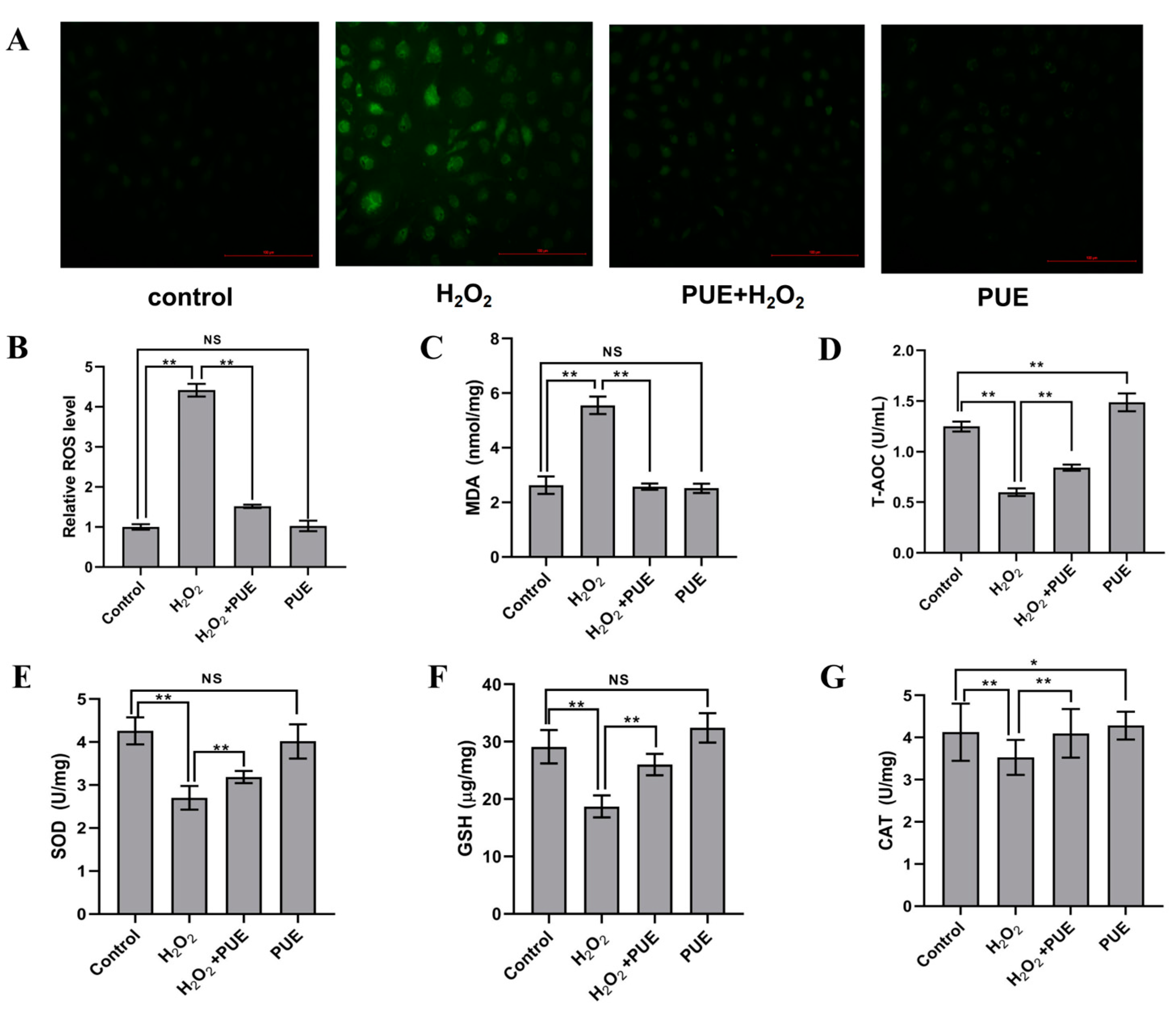
2.2. PUE Attenuates Oxidative Stress in H2O2-Treated BMECs
2.3. Effect of PUE on the Expression of Tight Junction Genes in H2O2-Treated BMECs
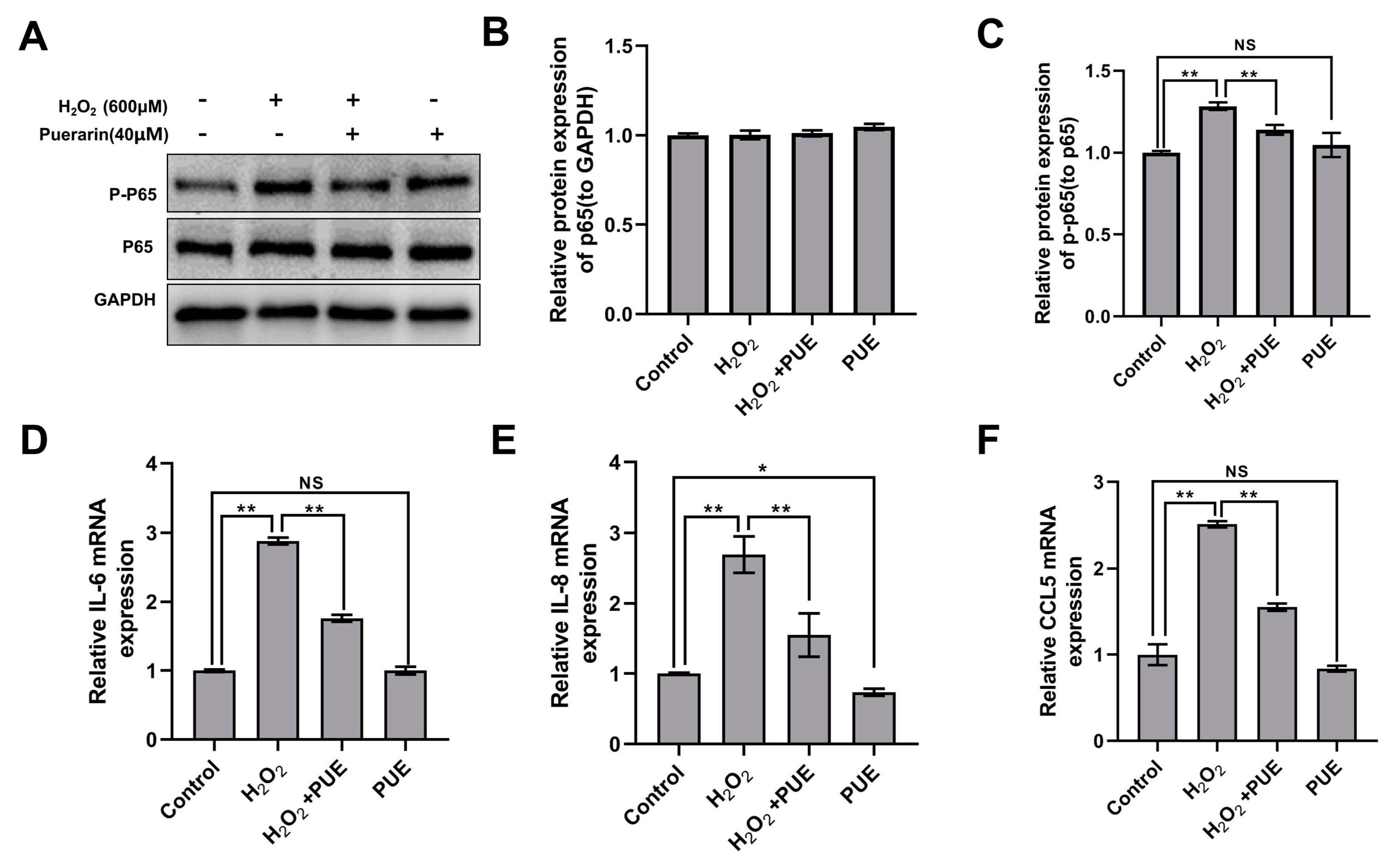
2.4. Effects of PUE on the NF-κB Signaling Pathway in BMECs Treated with H2O2
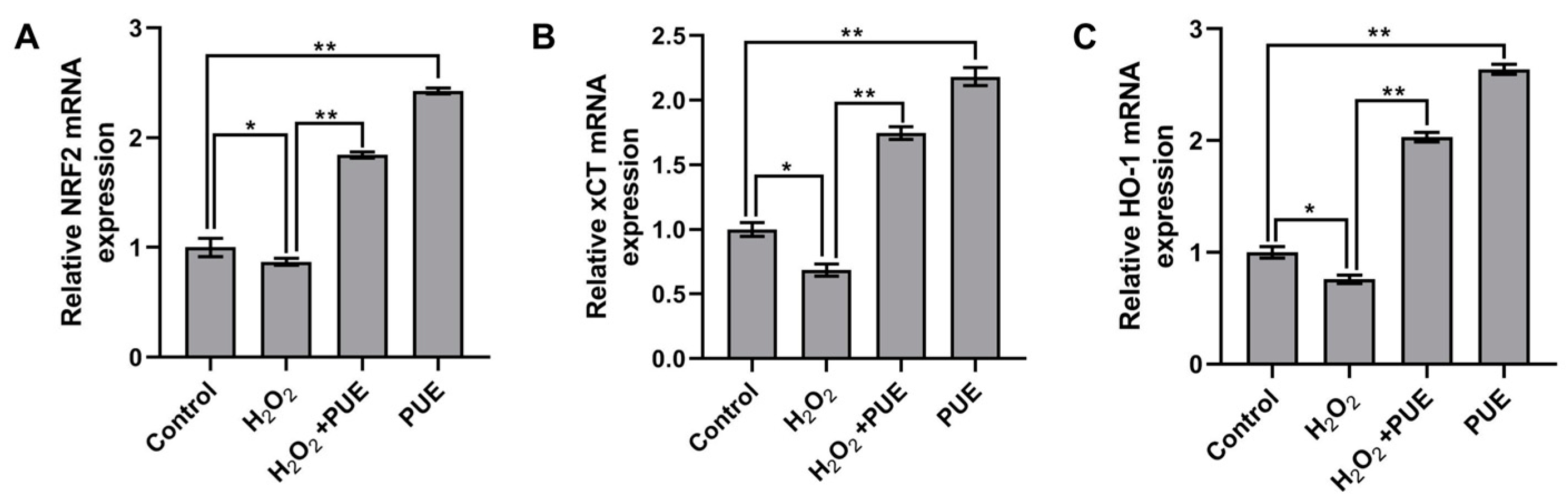
2.5. Effects of PUE on Nrf2 and Its Downstream Genes in BMECs Treated with H2O2
2.6. The Effect of PUE on the Prevention and Treatment of Mastitis in Dairy Cows
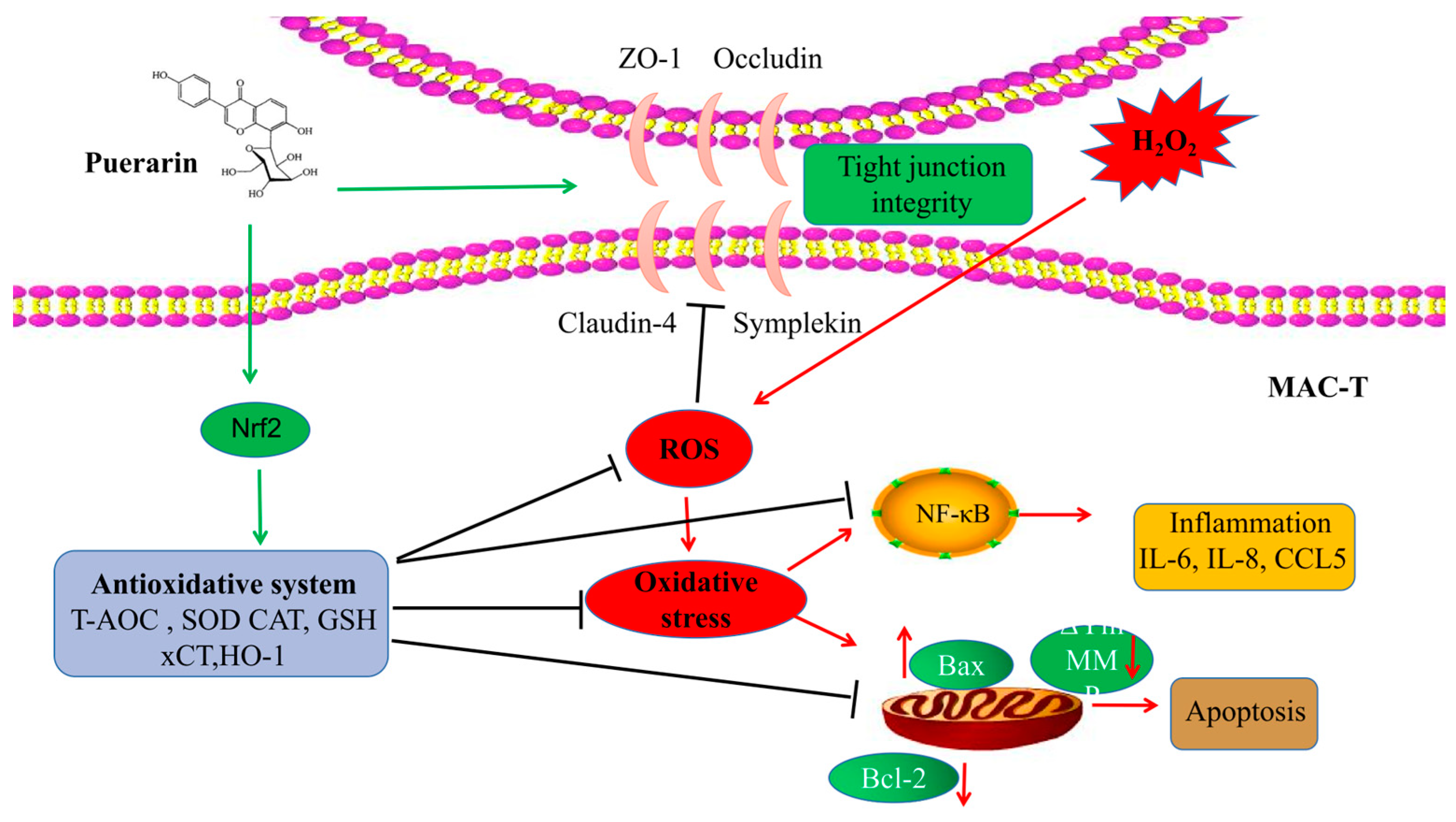
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Processing
4.2. Animals and Design
4.3. Immunofluorescence
4.4. Cell Viability Determination
4.5. ROS Determination
4.6. Detection of Oxidative Stress and Antioxidative Stress Indicators
4.7. RNA Extraction and Real-Time Fluorescent Quantitative PCR (qRT–PCR)
4.8. Western Blot Analysis
4.9. Enzyme-Linked Immunosorbent Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Li, C.; Ali, I.; Li, L.; Wang, G. N-acetylcysteine modulates non-esterified fatty acid-induced pyroptosis and inflammation in granulosa cells. Mol. Immunol. 2020, 127, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 2013, 7, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Messina, V.; Schembari, A.; Casella, S.; Giannetto, C.; Alberghina, D. Pattern of serum protein fractions in dairy cows during different stages of gestation and lactation. J. Dairy Res. 2011, 78, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Piccione, G.; Perillo, L.; Barberio, A.; Manuali, E.; Morgante, M.; Gianesella, M. Hepatic lipidosis in high-yielding dairy cows during the transition period: Haematochemical and histopathological findings. Anim. Prod. Sci. 2015, 57, 74. [Google Scholar] [CrossRef]
- Fiore, E.; Gianesella, M.; Arfuso, F.; Giudice, E.; Piccione, G.; Lora, M.; Stefani, A.; Morgante, M. Glucose infusion response on some metabolicparameters in dairy cows during transition period. Archiv. Tierzucht. 2014, 57, 3. [Google Scholar]
- Arfuso, F.; Minuti, A.; Liotta, L.; Giannetto, C.; Trevisi, E.; Piccione, G.; Lopreiato, V. Stress and inflammatory response of cows and their calves during peripartum and early neonatal period. Theriogenology 2023, 196, 157–166. [Google Scholar] [CrossRef]
- Song, Y.; Loor, J.J.; Li, C.; Liang, Y.; Li, N.; Shu, X.; Yang, Y.; Feng, X.; Du, X.; Wang, Z.; et al. Enhanced mitochondrial dysfunction and oxidative stress in the mammary gland of cows with clinical ketosis. J. Dairy Sci. 2021, 104, 6909–6918. [Google Scholar] [CrossRef]
- Yue, K.; Pu, X.; Loor, J.J.; Jiang, Q.; Dong, J.; Shen, T.; Li, G.; Gao, W.; Lei, L.; Du, X.; et al. Impaired autophagy aggravates oxidative stress in mammary gland of dairy cows with clinical ketosis. J. Dairy Sci. 2022, 105, 6030–6040. [Google Scholar] [CrossRef]
- Chalimeswamy, A.; Thanuja, M.Y.; Ranganath, S.H.; Pandya, K.; Kompella, U.B.; Srinivas, S.P. Oxidative Stress Induces a Breakdown of the Cytoskeleton and Tight Junctions of the Corneal Endothelial Cells. J. Ocul. Pharmacol. Ther. 2022, 38, 74–84. [Google Scholar] [CrossRef]
- Ali, I.; Yang, M.; Wang, Y.; Yang, C.; Shafiq, M.; Wang, G.; Li, L. Sodium propionate protect the blood-milk barrier integrity, relieve lipopolysaccharide-induced inflammatory injury and cells apoptosis. Life Sci. 2021, 270, 119138. [Google Scholar] [CrossRef]
- Rizzo, A.; Ceci, E.; Pantaleo, M.; Mutinati, M.; Spedicato, M.; Minoia, G.; Sciorsci, R.L. Evaluation of blood and milk oxidative status during early postpartum of dairy cows. Animal 2013, 7, 118–123. [Google Scholar] [CrossRef]
- Bae, H.; Jeong, C.H.; Cheng, W.N.; Hong, K.; Seo, H.G.; Han, S.G. Oxidative stress-induced inflammatory responses and effects of N-acetylcysteine in bovine mammary alveolar cells. J. Dairy Res. 2017, 84, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Graugnard, D.; Bionaz, M.; Trevisi, E.; Moyes, K.; Salak-Johnson, J.; Wallace, R.; Drackley, J.; Bertoni, G.; Loor, J. Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J. Dairy Sci. 2012, 95, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Domènech, A.; Parés, S.; Bach, A.; Arís, A. Mammary serum amyloid A3 activates involution of the mammary gland in dairy cows. J. Dairy Sci. 2014, 97, 7595–7605. [Google Scholar] [CrossRef]
- Lee, S.; Zhou, Y.; Gill, D.L.; Kelleher, S.L. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci. Rep. 2018, 8, 3542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, X.; Zhang, J.; Dong, G.; Xiao, W.; Xu, X. Hydrogen Sulfide Alleviates Skeletal Muscle Fibrosis via Attenuating Inflammation and Oxidative Stress. Front. Physiol. 2020, 11, 533690. [Google Scholar] [CrossRef] [PubMed]
- Laliotis, G.P.; Koutsouli, P.; Sotirakoglou, K.; Savoini, G.; Politis, I. Association of Oxidative Stress Biomarkers and Clinical Mastitis Incidence in Dairy Cows During the Periparturient Period. J. Vet. Res. 2020, 64, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.L.; Karcher, E.L.; Rezamand, P.; Gandy, J.C.; VandeHaar, M.J.; Capuco, A.V.; Sordillo, L.M. Evaluation of antioxidant and proinflammatory gene expression in bovine mammary tissue during the periparturient period. J. Dairy Sci. 2009, 92, 589–598. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Jia, H.; Wang, H.; Shui, G.; Qin, Y.; Shu, X.; Wang, Y.; Dong, J.; Liu, G.; et al. Nuclear Factor E2-Related Factor 2 Mediates Oxidative Stress-Induced Lipid Accumulation in Adipocytes by Increasing Adipogenesis and Decreasing Lipolysis. Antioxid. Redox Signal. 2019, 32, 173–192. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Ma, Y.F.; Wu, Z.H.; Gao, M.; Loor, J.J. Nuclear factor erythroid 2-related factor 2 antioxidant response element pathways protect bovine mammary epithelial cells against H2O2-induced oxidative damage in vitro. J. Dairy. Sci. 2018, 101, 5329–5344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Aabdin, Z.U.; Wang, Y.; Ma, N.; Dai, H.; Shi, X.; Shen, X. Glutamine pretreatment protects bovine mammary epithelial cells from inflammation and oxidative stress induced by γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP). J. Dairy Sci. 2021, 104, 2123–2139. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, G.; Wang, Y.; Zhang, Y. Moringa oleifera leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative stress in vitro. Reprod. Domest. Anim. 2020, 55, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, L.; Gao, M.; Loor, J.J. Tea polyphenols protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative damage in vitro. J. Anim. Sci. 2018, 96, 4159–4172. [Google Scholar] [CrossRef]
- Sun, X.; Jia, H.; Xu, Q.; Zhao, C.; Xu, C. Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Food Funct. 2019, 10, 6276–6285. [Google Scholar] [CrossRef]
- Ciampi, F.; Sordillo, L.; Gandy, J.; Caroprese, M.; Sevi, A.; Albenzio, M.; Santillo, A. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Dairy Sci. 2020, 103, 8938–8947. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef]
- Xiao, C.; Li, J.; Dong, X.; He, X.; Niu, X.; Liu, C.; Zhong, G.; Bauer, R.; Yang, D.; Lu, A. Anti-oxidative and TNF-α suppressive activities of puerarin derivative (4AC) in RAW264.7 cells and collagen-induced arthritic rats. Eur. J. Pharmacol. 2011, 666, 242–250. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Q.; Yi, D.; Wu, T.; Chen, H.; Guo, S.; Li, S.; Ji, C.; Wang, L.; Zhao, D.; et al. Quantitative Proteomic Analysis Reveals Antiviral and Anti-inflammatory Effects of Puerarin in Piglets Infected with Porcine Epidemic Diarrhea Virus. Front. Immunol. 2020, 11, 169. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Ge, X.; Zhang, F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019, 109, 726–733. [Google Scholar] [CrossRef]
- Lian, D.; Yuan, H.; Yin, X.; Wu, Y.; He, R.; Huang, Y.; Chen, Y. Puerarin inhibits hyperglycemia-induced inter-endothelial junction through suppressing endothelial Nlrp3 inflammasome activation via ROS-dependent oxidative pathway. Phytomedicine 2019, 55, 310–319. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, Y.Y.; Fan, W.; Yang, C.B.; Ye, S.F.; Cui, W.; Wei, W.; Lao, L.X.; Cai, J.; Han, Y.F.; et al. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci. Ther. 2015, 21, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.M.; Kim, C.-S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free. Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.F.; Jiang, L.Y.; Wang, D.M.; Zhao, F.Q.; Liu, J.X. Supplementation with N-carbamoylglutamate during the transition period improves the function of neutrophils and reduces inflammation and oxidative stress in dairy cows. J. Dairy Sci. 2022, 105, 5786–5795. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, K.; Xi, B.; Xie, J.; Bing, X. Protective effects of paeonol against lipopolysaccharide-induced liver oxidative stress and inflammation in gibel carp (Carassius auratus gibelio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 257, 109339. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Li, F.; Adhikari, B.K.; Liu, Y.; Sun, J.; Zhang, R.; Cai, L.; Liu, Q.; Wang, Y. Curcuminoids: Implication for inflammation and oxidative stress in cardiovascular diseases. Phytother. Res. 2019, 33, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free. Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Du, X.; Shen, T.; Wang, H.; Qin, X.; Xing, D.; Ye, Q.; Shi, Z.; Fang, Z.; Zhu, Y.; Yang, Y.; et al. Adaptations of hepatic lipid metabolism and mitochondria in dairy cows with mild fatty liver. J. Dairy Sci. 2018, 101, 9544–9558. [Google Scholar] [CrossRef]
- Gao, W.; Du, X.; Lei, L.; Wang, H.; Zhang, M.; Wang, Z.; Li, X.; Liu, G.; Li, X. NEFA-induced ROS impaired insulin signalling through the JNK and p38MAPK pathways in non-alcoholic steatohepatitis. J. Cell. Mol. Med. 2018, 22, 3408–3422. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Xing, G.D.; Qian, Y.; Sun, X.F.; Zhong, J.F.; Chen, K.L. Dihydromyricetin attenuates heat stress-induced apoptosis in dairy cow mammary epithelial cells through suppressing mitochondrial dysfunction. Ecotoxicol. Environ. Saf. 2021, 214, 112078. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Y.; Wang, Z.; Chen, J.; Yang, Y.; Dong, G. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 496. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; He, C. An isoflavonoid-enriched extract from Pueraria lobata (kudzu) root protects human umbilical vein endothelial cells against oxidative stress induced apoptosis. J. Ethnopharmacol. 2016, 193, 524–530. [Google Scholar] [CrossRef]
- Phyn, C.V.C.; Stelwagen, K.; Davis, S.R.; McMahon, C.D.; Dobson, J.M.; Singh, K. Tight Junction Protein Abundance and Apoptosis During Involution of Rat Mammary Glands. J. Cell. Physiol. 2017, 232, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Stelwagen, K.; Singh, K. The role of tight junctions in mammary gland function. J. Mammary Gland. Biol. Neoplasia. 2014, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.K.; Rudolph, M.C.; Ramanathan, P.; Burns, V.; Webb, P.; Bitler, B.G.; Stein, T.; Kobayashi, K.; Neville, M.C. Developmental Expression of Claudins in the Mammary Gland. J. Mammary Gland. Biol. Neoplasia. 2017, 22, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Weng, X.; Monteiro, A.P.A.; Guo, J.; Li, C.; Orellana, R.M.; Marins, T.N.; Bernard, J.K.; Tomlinson, D.J.; DeFrain, J.M.; Wohlgemuth, S.E.; et al. Effects of heat stress and dietary zinc source on performance and mammary epithelial integrity of lactating dairy cows. J. Dairy. Sci. 2018, 101, 2617–2630. [Google Scholar] [CrossRef]
- Xu, T.; Dong, Z.; Wang, X.; Qi, S.; Li, X.; Cheng, R.; Liu, X.; Zhang, Y.; Gao, M.Q. IL-1β induces increased tight junction permeability in bovine mammary epithelial cells via the IL-1β-ERK1/2-MLCK axis upon blood-milk barrier damage. J. Cell. Biochem. 2018, 119, 9028–9041. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Yamamoto, M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002, 348, 182–190. [Google Scholar] [PubMed]
- Facchinetti, M.M. Heme-Oxygenase-1. Antioxid. Redox Signal. 2020, 32, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Rezaie, T.; Seki, M.; Sunico, C.R.; Tabuchi, T.; Kitagawa, T.; Yanagitai, M.; Senzaki, M.; Kosegawa, C.; Taira, H.; et al. Dual neuroprotective pathways of a pro-electrophilic compound via HSF-1-activated heat-shock proteins and Nrf2-activated phase 2 antioxidant response enzymes. J. Neurochem. 2011, 119, 569–578. [Google Scholar] [CrossRef]
- Godden, S.M.; Royster, E.; Timmerman, J.; Rapnicki, P.; Green, H. Evaluation of an automated milk leukocyte differential test and the California Mastitis Test for detecting intramammary infection in early- and late-lactation quarters and cows. J. Dairy Sci. 2017, 100, 6527–6544. [Google Scholar] [CrossRef] [PubMed]


| Time | Control 1 | Control + PUE 2 | Mastitis 3 | Mastitis + PUE 4 | |
|---|---|---|---|---|---|
| IL-6 | 0 d | 130.58 ± 42.99 | 146.54 ± 36.02 | 513.82 ± 32.66 b | 492.21 ± 43.31 b |
| 7 d | 120.29 ± 34.55 | 116.23 ± 33.79 a | 489.86 ± 39.86 b | 303.18 ± 27.93 abc | |
| IL-1β | 0 d | 10.54 ± 0.89 | 10.21 ± 1.38 | 37.88 ± 2.25 b | 36.67 ± 0.75 b |
| 7 d | 10.58 ± 0.90 | 9.87 ± 0.78 a | 35.71 ± 2.14 b | 21.42 ± 0.85 abc | |
| TNFα | 0 d | 97.15 ± 8.83 | 103.15 ± 2.51 | 175.61 ± 7.95 b | 163.75 ± 6.01 b |
| 7 d | 98.20 ± 1.14 | 98.20 ± 6.74 a | 182.37 ± 20.24 b | 128.35 ± 4.51 abc |
| Time | Control 1 | Control + PUE 2 | Mastitis 3 | Mastitis + PUE 4 | |
|---|---|---|---|---|---|
| IL-6 | 0 d | 124.62 ± 8.76 | 142.74 ± 11.42 | 530.47 ± 24.15 b | 516.74 ± 44.23 b |
| 7 d | 134.12 ± 2.55 | 115.66 ± 9.94 a | 522.67 ± 26.65 b | 332.03 ± 19.09 abc | |
| IL-1β | 0 d | 14.54 ± 4.33 | 15.08 ± 3.00 | 49.83 ± 3.58 b | 50.92 ± 2.58 b |
| 7 d | 17.08 ± 0.90 | 13.92 ± 3.19 | 53.29 ± 2.06 b | 30.10 ± 3.12 abc | |
| TNFα | 0 d | 149.63 ± 10.89 | 157.00 ± 3.08 | 239.60 ± 7.23 b | 234.49 ± 7.45 b |
| 7 d | 146.82 ± 7.50 | 142.72 ± 15.16 | 240.50 ± 0.87 b | 186.79 ± 11.87 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, C.; Yuan, B.; Meng, Y.; Cong, S.; Che, H.; Ji, X.; Wang, H.; Chen, C.; Li, X.; Jiang, H.; et al. Puerarin Alleviates H2O2-Induced Oxidative Stress and Blood–Milk Barrier Impairment in Dairy Cows. Int. J. Mol. Sci. 2023, 24, 7742. https://doi.org/10.3390/ijms24097742
Lyu C, Yuan B, Meng Y, Cong S, Che H, Ji X, Wang H, Chen C, Li X, Jiang H, et al. Puerarin Alleviates H2O2-Induced Oxidative Stress and Blood–Milk Barrier Impairment in Dairy Cows. International Journal of Molecular Sciences. 2023; 24(9):7742. https://doi.org/10.3390/ijms24097742
Chicago/Turabian StyleLyu, Chenchen, Bao Yuan, Yu Meng, Shuai Cong, Haoyu Che, Xingyu Ji, Haoqi Wang, Chengzhen Chen, Xinwei Li, Hao Jiang, and et al. 2023. "Puerarin Alleviates H2O2-Induced Oxidative Stress and Blood–Milk Barrier Impairment in Dairy Cows" International Journal of Molecular Sciences 24, no. 9: 7742. https://doi.org/10.3390/ijms24097742
APA StyleLyu, C., Yuan, B., Meng, Y., Cong, S., Che, H., Ji, X., Wang, H., Chen, C., Li, X., Jiang, H., & Zhang, J. (2023). Puerarin Alleviates H2O2-Induced Oxidative Stress and Blood–Milk Barrier Impairment in Dairy Cows. International Journal of Molecular Sciences, 24(9), 7742. https://doi.org/10.3390/ijms24097742







