Evaluating Back-to-Back and Day-to-Day Reproducibility of Cortical GABA+ Measurements Using Proton Magnetic Resonance Spectroscopy (1H MRS)
Abstract
1. Introduction
2. Results
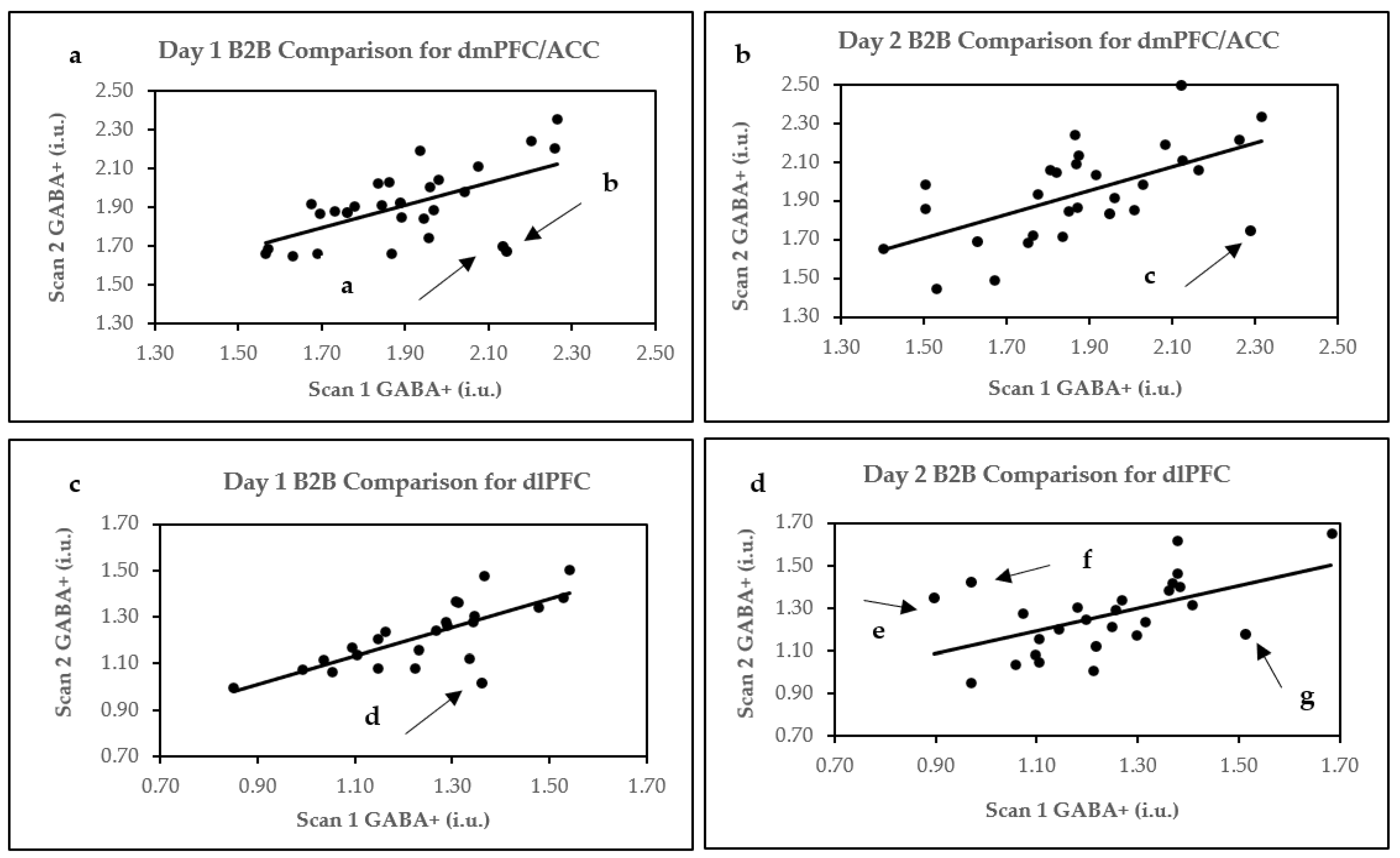
2.1. Within Session/B2B Reproducibility
2.1.1. dmPFC/ACC
2.1.2. dlPFC
2.2. D2D Reproducibility of 5.2 Min Scans
dmPFC/ACC
2.3. dlPFC
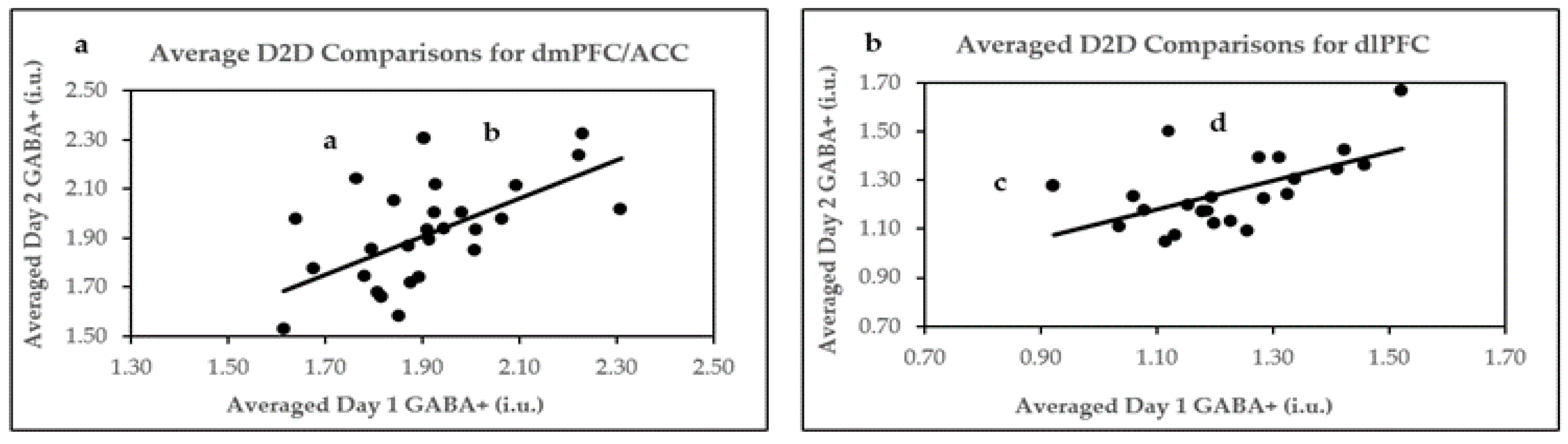
2.4. D2D Reproducibility of Two Averaged B2B 5.2 Min Scans
2.5. Assessment of D2D Voxel Overlaps, Tissue Heterogeneity, and GABA+ Concentrations
2.6. Sex Differences
3. Discussion
3.1. B2B Reproducibility
3.2. D2D Reproducibility
3.3. Sex Differences
3.4. Limitations
4. Materials and Methods
4.1. Participants
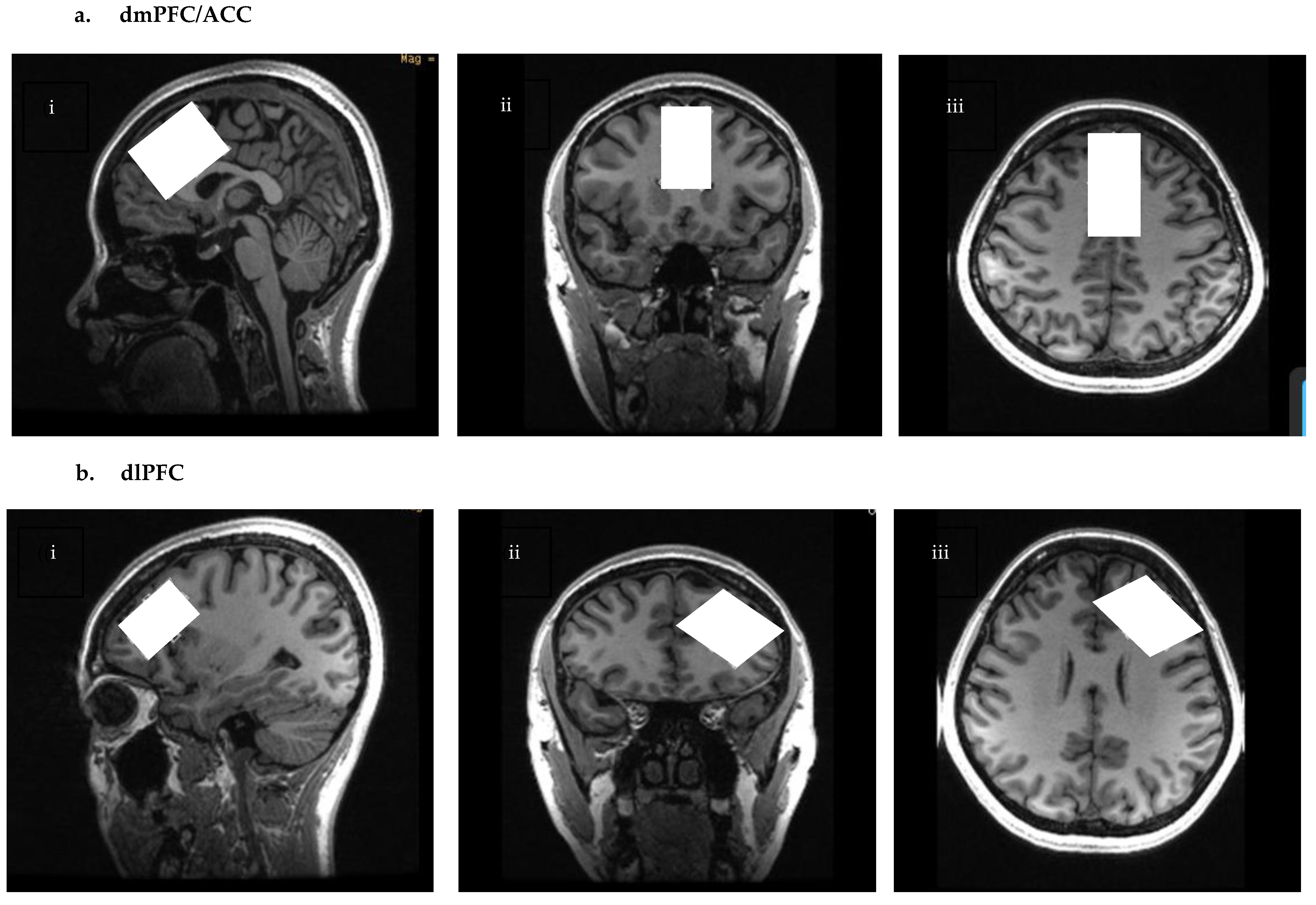
4.2. Scanning Procedures
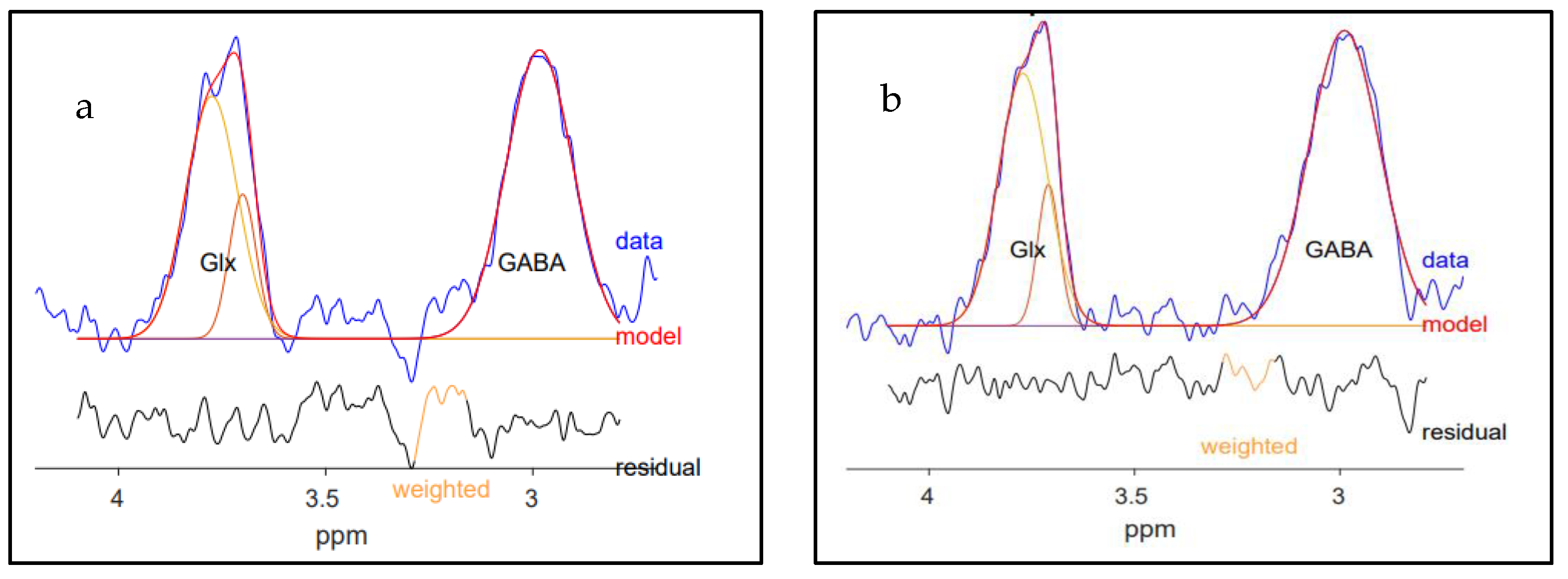
4.3. MRS Data Processing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rae, C.D. A Guide to the Metabolic Pathways and Function of Metabolites Observed in Human Brain 1H Magnetic Resonance Spectra. Neurochem. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Sriram, K. Molecular Mechanisms Underlying Neuroinflammation Elicited by Occupational Injuries and Toxicants. Int. J. Mol. Sci. 2023, 24, 2272. [Google Scholar] [CrossRef]
- Pham, T.H.; Gardier, A.M. Fast-acting antidepressant activity of ketamine: Highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol. Ther. 2019, 199, 58–90. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.B.; Waagepetersen, H.S.; Bak, L.K.; Schousboe, A.; Sonnewald, U. The Glutamine–Glutamate/GABA Cycle: Function, Regional Differences in Glutamate and GABA Production and Effects of Interference with GABA Metabolism. Neurochem. Res. 2015, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.M.; Mercier, L.J.; Stokoe, M.; La, P.L.; Bell, T.; Batycky, J.M.; Debert, C.T.; Harris, A.D. Glutamate, GABA and glutathione in adults with persistent post-concussive symptoms. NeuroImage Clin. 2022, 36, 103152. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Puts, N.A.; Edden, R.A. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef]
- Epperson, C.N.; Gueorguieva, R.; Czarkowski, K.A.; Stiklus, S.; Sellers, E.; Krystal, J.H.; Rothman, D.L.; Mason, G.F. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: A 1H-MRS study. Psychopharmacology 2006, 186, 425–433. [Google Scholar] [CrossRef]
- Gao, F.; Edden, R.A.; Li, M.; Puts, N.A.; Wang, G.; Liu, C.; Zhao, B.; Wang, H.; Bai, X.; Zhao, C.; et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. NeuroImage 2013, 78, 75–82. [Google Scholar] [CrossRef]
- O’Gorman, R.L.; Michels, L.; Edden, R.A.; Murdoch, J.B.; Martin, E. In vivo detection of GABA and glutamate with MEGA-PRESS: Reproducibility and gender effects. J. Magn. Reson. Imaging JMRI 2011, 33, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Dina, C.Z.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.S.; Markou, A. The “Stop” and “Go” of Nicotine Dependence: Role of GABA and Glutamate. Cold Spring Harb. Perspect. Med. 2013, 3, a012146. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Yang, G.; Ren, J.; Zhao, J.; Li, S. Caffeine Suppresses GABA Receptor-Mediated Current in Rat Primary Sensory Neurons via Inhibition of Intracellular Phosphodiesterase. Neurophysiology 2015, 47, 108–114. [Google Scholar] [CrossRef]
- Isokawa, M. Caffeine-Induced Suppression of GABAergic Inhibition and Calcium-Independent Metaplasticity. Neural Plast. 2016, 2016, 1239629. [Google Scholar] [CrossRef]
- Hasler, G.; van der Veen, J.W.; Geraci, M.; Shen, J.; Pine, D.; Drevets, W.C. Prefrontal cortical gamma-aminobutyric Acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol. Psychiatry 2009, 65, 273–275. [Google Scholar] [CrossRef]
- Hasler, G.; van der Veen, J.W.; Grillon, C.; Drevets, W.C.; Shen, J. Effect of Acute Psychological Stress on Prefrontal GABA Concentration Determined by Proton Magnetic Resonance Spectroscopy. Am. J. Psychiatry 2010, 167, 1226–1231. [Google Scholar] [CrossRef]
- Hattingen, E.; Lückerath, C.; Pellikan, S.; Vronski, D.; Roth, C.; Knake, S.; Kieslich, M.; Pilatus, U. Frontal and thalamic changes of GABA concentration indicate dysfunction of thalamofrontal networks in juvenile myoclonic epilepsy. Epilepsia 2014, 55, 1030–1037. [Google Scholar] [CrossRef]
- Sarlo, G.L.; Holton, K.F. Brain concentrations of glutamate and GABA in human epilepsy: A review. Seizure 2021, 91, 213–227. [Google Scholar] [CrossRef]
- Tayoshi, S.; Nakataki, M.; Sumitani, S.; Taniguchi, K.; Shibuya-Tayoshi, S.; Numata, S.; Iga, J.-I.; Ueno, S.-I.; Harada, M.; Ohmori, T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: A proton magnetic resonance spectroscopy study. Schizophr. Res. 2010, 117, 83–91. [Google Scholar] [CrossRef]
- Glausier, J.R.; Lewis, D.A. GABA and schizophrenia: Where we stand and where we need to go. Schizophr. Res. 2017, 181, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.H.; Jensen, J.E.; Simon, N.M.; Kaufman, R.E.; Renshaw, P.F. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: Response to treatment with levetiracetam. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Grills, R. Brain Neurochemicals in Generalized Social Anxiety Disorder: A Proton Magnetic Resonance Spectroscopy Study. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2011. [Google Scholar]
- Strawn, J.R.; Levine, A. Treatment response biomarkers in anxiety disorders: From neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark. Neuropsychiatry 2020, 3, 100024. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- de Graaf, R.A. In Vivo NMR Spectroscopy: Principles and Techniques, 2nd ed.; John Wiley & Sons Ltd.: London, UK, 2007. [Google Scholar]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. What are we measuring with GABA Magnetic Resonance Spectroscopy? Commun. Integr. Biol. 2011, 4, 573–575. [Google Scholar] [CrossRef]
- Mullins, P.G.; McGonigle, D.J.; O’Gorman, R.L.; Puts, N.A.J.; Vidyasagar, R.; Evans, C.J.; Cardiff Symposium on MRS of GABA; Edden, R.A.E. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage 2014, 86, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mescher, M.; Merkle, H.; Kirsch, J.; Garwood, M.; Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998, 11, 266–272. [Google Scholar] [CrossRef]
- Gruber, B.; Froeling, M.; Leiner, T.; Klomp, D.W. RF coils: A practical guide for nonphysicists. J. Magn. Reson. Imaging 2018, 48, 590–604. [Google Scholar] [CrossRef]
- Shokrollahi, P.; Drake, J.M.; Goldenberg, A.A. Signal-to-noise ratio evaluation of magnetic resonance images in the presence of an ultrasonic motor. Biomed. Eng. Online 2017, 16, 45. [Google Scholar] [CrossRef]
- Alger, J.R. Quantitative proton magnetic resonance spectroscopy and spectroscopic imaging of the brain: A didactic review. Top Magn Reson. Imaging 2010, 21, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Harris, A.D.; Gong, T.; Puts, N.A.J.; Wang, G.; Schär, M.; Barker, P.B.; Edden, R.A.E. Voxel Placement Precision for GABA-Edited Magnetic Resonance Spectroscopy. Open J. Radiol. 2017, 7, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.M.; Moser, A.D.; Zuo, C.S.; Du, F.; Chen, X.; Perlo, S.; Richards, C.E.; Nascimento, N.; Ironside, M.; Crowley, D.J.; et al. Repeatability and reliability of GABA measurements with magnetic resonance spectroscopy in healthy young adults. Magn. Reson. Med. 2021, 85, 2359–2369. [Google Scholar] [CrossRef]
- Brix, M.K.; Ersland, L.; Hugdahl, K.; Dwyer, G.E.; Grüner, R.; Noeske, R.; Beyer, M.K.; Craven, A.R. Within- and between-session reproducibility of GABA measurements with MR spectroscopy. J. Magn. Reson. Imaging JMRI 2017, 46, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, I.; Noah, S.; Maddock, R.J.; Ivry, R.B. Individual differences in GABA content are reliable but are not uniform across the human cortex. NeuroImage 2016, 139, 1–7. [Google Scholar] [CrossRef]
- Edden, R.A.; Puts, N.A.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging JMRI 2014, 40, 1445–1452. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Singh, K.D.; Brealy, J.A.; Linden, D.E.J.; Evans, C.J. Quantification of gamma-aminobutyric acid (GABA) in 1 H MRS volumes composed heterogeneously of grey and white matter. NMR Biomed. 2016, 29, 1644–1655. [Google Scholar] [CrossRef]
- Minarik, T.; Berger, B.; Althaus, L.; Bader, V.; Biebl, B.; Brotzeller, F.; Fusban, T.; Hegemann, J.; Jesteadt, L.; Kalweit, L.; et al. The Importance of Sample Size for Reproducibility of tDCS Effects. Front. Hum. Neurosci. 2016, 10, 453. [Google Scholar] [CrossRef]
- Motyka, S.; Moser, P.; Hingerl, L.; Hangel, G.; Heckova, E.; Strasser, B.; Eckstein, K.; Daniel Robinson, S.; Poser, B.A.; Gruber, S.; et al. The influence of spatial resolution on the spectral quality and quantification accuracy of whole-brain MRSI at 1.5T, 3T, 7T, and 9.4T. Magn. Reson. Med. 2019, 82, 551–565. [Google Scholar] [CrossRef]
- Kreis, R.; Boer, V.; Choi, I.; Cudalbu, C.; de Graaf, R.A.; Gasparovic, C.; Heerschap, A.; Krššák, M.; Lanz, B.; Maudsley, A.A.; et al. Terminology and concepts for the characterization of in vivo MR spectroscopy methods and MR spectra: Background and experts’ consensus recommendations. NMR Biomed. 2021, 34, e4347. [Google Scholar] [CrossRef]
- Jensen, J.E.; Frederick, B.D.; Renshaw, P.F. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005, 18, 570–576. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.W.; Shen, J. Regional difference in GABA levels between medial prefrontal and occipital cortices. J. Magn. Reson. Imaging JMRI 2013, 38, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Grachev, I.D.; Apkarian, A.V. Chemical Heterogeneity of the Living Human Brain: A Proton MR Spectroscopy Study on the Effects of Sex, Age, and Brain Region. NeuroImage 2000, 11, 554–563. [Google Scholar] [CrossRef]
- Aufhaus, E.; Weber-Fahr, W.; Sack, M.; Tunc-Skarka, N.; Oberthuer, G.; Hoerst, M.; Meyer-Lindenberg, A.; Boettcher, U.; Ende, G. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: A MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn. Reson. Med. 2013, 69, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Epperson, C.N.; O’Malley, S.; Czarkowski, K.A.; Gueorguieva, R.; Jatlow, P.; Sanacora, G.; Rothman, D.L.; Krystal, J.H.; Mason, G.F. Sex, GABA, and nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol. Psychiatry 2005, 57, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.W.; Listerud, J.; Kressel, H.Y. Combined chemical-shift and phase-selective imaging for fat suppression: Theory and initial clinical experience. Radiology 1991, 181, 41–47. [Google Scholar] [CrossRef]
- Gasparovic, C.; Song, T.; Devier, D.; Bockholt, H.J.; Caprihan, A.; Mullins, P.G.; Posse, S.; Jung, R.E.; Morrison, L.A. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006, 55, 1219–1226. [Google Scholar] [CrossRef]
- Kurcyus, K.; Annac, E.; Hanning, N.M.; Harris, A.D.; Oeltzschner, G.; Edden, R.; Riedl, V. Opposite Dynamics of GABA and Glutamate Levels in the Occipital Cortex during Visual Processing. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 9967–9976. [Google Scholar] [CrossRef]
- Zou, K.H.; Tuncali, K.; Silverman, S.G. Correlation and Simple Linear Regression. Radiology 2003, 227, 617–628. [Google Scholar] [CrossRef]
- Baeshen, A.; Wyss, P.O.; Henning, A.; O’Gorman, R.L.; Piccirelli, M.; Kollias, S.; Michels, L. Test-Retest Reliability of the Brain Metabolites GABA and Glx with JPRESS, PRESS, and MEGA-PRESS MRS Sequences in vivo at 3T. J. Magn. Reson. Imaging 2019, 51, 1181–1191. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Day 1 B2B | Day 2 B2B |
|---|---|---|
| n | 28 | 29 |
| Pearson’s correlation r | 0.59 *** | 0.60 *** |
| Within-subject mean CV% | 5.02 | 6.26 |
| ICC | 0.60 *** | 0.59 *** |
| Parameters | Day 1 B2B | Day 2 B2B |
|---|---|---|
| n | 24 | 25 |
| Pearson’s correlation r | 0.74 *** | 0.54 ** |
| Within-subject mean CV% | 5.15 | 6.89 |
| ICC | 0.73 *** | 0.54 ** |
| Parameters | S1D1 and S1D2 | S2D1 and S2D2 | S1D1 and S2D2 | S1D2 and S2D1 |
|---|---|---|---|---|
| n | 28 | 29 | 28 | 29 |
| Pearson’s correlation r | 0.71 *** | 0.37 * | 0.33 | 0.57 ** |
| Within-subject mean CV% | 5.44 | 6.63 | 7.08 | 6.73 |
| ICC | 0.70 *** | 0.36 * | 0.32 * | 0.56 ** |
| Parameters | S1D1 and S1D2 | S2D1 and S2D2 | S1D1 and S2D2 | S1D2 and S2D1 |
|---|---|---|---|---|
| n | 24 | 24 | 24 | 24 |
| Pearson’s correlation r | 0.53 ** | 0.52 ** | 0.44 * | 0.53 ** |
| Within-subject mean CV% | 8.15 | 6.85 | 8.68 | 7.69 |
| ICC | 0.54 ** | 0.49 ** | 0.44 * | 0.52 ** |
| Parameters | dmPFC/ACC D2D | dlPFC D2D |
|---|---|---|
| n | 28 | 23 |
| Pearson’s correlation r | 0.62 *** | 0.58 ** |
| Within-subject mean CV% | 4.95 | 5.85 |
| ICC | 0.61 *** | 0.58 ** |
| ROI | Tissue Fractions (M ± SD) | Day 1 | Day 2 | Average |
|---|---|---|---|---|
| dmPFC/ACC (n = 28) | GM | 0.62 ± 0.03 | 0.62 ± 0.03 | 0.62 ± 0.03 |
| WM | 0.15 ± 0.03 | 0.15 ± 0.03 | 0.15 ± 0.03 | |
| CSF | 0.23 ± 0.05 | 0.23 ± 0.05 | 0.23 ± 0.05 | |
| dlPFC (n = 23) | GM | 0.48 ± 0.04 | 0.49 ± 0.03 | 0.49 ± 0.03 |
| WM | 0.45 ± 0.06 | 0.45 ± 0.04 | 0.45 ± 0.04 | |
| CSF | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.02 |
| Sequence Parameters | dmPFC/ACC | dlPFC |
|---|---|---|
| Echo time (TE) | 68 ms | 68 ms |
| Repetition time (TR) | 1500 ms | 1500 ms |
| Number of acquisitions | 192 | 192 |
| Number of excitations (NEX) | 8 | 8 |
| Number of points | 4096 | 4096 |
| Spectral width | 5000 Hz | 5000 Hz |
| Scan time | 5.2 min | 5.2 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaid, S.; Truong, P.; Sailasuta, N.; Le Foll, B. Evaluating Back-to-Back and Day-to-Day Reproducibility of Cortical GABA+ Measurements Using Proton Magnetic Resonance Spectroscopy (1H MRS). Int. J. Mol. Sci. 2023, 24, 7713. https://doi.org/10.3390/ijms24097713
Elsaid S, Truong P, Sailasuta N, Le Foll B. Evaluating Back-to-Back and Day-to-Day Reproducibility of Cortical GABA+ Measurements Using Proton Magnetic Resonance Spectroscopy (1H MRS). International Journal of Molecular Sciences. 2023; 24(9):7713. https://doi.org/10.3390/ijms24097713
Chicago/Turabian StyleElsaid, Sonja, Peter Truong, Napapon Sailasuta, and Bernard Le Foll. 2023. "Evaluating Back-to-Back and Day-to-Day Reproducibility of Cortical GABA+ Measurements Using Proton Magnetic Resonance Spectroscopy (1H MRS)" International Journal of Molecular Sciences 24, no. 9: 7713. https://doi.org/10.3390/ijms24097713
APA StyleElsaid, S., Truong, P., Sailasuta, N., & Le Foll, B. (2023). Evaluating Back-to-Back and Day-to-Day Reproducibility of Cortical GABA+ Measurements Using Proton Magnetic Resonance Spectroscopy (1H MRS). International Journal of Molecular Sciences, 24(9), 7713. https://doi.org/10.3390/ijms24097713






