Exercise and Doxorubicin Modify Markers of Iron Overload and Cardiolipin Deficiency in Cardiac Mitochondria
Abstract
1. Introduction
2. Results
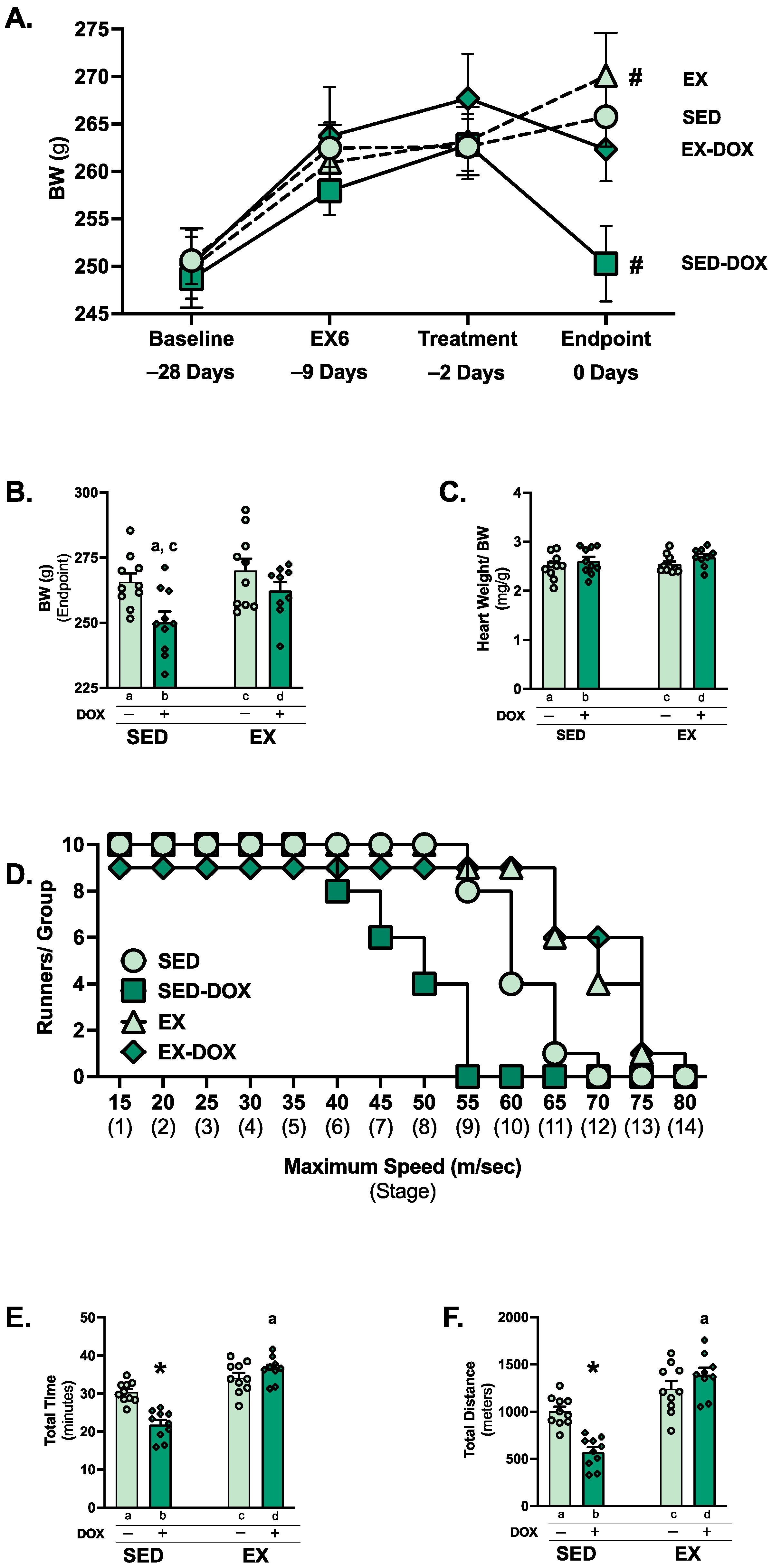
2.1. Exercise Preconditioning Limits Body Weight Loss and Exercise Intolerance in Response to DOX Treatment
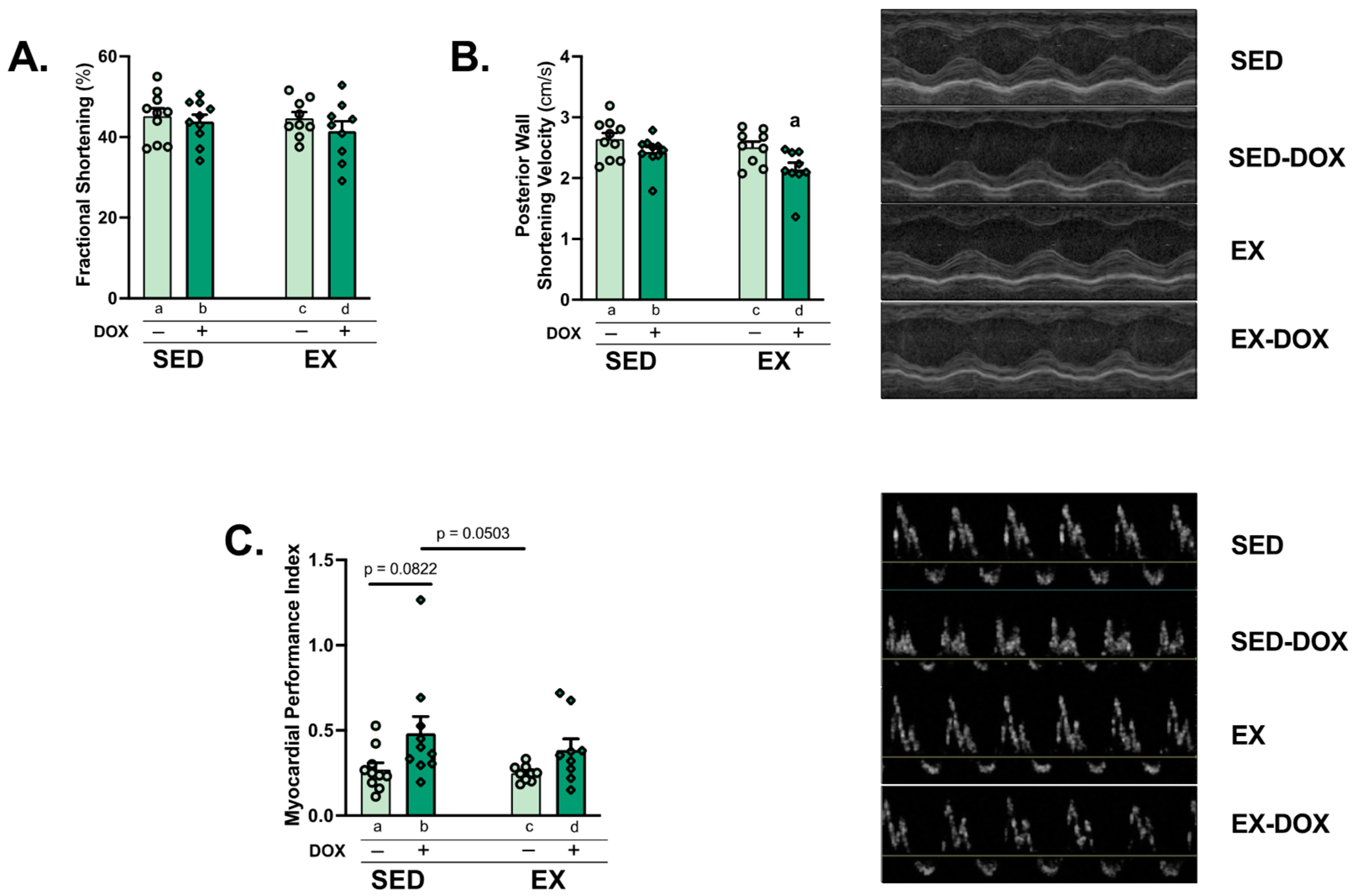
2.2. DOX Impairs Cardiac Function
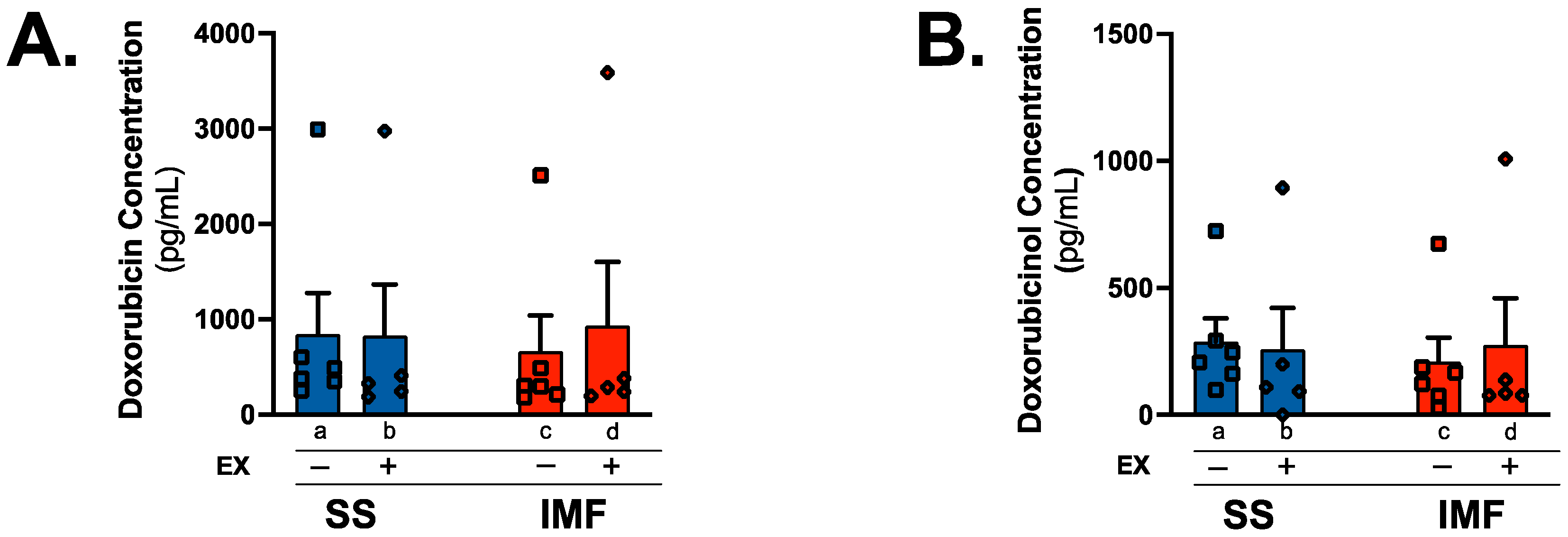
2.3. Exercise Does Not Alter DOX or Doxorubicinol Levels
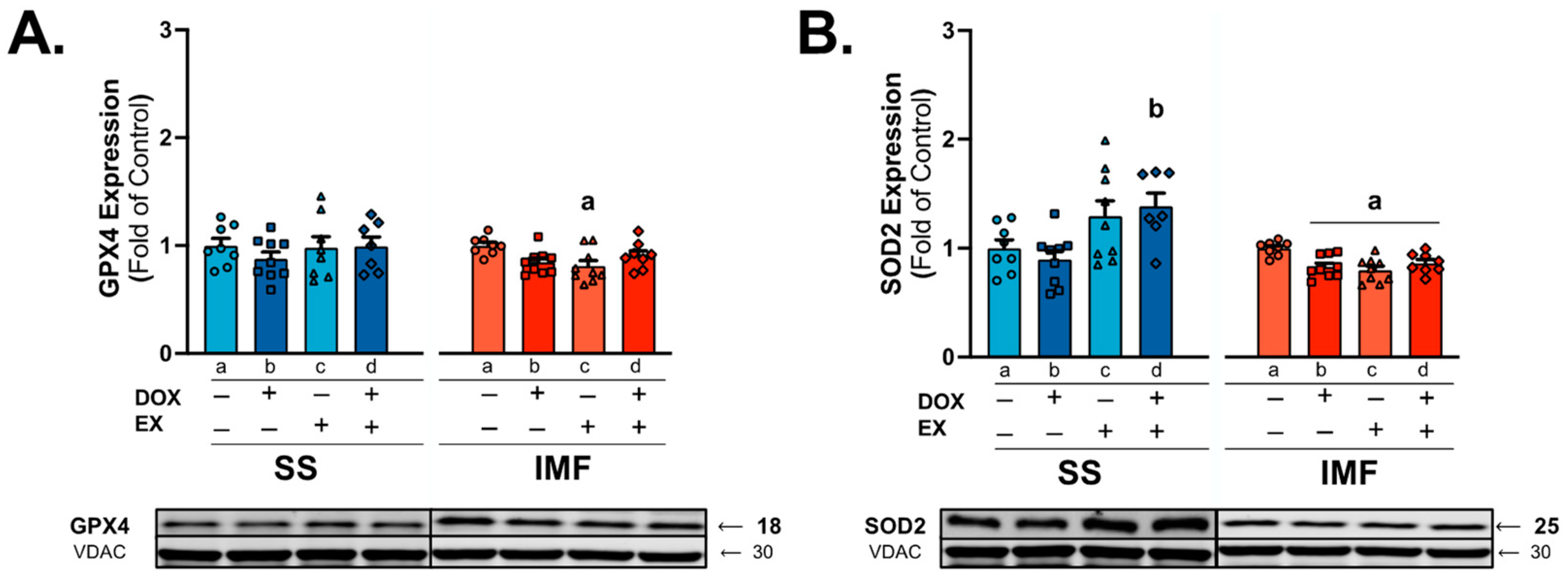
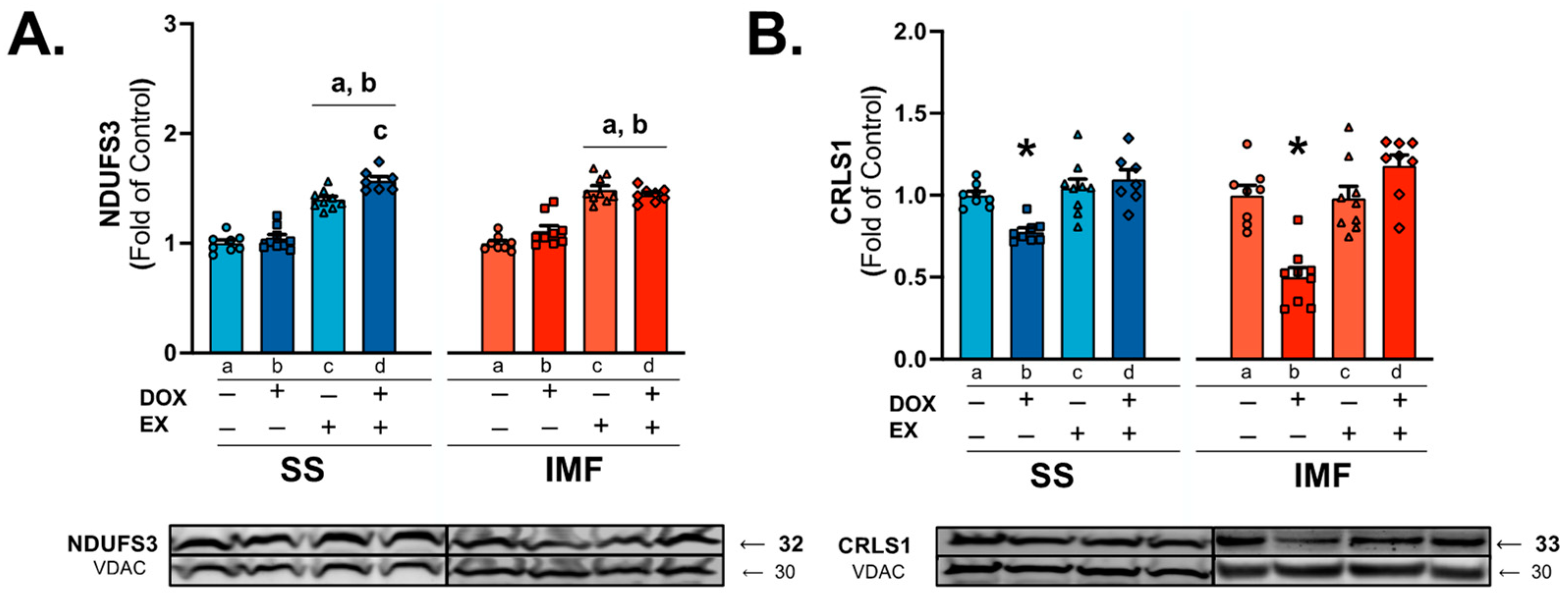
2.4. DOX and Exercise Increase Endogenous Antioxidant Expression in SS Mitochondria
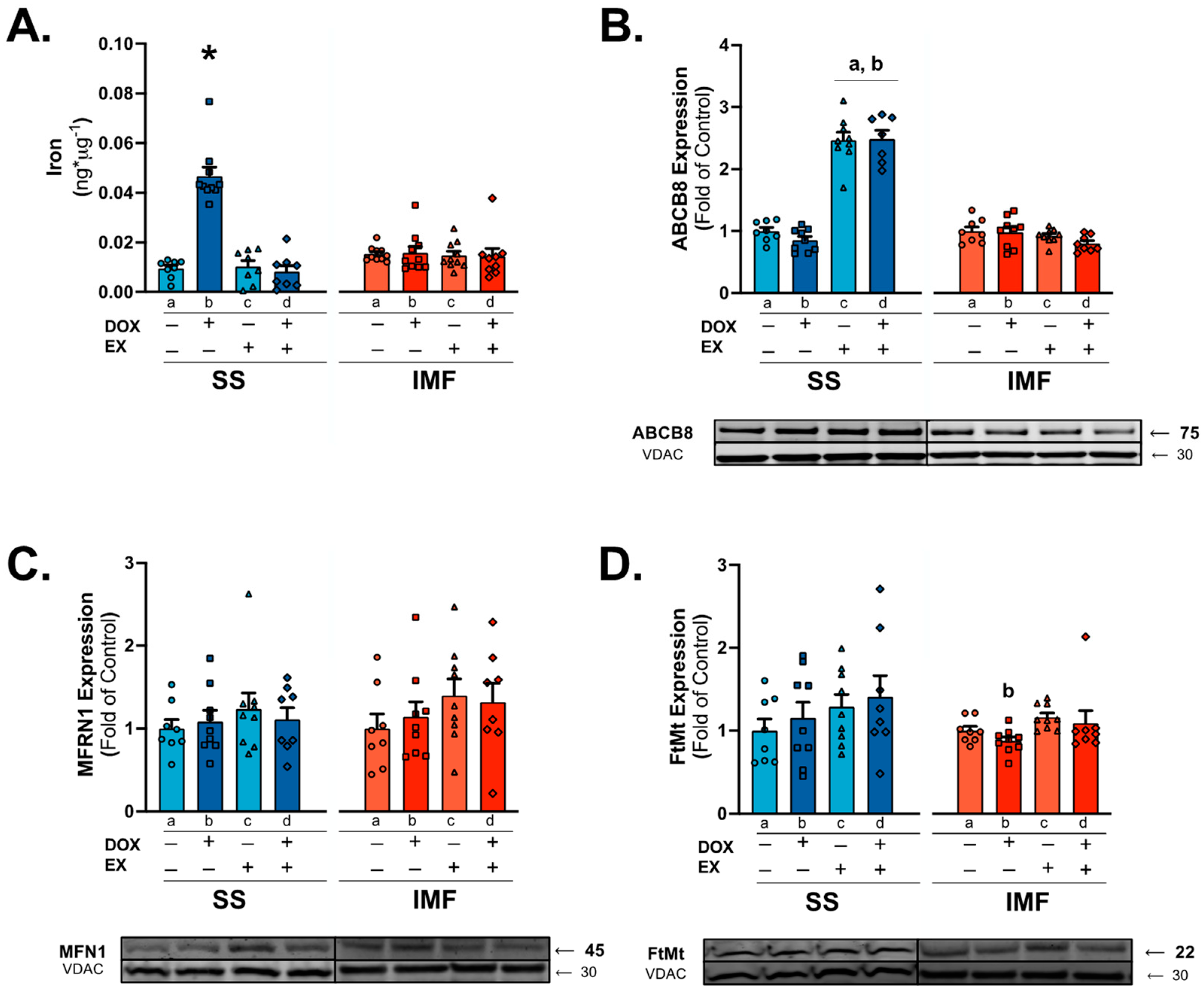
2.5. Mitochondrial Iron Levels and Signaling in SS Mitochondria Are Affected by DOX Treatment and Exercise
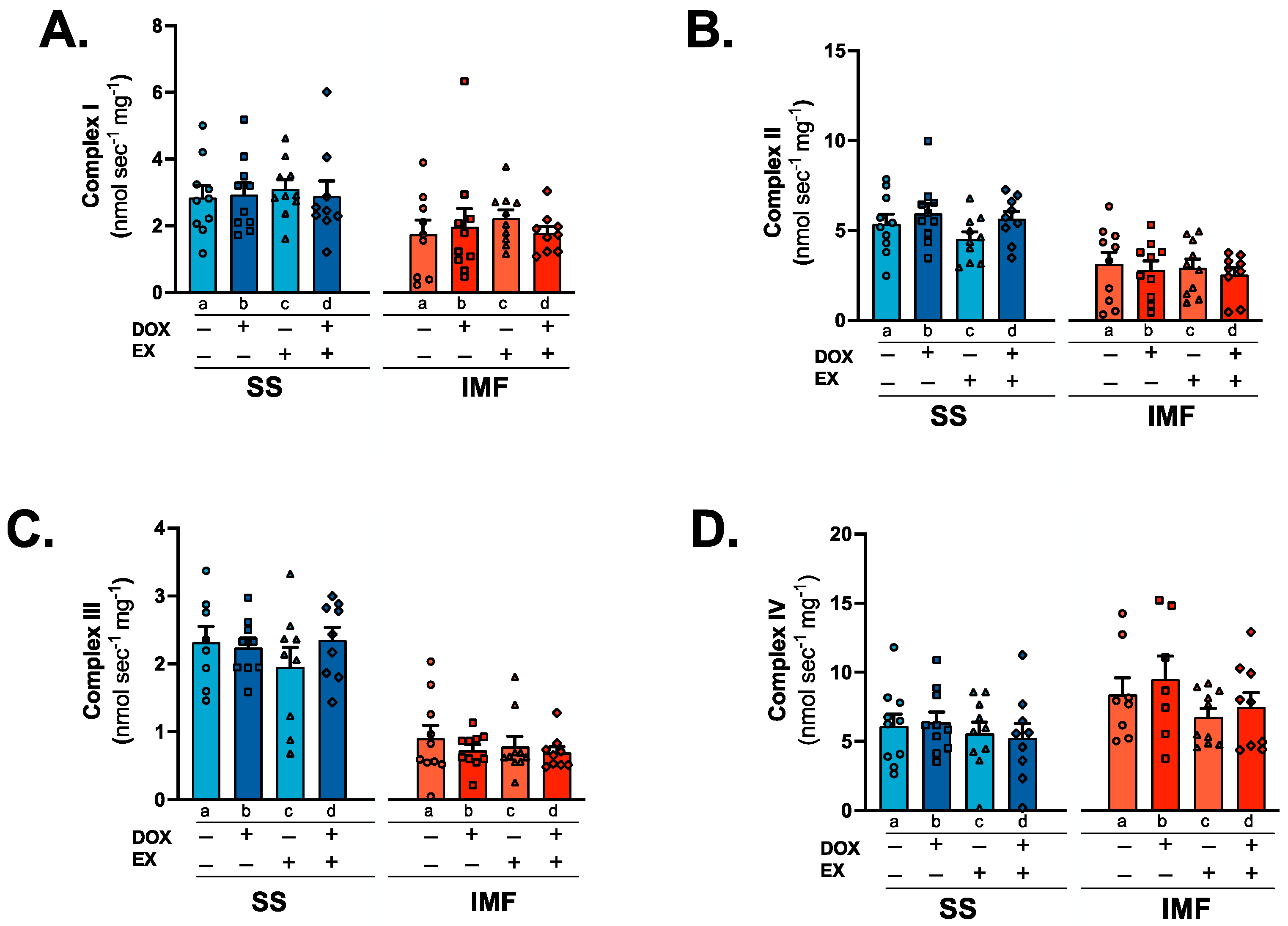
2.6. IMF Mitochondria Exhibit Increased Respiration Compared to SS Mitochondria
3. Discussion
3.1. Mitochondria Localization of DOX
3.2. Mitochondrial Antioxidant Enzymes
3.3. Mitochondrial Iron Handling
3.4. Mitochondrial Respiration and Complex Activity
4. Materials and Methods
4.1. Experimental Animals
4.2. Study Design
4.3. Exercise Tolerance Test
4.4. Echocardiography
4.5. Mitochondrial Isolation
4.6. Doxorubicin and Doxorubicinol Concentration
4.7. Mitochondrial Iron
4.8. Mitochondrial Respiration
4.9. Mitochondrial OXPHOS Complex Activity
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonadonna, G.; Monfardini, S.; De Lena, M.; Fossati-Bellani, F.; Beretta, G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res. 1970, 30, 2572–2582. [Google Scholar]
- Bonadonna, G.; De Lena, M.; Beretta, G. Preliminary clinical screening with adriamycin in lung cancer. Eur. J. Cancer 1971, 7, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Unverferth, D.V.; Magorien, R.D.; Unverferth, B.P.; Talley, R.L.; Balcerzak, S.P.; Baba, N. Human myocardial morphologic and functional changes in the first 24 hours after doxorubicin administration. Cancer Treat. Rep. 1981, 65, 1093–1097. [Google Scholar] [PubMed]
- Billingham, M.E.; Mason, J.W.; Bristow, M.R.; Daniels, J.R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 1978, 62, 865–872. [Google Scholar]
- Mallard, J.; Hucteau, E.; Charles, A.L.; Bender, L.; Baeza, C.; Pelissie, M.; Trensz, P.; Pflumio, C.; Kalish-Weindling, M.; Geny, B.; et al. Chemotherapy impairs skeletal muscle mitochondrial homeostasis in early breast cancer patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1896–1907. [Google Scholar] [CrossRef]
- Duarte-Karim, M.; Ruysschaert, J.M.; Hildebrand, J. Affinity of adriamycin to phospholipids. A possible explanation for cardiac mitochondrial lesions. Biochem. Biophys. Res. Commun. 1976, 71, 658–663. [Google Scholar] [CrossRef]
- Moulin, M.; Solgadi, A.; Veksler, V.; Garnier, A.; Ventura-Clapier, R.; Chaminade, P. Sex-specific cardiac cardiolipin remodelling after doxorubicin treatment. Biol. Sex Differ. 2015, 6, 20. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef] [PubMed]
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into Doxorubicin-induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol. Pharmacol. 2019, 96, 219–232. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Doerr, V.; Nguyen, B.L.; Kelley, R.C.; Smuder, A.J. Consideration of Sex as a Biological Variable in the Development of Doxorubicin Myotoxicity and the Efficacy of Exercise as a Therapeutic Intervention. Antioxidants 2021, 10, 343. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Santos-Alves, E.; Oliveira, P.J.; Moreira, P.I.; Magalhaes, J.; Ascensao, A. The beneficial role of exercise in mitigating doxorubicin-induced Mitochondrionopathy. Biochim. Biophys. Acta 2018, 1869, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol. Toxicol. 2003, 93, 105–115. [Google Scholar] [CrossRef]
- Wu, B.B.; Leung, K.T.; Poon, E.N. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef]
- Wallace, K.B.; Sardao, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Bayeva, M.; Gheorghiade, M.; Ardehali, H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 2013, 61, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Tandler, B.; Fujioka, H.; Riva, A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int. J. Biochem. Cell Biol. 2009, 41, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.M.; Thapa, D.; Shepherd, D.L. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1–H14. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E.S.; Benson, D.W.; Fry, D.E. Subpopulations of human heart mitochondria. J. Surg. Res. 1986, 40, 495–498. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Heterogeneous response of subsarcolemmal heart mitochondria to calcium. Am. J. Physiol. 1986, 250, H741–H748. [Google Scholar] [CrossRef]
- Dabkowski, E.R.; Williamson, C.L.; Bukowski, V.C.; Chapman, R.S.; Leonard, S.S.; Peer, C.J.; Callery, P.S.; Hollander, J.M. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H359–H369. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.L.; Dabkowski, E.R.; Baseler, W.A.; Croston, T.L.; Alway, S.E.; Hollander, J.M. Enhanced apoptotic propensity in diabetic cardiac mitochondria: Influence of subcellular spatial location. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H633–H642. [Google Scholar] [CrossRef] [PubMed]
- Adhihetty, P.J.; Ljubicic, V.; Hood, D.A. Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E748–E755. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977, 252, 8731–8739. [Google Scholar] [CrossRef]
- Timm, K.N.; Perera, C.; Ball, V.; Henry, J.A.; Miller, J.J.; Kerr, M.; West, J.A.; Sharma, E.; Broxholme, J.; Logan, A.; et al. Early detection of doxorubicin-induced cardiotoxicity in rats by its cardiac metabolic signature assessed with hyperpolarized MRI. Commun. Biol. 2020, 3, 692. [Google Scholar] [CrossRef]
- Kavazis, A.N.; Morton, A.B.; Hall, S.E.; Smuder, A.J. Effects of doxorubicin on cardiac muscle subsarcolemmal and intermyofibrillar mitochondria. Mitochondrion 2017, 34, 9–19. [Google Scholar] [CrossRef]
- Kavazis, A.N.; McClung, J.M.; Hood, D.A.; Powers, S.K. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H928–H935. [Google Scholar] [CrossRef]
- Naaktgeboren, W.R.; Binyam, D.; Stuiver, M.M.; Aaronson, N.K.; Teske, A.J.; van Harten, W.H.; Groen, W.G.; May, A.M. Efficacy of Physical Exercise to Offset Anthracycline-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis of Clinical and Preclinical Studies. J. Am. Heart Assoc. 2021, 10, e021580. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.B.; Mor Huertas, A.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Christou, D.D.; Smuder, A.J. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion 2019, 45, 52–62. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Doerr, V.; Min, K.; Szeto, H.H.; Smuder, A.J. Doxorubicin-induced oxidative stress differentially regulates proteolytic signaling in cardiac and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R227–R233. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, T.; Wang, Z.; Zhao, Y. The role of iron in doxorubicin-induced cardiotoxicity: Recent advances and implication for drug delivery. J. Mater. Chem. B 2021, 9, 4793–4803. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Santos-Alves, E.; Mariani, D.; Rizo-Roca, D.; Padrao, A.I.; Rocha-Rodrigues, S.; Viscor, G.; Torrella, J.R.; Ferreira, R.; Oliveira, P.J.; et al. Physical exercise prior and during treatment reduces sub-chronic doxorubicin-induced mitochondrial toxicity and oxidative stress. Mitochondrion 2015, 20, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kavazis, A.N.; Smuder, A.J.; Min, K.; Tumer, N.; Powers, S.K. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1515–H1524. [Google Scholar] [CrossRef] [PubMed]
- Goormaghtigh, E.; Chatelain, P.; Caspers, J.; Ruysschaert, J.M. Evidence of a complex between adriamycin derivatives and cardiolipin: Possible role in cardiotoxicity. Biochem. Pharmacol. 1980, 29, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Goormaghtigh, E.; Huart, P.; Praet, M.; Brasseur, R.; Ruysschaert, J.M. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys. Chem. 1990, 35, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Mushlin, P.S.; Brenner, D.E.; Fleischer, S.; Cusack, B.J.; Chang, B.K.; Boucek, R.J., Jr. Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc. Natl. Acad. Sci. USA 1988, 85, 3585–3589. [Google Scholar] [CrossRef]
- Smuder, A.J. Exercise stimulates beneficial adaptations to diminish doxorubicin-induced cellular toxicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R662–R672. [Google Scholar] [CrossRef]
- Powers, S.K.; Sollanek, K.J.; Wiggs, M.P.; Demirel, H.A.; Smuder, A.J. Exercise-induced improvements in myocardial antioxidant capacity: The antioxidant players and cardioprotection. Free Radic. Res. 2014, 48, 43–51. [Google Scholar] [CrossRef]
- Yen, H.C.; Oberley, T.D.; Vichitbandha, S.; Ho, Y.S.; St Clair, D.K. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J. Clin. Investig. 1996, 98, 1253–1260. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Quindry, J.C. Mechanisms of exercise-induced cardioprotection. Physiology 2014, 29, 27–38. [Google Scholar] [CrossRef]
- Ji, L.L.; Mitchell, E.W. Effects of Adriamycin on heart mitochondrial function in rested and exercised rats. Biochem. Pharmacol. 1994, 47, 877–885. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.D.; Lam, A.; Tham, S.; Dulhunty, A.F.; Beard, N.A. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 2014, 86, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Marcillat, O.; Zhang, Y.; Davies, K.J. Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. Biochem. J. 1989, 259, 181–189. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Cardiolipin and mitochondrial function in health and disease. Antioxid. Redox Signal. 2014, 20, 1925–1953. [Google Scholar] [CrossRef]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef]
- Kiebish, M.A.; Yang, K.; Sims, H.F.; Jenkins, C.M.; Liu, X.; Mancuso, D.J.; Zhao, Z.; Guan, S.; Abendschein, D.R.; Han, X.; et al. Myocardial regulation of lipidomic flux by cardiolipin synthase: Setting the beat for bioenergetic efficiency. J. Biol. Chem. 2012, 287, 25086–25097. [Google Scholar] [CrossRef]
- NRC. Guide for the Care and Use of Laboratory Animals; The National Academies Collection: Reports Funded by National Institutes of Health; National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; pp. 1–220. [Google Scholar]
- Thigpen, J.T.; Brady, M.F.; Homesley, H.D.; Malfetano, J.; DuBeshter, B.; Burger, R.A.; Liao, S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A gynecologic oncology group study. J. Clin. Oncol. 2004, 22, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Montalvo, R.N.; Doerr, V.; Kwon, O.S.; Talbert, E.E.; Yoo, J.K.; Hwang, M.H.; Nguyen, B.L.; Christou, D.D.; Kavazis, A.N.; Smuder, A.J. Protection against Doxorubicin-Induced Cardiac Dysfunction Is Not Maintained Following Prolonged Autophagy Inhibition. Int. J. Mol. Sci. 2020, 21, 8105. [Google Scholar] [CrossRef]
- Qin, F.; Dong, Y.; Wang, S.; Xu, M.; Wang, Z.; Qu, C.; Yang, Y.; Zhao, J. Maximum oxygen consumption and quantification of exercise intensity in untrained male Wistar rats. Sci. Rep. 2020, 10, 11520. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Copp, S.W.; Colburn, T.D.; Craig, J.C.; Allen, D.L.; Sturek, M.; O’Leary, D.S.; Zucker, I.H.; Musch, T.I. Guidelines for animal exercise and training protocols for cardiovascular studies. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1100–H1138. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.W.; Davis, R.T.; Poole, D.C.; Musch, T.I. Reproducibility of endurance capacity and VO2peak in male Sprague-Dawley rats. J. Appl. Physiol. 2009, 106, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Musch, T.I.; Moore, R.L.; Riedy, M.; Burke, P.; Zelis, R.; Leo, M.E.; Bruno, A.; Bradford, G.E. Glycogen concentrations and endurance capacity of rats with chronic heart failure. J. Appl. Physiol. (1985) 1988, 64, 1153–1159. [Google Scholar] [CrossRef]
- Doerr, V.; Montalvo, R.N.; Nguyen, B.L.; Boeno, F.P.; Sunshine, M.D.; Bindi, V.E.; Fuller, D.D.; Smuder, A.J. Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity. Antioxidants 2022, 11, 2073. [Google Scholar] [CrossRef]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Thome, T.; Kumar, R.A.; Burke, S.K.; Khattri, R.B.; Salyers, Z.R.; Kelley, R.C.; Coleman, M.D.; Christou, D.D.; Hepple, R.T.; Scali, S.T.; et al. Impaired muscle mitochondrial energetics is associated with uremic metabolite accumulation in chronic kidney disease. JCI Insight 2020, 6, e139826. [Google Scholar] [CrossRef]
- Kumar, R.A.; Thome, T.; Sharaf, O.M.; Ryan, T.E.; Arnaoutakis, G.J.; Jeng, E.I.; Ferreira, L.F. Reversible Thiol Oxidation Increases Mitochondrial Electron Transport Complex Enzyme Activity but Not Respiration in Cardiomyocytes from Patients with End-Stage Heart Failure. Cells 2022, 11, 2292. [Google Scholar] [CrossRef]
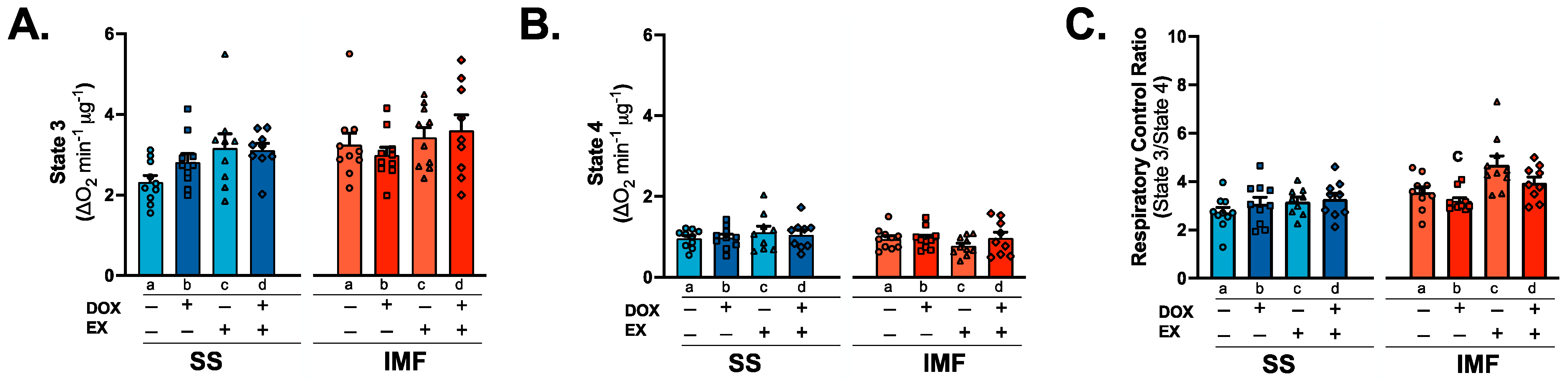
| SED | SED-DOX | EX | EX-DOX | |
|---|---|---|---|---|
| PWTd (mm) | 1.79 ± 0.10 | 1.80 ± 0.08 | 1.72 ± 0.03 | 2.02 ± 0.14 |
| PWTs (mm) | 2.79 ± 0.16 | 2.72 ± 0.10 | 2.62 ± 0.07 | 2.88 ± 0.13 |
| AWTd (mm) | 1.70 ± 0.11 | 1.71 ± 0.08 | 1.78 ± 0.12 | 1.78 ± 0.09 |
| AWTs (mm) | 2.75 ± 0.17 | 2.67 ± 0.10 | 2.75 ± 0.20 | 2.69 ± 0.14 |
| HR (bpm) | 363.9 ± 7.01 | 347.0 ± 4.80 ^ | 380.8 ± 15.39 | 336.2 ± 5.86 ^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo, R.N.; Boeno, F.P.; Dowllah, I.M.; Moritz, C.E.J.; Nguyen, B.L.; Doerr, V.; Bomkamp, M.P.; Smuder, A.J. Exercise and Doxorubicin Modify Markers of Iron Overload and Cardiolipin Deficiency in Cardiac Mitochondria. Int. J. Mol. Sci. 2023, 24, 7689. https://doi.org/10.3390/ijms24097689
Montalvo RN, Boeno FP, Dowllah IM, Moritz CEJ, Nguyen BL, Doerr V, Bomkamp MP, Smuder AJ. Exercise and Doxorubicin Modify Markers of Iron Overload and Cardiolipin Deficiency in Cardiac Mitochondria. International Journal of Molecular Sciences. 2023; 24(9):7689. https://doi.org/10.3390/ijms24097689
Chicago/Turabian StyleMontalvo, Ryan N., Franccesco P. Boeno, Imtiaz M. Dowllah, Cesar E. Jacintho Moritz, Branden L. Nguyen, Vivian Doerr, Matthew P. Bomkamp, and Ashley J. Smuder. 2023. "Exercise and Doxorubicin Modify Markers of Iron Overload and Cardiolipin Deficiency in Cardiac Mitochondria" International Journal of Molecular Sciences 24, no. 9: 7689. https://doi.org/10.3390/ijms24097689
APA StyleMontalvo, R. N., Boeno, F. P., Dowllah, I. M., Moritz, C. E. J., Nguyen, B. L., Doerr, V., Bomkamp, M. P., & Smuder, A. J. (2023). Exercise and Doxorubicin Modify Markers of Iron Overload and Cardiolipin Deficiency in Cardiac Mitochondria. International Journal of Molecular Sciences, 24(9), 7689. https://doi.org/10.3390/ijms24097689








