Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach
Abstract
1. Introduction
2. Cognitive Function: Attention, Learning, and Memory
- Encoding: capturing details about stimuli and the environment for subsequent consolidation (processing the information to store it).
- Storage: retention of information over time.
- Retrieval: using the retained information to create a conscious representation of the event or execute a learned motor response.
3. Human Cognitive Assessment
3.1. Anamnesis
3.2. Physical Examination
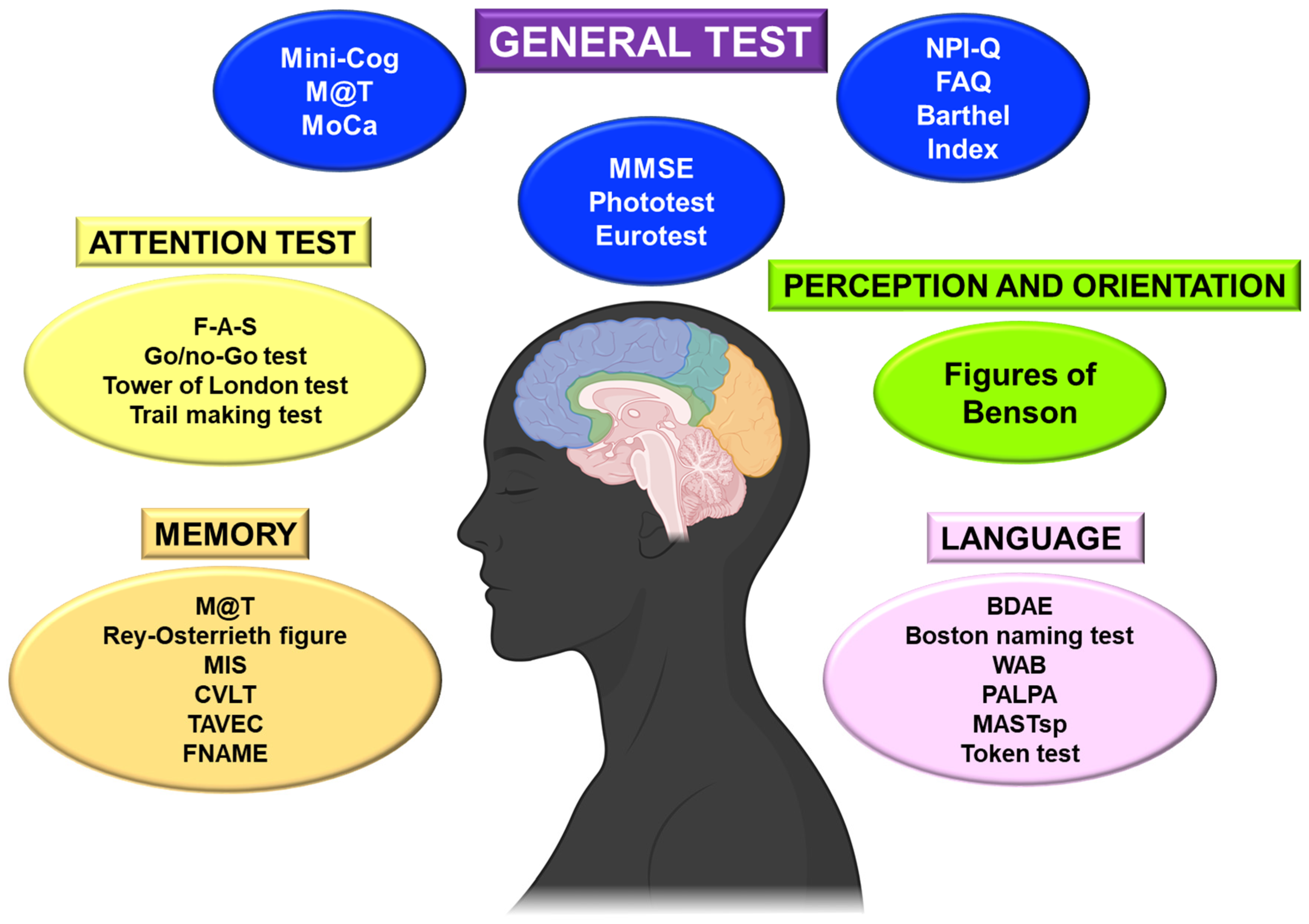
4. Cognitive Function Screening Tools
4.1. Basic Cognitive and Functional Tests
4.1.1. MMSE
4.1.2. Phototest
4.1.3. Eurotest
4.1.4. Brief Cognitive Screening Test (Mini-Cog)
4.1.5. Memory Alteration Test (M@T)
4.1.6. Montreal Cognitive Assessment (MoCa)
4.1.7. Neuropsychiatric Inventory Questionnaire (NPI-Q)
4.1.8. Functional Activities Questionnaire (FAQ)
4.1.9. Lawton and Brody Scale for Instrumental Activities of Daily Living
4.1.10. Barthel Index
5. Advanced Cognitive Evaluation and Specific Tests
5.1. Attention and Executive Function
- Assessment of mental flexibility: a test of execution of alternating sequences (mental flexibility evaluation) such as alternating graphic series, alternating motor series (example, fist-palm-singing sequence), or alternation of two automated series (Trail making test) and controlled verbal fluency (F-A-S).
- Evaluating the ability to inhibit irrelevant automatisms: go/no-go test, repetition of inverse digits, Stroop test.
- In the first section, the patient is asked to show one finger when the evaluator shows one finger and two fingers when the evaluator shows two.
- In the second section, the patient is asked to show two fingers when the evaluator shows one and one finger when the evaluator shows two.
- In the third section, the patient is asked to show one finger when the evaluator shows two, and none when the evaluator shows one.
- Assessment of the ability to inhibit impulsivity [39]: impulsivity is expressed as short reaction times and the absence of a work plan. Several tests help us to assess impulsivity, such as the Tower of London test.
- Assessment of distributed attention through dual-task evaluations [39]. Executive function (abstraction, task planning, organization, sequential execution) can be evaluated by different neuropsychological tests that assess mental flexibility, the ability to inhibit responses, the ability to perform alternating responses, abstraction, and processing speed.
- Executive function tests: the most widely used are the Luria sequence, the F-A-S, verbal fluency (phonetic fluency is related to prefrontal function [23]), Trail making test part B (sequence letters and numbers), the Stroop test, the Tower of London and Hanoy, the Wisconsin Card Sorting Test (WCST) and frontal assessment batteries, face processing, and body schema assessment (right/left orientation, digital gnosis, etc.).
5.2. Perception and Orientation
5.3. Praxias
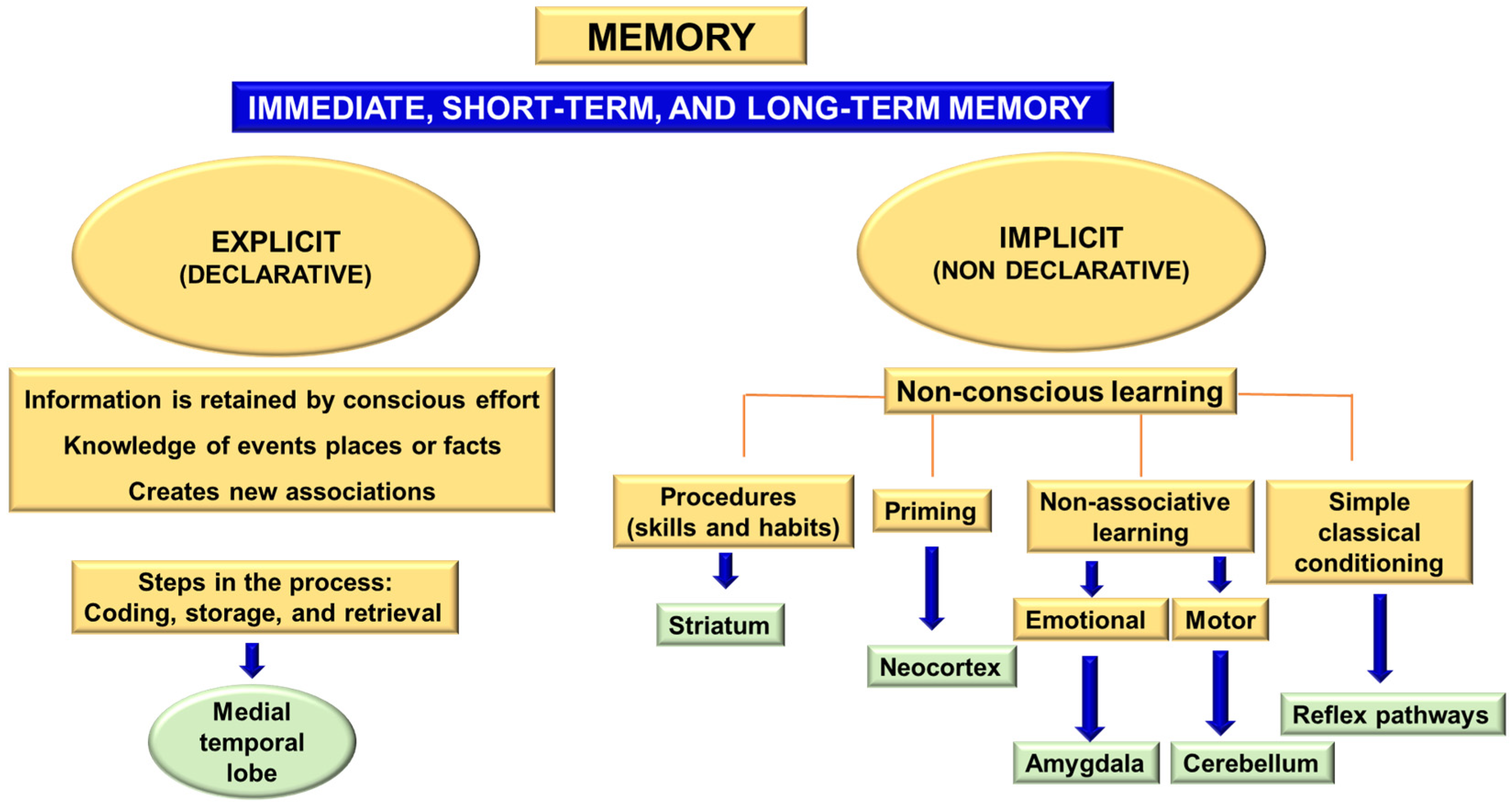
5.4. Memory
- Working memory, short-term memory, and long-term memory. The distinguishing feature of the working memory is that it uses executive functions, whereas short-term memory requires neither attention nor active maintenance.
- Declarative memory or non-declarative memory.
- Episodic (or autobiographical) memory. It depends mainly on the hippocampus, entorhinal cortex, amygdala, and diencephalic structures.
- Semantic memory. It is associated with the lateral temporal associative cortex.
5.5. Language
6. Cognitive Assessment in Special Populations
6.1. Cognitive Assessment of the Very Elderly Population
6.2. Cognitive Assessment in Low-Literacy Patients
6.3. Cognitive Assessment of Patients with Advanced Dementia
6.4. Selective Neuropsychological Studies in Psychiatric Populations
7. Experimental Animal Assessment
Types of Animal Models of Cognitive Disorders
- Pharmacological models: allowing the evaluation of drugs.
- Toxicological models: to determine the toxicity of heavy metals, toxicants, and neurotoxins [57].
8. Behavioral Tests for Evaluating Cognitive Disorders in Animal Models
8.1. Assessment of Attention
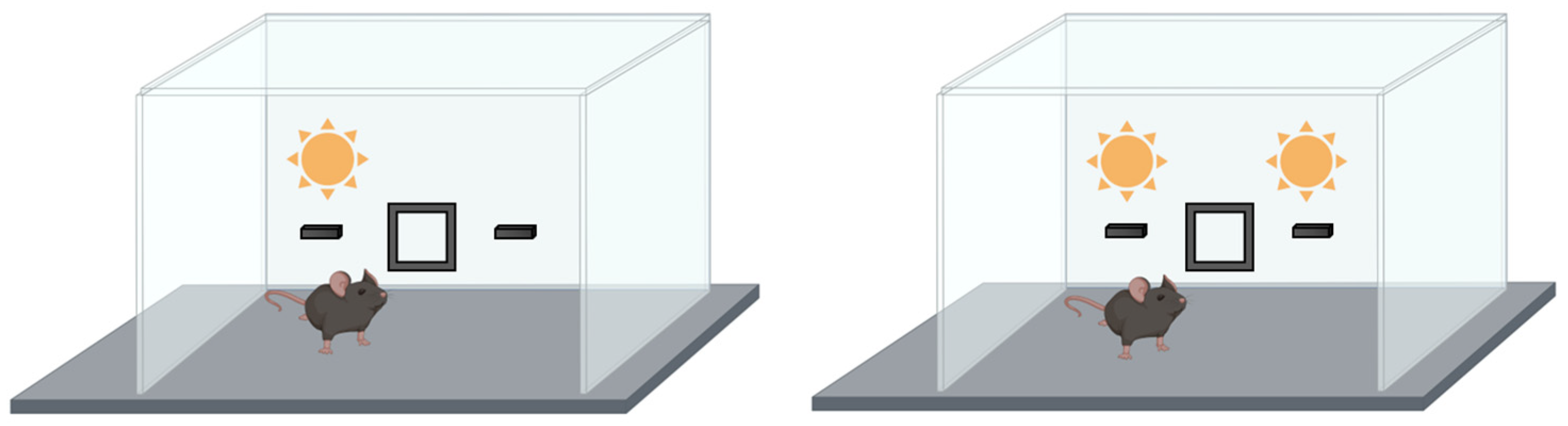
8.1.1. Pre-Attention: Prepulse Inhibition
8.1.2. Attention
Sustained (Vigilance) and Selective Attention: Five-Choice Serial Reaction Time Task (5-CSRTT)
Go/No-Go Test: This Test Is Divided into Go and No-Go Tests
- Training: the mouse learns to perform a task to obtain reinforcement, and after this, the go cue is applied for five days, in which 40 trials per day are performed.
- Go test: begins with the presentation of the go signal, and when the mouse responds to the stimulus within a certain period (e.g., 60 s), the reward is delivered. The mouse then has a set time, 20 s, to locate and consume the food. When the mouse completes 85% of the go trials for at least three consecutive days, it enters the go/no go test. Failure on any part of the test causes interruption before the next no-go test.
- Go/no-go test: this phase lasts approximately 10 days, during which go and no-go tests are randomly interspersed. After the no-go signal, the mouse must learn to hold its response for about 15 s to receive the reward. If the mouse fails to delay its response for a given period (15 s), the test should be repeated with a shorter time interval (2–10 s) to determine whether the deficit is due to an inability to delay its response for a prolonged period or to a failure to learn the no-go task. If the mouse responds prematurely or while the signal is being applied, it is considered a failure, and the reward is not provided.
Selective Attention: Latent Inhibition Test
Orientation Attention: Orientation and Habituation
8.2. Evaluation of Learning and Memory
8.2.1. Non-Spatial Memory
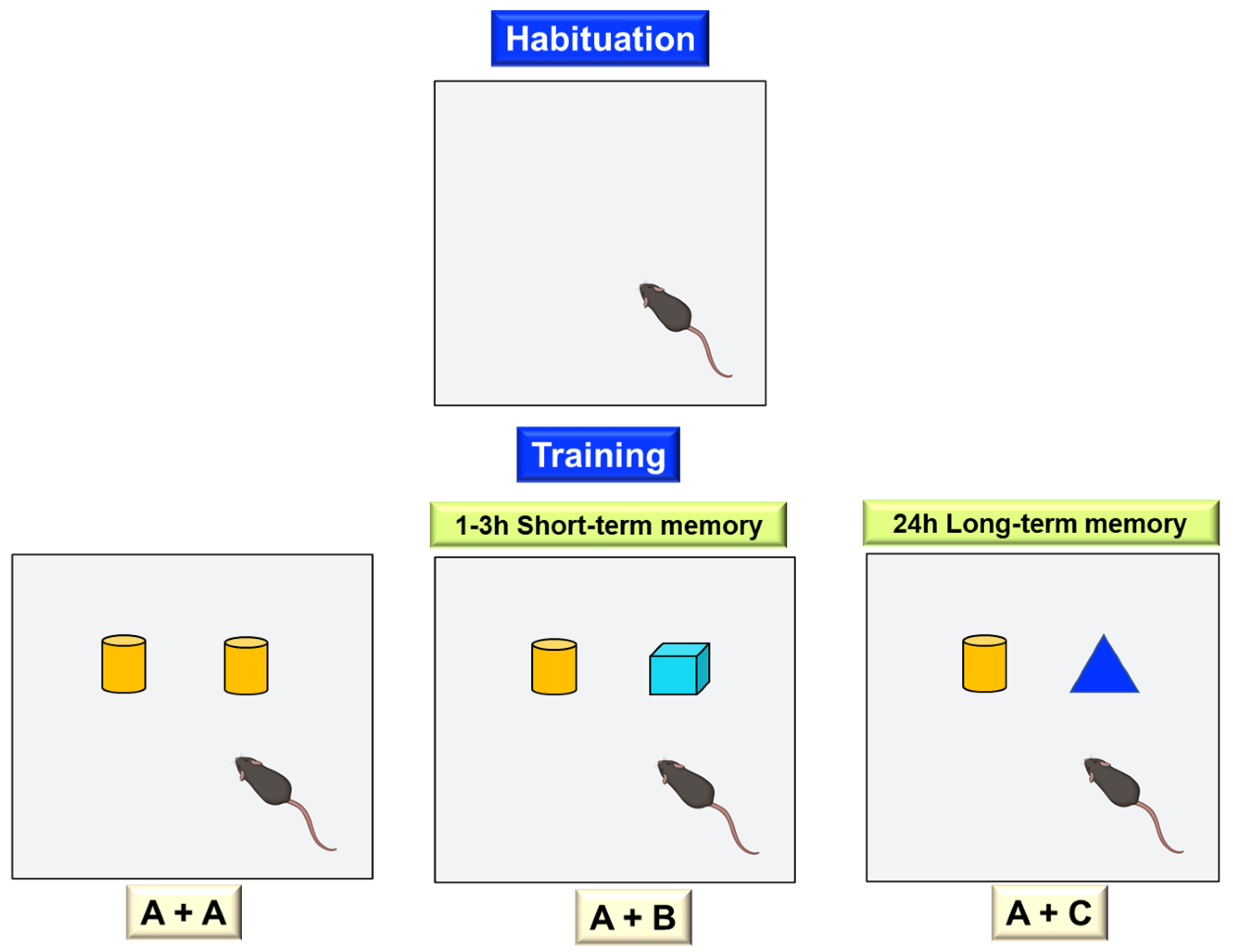
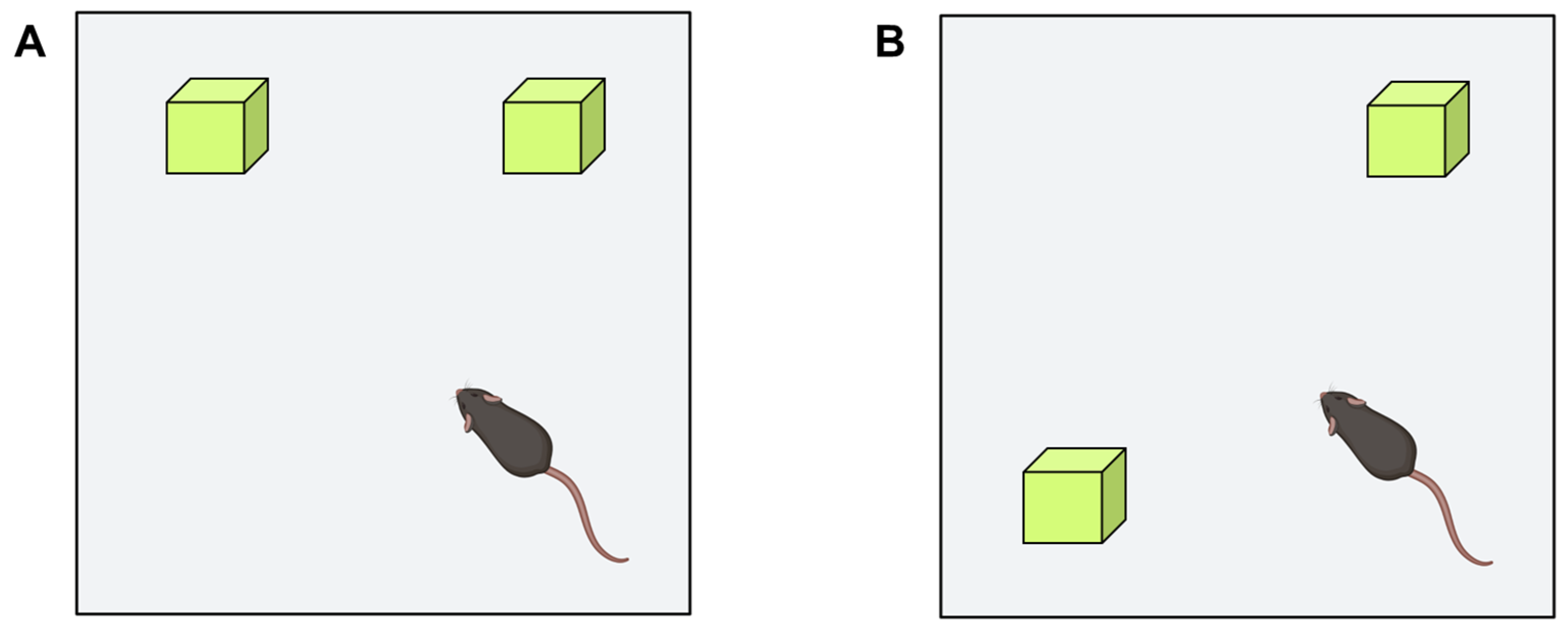
Object Recognition Test
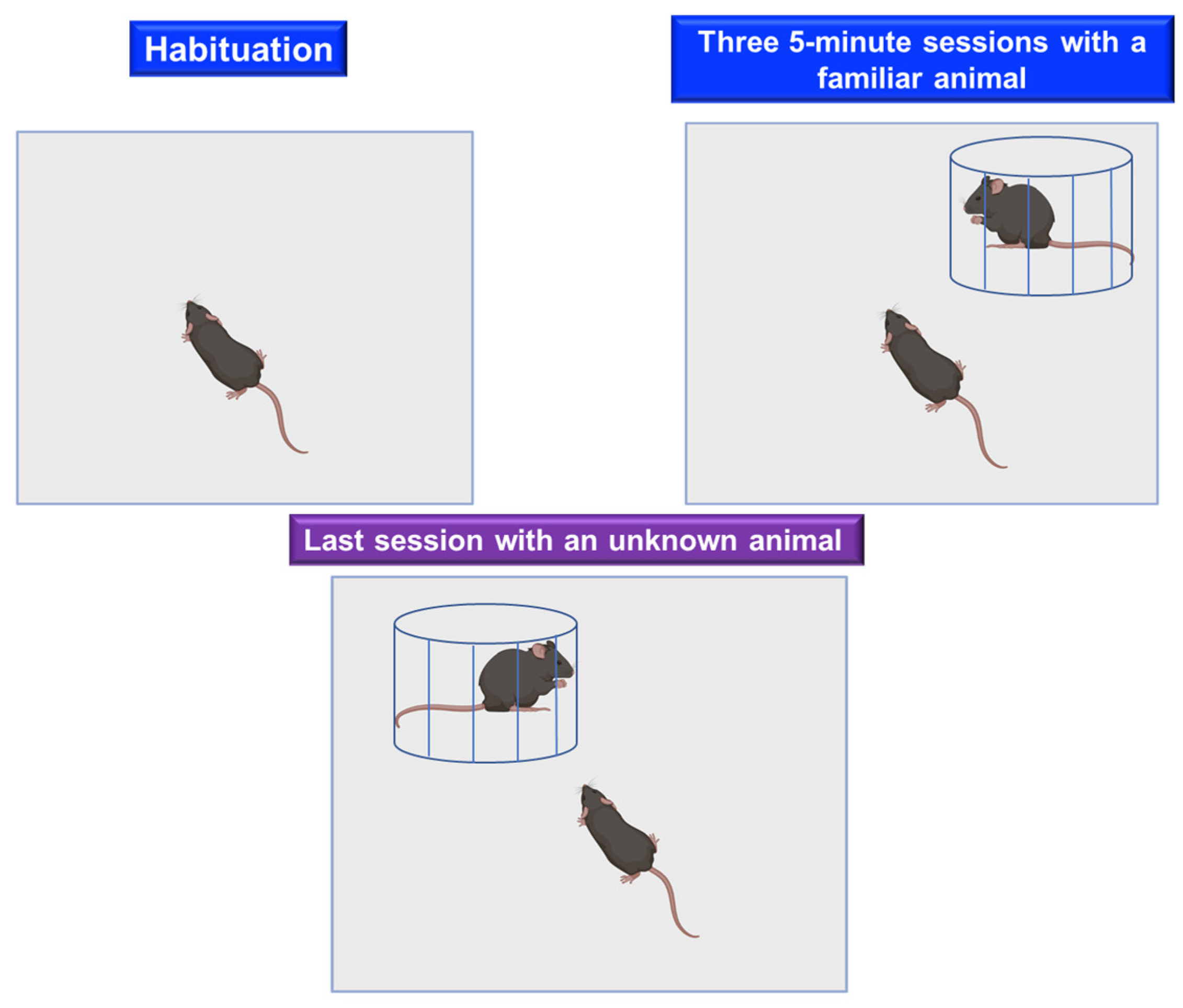
Social Recognition Test
8.2.2. Spatial Memory
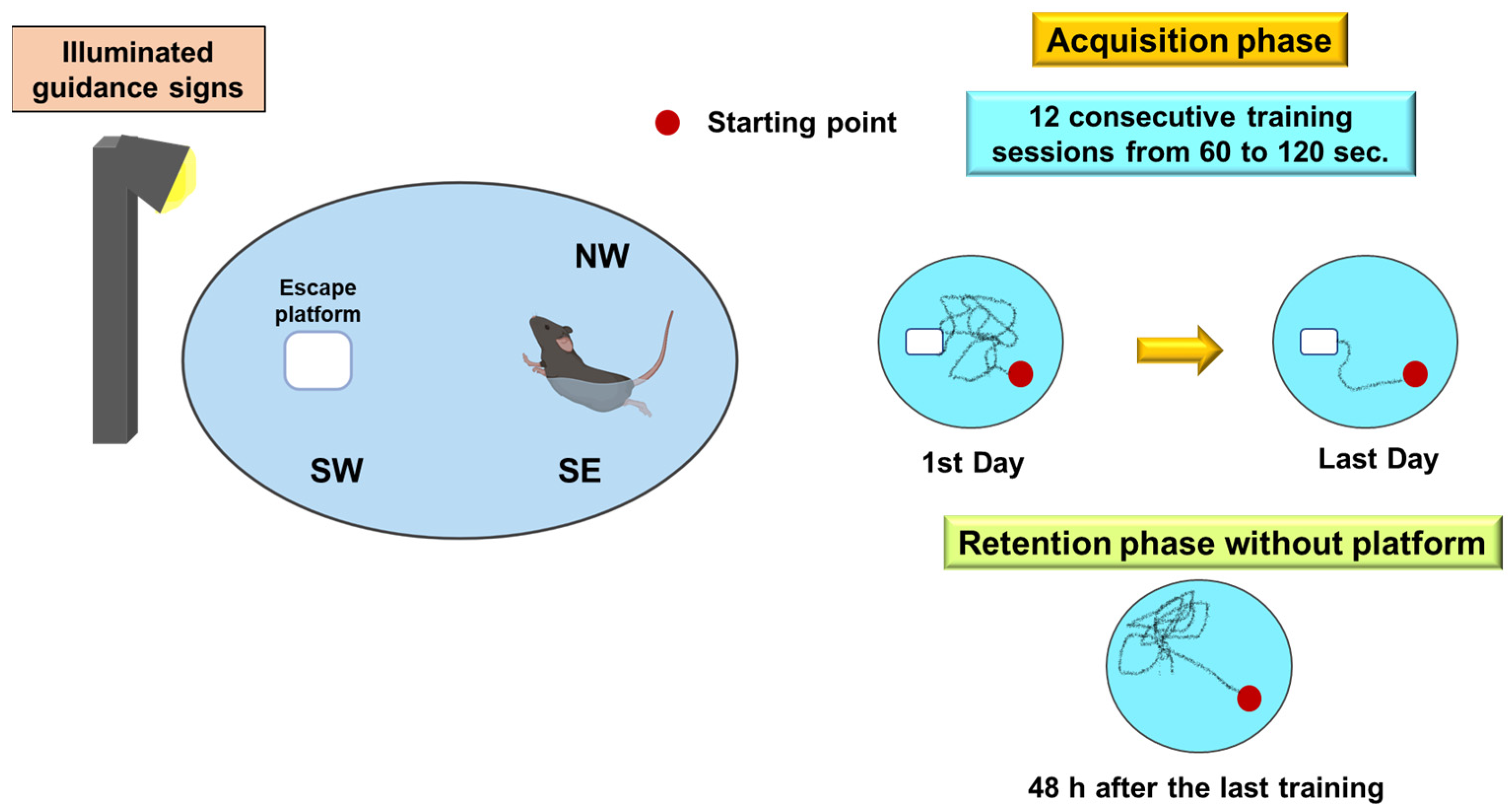
Morris Water Maze
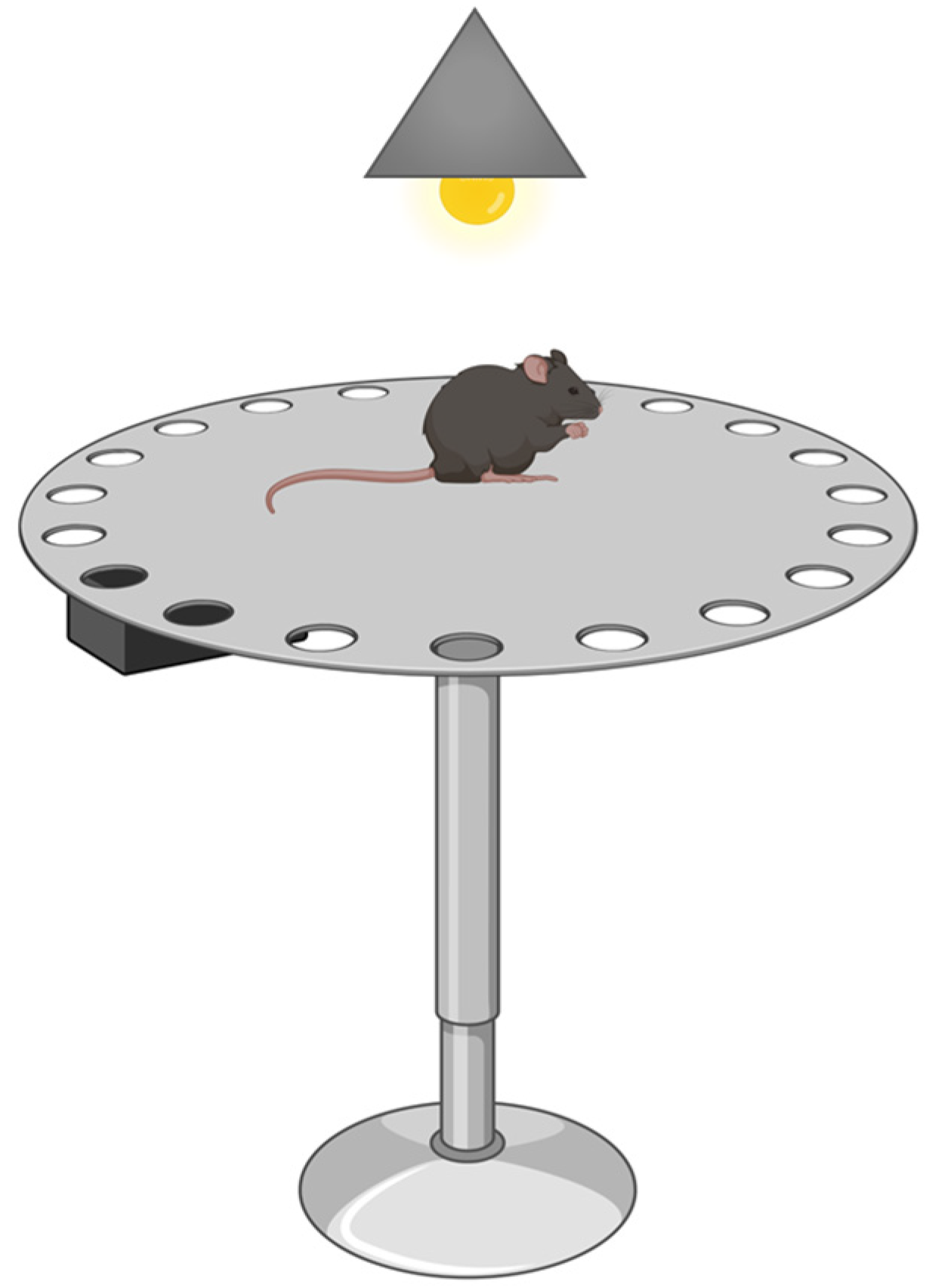
Barnes Maze
Object Location Test
8.3. Working Memory
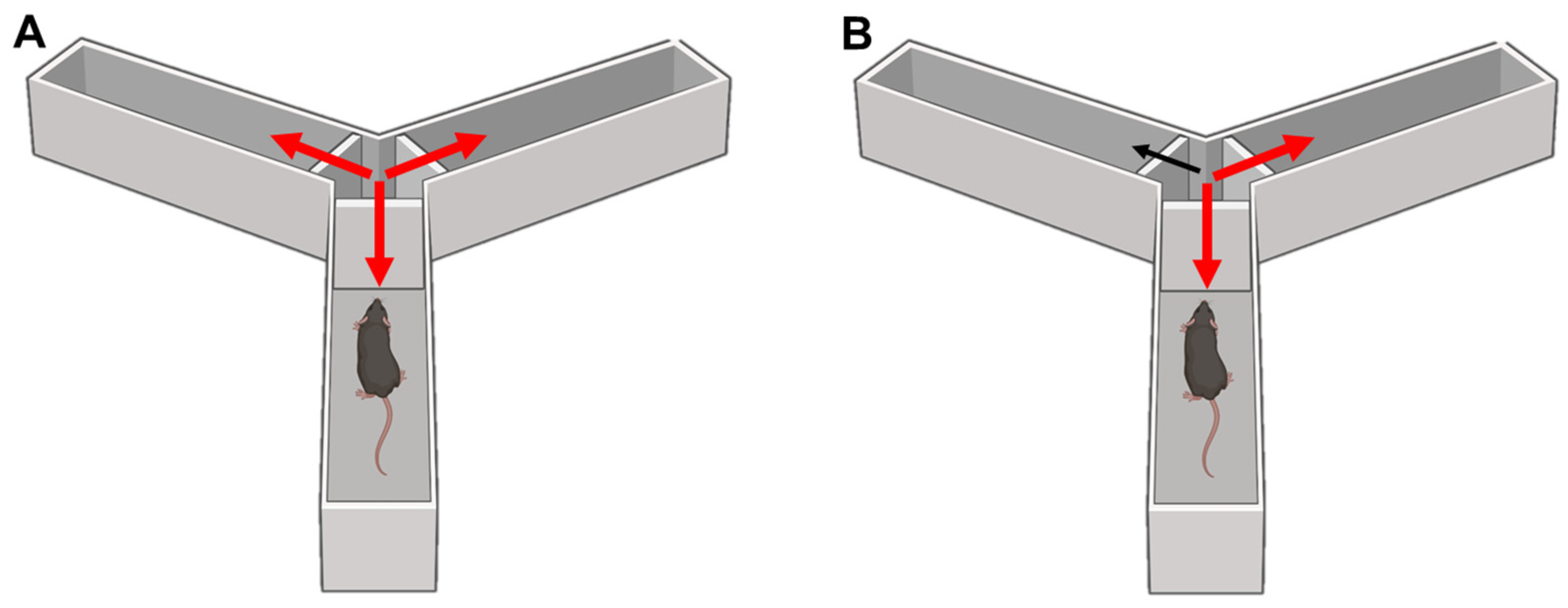
8.3.1. T-Maze
8.3.2. Y-Maze
8.3.3. Eight-Arm Radial Maze Test
8.4. Associative Learning
8.4.1. Contextual Fear Conditioning
8.4.2. Contextual and Cues Fear Conditioning Test
8.4.3. Step-Down Inhibitory Avoidance
8.4.4. Passive Avoidance Test
8.4.5. Active Avoidance Testing
8.5. Emotional Memory
8.5.1. Aversive Conditioning to Taste
8.5.2. Fear-Potentiated Overreaction
9. Conclusions
10. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bird, T.; Miller, B. Alzheimer’s disease and other dementias. In Harrison’s Principles of Internal Medicine; Kasper, D.L., Braunwald, E., Fauci, A.S., Hauser, S.L., Longo, D.L., Jameson, J., Loscalzo, J., Eds.; McGraw Hill: New York, NY, USA, 2005; pp. 2393–2406. [Google Scholar]
- Sarasa, M. Modelos experimentales de la enfermedad de Alzheimer. Rev. Neurol. 2006, 42, 297–301. [Google Scholar] [CrossRef]
- Campos-Romo, A. Evaluación de alteraciones motoras en modelos animales de enfermedad de Parkinson. Rev. Neurol. 2008, 46, 167–174. [Google Scholar] [CrossRef]
- Ostrosky-Solís, F. Características neuropsicológicas de la enfermedad de Parkinson. Rev. Neurol. 2000, 30, 788–796. [Google Scholar] [CrossRef]
- Benito-Leon, J.; Louis, E.D.; Bermejo-Pareja, F. Neurological Disorders in Central Spain Study, G. Elderly-onset essential tremor is associated with dementia. Neurology 2006, 66, 1500–1505. [Google Scholar] [CrossRef]
- Garcia-Ramos, R.; del Val-Fernandez, J.; Catalan-Alonso, M.J.; Barcia-Albacar, J.A.; Matias-Guiu, J. Experimental models of Huntington’s disease. Rev. Neurol. 2007, 45, 437–441. [Google Scholar]
- Ward, J.; Sheppard, J.M.; Shpritz, B.; Margolis, R.L.; Rosenblatt, A.; Brandt, J. A four-year prospective study of cognitive functioning in Huntington’s disease. J. Int. Neuropsychol. Soc. 2006, 12, 445–454. [Google Scholar] [CrossRef]
- Millar, D.; Griffiths, P.; Zermansky, A.J.; Burn, D.J. Characterizing behavioral and cognitive dysexecutive changes in progressive supranuclear palsy. Mov. Disord. 2006, 21, 199–207. [Google Scholar] [CrossRef]
- Mantovan, M.C.; Martinuzzi, A.; Squarzanti, F.; Bolla, A.; Silvestri, I.; Liessi, G.; Macchi, C.; Ruzza, G.; Trevisan, C.P.; Angelini, C. Exploring mental status in Friedreich’s ataxia: A combined neuropsychological, behavioral and neuroimaging study. Eur. J. Neurol. 2006, 13, 827–835. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.M.; Duque, P.; Izquierdo, G. Neuropsychiatric disorders in multiple sclerosis. Rev. Neurol. 2001, 33, 560–567. [Google Scholar]
- D’Angelo, M.G.; Bresolin, N. Cognitive impairment in neuromuscular disorders. Muscle Nerve 2006, 34, 16–33. [Google Scholar] [CrossRef]
- Mosconi, L.; Tsui, W.H.; Herholz, K.; Pupi, A.; Drzezga, A.; Lucignani, G.; Reiman, E.M.; Holthoff, V.; Kalbe, E.; Sorbi, S.; et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med. 2008, 49, 390–398. [Google Scholar] [CrossRef][Green Version]
- Branca, B. Neuropsychologic aspects of post-traumatic headache and chronic daily headache. Curr. Pain Headache Rep. 2006, 10, 54–66. [Google Scholar] [CrossRef]
- Hanly, J.G.; Fisk, J.D.; McCurdy, G.; Fougere, L.; Douglas, J.A. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and rheumatoid arthritis. J. Rheumatol. 2005, 32, 1459–1466. [Google Scholar]
- Lackner, J.M.; Lou Coad, M.; Mertz, H.R.; Wack, D.S.; Katz, L.A.; Krasner, S.S.; Firth, R.; Mahl, T.C.; Lockwood, A.H. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav. Res. Ther. 2006, 44, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I. Attention in Cognitive Neuroscience: An Overview; The MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- McGhie, A.; Chapman, J. Disorders of attention and perception in early schizophrenia. Br. J. Med. Psychol. 1961, 34, 103–116. [Google Scholar] [CrossRef]
- Dudai, Y. The Neurobiology of Memory: Concepts, Findings, Trends; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Baddeley, A.D.; Hitch, G. The recency effect: Implicit learning with explicit retrieval? Mem. Cognit. 1993, 21, 146–155. [Google Scholar] [CrossRef][Green Version]
- Kandel, E.R.; Kupfermann, I.; Iversen, S. Learning and Memory. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Haberlandt, K.; Thomas, J.G.; Lawrence, H.; Krohn, T. Transposition asymmetry in immediate serial recall. Memory 2005, 13, 274–282. [Google Scholar] [CrossRef]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef]
- Tang-Wai, D.F.; Freedman, M. Bedside Approach to the Mental Status Assessment. Continuum 2018, 24, 672–703. [Google Scholar] [CrossRef]
- Graham, S.A.; Lee, E.E.; Jeste, D.V.; Van Patten, R.; Twamley, E.W.; Nebeker, C.; Yamada, Y.; Kim, H.C.; Depp, C.A. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020, 284, 112732. [Google Scholar] [CrossRef][Green Version]
- Grossman, M.; Irwin, D.J. The Mental Status Examination in Patients With Suspected Dementia. Continuum 2016, 22, 385–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Llamas-Velasco, S.; Llorente-Ayuso, L.; Contador, I.; Bermejo-Pareja, F. Spanish versions of the Minimental State Examination (MMSE). Questions for their use in clinical practice. Rev. Neurol. 2015, 61, 363–371. [Google Scholar] [PubMed]
- McCollum, L.; Karlawish, J. Cognitive Impairment Evaluation and Management. Med. Clin. N. Am. 2020, 104, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Carnero-Pardo, C.; Montoro-Rios, M.T. The photo test. Rev. Neurol. 2004, 39, 801–806. [Google Scholar] [PubMed]
- Carnero-Pardo, C.; Montoro-Rios, M.T. Preliminary evaluation of a new screening test for dementia (Eurotest). Rev. Neurol. 2004, 38, 201–209. [Google Scholar]
- Rami, L.; Bosch, B.; Valls-Pedret, C.; Caprile, C.; Sanchez-Valle Diaz, R.; Molinuevo, J.L. Discriminatory validity and association of the mini-mental test (MMSE) and the memory alteration test (M@T) with a neuropsychological battery in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Rev. Neurol. 2009, 49, 169–174. [Google Scholar]
- Rami, L.; Bosch, B.; Sanchez-Valle, R.; Molinuevo, J.L. The memory alteration test (M@T) discriminates between subjective memory complaints, mild cognitive impairment and Alzheimer’s disease. Arch. Gerontol. Geriatr. 2010, 50, 171–174. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef][Green Version]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H., Jr.; Chance, J.M.; Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Graf, C. The Lawton instrumental activities of daily living scale. Am. J. Nurs. 2008, 108, 52–62; quiz 62–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Fillenbaum, G.G.; van Belle, G.; Morris, J.C.; Mohs, R.C.; Mirra, S.S.; Davis, P.C.; Tariot, P.N.; Silverman, J.M.; Clark, C.M.; Welsh-Bohmer, K.A.; et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The first twenty years. Alzheimers Dement. 2008, 4, 96–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Posner, M.I.; Bourke, P. The Blackwell Dicitionary of Neuropsychology; Beaumont, J.G., Kenealy, P.M., Rogers, M.J.C., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1999; pp. 122–127. [Google Scholar]
- Moscovitch, M.; Umiltà, C. Modularity and Neurospycholgy: Modules and central processes in attention and memory. In Modular deficits in Alzheimer-Type Dementia; The MIT Press: Cambridge, MA, USA, 1990; pp. 1–59. [Google Scholar]
- Farah, M.J. Specialization with visual object recognition: Clues for prosopagnosia and alexia. In The Neuropsychology of High-Level Vision; Psychology Press: New York, NY, USA, 1994; pp. 133–146. [Google Scholar]
- Packard, M.G.; Knowlton, B.J. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef][Green Version]
- Foerde, K.; Shohamy, D. The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiol. Learn. Mem. 2011, 96, 624–636. [Google Scholar] [CrossRef][Green Version]
- McCarthy, R.; Warrington, E.K. The dissolution of semantics. Nature 1990, 343, 599. [Google Scholar] [CrossRef]
- Cohen, N.J.; Squire, L.R. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science 1980, 210, 207–210. [Google Scholar] [CrossRef]
- Patnode, C.D.; Perdue, L.A.; Rossom, R.C.; Rushkin, M.C.; Redmond, N.; Thomas, R.G.; Lin, J.S. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2020. [Google Scholar]
- Paddick, S.M.; Gray, W.K.; McGuire, J.; Richardson, J.; Dotchin, C.; Walker, R.W. Cognitive screening tools for identification of dementia in illiterate and low-educated older adults, a systematic review and meta-analysis. Int. Psychogeriatr. 2017, 29, 897–929. [Google Scholar] [CrossRef]
- Devi-Bastida, J.; Dominguez-Luque, P.; Jofre-Font, S. Neuropsychological evaluation of advanced dementia: Are cognitive assessment psychometric instruments useful? A systematic review. Rev. Neurol. 2021, 72, 239–249. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lee, Y.; Lee, C.Y.; Lin, P.Y.; Hung, C.F.; Lee, S.Y.; Wang, L.J. Defining cognitive and functional profiles in schizophrenia and affective disorders. BMC Psychiatry 2020, 20, 39. [Google Scholar] [CrossRef][Green Version]
- Gomez-Benito, J.; Guilera, G.; Pino, O.; Rojo, E.; Tabares-Seisdedos, R.; Safont, G.; Martinez-Aran, A.; Franco, M.; Cuesta, M.J.; Crespo-Facorro, B.; et al. The screen for cognitive impairment in psychiatry: Diagnostic-specific standardization in psychiatric ill patients. BMC Psychiatry 2013, 13, 127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ott, C.V.; Knorr, U.; Jespersen, A.; Obenhausen, K.; Roen, I.; Purdon, S.E.; Kessing, L.V.; Miskowiak, K.W. Norms for the Screen for Cognitive Impairment in Psychiatry and cognitive trajectories in bipolar disorder. J. Affect. Disord. 2021, 281, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lena, C.; Changeux, J.P. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J. Neurosci. 1997, 17, 576–585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- LaFerla, F.M.; Tinkle, B.T.; Bieberich, C.J.; Haudenschild, C.C.; Jay, G. The Alzheimer’s A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat. Genet. 1995, 9, 21–30. [Google Scholar] [CrossRef]
- Mangiarini, L.; Sathasivam, K.; Seller, M.; Cozens, B.; Harper, A.; Hetherington, C.; Lawton, M.; Trottier, Y.; Lehrach, H.; Davies, S.W.; et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 1996, 87, 493–506. [Google Scholar] [CrossRef][Green Version]
- Matsui, M.; Yamada, S.; Oki, T.; Manabe, T.; Taketo, M.M.; Ehlert, F.J. Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci. 2004, 75, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Quon, D.; Wang, Y.; Catalano, R.; Scardina, J.M.; Murakami, K.; Cordell, B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature 1991, 352, 239–241. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Bymaster, F.P.; Felder, C.C.; Wade, M.; Gomeza, J.; Wess, J.; McKinzie, D.L.; Nomikos, G.G. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol. Psychiatry 2003, 8, 673–679. [Google Scholar] [CrossRef][Green Version]
- Luquin, M. Modelos experimentales de enfermedad de Parkinson. Rev. Neurol. 2000, 31, 60–66. [Google Scholar] [CrossRef]
- Gower, A.J.; Lamberty, Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav. Brain Res. 1993, 57, 163–173. [Google Scholar] [CrossRef]
- Ingram, D.K.; Spangler, E.L.; Iijima, S.; Ikari, H.; Kuo, H.; Greig, N.H.; London, E.D. Rodent models of memory dysfunction in Alzheimer’s disease and normal aging: Moving beyond the cholinergic hypothesis. Life Sci. 1994, 55, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Spangler, E.L.; Iijima, S.; Kuo, H.; Bresnahan, E.L.; Greig, N.H.; London, E.D. New pharmacological strategies for cognitive enhancement using a rat model of age-related memory impairment. Ann. N. Y. Acad. Sci. 1994, 717, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.B.; LeVasseur, R.A.; Faden, A.I. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: Implications for gene targeting approaches to neurotrauma. J. Neurotrauma 1999, 16, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Sarti, C.; Pantoni, L.; Bartolini, L.; Inzitari, D. Cognitive impairment and chronic cerebral hypoperfusion: What can be learned from experimental models. J. Neurol. Sci. 2002, 203, 263–266. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef]
- Robertson, I.H.; Ward, T.; Ridgeway, V.; Nimmo-Smith, I. The structure of normal human attention: The Test of Everyday Attention. J. Int. Neuropsychol. Soc. 1996, 2, 525–534. [Google Scholar] [CrossRef]
- Robbins, T.W. The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology 2002, 163, 362–380. [Google Scholar] [CrossRef]
- Levin, E.D.; Bushnell, P.J.; Rezvani, A.H. Attention-modulating effects of cognitive enhancers. Pharmacol. Biochem. Behav. 2011, 99, 146–154. [Google Scholar] [CrossRef][Green Version]
- Higgins, G.A.; Breysse, N. Rodent model of attention: The 5-choice serial reaction time task. Curr. Protoc. Pharmacol. 2008, 41, 5–49. [Google Scholar] [CrossRef]
- Young, J.W.; Light, G.A.; Marston, H.M.; Sharp, R.; Geyer, M.A. The 5-choice continuous performance test: Evidence for a translational test of vigilance for mice. PLoS ONE 2009, 4, e4227. [Google Scholar] [CrossRef][Green Version]
- Asinof, S.K.; Paine, T.A. The 5-choice serial reaction time task: A task of attention and impulse control for rodents. J. Vis. Exp. 2014, 10, e51574. [Google Scholar] [CrossRef]
- Callahan, P.M.; Terry, A.V., Jr. Attention. Handb. Exp. Pharmacol. 2015, 228, 161–189. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain. Res. 1997, 113, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Holloway, W.R., Jr.; Thor, D.H. Social memory deficits in adult male rats exposed to cadmium in infancy. Neurotoxicol. Teratol. 1988, 10, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gheusi, G.; Bluthe, R.M.; Goodall, G.; Dantzer, R. Ethological study of the effects of tetrahydroaminoacridine (THA) on social recognition in rats. Psychopharmacology 1994, 114, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Choleris, E.; Gustafsson, J.A.; Korach, K.S.; Muglia, L.J.; Pfaff, D.W.; Ogawa, S. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc. Natl. Acad. Sci. USA 2003, 100, 6192–6197. [Google Scholar] [CrossRef][Green Version]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Barnes, C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979, 93, 74–104. [Google Scholar] [CrossRef]
- Vogel-Ciernia, A.; Wood, M.A. Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci. 2014, 69, 8–31. [Google Scholar] [CrossRef][Green Version]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 20, e58593. [Google Scholar] [CrossRef]
- Gaspary, K.V.; Reolon, G.K.; Gusso, D.; Bonan, C.D. Novel object recognition and object location tasks in zebrafish: Influence of habituation and NMDA receptor antagonism. Neurobiol. Learn. Mem. 2018, 155, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Sasaki, T.; Ikegaya, Y. Hippocampal beta oscillations predict mouse object-location associative memory performance. Hippocampus 2021, 31, 503–511. [Google Scholar] [CrossRef]
- Bocchi, A.; Palermo, L.; Boccia, M.; Palmiero, M.; D’Amico, S.; Piccardi, L. Object recognition and location: Which component of object location memory for landmarks is affected by gender? Evidence from four to ten year-old children. Appl. Neuropsychol. Child 2020, 9, 31–40. [Google Scholar] [CrossRef]
- Bayraktar, G.; Hojgaard, K.; Nijssen, L.; Takeuchi, T. A Within-Subject Experimental Design using an Object Location Task in Rats. J. Vis. Exp. 2021, 6, e62458. [Google Scholar] [CrossRef]
- Olton, D.; Samuelson, R. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Darcet, F.; Gardier, A.M.; Gaillard, R.; David, D.J.; Guilloux, J.P. Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease. Pharmaceuticals 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Small, W. Experimental Study of the Mental Processes of the Rat. II. Am. J. Psychol. 1901, 12, 206–239. [Google Scholar] [CrossRef]
- Guest, P.C. Pre-clinical Models: Techniques and Protocols; Guest, P.C., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Bak, J.; Pyeon, H.I.; Seok, J.I.; Choi, Y.S. Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation. Behav. Brain Res. 2017, 320, 219–224. [Google Scholar] [CrossRef]
- Cleal, M.; Fontana, B.D.; Ranson, D.C.; McBride, S.D.; Swinny, J.D.; Redhead, E.S.; Parker, M.O. The Free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behav. Res. Methods 2021, 53, 536–557. [Google Scholar] [CrossRef]
- Lainiola, M.; Procaccini, C.; Linden, A.M. mGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behav. Brain Res. 2014, 266, 94–103. [Google Scholar] [CrossRef]
- Olton, D.S.; Collison, C.; Werz, M.A. Spatial memory and radial arm maze performance of rats. Learn. Motiv. 1977, 8, 289–314. [Google Scholar] [CrossRef]
- Patil, S.S.; Sunyer, B.; Hoger, H.; Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav. Brain Res. 2009, 198, 58–68. [Google Scholar] [CrossRef]
- Kim, J.J.; Jung, M.W. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci. Biobehav. Rev. 2006, 30, 188–202. [Google Scholar] [CrossRef][Green Version]
- Fanselow, M.S.; Tighe, T.J. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J. Exp. Psychol. Anim. Behav. Process. 1988, 14, 187–199. [Google Scholar] [CrossRef]
- Fanselow, M.S. Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1994, 1, 429–438. [Google Scholar] [CrossRef][Green Version]
- Curzon, P.; Rustay, N.R.; Browman, K.E. Cued and Contextual Fear Conditioning for Rodents. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Contextual and cued fear conditioning test using a video analyzing system in mice. J. Vis. Exp. 2014, 85, e50871. [Google Scholar] [CrossRef][Green Version]
- Izquierdo, I.; Izquierdo, L.A.; Barros, D.M.; Mello e Souza, T.; de Souza, M.M.; Quevedo, J.; Rodrigues, C.; Sant’Anna, M.K.; Madruga, M.; Medina, J.H. Differential involvement of cortical receptor mechanisms in working, short-term and long-term memory. Behav. Pharmacol. 1998, 9, 421–427. [Google Scholar] [CrossRef]
- Kameyama, T.; Nabeshima, T.; Kozawa, T. Step-down-type passive avoidance- and escape-learning method. Suitability for experimental amnesia models. J. Pharmacol. Methods 1986, 16, 39–52. [Google Scholar] [CrossRef]
- Weinberger, S.B.; Koob, G.F.; Martinez, J.L., Jr. Differences in one-way active avoidance learning in mice of three inbred strains. Behav. Genet. 1992, 22, 177–188. [Google Scholar] [CrossRef]
- Clincke, G.H.; Wauquier, A. Pharmacological protection against hypoxia-induced effects on medium-term memory in a two-way avoidance paradigm. Behav. Brain Res. 1984, 14, 139–142. [Google Scholar] [CrossRef]
- Thomas, S.A.; Palmiter, R.D. Disruption of the dopamine beta-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav. Neurosci. 1997, 111, 579–589. [Google Scholar] [CrossRef]
- Garcia, J.; Kimeldorf, D.J.; Koelling, R.A. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 1955, 122, 157–158. [Google Scholar] [CrossRef]
- Brown, J.S.; Kalish, H.I.; Farber, I.E. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J. Exp. Psychol. 1951, 41, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Astrachan, D.I. Conditioned fear and startle magnitude: Effects of different footshock or backshock intensities used in training. J. Exp. Psychol. Anim. Behav. Process. 1978, 4, 95–103. [Google Scholar] [CrossRef] [PubMed]

| Purpose | Specie | Methods | Specific Field |
|---|---|---|---|
| Evaluation of global assessment of the patient’s basic cognitive situation | Human | MMSE | Attention, orientation, language, and praxis |
| Phototest | Verbal fluency | ||
| Eurotest | Complement of other more complete tests | ||
| Mini-Cog | Psychometric test of memory, executive function, language, and praxis | ||
| M@T | Memory | ||
| MoCa | Memory, executive function, language, and praxis | ||
| NPI-Q | Symptoms associated with different types of dementia | ||
| FAQ | Information on the patient’s performance of activities of daily living | ||
| Lawton and Brody scale | Assesses the patient’s ability to perform eight instrumental activities of daily living | ||
| Barthel Index | Assessing activities of daily living | ||
| Specific test | |||
| Attention and executive function | Human | Trail making test | Mental flexibility |
| F-A-S | |||
| Go/no go test | Evaluating the ability to inhibit irrelevant automatisms | ||
| Stroop test | |||
| Tower of London Test | Assessment of the ability to inhibit impulsivity | ||
| Luria sequence | Specific executive function tests | ||
| Trail making test | |||
| Wisconsin card sorting test | |||
| Figure of Benson | Perception and orientation | ||
| Rodents | Go/no-go test | Pre-attention | |
| Pre-pulse inhibition | |||
| Five-choice serial reaction | Vigilance and selective attention | ||
| Latent inhibition test | Selective attention Orientation attention | ||
| Orientation and habituation | |||
| Memory | Human | M@T | Declarative memory |
| Rey Osterrieth complex | Declarative memory and visuospatial domain | ||
| MIS | Verbal and visual memory | ||
| Wechsler memory scale | |||
| CVLT | |||
| TAVEC | |||
| FNAME | |||
| Rodents | Object recognition test | Non-spatial memory | |
| Social recognition test | |||
| Morris water maze | Spatial memory | ||
| Barnes maze | |||
| Object location test | |||
| T-maze | Working memory | ||
| Y-maze | |||
| Eight-arm radial maze test | |||
| Aversive conditioning to taste | Emotional memory | ||
| Fear potential overreaction | |||
| Learning | Rodents | Contextual fear conditioning | Evaluates aversive learning |
| Contextual and cues fear conditioning test | Measures cue and contextual learning | ||
| Step-down inhibitory avoidance | Evaluates aversive memory retention in rodents | ||
| Passive avoidance test | Studies acquired learning and memory | ||
| Active avoidance testing | Associative learning | ||
| Language | Humans | BDAE | Fluency, naming, comprehension, and repetition |
| Boston naming test | |||
| WAB | |||
| PALPA | |||
| MASTsp | |||
| Token test |
| Sensations | Sensory Modalities |
|---|---|
| Perceptions | Object recognition Organizational strategies |
| Motor and constructive tasks | Copy Drawing Other praxias |
| Attention and concentration | Selective attention Sustained attention/surveillance |
| Memory | Working memory: verbal, spatial, object, location Components of memory: central executive, maintenance, manipulation Episodic/declarative memory: Verbal and nonverbal (encoding, storage, free retrieval, cued retrieval, forced recognition by choice) Procedural memory Semantic memory Prospective memory: time-based or event-based |
| Executive functions | Reasoning Problem-solving Skills management |
| Processing speed | Semantic fluency Encoding and decoding |
| Language and verbal skills | Nomination Fluency Reading and comprehension |
| Cognitive Domains | Neural Networks |
|---|---|
| Memory | Temporomedial and limbic network: memory and emotional responses |
| Language | Perisylvian network |
| Perception | Occipitotemporal network |
| Praxias | Parieto-frontal network: spatial perception and orientation |
| Executive-attentional function | Prefrontal-subcortical network: involved in attention and planning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, D.; Gasparyan, A.; Martí Martínez, S.; Díaz Marín, C.; Navarrete, F.; García Gutiérrez, M.S.; Manzanares, J. Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach. Int. J. Mol. Sci. 2023, 24, 7653. https://doi.org/10.3390/ijms24087653
Navarro D, Gasparyan A, Martí Martínez S, Díaz Marín C, Navarrete F, García Gutiérrez MS, Manzanares J. Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach. International Journal of Molecular Sciences. 2023; 24(8):7653. https://doi.org/10.3390/ijms24087653
Chicago/Turabian StyleNavarro, Daniela, Ani Gasparyan, Silvia Martí Martínez, Carmen Díaz Marín, Francisco Navarrete, María Salud García Gutiérrez, and Jorge Manzanares. 2023. "Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach" International Journal of Molecular Sciences 24, no. 8: 7653. https://doi.org/10.3390/ijms24087653
APA StyleNavarro, D., Gasparyan, A., Martí Martínez, S., Díaz Marín, C., Navarrete, F., García Gutiérrez, M. S., & Manzanares, J. (2023). Methods to Identify Cognitive Alterations from Animals to Humans: A Translational Approach. International Journal of Molecular Sciences, 24(8), 7653. https://doi.org/10.3390/ijms24087653










