Transcriptomic Analysis Reveals Key Roles of (p)ppGpp and DksA in Regulating Metabolism and Chemotaxis in Yersinia enterocolitica
Abstract
1. Introduction
2. Results
2.1. (p)ppGpp- and DksA-Dependent Genes in Y. enterocolitica Identified Using RNA-Seq
2.2. (p)ppGpp- and DksA- Dependent Cell Functions in Y. enterocolitica
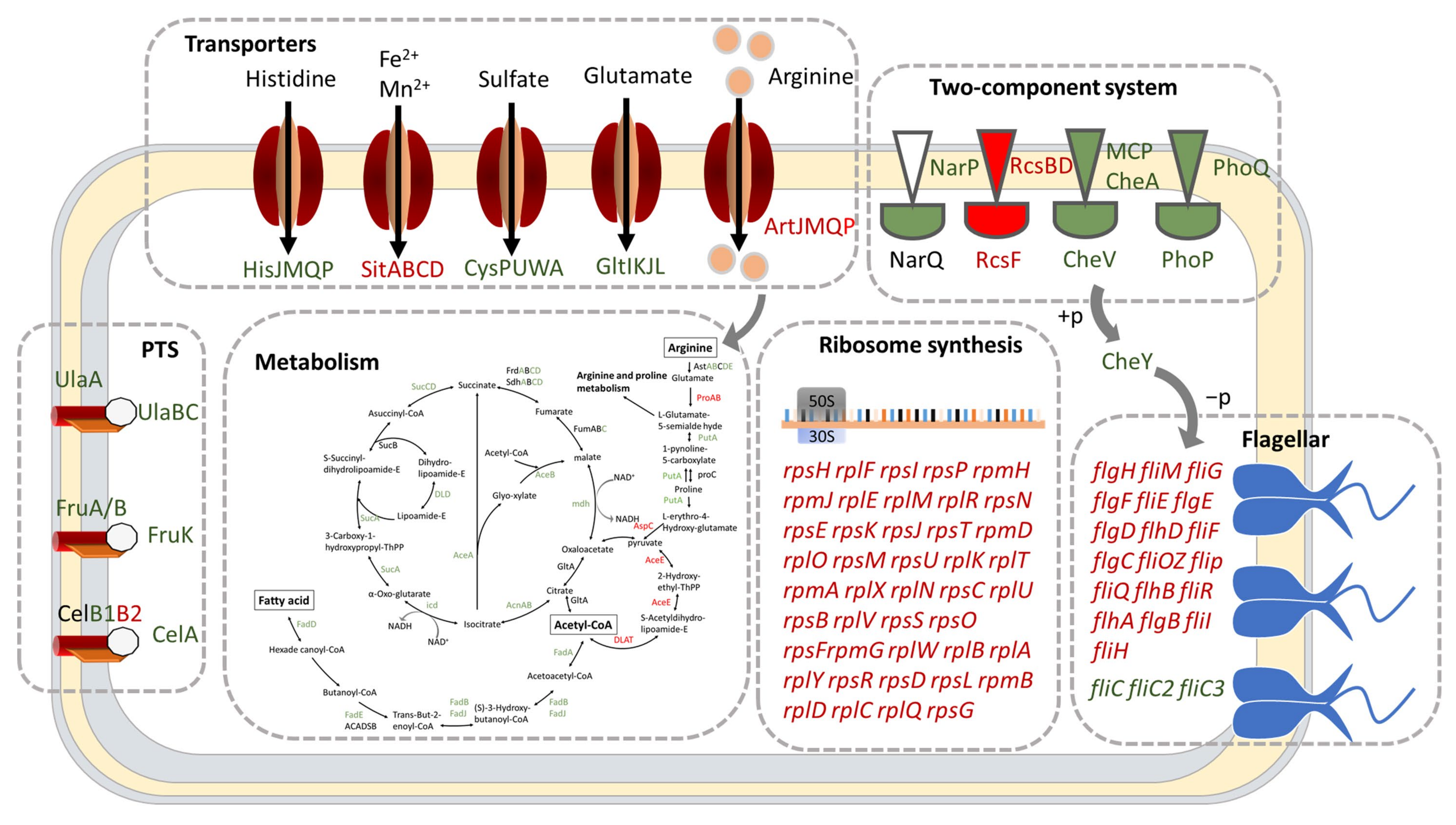
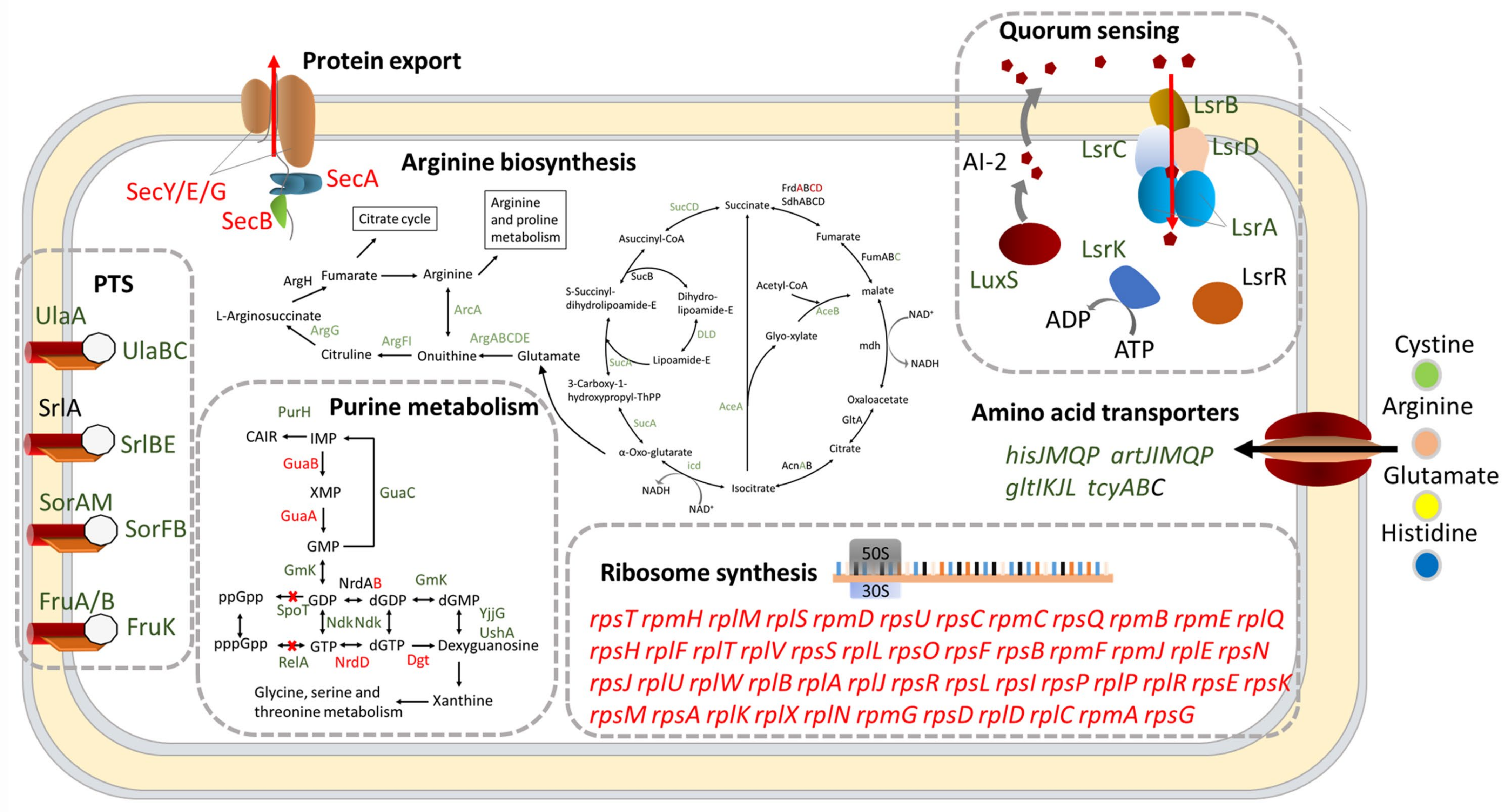
2.3. DksA and (p)ppGpp Inhibit Ribosomal Synthesis and Regulate Metabolic Networks
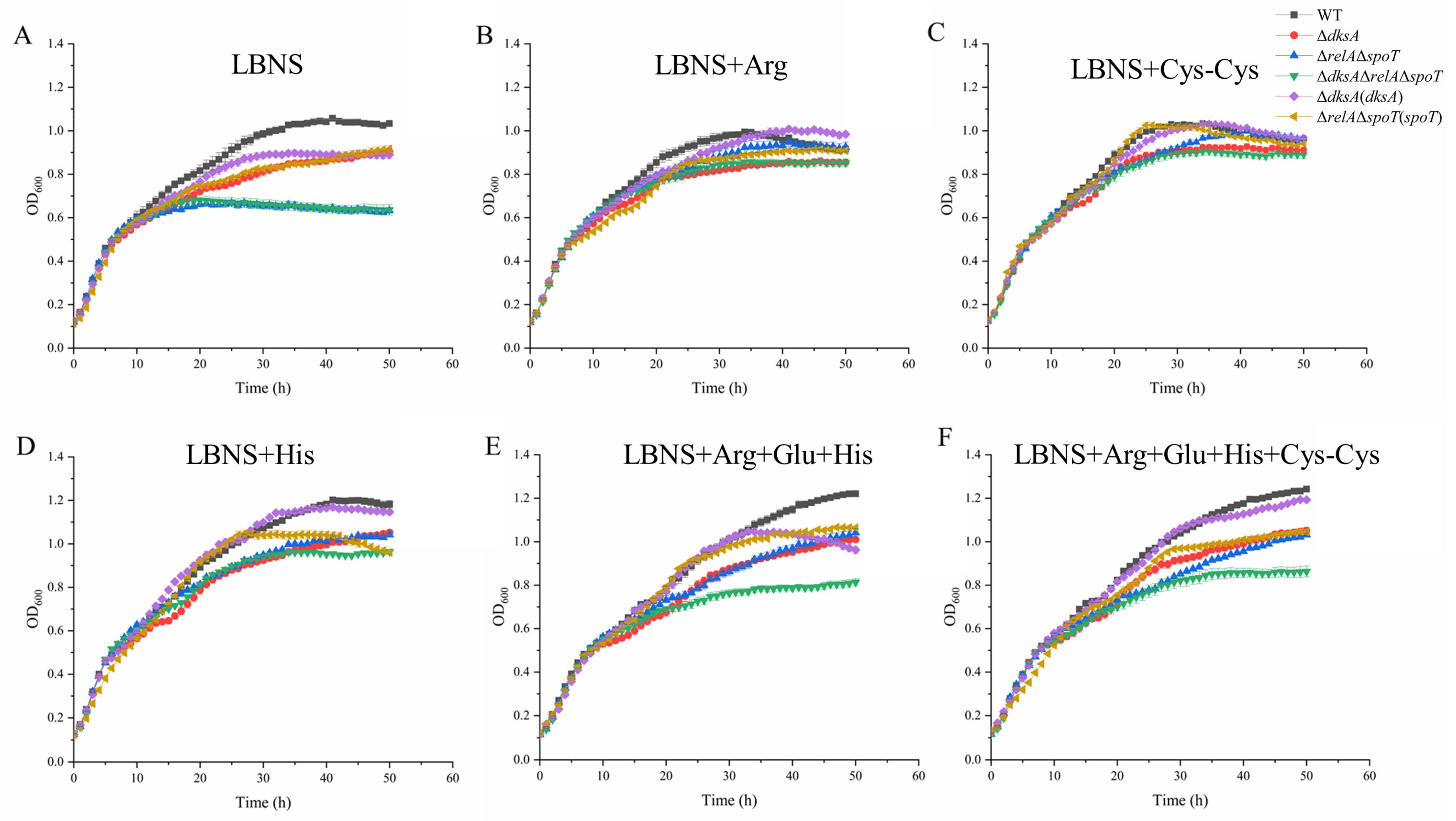
2.4. DksA and (p)ppGpp Are Required for Amino Acid Transport and Utilization
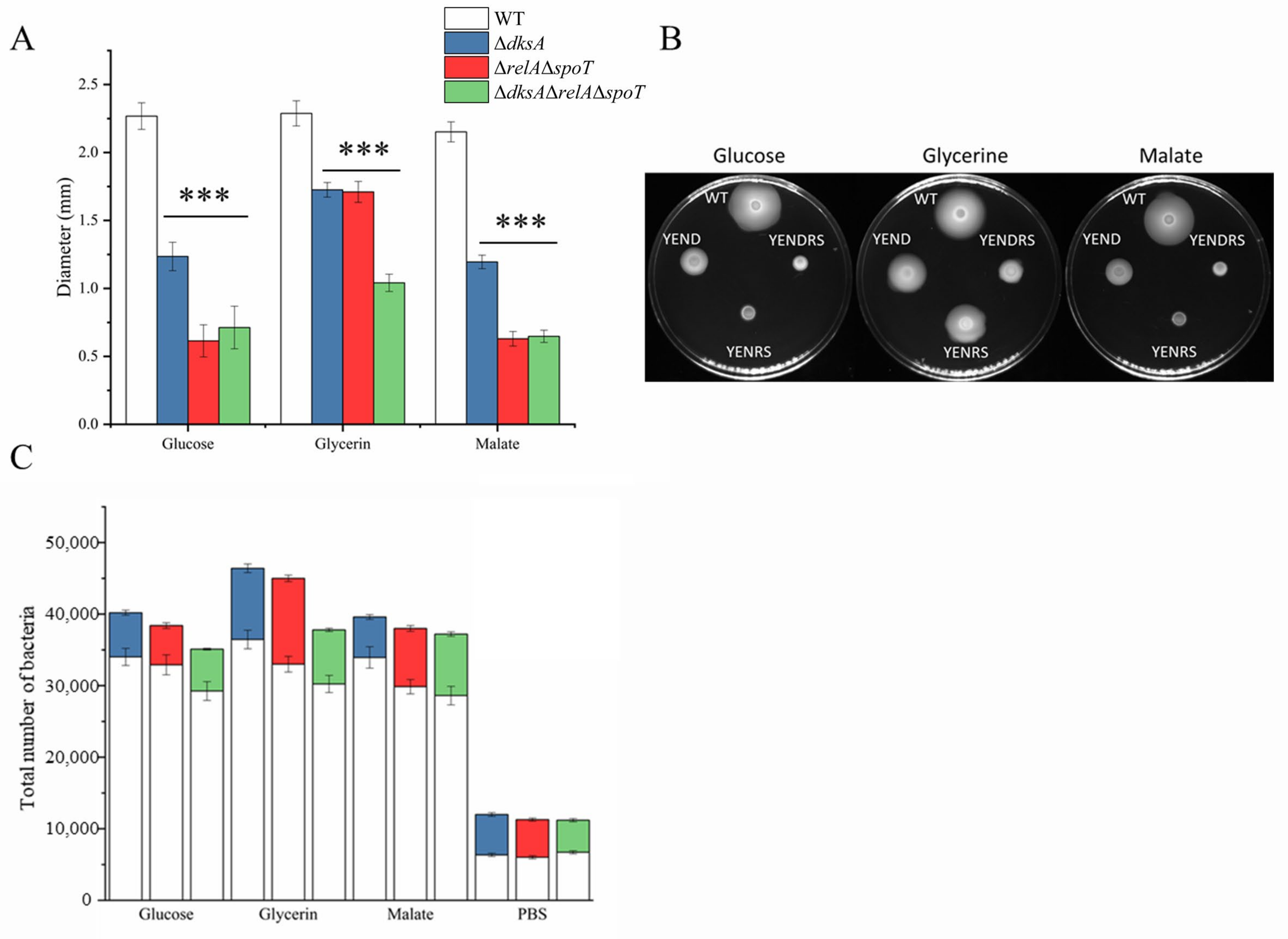
2.5. DksA and ppGpp Positively Affect Bacterial Chemotaxis
3. Discussion
4. Material and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. RNA Extraction, Library Construction, and Sequencing
4.3. Gene Annotation and Data Analysis
4.4. RT-qPCR
4.5. In Vitro Bacterial Growth
4.6. Swim Plate and Competitive Capillary Assays for Chemotaxis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pesavento, C.; Hengge, R. Bacterial nucleotide-based second messengers. Curr. Opin. Microbiol. 2009, 12, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Swanson, M.S. ppGpp: Magic beyond RNA polymerase. Nat. Rev. Microbiol. 2012, 10, 203–212. [Google Scholar] [CrossRef]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still Magical?*. Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef]
- Atkinson, G.C.; Tanel, T.; Vasili, H.; Stiller, J.W. The RelA/SpoT Homolog (RSH) Superfamily: Distribution and Functional Evolution of ppGpp Synthetases and Hydrolases across the Tree of Life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef]
- Fung, D.K.; Anderson, B.W.; Tse, J.L.; Wang, J.D. Nucleotide Second Messengers: (p)ppGpp and Cyclic Dinucleotides. In Bacillus: Cellular and Molecular Biology; Caister Academic Press: Poole, UK, 2017; pp. 439–465. [Google Scholar]
- Murdeshwar, M.S.; Chatterji, D. MS_RHII-RSD, a Dual-Function RNase HII-(p)ppGpp Synthetase from Mycobacterium smegmatis. J. Bacteriol. 2012, 194, 4003–4014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanchez-Vazquez, P.; Dewey, C.N.; Kitten, N.; Ross, W.; Gourse, R.L. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA 2019, 116, 8310–8319. [Google Scholar] [CrossRef]
- Aberg, A.; Fernandez-Vazquez, J.; Cabrer-Panes, J.D.; Sanchez, A.; Balsalobre, C. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J. Bacteriol. 2009, 191, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Teper, D.; Xu, J.; Wang, N. Stringent response regulators (p)ppGpp and DksA positively regulate virulence and host adaptation of Xanthomonas citri. Mol. Plant Pathol. 2019, 20, 1550–1565. [Google Scholar] [CrossRef]
- Tagami, S.; Sekine, S.; Kumarevel, T.; Hino, N.; Murayama, Y.; Kamegamori, S.; Yamamoto, M.; Sakamoto, K.; Yokoyama, S. Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature 2010, 468, 978-U282. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Vrentas, C.E.; Sanchez-Vazquez, P.; Gaal, T.; Gourse, R.L. The Magic Spot: A ppGpp Binding Site on E. coli RNA Polymerase Responsible for Regulation of Transcription Initiation. Mol. Cell 2013, 50, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Sanchez-Vazquez, P.; Chen, A.Y.; Lee, J.-H.; Burgos, H.L.; Gourse, R.L. ppGpp Binding to a Site at the RNAP-DksA Interface Accounts for Its Dramatic Effects on Transcription Initiation during the Stringent Response. Mol. Cell 2016, 62, 811–823. [Google Scholar] [CrossRef]
- Wang, J.D.; Sanders, G.M.; Grossman, A.D. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 2007, 128, 865–875. [Google Scholar] [CrossRef]
- Milon, P.; Tischenko, E.; Tomšic, J.; Caserta, E.; Folkers, G.; La Teana, A.; Rodnina, M.V.; Pon, C.L.; Boelens, R.; Gualerzi, C.O. The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13962–13967. [Google Scholar] [CrossRef]
- Mitkevich, V.A.; Ermakov, A.; Kulikova, A.A.; Tankov, S.; Shyp, V.; Soosaar, A.; Tenson, T.; Makarov, A.A.; Ehrenberg, M.; Hauryliuk, V. Thermodynamic Characterization of ppGpp Binding to EF-G or 1F2 and of Initiator tRNA Binding to Free 1F2 in the Presence of GDP, GTP, or ppGpp. J. Mol. Biol. 2010, 402, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Czech, L.; Mais, C.-N.; Kratzat, H.; Sarmah, P.; Giammarinaro, P.; Freibert, S.-A.; Esser, H.F.; Musial, J.; Berninghausen, O.; Steinchen, W.; et al. Inhibition of SRP-dependent protein secretion by the bacterial alarmone (p)ppGpp. Nat. Commun. 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Wolz, C. Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int. J. Med. Microbiol. 2014, 304, 150–155. [Google Scholar] [CrossRef]
- Kruger, L.; Herzberg, C.; Wicke, D.; Bahre, H.; Heidemann, J.L.; Dickmanns, A.; Schmitt, K.; Ficner, R.; Stulke, J. A meet-up of two second messengers: The c-di-AMP receptor DarB controls (p)ppGpp synthesis in Bacillus subtilis. Nat. Commun. 2021, 12, 1210. [Google Scholar] [CrossRef]
- Osterberg, S.; del Peso-Santos, T.; Shingler, V. Regulation of Alternative Sigma Factor Use. Annu. Rev. Microbiol. 2011, 65, 37–55. [Google Scholar] [CrossRef]
- Lennon, C.W.; Lemmer, K.C.; Irons, J.L.; Sellman, M.I.; Donohue, T.J.; Gourse, R.L.; Ross, W. A Rhodobacter sphaeroides Protein Mechanistically Similar to Escherichia coli DksA Regulates Photosynthetic Growth. Mbio 2014, 5, e01105–e01114. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Furman, R.; Rodionov, D.A.; Artsimovitch, I.; de Crecy-Lagard, V. Role of a Zn-independent DksA in Zn homeostasis and stringent response. Mol. Microbiol. 2011, 79, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Perederina, A.; Svetlov, V.; Vassylyeva, M.N.; Tahirov, T.H.; Yokoyama, S.; Artsimovitch, I.; Vassylyev, D.G. Regulation through the secondary channel-structural framework for ppGpp-DksA synergism during transcription. Cell 2004, 118, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Gourse, R.L.; Chen, A.Y.; Gopalkrishnan, S.; Sanchez-Vazquez, P.; Myers, A.; Ross, W. Transcriptional Responses to ppGpp and DksA. Annu. Rev. Microbiol. 2018, 72, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Lyzen, R.; Maitra, A.; Milewska, K.; Kochanowska-Lyzen, M.; Hernandez, J.; Szalewska-Palasz, A. The dual role of DksA protein in the regulation of Escherichia coli pArgX promoter. Nucleic Acids Res. 2016, 44, 10316–10325. [Google Scholar]
- Magnusson, L.U.; Gummesson, B.; Joksimovic, P.; Farewell, A.; Nystrom, T. Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J. Bacteriol. 2007, 189, 5193–5202. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Meng, J.; Li, W.; Chen, J. Similar and Divergent Roles of Stringent Regulator (p)ppGpp and DksA on Pleiotropic Phenotype of Yersinia enterocolitica. Microbiol. Spectr. 2022, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Azriel, S.; Goren, A.; Rahav, G.; Gal-Mor, O. The Stringent Response Regulator DksA Is Required for Salmonella enterica Serovar Typhimurium Growth in Minimal Medium, Motility, Biofilm Formation, and Intestinal Colonization. Infect. Immun. 2016, 84, 375–384. [Google Scholar] [CrossRef]
- Ancona, V.; Lee, J.H.; Chatnaparat, T.; Oh, J.; Hong, J.; Zhao, Y. The Bacterial Alarmone (p)ppGpp Activates the Type III Secretion System in Erwinia amylovora. J. Bacteriol. 2015, 197, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Haseltin, W.A.; Block, R.; Weber, K.; Gilbert, W. MSI and MSII made on ribosome in idling step of protein-synthesis. Nature 1972, 238, 381–384. [Google Scholar] [CrossRef]
- Cashel, M. Regulation of Bacterial ppGpp and pppGpp. Annu. Rev. Microbiol. 1975, 29, 301–318. [Google Scholar] [CrossRef]
- Young, G.M.; Badger, J.L.; Miller, V.L. Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 2000, 68, 4323–4326. [Google Scholar] [CrossRef]
- Lemke, J.J.; Durfee, T.; Gourse, R.L. DksA and ppGpp directly regulate transcription of the Escherichia coli flagellar cascade. Mol. Microbiol. 2009, 74, 1368–1379. [Google Scholar] [CrossRef]
- Meng, J.; Bai, J.; Chen, J. Transcriptomic analysis reveals the role of RcsB in suppressing bacterial chemotaxis, flagellar assembly and infection in Yersinia enterocolitica. Curr. Genet. 2020, 66, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Gunderson, C.W.; Mateescu, E.M.; Zhang, Z.G.; Hwa, T. Interdependence of Cell Growth and Gene Expression: Origins and Consequences. Science 2010, 330, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Das, S.; Biswas, A.; Bhadra, R.K.; Das, S. Small alarmones (p)ppGpp regulate virulence associated traits and pathogenesis of Salmonella enterica serovar Typhi. Cell. Microbiol. 2019, 21, e13034. [Google Scholar] [CrossRef]
- Lyzen, R.; Kochanowska, M.; Wegrzyn, G.; Szalewska-Palasz, A. Transcription from bacteriophage lambda pR promoter is regulated independently and antagonistically by DksA and ppGpp. Nucleic Acids Res. 2009, 37, 6655–6664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Liu, L.; Fitzsimmons, L.F.; Wang, Y.; Crawford, M.A.; Mastrogiovanni, M.; Trujillo, M.; Till, J.K.A.; Radi, R.; Dai, S.; et al. DksA-DnaJ redox interactions provide a signal for the activation of bacterial RNA polymerase. Proc. Natl. Acad. Sci. USA 2018, 115, E11780–E11789. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.J.; Berkmen, M.B.; Gourse, R.L. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc. Natl. Acad. Sci. USA 2005, 102, 7823–7828. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Tyulenev, A.V.; Bezmaternykh, K.V.; Muzyka, N.G.; Ushakov, V.Y.; Oktyabrsky, O.N. Cysteine homeostasis under inhibition of protein synthesis in Escherichia coli cells. Amino Acids 2019, 51, 1577–1592. [Google Scholar] [CrossRef]
- Adler, J. Chemotaxis in bacteria. Harvey Lect. 1978, 72, 195–230. [Google Scholar]
- Jiang, N.; Liu, W.; Li, Y.; Wu, H.; Zhang, Z.; Alexandre, G.; Elmerich, C.; Xie, Z. A Chemotaxis Receptor Modulates Nodulation during the Azorhizobium caulinodans-Sesbania rostrata Symbiosis. Appl. Environ. Microbiol. 2016, 82, 3174–3184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Sun, Y.; Xia, C.; Elmerich, C.; Xie, Z. A cheZ-Like Gene in Azorhizobium caulinodans is a Key Gene in the Control of Chemotaxis and Colonization of the Host Plant. Appl. Environ. Microbiol. 2018, 84, 3. [Google Scholar] [CrossRef] [PubMed]
- Soutourina, O.A.; Bertin, P.N. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 2003, 27, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Huang, C.; Huang, X.; Liu, D.; Chen, J. Osmoregulated Periplasmic Glucans Transmit External Signals Through Rcs Phosphorelay Pathway in Yersinia enterocolitica. Front. Microbiol. 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
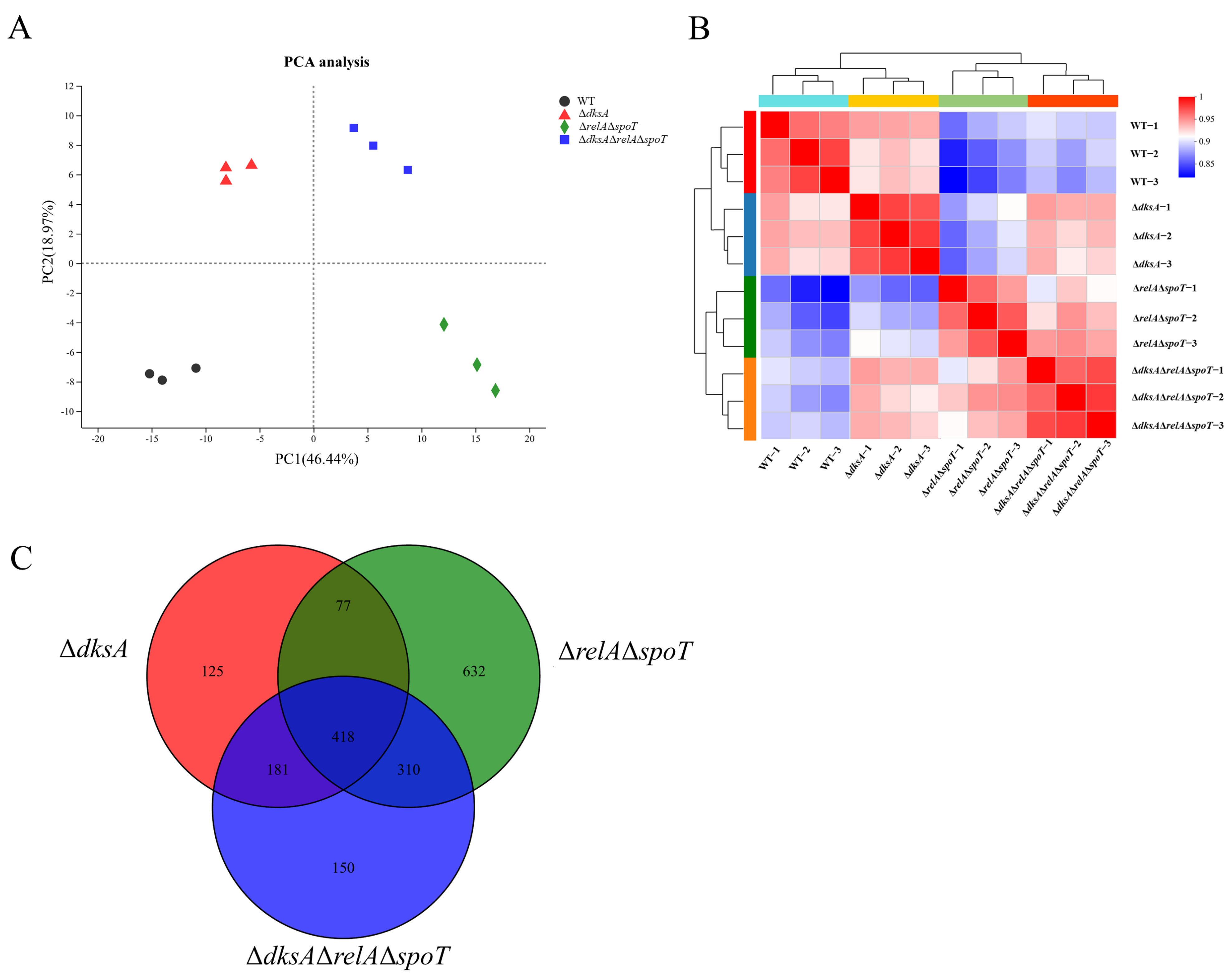
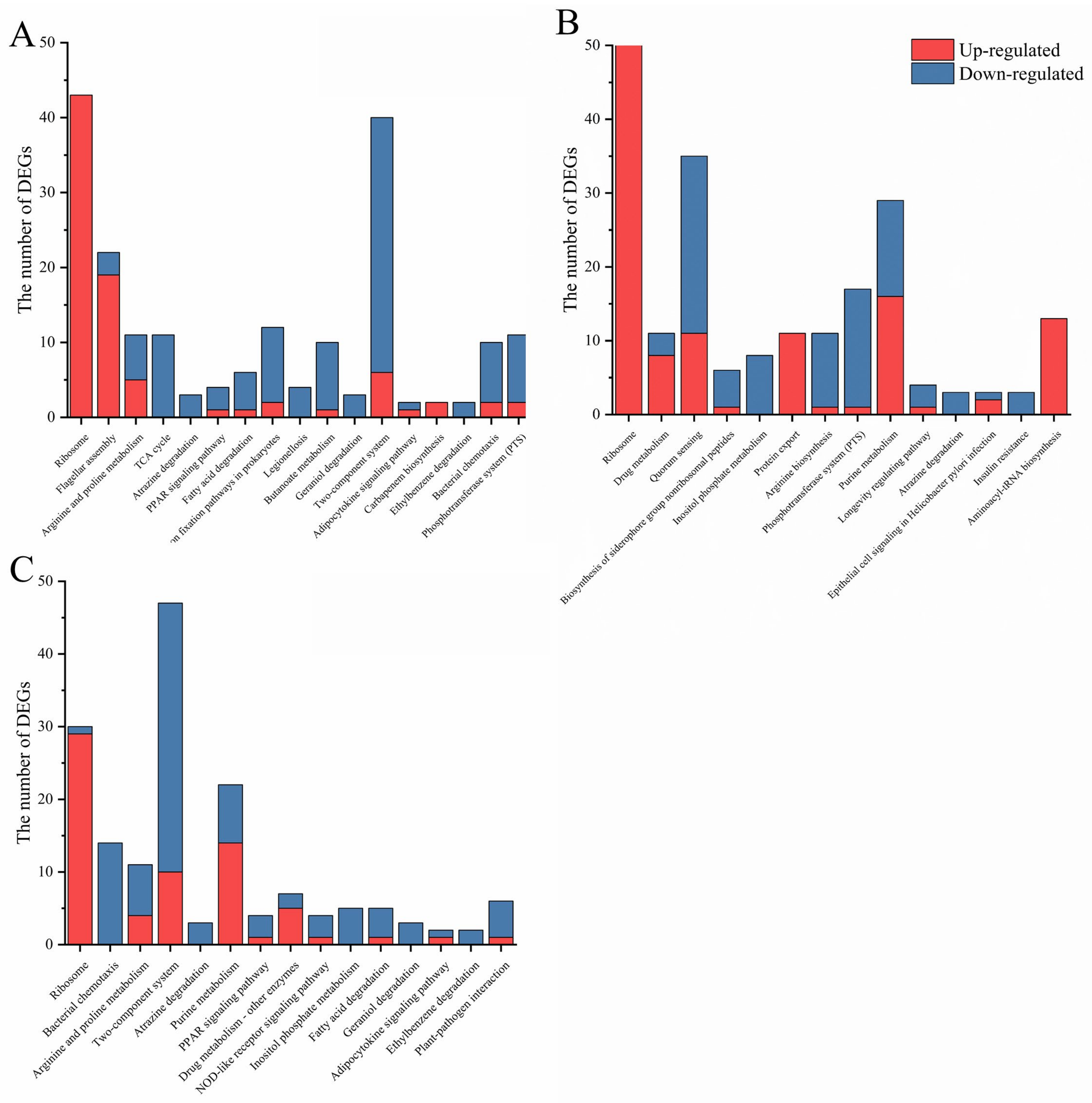
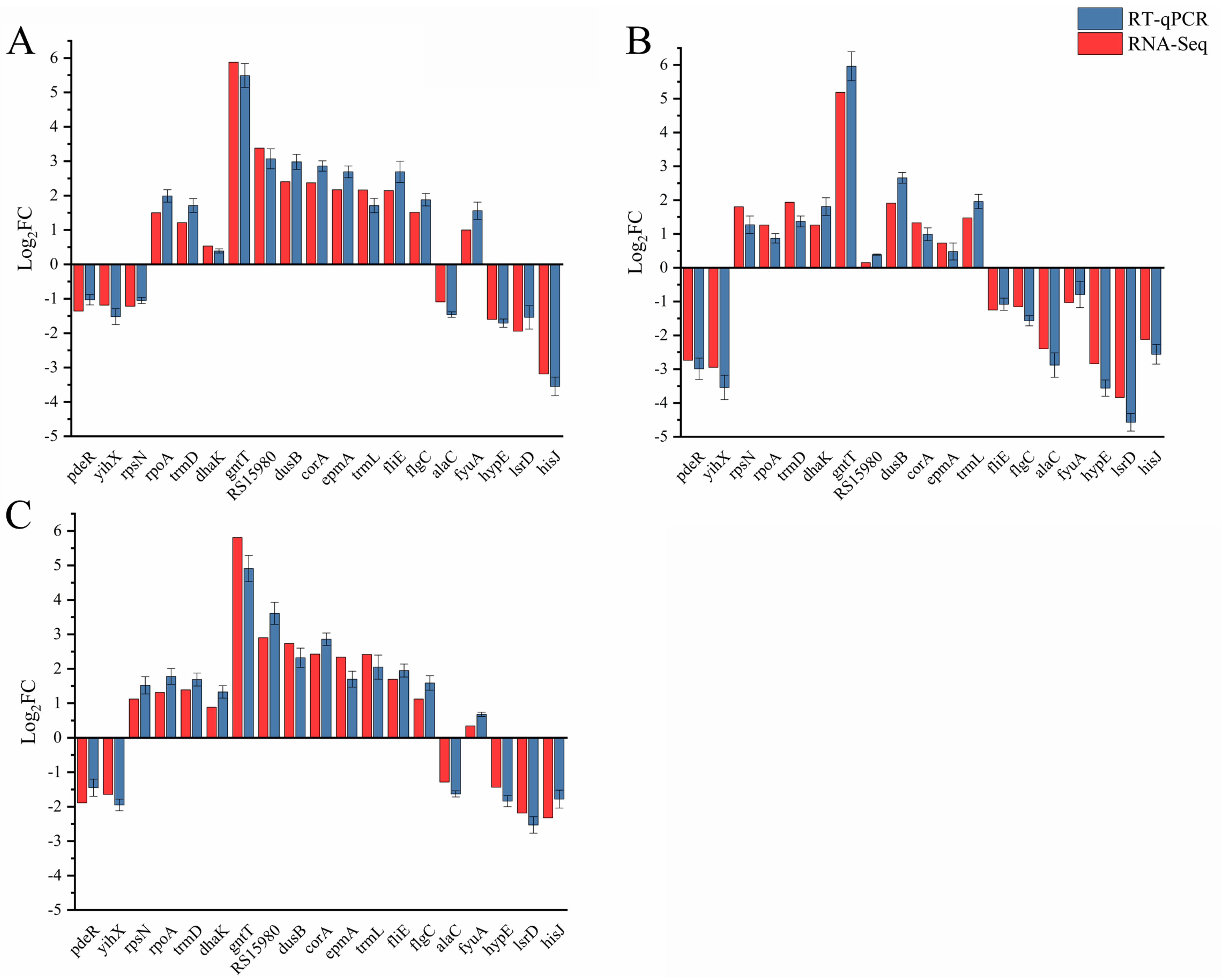
| Samples | Genetic Number | Ratios | Number of DEGs | DEGs | |
|---|---|---|---|---|---|
| Upregulated | Downregulated | ||||
| WT | 3651 | 84.65% | 0 | 0 | 0 |
| ΔdksA | 3644 | 84.49% | 801 | 384 | 417 |
| ΔrelAΔspoT | 3434 | 79.62% | 1437 | 695 | 742 |
| ΔdksAΔrelAΔspoT | 3614 | 83.79% | 1059 | 560 | 499 |
| Strain | Genotype | Sources |
|---|---|---|
| WT | Y. enterocolitica ATCC 23715; Serotype O:8; Biotype 1B; pYV- | Lab stored |
| ΔdksA | Y. enterocolitica ATCC 23715 derived, Δ dksA | Lab stored |
| ΔrelAΔspoT | Y. enterocolitica ATCC 23715 derived, Δ relA Δ spoT | Lab stored |
| ΔdksAΔrelAΔspoT | ΔrelAΔspoT derived, Δ dksA | Lab stored |
| ΔdksA(dksA) | ΔdksA harboring pBAD24—dksA, AMPr | Lab stored |
| ΔrelAΔspoT(spoT) | ΔrelAΔspoT harboring pBAD24—spoT, AMPr | Lab stored |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Li, W.; Chen, J. Transcriptomic Analysis Reveals Key Roles of (p)ppGpp and DksA in Regulating Metabolism and Chemotaxis in Yersinia enterocolitica. Int. J. Mol. Sci. 2023, 24, 7612. https://doi.org/10.3390/ijms24087612
Huang C, Li W, Chen J. Transcriptomic Analysis Reveals Key Roles of (p)ppGpp and DksA in Regulating Metabolism and Chemotaxis in Yersinia enterocolitica. International Journal of Molecular Sciences. 2023; 24(8):7612. https://doi.org/10.3390/ijms24087612
Chicago/Turabian StyleHuang, Can, Wenqian Li, and Jingyu Chen. 2023. "Transcriptomic Analysis Reveals Key Roles of (p)ppGpp and DksA in Regulating Metabolism and Chemotaxis in Yersinia enterocolitica" International Journal of Molecular Sciences 24, no. 8: 7612. https://doi.org/10.3390/ijms24087612
APA StyleHuang, C., Li, W., & Chen, J. (2023). Transcriptomic Analysis Reveals Key Roles of (p)ppGpp and DksA in Regulating Metabolism and Chemotaxis in Yersinia enterocolitica. International Journal of Molecular Sciences, 24(8), 7612. https://doi.org/10.3390/ijms24087612







