Exploiting Enzyme in the Polymer Synthesis for a Remarkable Increase in Thermal Conductivity
Abstract
1. Introduction
2. Results and Discussion
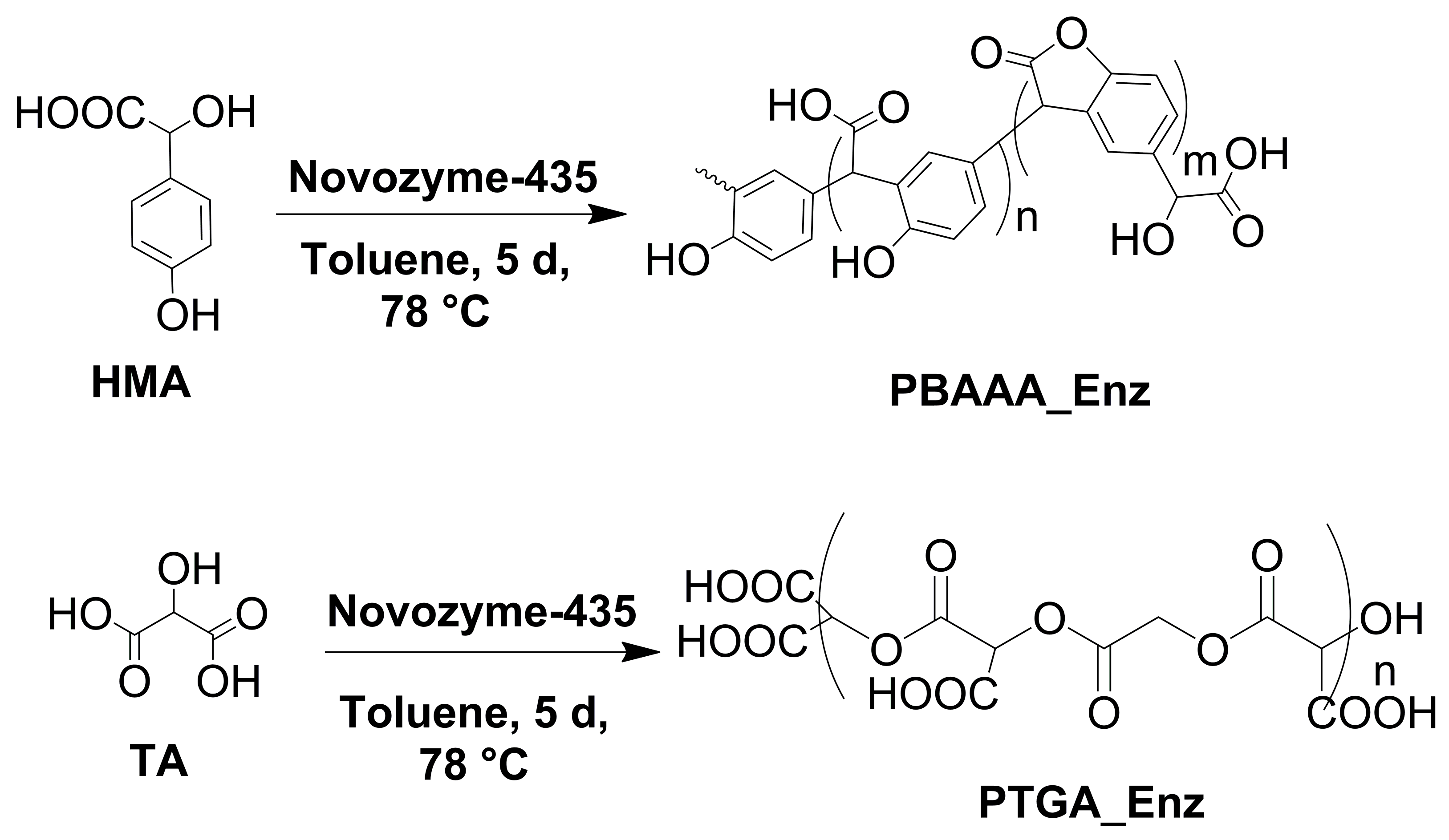
2.1. Enzymatic Synthesis of Polymers
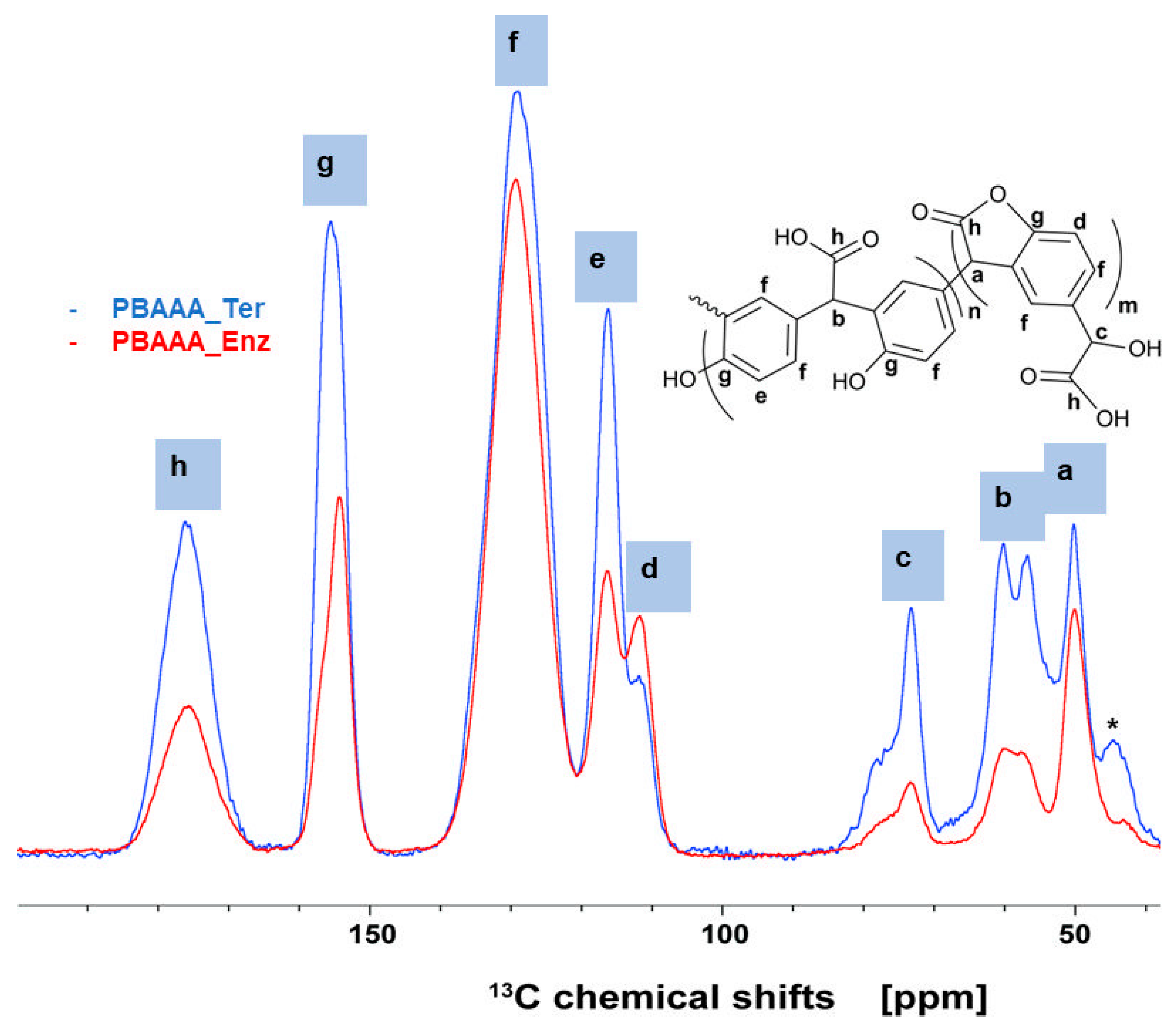
2.2. NMR Spectroscopy of Polymers
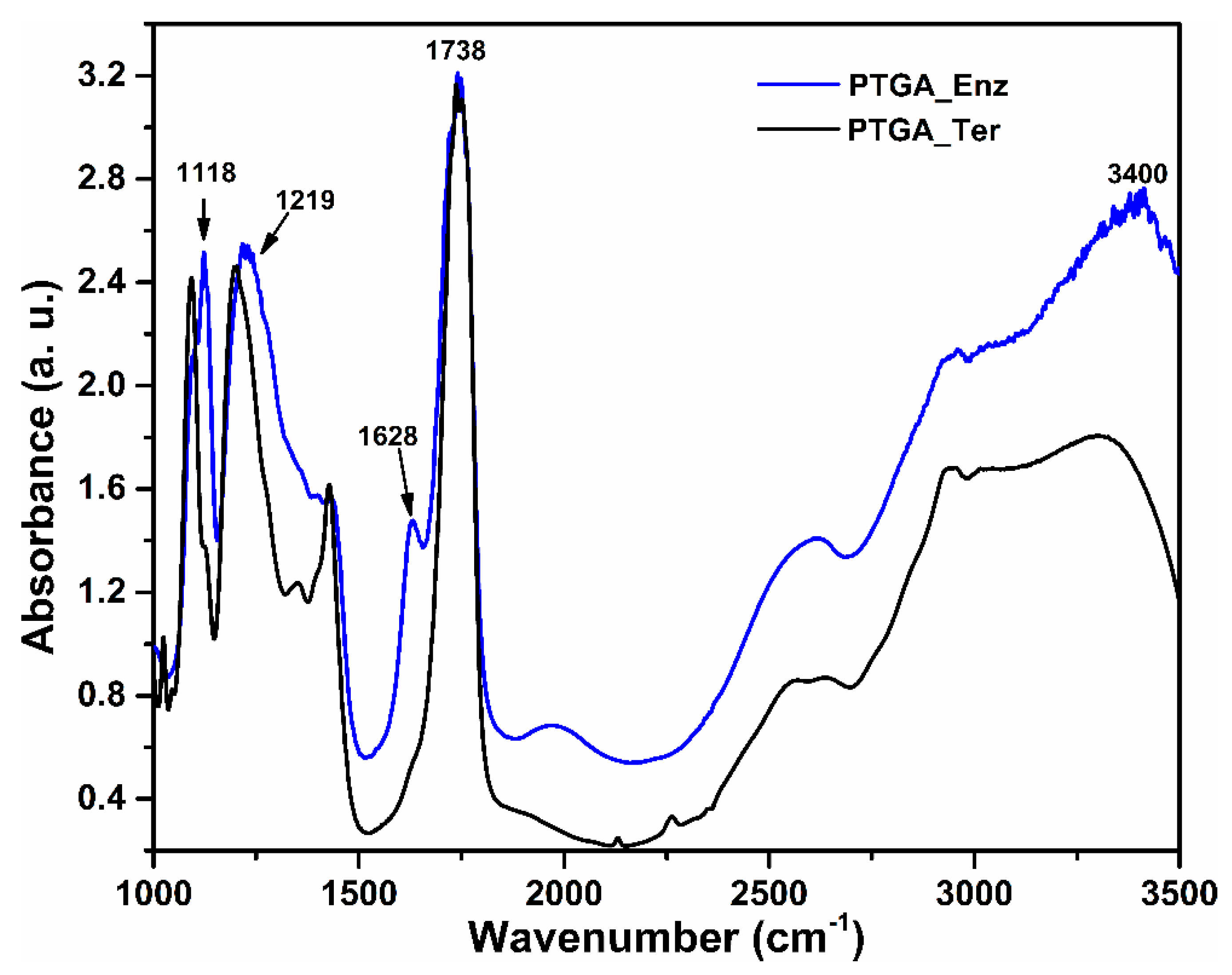
2.3. FTIR Spectroscopy of the Polymers
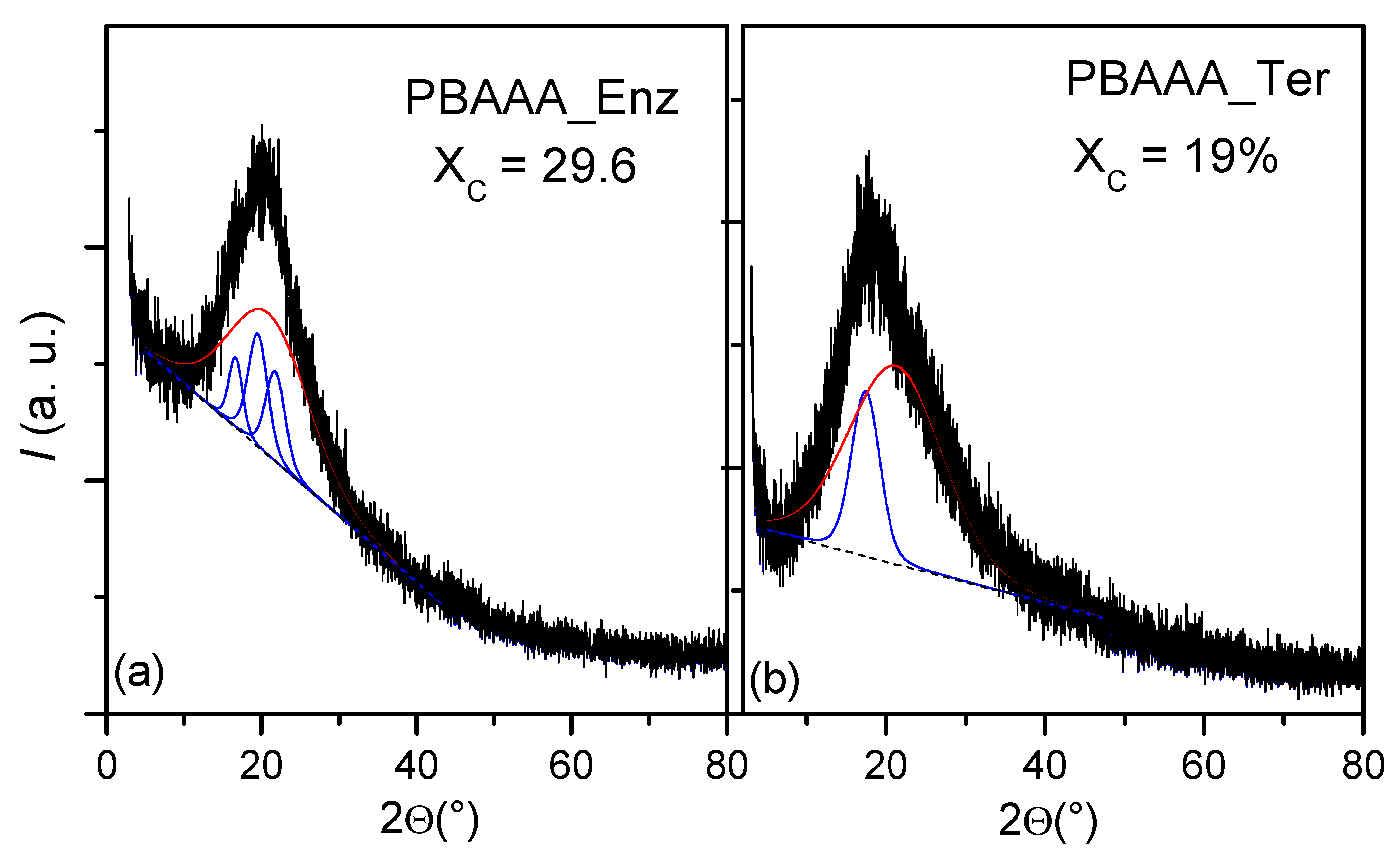
2.4. Powder XRD Analysis
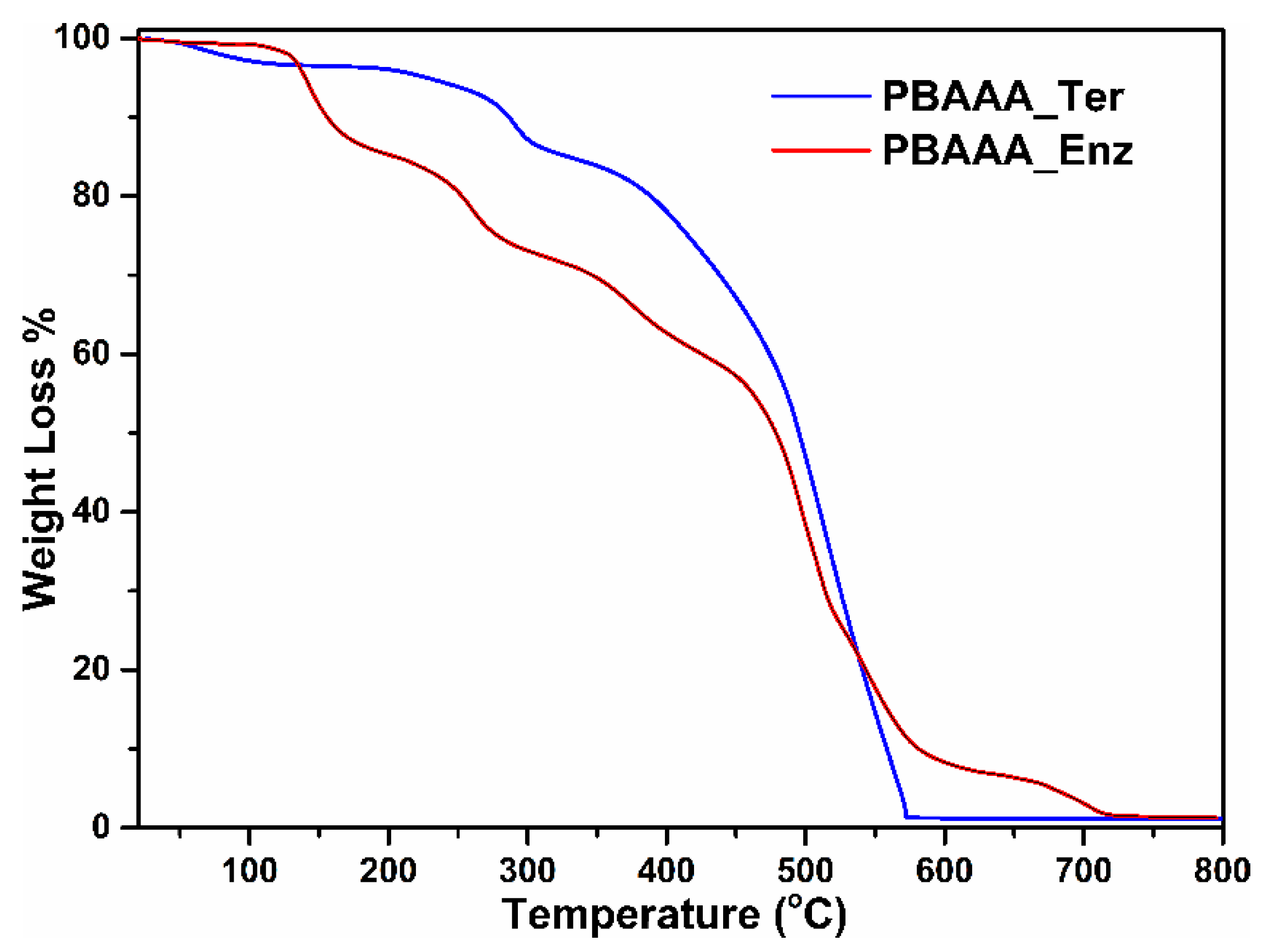
2.5. Thermogravimetric Analyses of Polymers
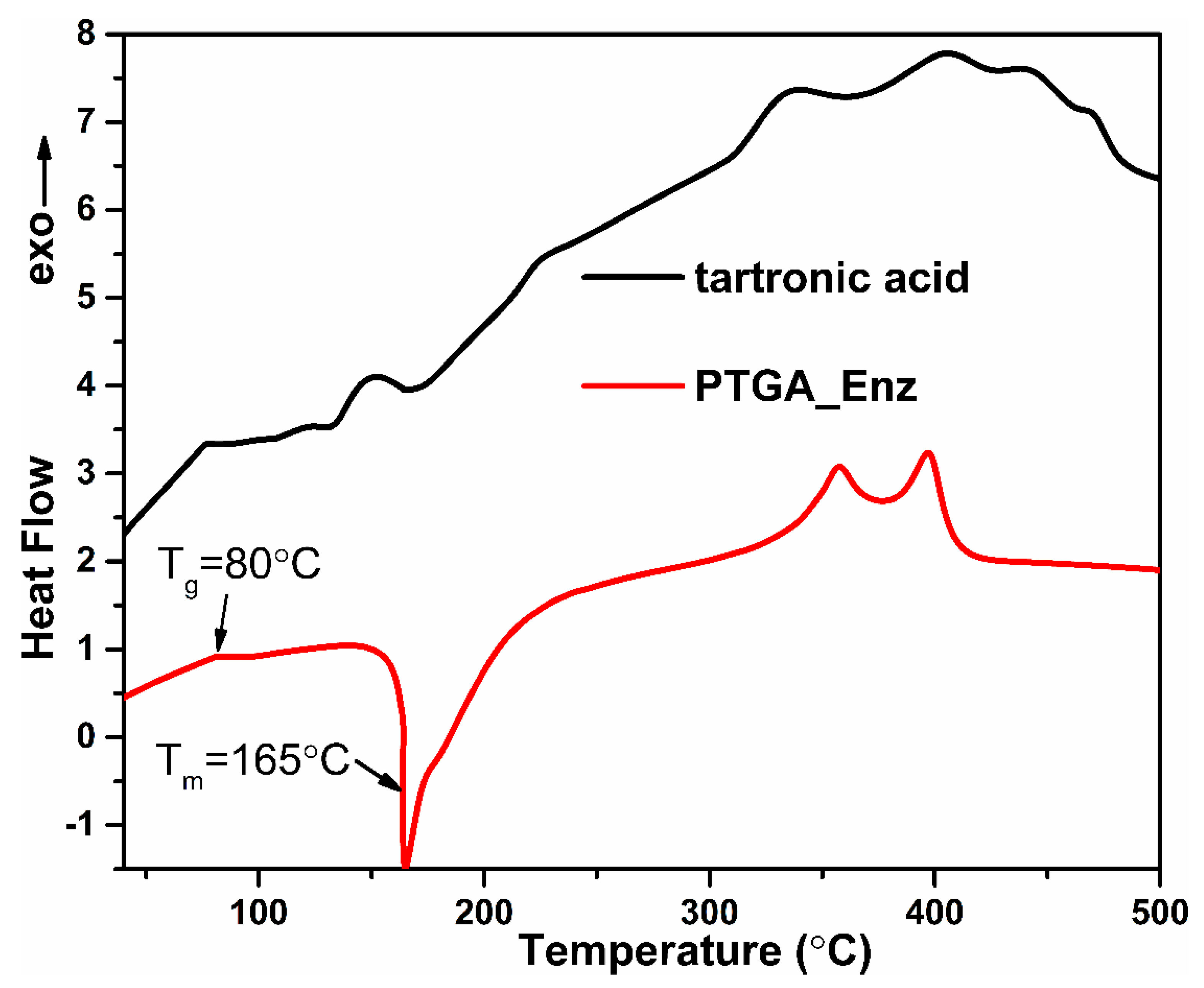
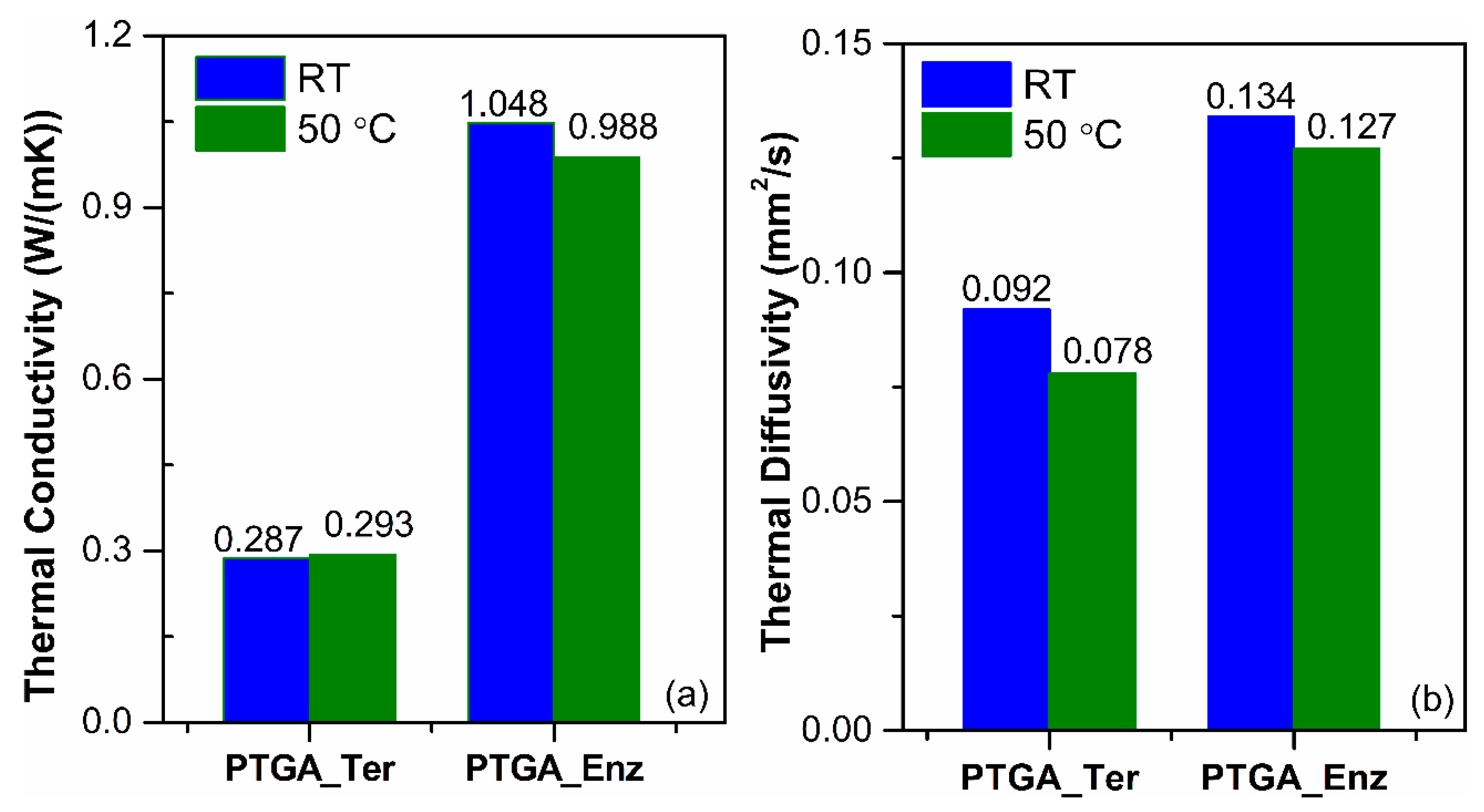
2.6. Thermal Analysis of Polymers
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Enzymatic Synthesis of Polymers
3.2.1. Enzymatic Synthesis of PBAAA_Enz
3.2.2. Enzymatic Synthesis of PTGA_Enz
3.3. NMR Spectroscopy
3.4. FTIR Spectroscopy
3.5. Thermogravimetric Analysis
3.6. Powder XRD
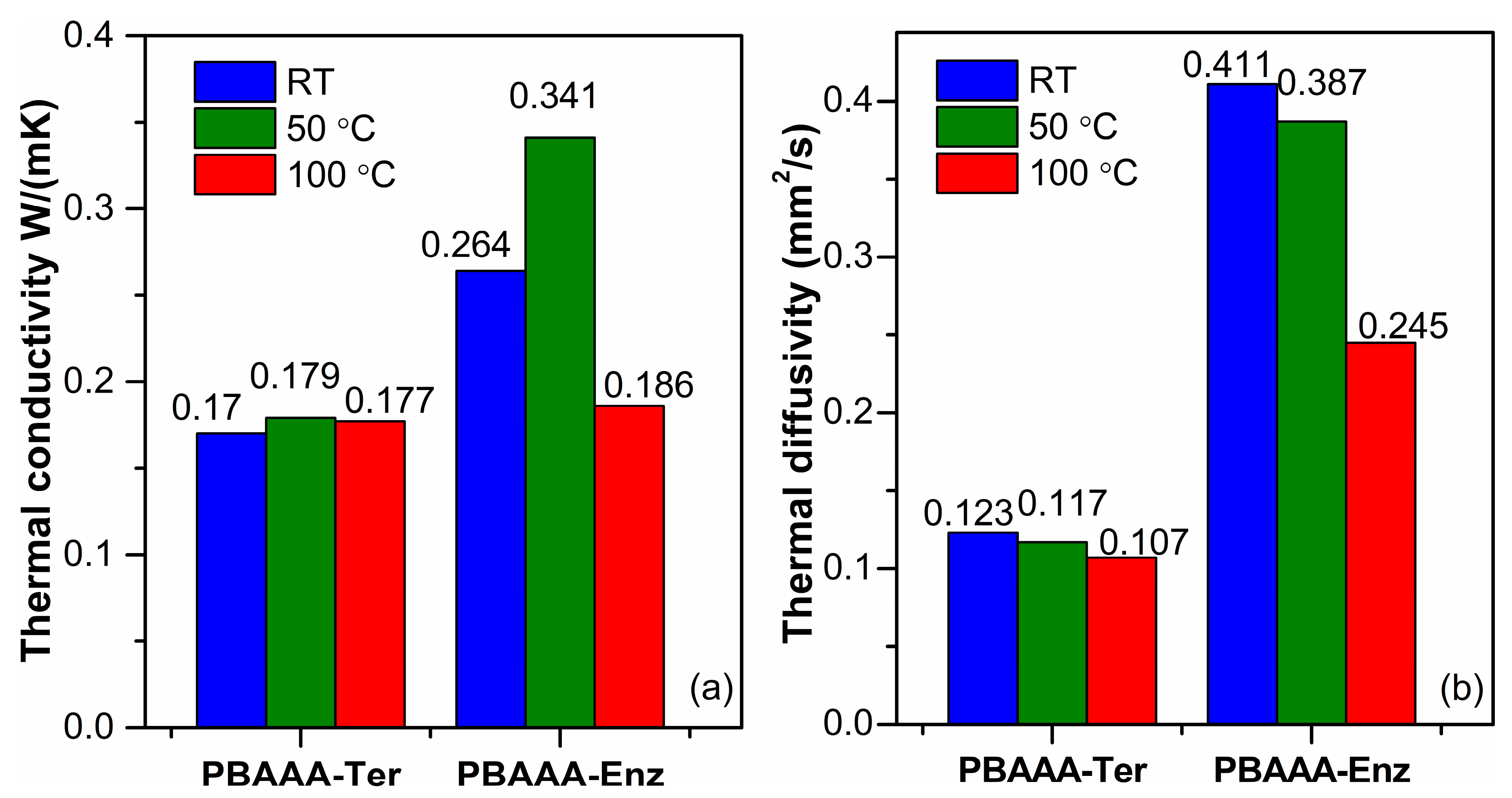
3.7. Thermal Conductivity and Diffusivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal Conductivity of Polymer-Based Composites: Fundamentals and Applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal Conductivity of Polymers and Polymer Nanocomposites. Mater. Sci. Eng. R 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.J.; Son, Y.R.; In, I.; An, K.H.; Kim, B.J.; Park, S.J. Recent Advanced Thermal Interfacial Materials: A Review of Conducting Mechanisms and Parameters of Carbon Materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Shanker, A.; Li, C.; Kim, G.; Gidley, D.; Pipe, K.P.; Kim, J. High Thermal Conductivity in Electrostatically Engineered Amorphous Polymers. Sci. Adv. 2017, 3, e1700342. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal Conductivity of Polymers and Their Nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Chen, J. Thermal Transport in Conductive Polymer-Based Materials. Adv. Funct. Mater. 2020, 30, 1904704. [Google Scholar] [CrossRef]
- Choy, C.L.; Chen, F.C.; Luk, W.H. Thermal conductivity of oriented crystalline polymers. J. Polym. Sci. Polym. Lett. Ed. 1980, 18, 1187–1207. [Google Scholar] [CrossRef]
- Choy, C.L.; Luk, W.H.; Chen, F.C. Thermal conductivity of highly oriented polyethylene. Polymer 1978, 19, 155–162. [Google Scholar] [CrossRef]
- Choy, C.L.; Wong, Y.W.; Yang, G.W.; Kanamoto, T. Elastic modulus and thermal conductivity of ultradrawn polyethylene. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3359–3367. [Google Scholar] [CrossRef]
- Wang, X.; Ho, V.; Segalman, R.A.; Cahill, D.G. Thermal conductivity of high-modulus polymer fibers. Macromolecules 2013, 46, 4937–4943. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Feng, N.; Jiang, W.; Jin, Y.; Li, X. Recent advances in the synthesis of biodegradable polyesters by sustainable polymerization: Lipase-catalyzed polymerization. RSC Adv. 2020, 10, 36230. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Khani, M.-M.; Rabbani, S.; Mashaghi, A.; Noorizadeh, F.; Faridi-Majidi, R.; Ghanbari, H. Development of poly (mannitol sebacate)/poly (lactic acid) nanofibrous scaffolds with potential applications in tissue engineering. Mater. Sci. Eng. C 2020, 110, 110626. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Recent Developments in Lipase-Catalyzed Synthesis of Polyesters. Macromol. Rapid Commun. 2009, 30, 237. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef]
- Kobayashi, S. Lipase-catalyzed polyester synthesis: A green polymer chemistry. Proc. Jpn. Acad. Ser. B 2010, 86, 338–365. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kobayashi, S. Polymer synthesis by enzymatic catalysis. Curr. Opin. Chem. Biol. 2010, 14, 145–153. [Google Scholar] [CrossRef]
- Ma, J.; Li, Q.; Song, B.; Liu, D.; Zheng, B.; Zhang, Z.; Feng, Y. Ring-opening polymerization of ε-caprolactone catalyzed by a novel thermophilic esterase from the archaeon Archaeoglobus fulgidus. J. Mol. Catal. B Enzym. 2009, 56, 151–157. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Yu, S.; Zhang, Z.; Ma, F.; Feng, Y. Ring-opening polymerization of -caprolactone catalyzed by a novel thermophilic lipase from Fervidobacteriumnodosum. Process Biochem. 2011, 46, 253–257. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Shi, W. Thermophilic lipase and recombinant Escherichia coli whole-cell: Novel biocatalysts for the synthesis of biodegradable polyesters. J. Control. Release 2011, 152, e221–e223. [Google Scholar] [CrossRef]
- Perin, G.B.; Felisberti, M.I. Mechanism and kinetics of lipase-catalyzed polycondensation of glycerol and sebacic acid: Influence of solvent and temperature. Biomacromolecules 2022, 23, 2968–2975. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Han, H.; Li, G.; Yang, J.; Zhang, L.; Yang, Y.; Fang, X.; Li, Q. Biocatalytic synthesis of poly(ε-valerolactone) using a thermophilic esterase from Archaeoglobusfulgidus as catalyst. Int. J. Mol. Sci. 2012, 13, 12232–12241. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Lang, K.; Xia, K.; Linhardt, R.J.; Gross, R.A. Lipase-Catalyzed Synthesis and Characterization of Poly(glycerol sebacate). Biomacromolecules 2022, 23, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Todea, A.; Dreava, D.M.; Benea, I.C.; Bîtcan, I.; Peter, F.; Boeriu, C.G. Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases. Processes 2021, 9, 646. [Google Scholar] [CrossRef]
- Fabbri, F.; Bertolini, F.A.; Guebitz, G.M.; Pellis, A. Biocatalyzed Synthesis of Flavor Esters and Polyesters: A Design of Experiments (DoE) Approach. Int. J. Mol. Sci. 2021, 22, 8493. [Google Scholar] [CrossRef]
- Miletić, N.; Nastasović, A.; Loos, K. Immobilization of biocatalysts for enzymatic polymerizations: Possibilities, advantages, applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef]
- Mespouille, L.; Coulembier, O.; Kawalec, M.; Dove, A.P.; Dubois, P. Implementation of metal-free ring-opening polymerization in the preparation of aliphatic polycarbonate materials. Prog. Polym. Sci. 2014, 39, 1144–1164. [Google Scholar] [CrossRef]
- Siódmiak, T.; Mangelings, D.; Heyden, Y.V.; Ziegler-Borowska, M.; Marszałł, M.P. High Enantioselective Novozym 435-Catalyzed Esterification of (R,S)-Flurbiprofen Monitored with a Chiral Stationary Phase. Appl. Biochem. Biotechnol. 2015, 175, 2769–2785. [Google Scholar] [CrossRef]
- Nan, A.; Bunge, A.; Cîrcu, M.; Petran, A.; Hădade, N.D.; Filip, X. Poly(benzofuran-co-arylacetic acid)—A new type of highly functionalized polymers. Polym. Chem. 2017, 8, 3504–3514. [Google Scholar] [CrossRef]
- Cîrcu, M.; Bunge, A.; Vasilescu, C.; Porav, S.; Nan, A. Non-catalytic, solvent-free synthesis of poly(tartronic-co-glycolic acid) as a versatile coating for different surfaces. Polym. Int. 2018, 67, 212–219. [Google Scholar] [CrossRef]
- Poojari, Y.; Clarso, S.J. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles inorganic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Al-Mesfer, H.; Tighe, B.J. Polymers for biodegradable medical devices: III. Polymerization and copolymerization of cyclic derivatives of tartronic acid. Biomaterials 1987, 8, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Poly (ketomalonate) by Catalytic Oxidation of Glycerol Anionic Polymerization. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 195–205. [Google Scholar] [CrossRef]
- Lee, W.A.; Knight, G.J. Ratio of the Glass Transition Temperature to the Melting point in Polymers. Br. Polym. J. 1970, 2, 73–80. [Google Scholar]
- Fujishiro, H.; Ikebe, M.; Kashima, T.; Yamanaka, A. Thermal conductivity and diffusivity of high-strength polymer fibers. Jpn. J. Appl. Phys. 1997, 36, 5633–5637. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Q.; Mayo, A.; Ni, Z.; Yi, H.; Chen, Y.; Mu, R.; Bellan, L.M.; Li, D. Thermal conductivity of electrospun polyethylene nanofibers. Nanoscale 2015, 7, 16899–16908. [Google Scholar] [CrossRef]
- Choy, C.L. Thermal conductivity of polymers. Polymer 1977, 18, 984–1004. [Google Scholar] [CrossRef]
- Mehra, N.; Mu, L.; Ji, T.; Yang, X.; Kong, J.; Gu, J.; Zhu, J. Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 2018, 12, 92–130. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, L.; Yang, X.; Ruan, K.; Han, Y.; Kong, J.; Gu, J. Simultaneous improvement of thermal conductivities and electromagnetic interference shielding performances in polystyrene composites via constructing interconnection oriented networks based on electrospinning technology. Compos. Appl. Sci. Manuf. Part A Appl. S. 2019, 124, 105484. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]

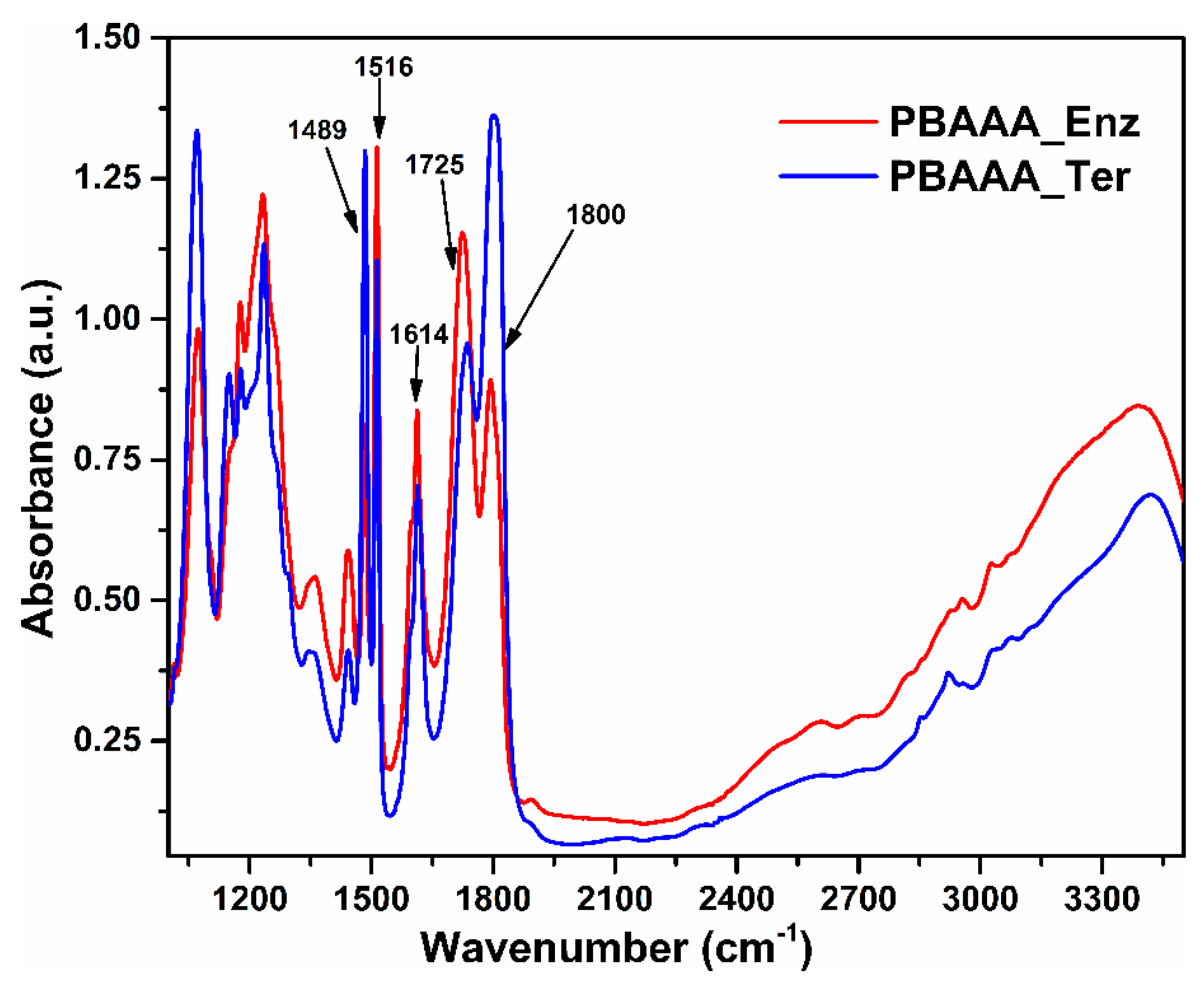

| Polymer | C=O Bond from the Carboxyl Group | C=O Bond from Lactone Ring (1800 cm−1) |
|---|---|---|
| PBAAA_Ter | 61% (1735 cm−1) | 57% |
| PBAAA_Enz | 58% (1725 cm−1) | 25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petran, A.; Radu, T.; Dan, M.; Nan, A. Exploiting Enzyme in the Polymer Synthesis for a Remarkable Increase in Thermal Conductivity. Int. J. Mol. Sci. 2023, 24, 7606. https://doi.org/10.3390/ijms24087606
Petran A, Radu T, Dan M, Nan A. Exploiting Enzyme in the Polymer Synthesis for a Remarkable Increase in Thermal Conductivity. International Journal of Molecular Sciences. 2023; 24(8):7606. https://doi.org/10.3390/ijms24087606
Chicago/Turabian StylePetran, Anca, Teodora Radu, Monica Dan, and Alexandrina Nan. 2023. "Exploiting Enzyme in the Polymer Synthesis for a Remarkable Increase in Thermal Conductivity" International Journal of Molecular Sciences 24, no. 8: 7606. https://doi.org/10.3390/ijms24087606
APA StylePetran, A., Radu, T., Dan, M., & Nan, A. (2023). Exploiting Enzyme in the Polymer Synthesis for a Remarkable Increase in Thermal Conductivity. International Journal of Molecular Sciences, 24(8), 7606. https://doi.org/10.3390/ijms24087606








