Colorectal Cancer Chemoprevention: A Dream Coming True?
Abstract
1. Introduction
2. Precancerous Colorectal Lesions
3. Target Populations for Chemoprevention
4. Chemopreventive Agents
4.1. Anti-Inflammatory Agents
4.1.1. Aspirin
Evaluation of Aspirin Treatment with ACF or Adenoma Lesions as Endpoints
Evaluation of Aspirin Treatment with CRC as an Endpoint
Biomarkers for Aspirin Efficacy as a CRC Chemopreventive Agent
4.1.2. Non-Aspirin NSAIDs
4.1.3. 5-Aminosalicylates
4.1.4. Ursodeoxycholic Acid
4.2. Metabolic Agents
4.2.1. Metformin
4.2.2. Statins
4.2.3. Long-Chain Omega-3 Polyunsaturated Fatty Acids
4.2.4. Folic Acid
4.3. Antioxidants
4.3.1. Selenium
4.3.2. Vitamins A, C, E and β-Carotene
4.3.3. Curcumin
4.4. Minerals and Vitamin D
4.4.1. Magnesium
4.4.2. Calcium
4.4.3. Vitamin D
4.5. Hormone Replacement Therapy
4.6. Dietary Products
4.7. Vaccine Strategy
4.8. Target Therapy
| Agent | Primary Target | Mechanism | Endpoint | Study or Trial (Years) | Participants (n) | Age of Participants | CRC Risk Level | Dose | Median Time of Follow-Up | Results | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-inflammatory agents | |||||||||||
| Aspirin | COX-1 and COX-2 (irreversible inhibition) | Inhibits prostaglandin synthesis and the β-catenin WNT pathway | ACF | In vivo studies | AOM-treated rats | N.A. | N.A. | 0.2–0.6% | N.A. | Reduced ACF number and size | [35,36] |
| Adenoma | CALGB (1993–2000) | Individuals with prior CRC (517) | 30–80 years | High | 325 mg daily | 13 months | Reduced adenoma risk | [38] | |||
| CAPP1 (1993–2005) | FAP patients (133) | 10–21 years | High | 600 mg twice daily | After 1 year and then annually | Reduced adenoma largest size | [41] | ||||
| AFPPS (1994–1998) | Individuals with prior adenomas (1121) | 21–80 years | Moderate | 81 mg or 325 mg daily | 3 years | Low dose but not high dose reduced the risk of adenoma recurrence | [37] | ||||
| ukCAP (1997–2005) | Individuals with prior adenomas (945) | Younger than 75 years | Moderate | 300 mg daily | 3 years | Reduced adenoma recurrence risk | [39] | ||||
| APACC (1997–2001) | Individuals with prior adenomas (272) | 18–75 years | Moderate | 160 mg or 300 mg daily | 1 and 4 years | Reduced adenoma risk after 1 year, but not after 4 years | [40,42] | ||||
| seAFOod (2010–2017) | Individuals with prior adenomas (709) | 55–73 years | Moderate | 300 mg daily | 1 year | Reduced number of conventional and serrated adenomas in the right colon at secondary analysis | [43] | ||||
| Cross-sectional studies (2011–2014) | General population divided into smokers and non-smokers and people with CRC family history (2918) | 45–65 years | Average and moderate | 81 mg | 30 months | Reduced adenoma risk only in non-smoker users | [45] | ||||
| CRC | NHS (1980–2000) | General population (82,911 female) | 30–55 years | Average | 325 mg 2 times per week | Every 2 years | Reduced CRC risk | [46] | |||
| PHS (1982–1995) | General population (22,071 male) | 40–84 years | Average | 325 mg on alternate days | 5–12 years | No reduced CRC incidence | [47] | ||||
| CPS II (1982–1988) | General population (662,424) | 57 years (mean) | Average | 100 mg on alternate days | 10–18 years | Reduced CRC risk | [48] | ||||
| HPFS (1986–1990) | General population (47,900 male) | 40–75 years | Average | 100 mg 2 times per week | 2–4 years | Reduced CRC risk and metastatic CRC | [49,50] | ||||
| WHS (1993–2004) | General population (39,876 female) | 45 years or older | Average | 100 mg on alternate days | 1-10-18 years | Reduced CRC incidence only after 10 or 18 years of follow-up | [51,52] | ||||
| CCFR (1997–2012) | Lynch syndrome patients (1858) | 43 years (mean) | High | Twice a week | Not reported | Reduced CRC risk | [53] | ||||
| CAPP2 (2001–2008) | Lynch syndrome patients (861) | 45 years (mean) | High | 600 mg daily | 2–10 years | No reduced CRC risk after two years of follow-up, a strong reduction in CRC risk at 10 years | [54,55] | ||||
| USPSTF (2004–2015) | General population and individuals with prior adenomas | 40–79 years | Average and moderate | 75 mg daily or on alternate days | 10–20 years | Reduced CRC risk and mortality | [56,57] | ||||
| J-CAPP (2007–2012) | Individuals with prior adenomas (311) | 40–70 years | Moderate | 100 mg daily | 2 years | Reduced CRC risk in the non-smoker population | [58] | ||||
| ASCOLT (2008-ongoing) | Dukes’ B and C CRC (1587) | 18 years and older | High | 200 mg daily | Every 3 months for 3 years + every 6 months for another 2 years | Final results pending | [59] | ||||
| Pooled analysis derived from 4 RCTs and 1 study of different doses of aspirin (2010) | General population (13,500) | 45–69 years | Average | 75 mg or 300 mg daily | 20 years | Reduced CRC risk and mortality | [60] | ||||
| ASPIRED (2010-ongoing) | Individuals with prior adenomas (180) | 50–69 years | Moderate | 81 mg or 325 mg daily | Every 6 months | Final results pending | [61] | ||||
| CAPP3 (2014–2019) | Lynch syndrome patients (1500) | Not reported | High | 100 mg or 300 mg or 600 mg daily | 5 years | Final results pending | [62] | ||||
| J-FAPP (2015–2017) | FAP patients (311) | 40–70 years | High | 100 mg and/or mesalazine daily | 8 months | Reduced adenoma and CRC risk | [58] | ||||
| NA-NSAIDs | COX-1 and COX-2 (reversible inhibition) | Inhibit prostaglandin synthesis and WNT signaling pathway | ACF | Sulindac | General population and individuals with a CRC family history (304) | 55–75 years | Average and moderate | 150 mg | 2 months and 1 year | Reduced ACF number | [88] |
| Adenoma | Sulindac | FAP patients (46) | 14–46 years | High | 300 mg daily | 1 year | Reduced adenoma risk | [89,90,91] | |||
| Double-blind, placebo-controlled study (celecoxib) (1996–1998) | FAP patients (77) | 18–65 years | High | 100 mg or 400 mg twice daily | 6 months | Reduced adenoma risk | [93] | ||||
| Double-blind, placebo-controlled study (rofecoxib) | FAP patients (21) | Not reported | High | 25 mg | 3-6-9 months | Reduced adenoma risk | [94] | ||||
| Nested case-control study (rofecoxib and celecoxib) | General population (3477) | 65 years or older | Average | Not reported | 3 months | Reduced adenoma risk | [95] | ||||
| APC trial (1999–2002) | Individuals with prior adenomas (2035) | 31–88 years | Moderate | Celecoxib 200 mg or 400 mg twice daily | 3–5 years | Reduced adenoma risk | [96,97] | ||||
| Pre-Sap (2001–2005) | Individuals with prior adenomas (1561) | 30 years or older | Moderate | Celecoxib 400 mg daily | 1–3 years | Reduced adenoma risk | [99] | ||||
| APPROVe (2001–2004) | Individuals with prior adenomas (2586) | 40–96 years | Moderate | Rofecoxib 25 mg daily | 1–3 years | Reduced adenoma risk | [101] | ||||
| 5-ASAs | Derivatives of aspirin | Inhibit prostaglandin synthesis | Adenoma and CRC | Observational studies (1972–2002) | Ulcerative colitis patients | Not reported | High | Mesalamine >1.2 g/day Sulfasalazine >2.4 g/day | 10-20-30 years | Reduced adenoma and CRC risk | [106,107,108] |
| UDCA | Secondary bile acids | Disruption of the balance between colorectal crypt cell proliferation, differentiation, and apoptosis | ACF | In vivo studies | AOM-treated Fisher male rats (344) | N.A. | N.A. | UDCA 0.2% or 0.4% for 2 weeks | 28 weeks | Reduced ACF number | [109,110] |
| Adenoma and CRC | Phase III clinical trial | Individuals with prior adenomas and ulcerative colitis patients (1285) | 40–80 years | Moderate and high | 300 mg | 3 years | Reduced adenoma and CRC risk | [111] | |||
| Cross-sectional study | Ulcerative colitis and primary sclerosing cholangitis patients (59) | Not reported | High | 9.9 mg/kg daily | 3 years | Reduced adenoma and CRC risk | [112,113] | ||||
| Metabolic agents | |||||||||||
| Metformin | Inhibits mitochondrial complex I to prevent the production of mitochondrial ATP | Activates AMPK, reduces cyclin D1 expression and RB phosphorylation | ACF and adenoma | In vivo studies | AOM-BALB/c mice | N.A. | N.A. | 250 mg/kg daily | 6 weeks for ACF and 32 weeks for adenomas | Reduced ACF and adenoma risk | [120] |
| Adenoma | In vivo studies | APCMin/+ mice | N.A. | N.A. | 250 mg/kg | N.A. | Reduced number of intestinal polyps larger than 2 mm | [121] | |||
| ACF, adenoma | Short-term randomized study | Non-diabetic patients (26) | 65–75 years | Average | 250 mg daily | 1 month | Reduced ACF and adenoma risk | [122] | |||
| ACF | RCT | Non-diabetic patients (60) | Not reported | Average | 250 mg and/or aspirin 100 mg daily | 8 weeks | Final results pending | [123] | |||
| Adenoma | Multicenter double-blind, placebo-controlled, randomized phase 3 trial (2011–2014) | Non-diabetic patients (498) | 20 years or older | Average | 250 mg daily | 1 year | Reduced prevalence and number of metachronous adenomas or polyps after polypectomy | [124] | |||
| Adenoma and CRC | Case-control studies and RCT (2008–2016) | Non-diabetic and diabetic patients, individuals with prior adenomas and CRC (8726) | 40–89 years | Average, moderate, and high | ≥250 mg daily | 4–15 years | Reduced adenoma and CRC risk | [125] | |||
| Epidemiology studies | Non-diabetic and diabetic patients | 20–80 years | Average and high | 250 mg or 500 mg daily | 1–3 years | Conflicting results | [126,127,128,129,130,131,132,133,134,135,136,137] | ||||
| CRC | Retrospective cohort study | Diabetic patients (60,520) | 40 years or older | High | 750–4000 mg daily | 5 years | Reduced CRC risk | [138] | |||
| Statins | HMG-CoA reductase (reversible inhibition) | Disruption of the mevalonate pathway | Adenoma and CRC | In vivo studies | APCMin/+ mice | 6-week-old | N.A. | Pitavastatin at doses of 20 and 40 ppm | 14 weeks | Reduced adenomas in a dose-dependent way | [142] |
| In vivo studies | AOM-treated F344 rats | 5-week-old | N.A. | Atorvastatin 100–200 ppm and/or sulindac 100 ppm or naproxen 150 ppm | 45 weeks | Reduced CRC risk | [143] | ||||
| In vivo studies | APCMin/+ mice | 6-week-old | N.A. | Atorvastatin 100 ppm and/or celecoxib 300 ppm | 80 days | Reduced adenoma and CRC risk | [144] | ||||
| Adenoma | Review of endoscopy and pathology databases | Individuals with prior adenomas (2626) | 63 years (mean) | Moderate | Not reported | 3–5 years | Reduced adenoma risk | [145] | |||
| Secondary analysis of data from three large colorectal adenoma chemoprevention trials | General population (2915) | Not reported | Average | Not reported | Not reported | No reduced adenoma risk | [146] | ||||
| CRC | Molecular Epidemiology of Colorectal Cancer Study (1998–2004) | Individuals with prior CRC (3968) | 58–80 years | High | Not reported | 5 years | Reduced CRC risk | [147] | |||
| Double-blind trial | Patients with myocardial infarction who had plasma total cholesterol levels below 240 mg/dL and low-density lipoprotein (LDL) cholesterol levels of 115 to 174 mg/dL (4159) | 50–70 years | Moderate | Pravastatin 40 mg daily | 5 years | Reduced CRC risk | [148] | ||||
| Survival study | Patients with angina pectoris or previous myocardial infarction and serum cholesterol levels of 5.5 to 8.0 mmol/L (4444) | 35–70 years | Moderate | Simvastatin 20–40 mg daily | 5 years | Reduced CRC risk | [149] | ||||
| Systematic review and meta-analysis | General population | 40–80 years | Average | Not reported | 3–6 years | Conflicting results | [150] | ||||
| Long-Chain Omega-3 PUFAs | Components of phospholipids that form cell membranes | Anti-proliferative, apoptotic, and anti-angiogenic properties | ACF | In vivo studies | Wistar rats | N.A. | N.A. | EPA 18.7%; DHA 8% | 48 h | Reduced ACF number | [158] |
| ACF, adenoma, and CRC | In vivo studies | APCMin/+ mice, AOM-treated mice, xenograft mice | N.A. | N.A. | EPA 4–16%; DHA 0.75–6% | 1 day-32 weeks | Reduced ACF number, adenoma, and CRC risk | [159] | |||
| Adenoma | Prospective study (2006–2007) | FAP patients (55) | 18 years or older | High | EPA 500 mg twice daily | 6 months | Reduced adenoma risk | [161] | |||
| seAFOod (2010–2017) | Individuals with prior adenomas (709) | 55–73 years | High | EPA 2 g daily | 1 year | Reduced number of conventional and left-sided adenomas at secondary analysis | [43] | ||||
| CRC | Prospective study (2000–2008) | General population (68,109) | 50–76 years | Average | Fish oil more than 4 days per week | 3 years | Reduced CRC risk | [162] | |||
| RCTs (2001–2011) | General population, FAP patients | 40–75 years | Average and high | EPA 0.09 vs. 0.03 g daily DHA 0.18 vs. 0.08 g daily | 3–22 years | Conflicting results | [163,164,165] | ||||
| Folic acid | Coenzyme in single transfers in the synthesis of nucleic acid and amino acid metabolism | Maintaining normal DNA methylation required for synthesis and repair | ACF and CRC | In vivo studies | AOM-treated rats (159) | 6-week old | N.A. | 0, 2, 5, or 8 mg/kg | 34 weeks | Conflicting results | [166,171] |
| Adenoma and CRC | Epidemiology studies | General population | Not reported | Average | 100 μg or 600 μg daily | Not reported | Reduced CRC risk | [172,173,174] | |||
| Adenoma | RCT | General population, individuals with prior adenoma | 65 years (mean) | Average and high | 0.5 to 2.5 mg daily | 36–88 months | No reduced adenoma risk | [175] | |||
| CRC | NHS (1980–1994) | General population (88,756 female) | 30–55 years | Average | 200 μg or 400 μg daily | Every 2 years | Reduced risk of CRC | [176] | |||
| Canadian National Breast Screening Study | General population (5681) | Not reported | Average | 200 μg or 400 μg daily | 10 years | Reduced risk of CRC | [177] | ||||
| Case-control studies | Ulcerative colitis patients | Not reported | High | 0.4–1.0 mg daily | Not reported | Reduced risk of CRC | [178,179] | ||||
| Antioxidant agents | |||||||||||
| Selenium | Trace minerals required to make selenium-containing proteins | Antioxidant properties | Adenoma and CRC | RCT | General population | 62 years (mean) | Average | 200 μg daily | 6–12 years | Conflicting results | [187,188,189] |
| Vitamin A | Combines with retinol-binding protein | Regulates nuclear receptors that are involved in tumor formation | CRC | Observational studies | General population | 34–80 years | Average | 1 μg daily | 8–10 years | Conflicting results | [192,193] |
| Vitamin C | Cofactor in collagen formation and tissue repair | Reduces oxidative stress | CRC | RCT | General population | 40–80 years | Average | 75 mg or 250 mg or 500 mg daily | 5–9 years | No reduced CRC risk | [188,192,193] |
| Vitamin E | Primarily ends up in cell and organelle membranes | Inhibits lipid peroxidation in membranes | CRC | RCT | General population | Not reported | Average | 30 mg or 50 mg or 600 mg daily | 6–12 years | No reduced CRC risk | [187,188,189,192,193,194,195] |
| β-carotene | Functions as a provitamin A | Antioxidant properties | CRC | RCT | General population | 55 years (mean) | Average | 20 mg or 30 mg daily | 2–12 years | No reduced CRC risk | [187,188,189,196,197] |
| Curcumin | Inhibits reactive oxygen-generating enzymes | Antioxidant properties | Adenoma | Prospective study | FAP patients (5) | Not reported | High | Curcumin 480 mg and quercetin 20 mg orally 3 times a day | Every 3 months | Reduced adenoma risk | [199] |
| RCT (2011-2016) | FAP patients (44) | 18–85 years | High | 3000 mg daily | 1 year | No reduced adenoma risk | [200] | ||||
| Minerals and vitamin D | |||||||||||
| Magnesium | Involved in metabolism, insulin resistance, and inflammation | Important for DNA synthesis and repair | ACF and CRC | In vivo studies | Methylazoxymethanol acetate-treated male F344 rats | N.A. | N.A. | 250 ppm or 500 ppm 1000 ppm | 4-6-8 weeks | Reduced ACF and CRC risk | [202,203] |
| CRC | In vivo studies | Methylazoxymethanol acetate-treated male F344 rats | N.A. | N.A. | 500 ppm or 1000 ppm | 227 days | Reduced CRC risk | [203] | |||
| CRC | Prospective studies (2005–2012) | General population (338,979) | 40–75 years | Average | 50 mg daily | 8–28 years | Reduced CRC risk | [204] | |||
| Adenoma | Case-control studies | General population, individuals with a CRC family history | 18–75 years | Average and moderate | 100 mg daily | Not reported | Reduced adenoma risk | [205] | |||
| Adenoma and CRC | Epidemiologic and prospective studies | General population (1,236,004) | Not reported | Average | 300–400 mg daily | Not reported | Reduced adenoma and CRC risk | [206] | |||
| Calcium | Incorporated into the skeleton | Bile acid-binding capacity | CRC | In vivo studies | 1,2-Dimethylhydrazine (DMH)-treated Slac mice (80) | N.A. | N.A. | 1.24–3.0% | 24 weeks | Reduced CRC risk | [210] |
| Adenoma | Calcium Polyp Prevention Study Group RCT | Individuals with prior adenomas (930) | 61 years (mean) | Moderate | 3 g daily | 1-4-9 years | Reduced advanced adenoma recurrence risk | [211,212,213] | |||
| The European Cancer Prevention Intervention Study | Individuals with prior adenomas (665) | 35–75 years | Moderate | 2 g daily | 3 years | No significant effect on adenoma risk | [214] | ||||
| Systematic review and meta-analysis of RCTs (2010) | General population, individuals with prior adenomas, FAP patients | 16–80 years | Average, moderate, and high | 500 mg−2 g−3 g daily | 6 months–7 years | No positive results for average- and high-risk populations, reduced adenoma risk in individuals with a history of adenomas | [215] | ||||
| CRC | Cancer Prevention Study II Nutrition Cohort (1992-1993) | General population (1,277,499) | 50–74 years | Average | 500 mg daily | 5 years | Reduced CRC risk | [216] | |||
| Prospective study (2000) | General population (61,463) | 53 years (mean) | Average | 900 mg daily | 12 years | Reduced CRC risk | [217] | ||||
| NHS and HPFS | General population (135,342) | 30–75 years | Average | 500–1250 mg daily | 10–16 years | Reduced distal colon cancer risk | [218] | ||||
| Prospective study | General population (34,702) | Not reported | Average | Not reported | 9 years | Reduced rectal cancer risk | [219] | ||||
| WHS | General population (36,282 female) | 50–79 years | Average | Calcium carbonate 500 mg and vitamin D 200 IU twice daily | 7 years | No reduced CRC risk | [220] | ||||
| Vitamin D | Regulates gene transcription by binding vitamin D receptors | Inhibits proliferation and angiogenesis | Adenoma, CRC, and rectal cancer | RCT | General population, individuals with prior adenomas | 50–79 years | Average and moderate | 400 IU daily | 7 years | Conflicting results | [220,225,226] |
| CRC | RCT | General population (25,871) | 50 years or older | Average | Vitamin D 2000 IU and omega-3 fatty acids 1 g daily | 5 years | No reduced CRC risk | [227,228] | |||
| Hormone replacement therapy | |||||||||||
| Hormones | Increase the production of insulin-like growth factor-I or secondary bile acids | Inhibit proliferation and promote cell cycle arrest and apoptosis | Adenoma | Prospective studies | Individuals with prior adenomas (411) | 30–74 years | Moderate | Not reported | Not reported | Reduced adenoma risk | [232,233,234,235] |
| CRC | The Molecular Epidemiology of Colorectal Cancer Study (1998–2006) | Individuals with prior CRC (1234) | 60 years or older | High | Not reported | 5 years | Reduced CRC risk | [236,237,238,239] | |||
| Women’s Health Initiative (WHI) RCT | General population (postmenopausal status) (10,739) | 50–79 years | Average | Conjugated equine estrogen 0.625 mg plus medroxyprogesterone acetate 2.5 mg daily | 7 years | No reduced CRC risk | [240,241,242] | ||||
| Dietary products | |||||||||||
| Fibers | Involved in the metabolism and catabolism of bioactive food components | Decrease the exposure of colonic cells to carcinogens | CRC | RCT | General population | 25–76 years | Average | 90 g daily increments | 6–16 years | Reduced CRC risk | [246,247,248,249,250] |
| Fruits and vegetables | Involved in the metabolism and catabolism of bioactive food components | Decrease the exposure of colonic cells to carcinogens | CRC | RCT | General population | 34–82 years | Average | 100 g daily increments | Not reported | Reduced CRC risk | [163,246,248,251,252,253,254,255,256] |
| Vaccines | |||||||||||
| FSP-based vaccines | TAF1B(−1), HT001(−1), and AIM2(−1) | Development of humoral and T-cell responses against FSPs | CRC | Phase I/IIa clinical trial (2011–2015) | Lynch syndrome (22) | 55 years (mean) | High | 3 cycles of subcutaneous vaccinations mixed with Montanide ISA-51 VG | 6 months | Enhanced immune response against FSP peptides | [259] |
| Nous 209 viral-vectored vaccine | 209 FSPs | Neoantigen-based vaccine for the treatment of MSI tumors | Immunogenic response | In vivo studies | CB6F1 mice | 6-week-old | N.A. | GAd-209-FSP and MVA-209-FSP were administered i.m. at the dosage of 4 × 108 vp and 4 × 107 ifu, respectively | 3 weeks | CD8 and CD4 T-cell responses | [260] |
| CRC | Phase I/II clinical trial (2019–2025) | Individuals with prior CRC (34) | 18 years or older | High | GAd-209-FSP low dose; MVA-209-FSP low dose; GAd-209-FSP high dose; MVA-209-FSP high dose; GAd20-209-FSP; RP2D; MVA-209-FSP, RP2D | Up to 110 weeks | Final results pending | [261] | |||
| Phase Ib/II clinical trial (2021–2025) | Lynch syndrome patients (45) | 18 years or older | High | GAd-209-FSP and MVA-209-FSP | Every 12 months | Final results pending | [262] | ||||
| Synthetic peptide | ERBB3 | Development of humoral and cellular immunity against FSPs | Adenoma | In vivo studies | APCMin/+ mice | 3-week-old | N.A. | 100 mg of EBX peptide, EB3IV, or KLH in 100 mL of a 50/50 mixture of antigen and CFA | 3 months | Reduced recurrent adenomas | [263] |
| TAA vaccine | MUC-1-derived peptides | Anti-MUC-1 IgG response | Adenoma | Phase II clinical trial—RCT (2008–2013) | Individuals with prior adenomas | 40–70 years | Moderate | 100 µg MUC1 + Hiltonol®at week 0, 2, 10, and 52 | 54 weeks | Reduced recurrent adenomas | [264,265] |
| Target therapy | |||||||||||
| DFMO | Ornithine decarboxylase (irreversible inhibition) | Inhibits polyamine synthesis | Adenoma | RCT | Individuals with prior adenomas (375) | 40–80 years | Average and high | DFMO 500 mg daily and/or sulindac 150 mg | 36 months | Reduced recurrent adenomas | [268] |
| RCT | FAP patients (171) | 18 years or older | High | DFMO 750 mg and/or sulindac150 mg | 48 months | Conflicting results | [269] | ||||
| RCT | FAP patients (112) | 38 years (mean) | High | DFMO 250 mg and/or celecoxib 400 mg | 6 months | Modest reduction in adenoma risk | [270] | ||||
| Erlotinib | EGFR tyrosine kinase inhibitor (reversible inhibition) | Inhibits EGFR signaling | Adenoma | RCT (2010–2014) | FAP patients (92) | 41 years (mean) | High | Erlotinib 75 mg daily and/or sulindac 150 mg twice daily | 6 months | Reduced recurrent adenomas | [277,278] |
| Guselkumab | Monoclonal antibody against IL-23 subunit alpha | Inhibits IL-23 signaling | Adenoma | RCT | FAP patients | Not reported | High | Not reported | Not reported | Final results pending | [280] |
| Biomarker | Type | Chemopreventive Agent | Study (Number of Participants) | Endpoint | Description | Outcome | Ref |
|---|---|---|---|---|---|---|---|
| rs2070959-G | Genetic variant | Aspirin | NHS (1062) | CRC | G genotype SNP | Protective against CRC | [69,70] |
| rs4365457-C | Genetic variant | Aspirin | NHS (1062) | CRC | C genotype SNP | Protective against CRC | [69,70] |
| rs2430420-GG | Genetic variant | Low-dose aspirin | AFFPS nested cohorts within an RCT (370) | Adenoma | GG genotype SNP | Protective against CRC | [71] |
| rs28362380-TT | Genetic variant | Low-dose aspirin | AFFPS nested cohorts within an RCT (370) | Adenoma | TT genotype SNP | Protective against CRC | [71] |
| PGE-M | Urine levels | Aspirin | NHS and AFPPS (748) | Adenoma | High levels | Protective against CRC | [72] |
| rs2920421-GA | Genetic variation | Aspirin | CCFR (1621) | CRC | GA genotype SNP | Protective against CRC | [73] |
| sTNFR-2 | Plasma levels | Aspirin | NHS (280) | CRC | High levels | Protective against CRC | [74] |
| MIC1 | Plasma levels | Aspirin | NHS and HPFS (618) | CRC | High levels | Promotes COX-2-positive CRC | [75] |
| COX-2 | Overexpression in tumor | Aspirin | NHS and HPFS (632) | CRC | High levels | Protective against CRC | [76,77] |
| PIK3CA | Mutation in tumor | Aspirin | NHS and HPFS (2190) | CRC | PIK3CA exons 9 and 20 mutated in tumor | Protective against CRC | [78] |
| BRAF | Mutation in tumor | Aspirin | NHS and HPFS (1226) | CRC | BRAF V600E mutated in tumor | Promotes CRC | [79] |
| rs6983267-T | Genetic variant | Aspirin | NHS and HPFS (840) | CRC | T genotype SNP | Protective against CRC | [83,84] |
| 15-PGDH | Colon mucosa levels | Aspirin | NHS and HPFS (270) | CRC | High levels | Protective against CRC | [85] |
| rs2965667-TT | Genetic variant | Aspirin | GWAS (8,634) | CRC | TT genotype SNP | Protective against CRC | [86] |
| rs16973225-AA | Genetic variant | Aspirin | GWAS (8,634) | CRC | AA genotype SNP | Protective against CRC | [86] |
| rs1057910-C | Genetic variant | Celecoxib | APC (2,035) | Adenoma | C genotype SNP | Protective against CRC | [98] |
| rs12654264-AA | Genetic variant | Statins | MECC (4,187) | CRC | AA genotype SNP | Protective against CRC | [154] |
| MTHFR 677TT | Genetic variant | Folic acid | GSEC (30,650) | CRC | TT genotype SNP | Protective against CRC | [181,182,183] |
| MSI | Microsatellite instability | Hormone replacement therapy (estrogen and progestin) | Case-control studies | CRC | MSI-low or stable tumors | Protective against CRC | [243] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Schilsky, R.L.; Alberts, D.S.; Day, R.W.; Guyton, K.Z.; Pearce, H.L.; Peck, J.C.; Phillips, R.; Sigman, C.C. Colorectal Adenomas: A Prototype for the Use of Surrogate End Points in the Development of Cancer Prevention Drugs. Clin. Cancer Res. 2004, 10, 3908–3918. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A Genetic Model for Colorectal Tumorigenesis. Cell. 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Chapelle, N.; Martel, M.; Toes-Zoutendijk, E.; Barkun, A.N.; Bardou, M. Recent Advances in Clinical Practice: Colorectal Cancer Chemoprevention in the Average-Risk Population. Gut 2020, 69, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Rex, D.K. Advances in CRC Prevention: Screening and Surveillance. Gastroenterology 2018, 154, 1970–1984. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B. Approaches to Prevention of Epithelial Cancer during the Preneoplastic Period. Cancer Res. 1976, 36, 2699–2702. [Google Scholar] [PubMed]
- Umezawa, S.; Higurashi, T.; Komiya, Y.; Arimoto, J.; Horita, N.; Kaneko, T.; Iwasaki, M.; Nakagama, H.; Nakajima, A. Chemoprevention of Colorectal Cancer: Past, Present, and Future. Cancer Sci. 2019, 110, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Weiss, J.M. Chemoprevention of Colorectal Cancer. Gastroenterology 2020, 158, 368–388. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.A.; Kelloff, G.J.; Gordon, G.B.; Dannenberg, A.J.; Hong, W.K.; Fabian, C.J.; Sigman, C.C.; Bertagnolli, M.M.; Stratton, S.P.; Lam, S.; et al. Treatment and Prevention of Intraepithelial Neoplasia: An Important Target for Accelerated New Agent Development. Clin. Cancer Res. 2002, 8, 314–346. [Google Scholar]
- Orlando, F.A.; Tan, D.; Baltodano, J.D.; Khoury, T.; Gibbs, J.F.; Hassid, V.J.; Ahmed, B.H.; Alrawi, S.J. Aberrant Crypt Foci as Precursors in Colorectal Cancer Progression. J. Surg. Oncol. 2008, 98, 207–213. [Google Scholar] [CrossRef]
- Siskova, A.; Cervena, K.; Kral, J.; Hucl, T.; Vodicka, P.; Vymetalkova, V. Colorectal Adenomas—Genetics and Searching for New Molecular Screening Biomarkers. Int. J. Mol. Sci. 2020, 21, 3260. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Sansonno, D.; Russi, S.; Dammacco, F. Precancerous Colorectal Lesions (Review). Int. J. Oncol. 2013, 43, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and Number of Cancer Cases and Deaths Attributable to Potentially Modifiable Risk Factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Lowery, J.T.; Ahnen, D.J.; Schroy, P.C.; Hampel, H.; Baxter, N.; Boland, C.R.; Burt, R.W.; Butterly, L.; Doerr, M.; Doroshenk, M.; et al. Understanding the Contribution of Family History to Colorectal Cancer Risk and Its Clinical Implications: A State-of-the-Science Review. Cancer 2016, 122, 2633–2645. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Mangu, P.B.; Gruber, S.B.; Hamilton, S.R.; Kalady, M.F.; Lau, M.W.Y.; Lu, K.H.; Roach, N.; Limburg, P.J.; American Society of Clinical Oncology; et al. Hereditary Colorectal Cancer Syndromes: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the Familial Risk-Colorectal Cancer: European Society for Medical Oncology Clinical Practice Guidelines. J. Clin. Oncol. 2015, 33, 209–217. [Google Scholar] [CrossRef]
- Duraturo, F.; Liccardo, R.; De Rosa, M.; Izzo, P. Genetics, Diagnosis and Treatment of Lynch Syndrome: Old Lessons and Current Challenges. Oncol. Lett. 2019, 17, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals with Colorectal Cancer. J. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef]
- Medina Pabón, M.A.; Babiker, H.M. A Review of Hereditary Colorectal Cancers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Disciglio, V.; Fasano, C.; Cariola, F.; Forte, G.; Grossi, V.; Sanese, P.; Lepore Signorile, M.; Resta, N.; Lotesoriere, C.; Stella, A.; et al. Gastric Polyposis and Desmoid Tumours as a New Familial Adenomatous Polyposis Clinical Variant Associated with APC Mutation at the Extreme 3′-End. J. Med. Genet. 2020, 57, 356–360. [Google Scholar] [CrossRef]
- Goral, D.; Highland, J.; Lovell, M.A.; Chan, K.H. Head and Neck Presentation of Gardner Syndrome: A Pediatric Case Series. Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Gay, J.T.; Troxell, T.; Gross, G.P. Muir-Torre Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Khattab, A.; Monga, D.K. Turcot Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Larsen Haidle, J.; MacFarland, S.P.; Howe, J.R. Juvenile Polyposis Syndrome. In GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Wu, M.; Krishnamurthy, K. Peutz-Jeghers Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sloot, Y.J.E.; Rabold, K.; Netea, M.G.; Smit, J.W.A.; Hoogerbrugge, N.; Netea-Maier, R.T. Effect of PTEN Inactivating Germline Mutations on Innate Immune Cell Function and Thyroid Cancer-Induced Macrophages in Patients with PTEN Hamartoma Tumor Syndrome. Oncogene 2019, 38, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Umeno, J.; Esaki, M. Gastrointestinal: Multiple Venous Malformations and Polyps of the Small Intestine in Cowden Syndrome. J. Gastroenterol. Hepatol. 2018, 33, 1819. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Pallone, F.; Monteleone, G.; Fantini, M.C. Intestinal Inflammation and Colorectal Cancer: A Double-Edged Sword? World J. Gastroenterol. 2011, 17, 3092–3100. [Google Scholar] [CrossRef]
- Fuster, V.; Sweeny, J.M. Aspirin: A Historical and Contemporary Therapeutic Overview. Circulation 2011, 123, 768–778. [Google Scholar] [CrossRef]
- Drew, D.A.; Cao, Y.; Chan, A.T. Aspirin and Colorectal Cancer: The Promise of Precision Chemoprevention. Nat. Rev. Cancer 2016, 16, 173–186. [Google Scholar] [CrossRef]
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; DuBois, R.N. Up-Regulation of Cyclooxygenase 2 Gene Expression in Human Colorectal Adenomas and Adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar] [CrossRef]
- Fujita, T.; Matsui, M.; Takaku, K.; Uetake, H.; Ichikawa, W.; Taketo, M.M.; Sugihara, K. Size- and Invasion-Dependent Increase in Cyclooxygenase 2 Levels in Human Colorectal Carcinomas. Cancer Res. 1998, 58, 4823–4826. [Google Scholar]
- Cebola, I.; Custodio, J.; Muñoz, M.; Díez-Villanueva, A.; Paré, L.; Prieto, P.; Aussó, S.; Coll-Mulet, L.; Boscá, L.; Moreno, V.; et al. Epigenetics Override Pro-Inflammatory PTGS Transcriptomic Signature towards Selective Hyperactivation of PGE2 in Colorectal Cancer. Clin. Epigenetics 2015, 7, 74. [Google Scholar] [CrossRef]
- Katsuki, S.; Oui, M.; Takayama, T.; Takahashi, Y.; Shuichi, N.; Niitsu, Y. [Aberrant crypt foci as biomarkers in chemoprevention for colorectal cancer]. Nihon. Geka. Gakkai. Zasshi. 1998, 99, 379–384. [Google Scholar]
- Shpitz, B.; Bomstein, Y.; Kariv, N.; Shalev, M.; Buklan, G.; Bernheim, J. Chemopreventive Effect of Aspirin on Growth of Aberrant Crypt Foci in Rats. Int. J. Colorectal Dis. 1998, 13, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Cole, B.F.; Sandler, R.S.; Haile, R.W.; Ahnen, D.; Bresalier, R.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.; Burke, C.A.; et al. A Randomized Trial of Aspirin to Prevent Colorectal Adenomas. N. Engl. J. Med. 2003, 348, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R.S.; Halabi, S.; Baron, J.A.; Budinger, S.; Paskett, E.; Keresztes, R.; Petrelli, N.; Pipas, J.M.; Karp, D.D.; Loprinzi, C.L.; et al. A Randomized Trial of Aspirin to Prevent Colorectal Adenomas in Patients with Previous Colorectal Cancer. N. Engl. J. Med. 2003, 348, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.F.A.; Grainge, M.J.; Shepherd, V.C.; Armitage, N.C.; Muir, K.R. ukCAP Trial Group Aspirin and Folic Acid for the Prevention of Recurrent Colorectal Adenomas. Gastroenterology 2008, 134, 29–38. [Google Scholar] [CrossRef]
- Benamouzig, R.; Deyra, J.; Martin, A.; Girard, B.; Jullian, E.; Piednoir, B.; Couturier, D.; Coste, T.; Little, J.; Chaussade, S. Daily Soluble Aspirin and Prevention of Colorectal Adenoma Recurrence: One-Year Results of the APACC Trial. Gastroenterology 2003, 125, 328–336. [Google Scholar] [CrossRef]
- Burn, J.; Bishop, D.T.; Chapman, P.D.; Elliott, F.; Bertario, L.; Dunlop, M.G.; Eccles, D.; Ellis, A.; Evans, D.G.; Fodde, R.; et al. A Randomized Placebo-Controlled Prevention Trial of Aspirin and/or Resistant Starch in Young People with Familial Adenomatous Polyposis. Cancer Prev. Res. Phila 2011, 4, 655–665. [Google Scholar] [CrossRef]
- Benamouzig, R.; Uzzan, B.; Deyra, J.; Martin, A.; Girard, B.; Little, J.; Chaussade, S.; Association pour la Prévention par l’Aspirine du Cancer Colorectal Study Group (APACC). Prevention by Daily Soluble Aspirin of Colorectal Adenoma Recurrence: 4-Year Results of the APACC Randomised Trial. Gut 2012, 61, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Sprange, K.; Hepburn, T.; Tan, W.; Shafayat, A.; Rees, C.J.; Clifford, G.; Logan, R.F.; Loadman, P.M.; Williams, E.A.; et al. Eicosapentaenoic Acid and Aspirin, Alone and in Combination, for the Prevention of Colorectal Adenomas (SeAFOod Polyp Prevention Trial): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, 2 × 2 Factorial Trial. Lancet 2018, 392, 2583–2594. [Google Scholar] [CrossRef]
- Parker, R.A.; Weir, C.J. Multiple Secondary Outcome Analyses: Precise Interpretation Is Important. Trials 2022, 23, 27. [Google Scholar] [CrossRef]
- Drew, D.A.; Goh, G.; Mo, A.; Grady, J.J.; Forouhar, F.; Egan, G.; Swede, H.; Rosenberg, D.W.; Stevens, R.G.; Devers, T.J. Colorectal Polyp Prevention by Daily Aspirin Use Is Abrogated among Active Smokers. Cancer Causes Control 2016, 27, 93–103. [Google Scholar] [CrossRef]
- At, C.; El, G.; Ja, M.; Es, S.; Gc, C.; Cs, F. Long-Term Use of Aspirin and Nonsteroidal Anti-Inflammatory Drugs and Risk of Colorectal Cancer. JAMA 2005, 294, 914–923. [Google Scholar] [CrossRef]
- Stürmer, T.; Glynn, R.J.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. Aspirin Use and Colorectal Cancer: Post-Trial Follow-up Data from the Physicians’ Health Study. Ann. Intern. Med. 1998, 128, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Namboodiri, M.M.; Heath, C.W. Aspirin Use and Reduced Risk of Fatal Colon Cancer. N. Engl. J. Med. 1991, 325, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Willett, W.C. Aspirin Use and the Risk for Colorectal Cancer and Adenoma in Male Health Professionals. Ann. Intern. Med. 1994, 121, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Giovannucci, E.L.; Meyerhardt, J.A.; Schernhammer, E.S.; Wu, K.; Fuchs, C.S. Aspirin Dose and Duration of Use and Risk of Colorectal Cancer in Men. Gastroenterology 2008, 134, 21–28. [Google Scholar] [CrossRef]
- Cook, N.R.; Lee, I.-M.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Low-Dose Aspirin in the Primary Prevention of Cancer: The Women’s Health Study: A Randomized Controlled Trial. JAMA 2005, 294, 47–55. [Google Scholar] [CrossRef]
- Cook, N.R.; Lee, I.-M.; Zhang, S.M.; Moorthy, M.V.; Buring, J.E. Alternate-Day, Low-Dose Aspirin and Cancer Risk: Long-Term Observational Follow-up of a Randomized Trial. Ann. Intern. Med. 2013, 159, 77–85. [Google Scholar] [CrossRef]
- Ait Ouakrim, D.; Dashti, S.G.; Chau, R.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Winship, I.M.; Young, J.P.; Giles, G.G.; Leggett, B.; et al. Aspirin, Ibuprofen, and the Risk of Colorectal Cancer in Lynch Syndrome. J. Natl. Cancer Inst. 2015, 107, djv170. [Google Scholar] [CrossRef]
- Burn, J.; Bishop, D.T.; Mecklin, J.-P.; Macrae, F.; Möslein, G.; Olschwang, S.; Bisgaard, M.-L.; Ramesar, R.; Eccles, D.; Maher, E.R.; et al. Effect of Aspirin or Resistant Starch on Colorectal Neoplasia in the Lynch Syndrome. N. Engl. J. Med. 2008, 359, 2567–2578. [Google Scholar] [CrossRef]
- Burn, J.; Gerdes, A.-M.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-Term Effect of Aspirin on Cancer Risk in Carriers of Hereditary Colorectal Cancer: An Analysis from the CAPP2 Randomised Controlled Trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef]
- Chubak, J.; Kamineni, A.; Buist, D.S.; Anderson, M.L.; Whitlock, E.P. Aspirin Use for the Prevention of Colorectal Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force; U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2015. [Google Scholar]
- Dehmer, S.P.; Maciosek, M.V.; Flottemesch, T.J. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: A Decision Analysis: Technical Report; U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2015. [Google Scholar]
- Ishikawa, H.; Mutoh, M.; Suzuki, S.; Tokudome, S.; Saida, Y.; Abe, T.; Okamura, S.; Tajika, M.; Joh, T.; Tanaka, S.; et al. The Preventive Effects of Low-Dose Enteric-Coated Aspirin Tablets on the Development of Colorectal Tumours in Asian Patients: A Randomised Trial. Gut 2014, 63, 1755–1759. [Google Scholar] [CrossRef]
- Ali, R.; Toh, H.-C.; Chia, W.-K. The Utility of Aspirin in Dukes C and High Risk Dukes B Colorectal Cancer—The ASCOLT Study: Study Protocol for a Randomized Controlled Trial. Trials 2011, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Elwin, C.-E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-Term Effect of Aspirin on Colorectal Cancer Incidence and Mortality: 20-Year Follow-up of Five Randomised Trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Chin, S.M.; Gilpin, K.K.; Parziale, M.; Pond, E.; Schuck, M.M.; Stewart, K.; Flagg, M.; Rawlings, C.A.; Backman, V.; et al. ASPirin Intervention for the REDuction of Colorectal Cancer Risk (ASPIRED): A Study Protocol for a Randomized Controlled Trial. Trials 2017, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.-P.; Möslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer Prevention with Aspirin in Hereditary Colorectal Cancer (Lynch Syndrome), 10-Year Follow-up and Registry-Based 20-Year Data in the CAPP2 Study: A Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the Management of Hereditary Colorectal Cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef]
- National Guideline Alliance (UK) Effectiveness of Aspirin in the Prevention of Colorectal Cancer in People with Lynch Syndrome: Colorectal Cancer (Update): Evidence Review A1; NICE Evidence Reviews Collection; National Institute for Health and Care Excellence (NICE): London, UK, 2020; ISBN 978-1-4731-3657-1.
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Sampaio Soares, A.; Jimenez-Rodriguez, R.; Sánchez-Guillén, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European Guidelines from the EHTG and ESCP for Lynch Syndrome: An Updated Third Edition of the Mallorca Guidelines Based on Gene and Gender. Br. J. Surg. 2021, 108, 484–498. [Google Scholar] [CrossRef]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of Cancer: Current Evidence and Future Prospects. F1000Research 2015, 4, 916. [Google Scholar] [CrossRef]
- Thorat, M.A.; Cuzick, J. Prophylactic Use of Aspirin: Systematic Review of Harms and Approaches to Mitigation in the General Population. Eur. J. Epidemiol. 2015, 30, 5–18. [Google Scholar] [CrossRef]
- Ciotti, M.; Marrone, A.; Potter, C.; Owens, I.S. Genetic Polymorphism in the Human UGT1A6 (Planar Phenol) UDP-Glucuronosyltransferase: Pharmacological Implications. Pharmacogenetics 1997, 7, 485–495. [Google Scholar] [CrossRef]
- Chan, A.T.; Tranah, G.J.; Giovannucci, E.L.; Hunter, D.J.; Fuchs, C.S. Genetic Variants in the UGT1A6 Enzyme, Aspirin Use, and the Risk of Colorectal Adenoma. J. Natl. Cancer Inst. 2005, 97, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Bigler, J.; Whitton, J.; Lampe, J.W.; Fosdick, L.; Bostick, R.M.; Potter, J.D. CYP2C9 and UGT1A6 Genotypes Modulate the Protective Effect of Aspirin on Colon Adenoma Risk. Cancer Res. 2001, 61, 3566–3569. [Google Scholar] [PubMed]
- Barry, E.L.; Mott, L.A.; Sandler, R.S.; Ahnen, D.J.; Baron, J.A. Variants Downstream of the Ornithine Decarboxylase Gene Influence Risk of Colorectal Adenoma and Aspirin Chemoprevention. Cancer Prev. Res. 2011, 4, 2072–2082. [Google Scholar] [CrossRef]
- Bezawada, N.; Song, M.; Wu, K.; Mehta, R.S.; Milne, G.L.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Urinary PGE-M Levels Are Associated with Risk of Colorectal Adenomas and Chemopreventive Response to Anti-Inflammatory Drugs. Cancer Prev. Res. 2014, 7, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Resler, A.J.; Makar, K.W.; Heath, L.; Whitton, J.; Potter, J.D.; Poole, E.M.; Habermann, N.; Scherer, D.; Duggan, D.; Wang, H.; et al. Genetic Variation in Prostaglandin Synthesis and Related Pathways, NSAID Use and Colorectal Cancer Risk in the Colon Cancer Family Registry. Carcinogenesis 2014, 35, 2121–2126. [Google Scholar] [CrossRef]
- Chan, A.T.; Ogino, S.; Giovannucci, E.L.; Fuchs, C.S. Inflammatory Markers Are Associated with Risk of Colorectal Cancer and Chemopreventive Response to Anti-Inflammatory Drugs. Gastroenterology 2011, 140, 799–808, quiz e11. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Bezawada, N.; Wu, K.; Garcia-Albeniz, X.; Morikawa, T.; Fuchs, C.S.; Ogino, S.; Giovannucci, E.L.; Chan, A.T. A Prospective Study of Macrophage Inhibitory Cytokine-1 (MIC-1/GDF15) and Risk of Colorectal Cancer. J. Natl. Cancer Inst. 2014, 106, dju016. [Google Scholar] [CrossRef]
- Thun, M.J.; Jacobs, E.J.; Patrono, C. The Role of Aspirin in Cancer Prevention. Nat. Rev. Clin. Oncol. 2012, 9, 259–267. [Google Scholar] [CrossRef]
- Chan, A.T.; Ogino, S.; Fuchs, C.S. Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of COX-2. N. Engl. J. Med. 2007, 356, 2131–2142. [Google Scholar] [CrossRef]
- Liao, X.; Lochhead, P.; Nishihara, R.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Imamura, Y.; Qian, Z.R.; Baba, Y.; Shima, K.; et al. Aspirin Use, Tumor PIK3CA Mutation, and Colorectal-Cancer Survival. N. Engl. J. Med. 2012, 367, 1596–1606. [Google Scholar] [CrossRef]
- Nishihara, R.; Lochhead, P.; Kuchiba, A.; Jung, S.; Yamauchi, M.; Liao, X.; Imamura, Y.; Qian, Z.R.; Morikawa, T.; Wang, M.; et al. Aspirin Use and Risk of Colorectal Cancer According to BRAF Mutation Status. JAMA 2013, 309, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser, W.K.; Lubin, I.M.; Monzon, F.A.; Zehnbauer, B.A.; Evans, J.P.; Ogino, S.; Nowak, J.A. Relevance, Pathogenesis, and Testing Algorithm for Mismatch Repair-Defective Colorectal Carcinomas: A Report of the Association for Molecular Pathology. J. Mol. Diagn. 2012, 14, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Teramoto, H.; Gutkind, J.S. Cyclooxygenase-2 and Colorectal Cancer Chemoprevention: The Beta-Catenin Connection. Cancer Res. 2006, 66, 11085–11088. [Google Scholar] [CrossRef] [PubMed]
- Bikkavilli, R.K.; Feigin, M.E.; Malbon, C.C. P38 Mitogen-Activated Protein Kinase Regulates Canonical Wnt-Beta-Catenin Signaling by Inactivation of GSK3beta. J. Cell. Sci. 2008, 121, 3598–3607. [Google Scholar] [CrossRef]
- Nan, H.; Morikawa, T.; Suuriniemi, M.; Imamura, Y.; Werner, L.; Kuchiba, A.; Yamauchi, M.; Hunter, D.J.; Kraft, P.; Giovannucci, E.L.; et al. Aspirin Use, 8q24 Single Nucleotide Polymorphism Rs6983267, and Colorectal Cancer According to CTNNB1 Alterations. J. Natl. Cancer Inst. 2013, 105, 1852–1861. [Google Scholar] [CrossRef]
- Tuupanen, S.; Turunen, M.; Lehtonen, R.; Hallikas, O.; Vanharanta, S.; Kivioja, T.; Björklund, M.; Wei, G.; Yan, J.; Niittymäki, I.; et al. The Common Colorectal Cancer Predisposition SNP Rs6983267 at Chromosome 8q24 Confers Potential to Enhanced Wnt Signaling. Nat. Genet. 2009, 41, 885–890. [Google Scholar] [CrossRef]
- Fink, S.P.; Yamauchi, M.; Nishihara, R.; Jung, S.; Kuchiba, A.; Wu, K.; Cho, E.; Giovannucci, E.; Fuchs, C.S.; Ogino, S.; et al. Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of 15-Hydroxyprostaglandin Dehydrogenase (HPGD). Sci. Transl. Med. 2014, 6, 233re2. [Google Scholar] [CrossRef]
- Nan, H.; Hutter, C.M.; Lin, Y.; Jacobs, E.J.; Ulrich, C.M.; White, E.; Baron, J.A.; Berndt, S.I.; Brenner, H.; Butterbach, K.; et al. Association of Aspirin and NSAID Use with Risk of Colorectal Cancer According to Genetic Variants. JAMA 2015, 313, 1133–1142. [Google Scholar] [CrossRef]
- Lora, M.; Denault, J.B.; Leduc, R.; de Brum-Fernandes, A.J. Systematic Pharmacological Approach to the Characterization of NSAIDs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 59, 55–62. [Google Scholar] [CrossRef]
- Takayama, T.; Nagashima, H.; Maeda, M.; Nojiri, S.; Hirayama, M.; Nakano, Y.; Takahashi, Y.; Sato, Y.; Sekikawa, H.; Mori, M.; et al. Randomized Double-Blind Trial of Sulindac and Etodolac to Eradicate Aberrant Crypt Foci and to Prevent Sporadic Colorectal Polyps. Clin. Cancer Res. 2011, 17, 3803–3811. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Hamilton, S.R.; Krush, A.J.; Piantadosi, S.; Hylind, L.M.; Celano, P.; Booker, S.V.; Robinson, C.R.; Offerhaus, G.J. Treatment of Colonic and Rectal Adenomas with Sulindac in Familial Adenomatous Polyposis. N. Engl. J. Med. 1993, 328, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Labayle, D.; Fischer, D.; Vielh, P.; Drouhin, F.; Pariente, A.; Bories, C.; Duhamel, O.; Trousset, M.; Attali, P. Sulindac Causes Regression of Rectal Polyps in Familial Adenomatous Polyposis. Gastroenterology 1991, 101, 635–639. [Google Scholar] [CrossRef]
- Nugent, K.P.; Farmer, K.C.; Spigelman, A.D.; Williams, C.B.; Phillips, R.K. Randomized Controlled Trial of the Effect of Sulindac on Duodenal and Rectal Polyposis and Cell Proliferation in Patients with Familial Adenomatous Polyposis. Br. J. Surg. 1993, 80, 1618–1619. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.J.; Elgendy, I.Y.; Bavry, A.A. Cardiovascular Safety and Bleeding Risk Associated with Nonsteroidal Anti-Inflammatory Medications in Patients with Cardiovascular Disease. Curr. Cardiol. Rep. 2017, 19, 8. [Google Scholar] [CrossRef]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Iwama, T.; Yoshinaga, K.; Toyooka, M.; Taketo, M.M.; Sugihara, K. A Randomized, Double-Blind, Placebo-Controlled Trial of the Effects of Rofecoxib, a Selective Cyclooxygenase-2 Inhibitor, on Rectal Polyps in Familial Adenomatous Polyposis Patients. Clin. Cancer Res. 2003, 9, 4756–4760. [Google Scholar]
- Rahme, E.; Barkun, A.N.; Toubouti, Y.; Bardou, M. The Cyclooxygenase-2-Selective Inhibitors Rofecoxib and Celecoxib Prevent Colorectal Neoplasia Occurrence and Recurrence. Gastroenterology 2003, 125, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Solomon, S.D.; Kim, K.; Tang, J.; Rosenstein, R.B.; Wittes, J.; Corle, D.; et al. Celecoxib for the Prevention of Sporadic Colorectal Adenomas. N. Engl. J. Med. 2006, 355, 873–884. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Breazna, A.; Kim, K.; Tang, J.; Rosenstein, R.B.; Umar, A.; Bagheri, D.; et al. Five-Year Efficacy and Safety Analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev. Res. 2009, 2, 310–321. [Google Scholar] [CrossRef]
- Chan, A.T.; Zauber, A.G.; Hsu, M.; Breazna, A.; Hunter, D.J.; Rosenstein, R.B.; Eagle, C.J.; Hawk, E.T.; Bertagnolli, M.M. Cytochrome P450 2C9 Variants Influence Response to Celecoxib for Prevention of Colorectal Adenoma. Gastroenterology 2009, 136, 2127–2136.e1. [Google Scholar] [CrossRef]
- Arber, N.; Eagle, C.J.; Spicak, J.; Rácz, I.; Dite, P.; Hajer, J.; Zavoral, M.; Lechuga, M.J.; Gerletti, P.; Tang, J.; et al. Celecoxib for the Prevention of Colorectal Adenomatous Polyps. N. Engl. J. Med. 2006, 355, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Arber, N.; Spicak, J.; Rácz, I.; Zavoral, M.; Breazna, A.; Gerletti, P.; Lechuga, M.J.; Collins, N.; Rosenstein, R.B.; Eagle, C.J.; et al. Five-Year Analysis of the Prevention of Colorectal Sporadic Adenomatous Polyps Trial. Am. J. Gastroenterol. 2011, 106, 1135–1146. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef]
- Baron, J.A.; Sandler, R.S.; Bresalier, R.S.; Quan, H.; Riddell, R.; Lanas, A.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Loftus, S.; et al. A Randomized Trial of Rofecoxib for the Chemoprevention of Colorectal Adenomas. Gastroenterology 2006, 131, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Dubé, C.; Lewin, G.; Tsertsvadze, A.; Barrowman, N.; Code, C.; Sampson, M.; Moher, D. U.S. Preventive Services Task Force Nonsteroidal Anti-Inflammatory Drugs and Cyclooxygenase-2 Inhibitors for Primary Prevention of Colorectal Cancer: A Systematic Review Prepared for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2007, 146, 376–389. [Google Scholar] [CrossRef]
- Jensen, A.B.; Larsen, M.; Gislum, M.; Skriver, M.V.; Jepsen, P.; Nørgaard, B.; Sørensen, H.T. Survival after Colorectal Cancer in Patients with Ulcerative Colitis: A Nationwide Population-Based Danish Study. Am. J. Gastroenterol. 2006, 101, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Herszényi, L.; Farinati, F.; Miheller, P.; Tulassay, Z. Chemoprevention of Colorectal Cancer: Feasibility in Everyday Practice? Eur. J. Cancer Prev. 2008, 17, 502–514. [Google Scholar] [CrossRef]
- Munkholm, P.; Loftus, E.V.; Reinacher-Schick, A.; Kornbluth, A.; Mittmann, U.; Esendal, B. Prevention of Colorectal Cancer in Inflammatory Bowel Disease: Value of Screening and 5-Aminosalicylates. Digestion 2006, 73, 11–19. [Google Scholar] [CrossRef]
- Velayos, F.S.; Terdiman, J.P.; Walsh, J.M. Effect of 5-Aminosalicylate Use on Colorectal Cancer and Dysplasia Risk: A Systematic Review and Metaanalysis of Observational Studies. Am. J. Gastroenterol. 2005, 100, 1345–1353. [Google Scholar] [CrossRef]
- Sandborn, W.J. Treatment of Ulcerative Colitis with Oral Mesalamine: Advances in Drug Formulation, Efficacy Expectations and Dose Response, Compliance, and Chemoprevention. Rev. Gastroenterol. Disord. 2006, 6, 97–105. [Google Scholar]
- Earnest, D.L.; Holubec, H.; Wali, R.K.; Jolley, C.S.; Bissonette, M.; Bhattacharyya, A.K.; Roy, H.; Khare, S.; Brasitus, T.A. Chemoprevention of Azoxymethane-Induced Colonic Carcinogenesis by Supplemental Dietary Ursodeoxycholic Acid. Cancer Res. 1994, 54, 5071–5074. [Google Scholar] [PubMed]
- Ikegami, T.; Matsuzaki, Y.; Shoda, J.; Kano, M.; Hirabayashi, N.; Tanaka, N. The Chemopreventive Role of Ursodeoxycholic Acid in Azoxymethane-Treated Rats: Suppressive Effects on Enhanced Group II Phospholipase A2 Expression in Colonic Tissue. Cancer Lett. 1998, 134, 129–139. [Google Scholar] [CrossRef]
- Alberts, D.S.; Martínez, M.E.; Hess, L.M.; Einspahr, J.G.; Green, S.B.; Bhattacharyya, A.K.; Guillen, J.; Krutzsch, M.; Batta, A.K.; Salen, G.; et al. Phase III Trial of Ursodeoxycholic Acid to Prevent Colorectal Adenoma Recurrence. J. Natl. Cancer Inst. 2005, 97, 846–853. [Google Scholar] [CrossRef]
- Tung, B.Y.; Emond, M.J.; Haggitt, R.C.; Bronner, M.P.; Kimmey, M.B.; Kowdley, K.V.; Brentnall, T.A. Ursodiol Use Is Associated with Lower Prevalence of Colonic Neoplasia in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis. Ann. Intern. Med. 2001, 134, 89–95. [Google Scholar] [CrossRef]
- Pardi, D.S.; Loftus, E.V.; Kremers, W.K.; Keach, J.; Lindor, K.D. Ursodeoxycholic Acid as a Chemopreventive Agent in Patients with Ulcerative Colitis and Primary Sclerosing Cholangitis. Gastroenterology 2003, 124, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Berster, J.M.; Göke, B. Type 2 Diabetes Mellitus as Risk Factor for Colorectal Cancer. Arch. Physiol. Biochem. 2008, 114, 84–98. [Google Scholar] [CrossRef]
- Tie, G.; Yan, J.; Khair, L.; Messina, J.A.; Deng, A.; Kang, J.; Fazzio, T.; Messina, L.M. Hypercholesterolemia Increases Colorectal Cancer Incidence by Reducing Production of NKT and Γδ T Cells from Hematopoietic Stem Cells. Cancer Res. 2017, 77, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Guo, Z.; Zeng, Y.; Zhang, W.; Wang, H. Metformin: Current Clinical Applications in Nondiabetic Patients with Cancer. Aging 2020, 12, 3993–4009. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, X.; Gao, P.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z.; Song, Y. The Potential Effect of Metformin on Cancer: An Umbrella Review. Front. Endocrinol. 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, M.N.A.; Sarker, M.R.; Zhou, J.-R.; Parhar, I. Metformin in Colorectal Cancer: Molecular Mechanism, Preclinical and Clinical Aspects. J. Exp. Clin. Cancer Res. 2019, 38, 491. [Google Scholar] [CrossRef]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Uchiyama, T.; Suzuki, K.; Nozaki, Y.; Yoneda, K.; Fujita, K.; Yoneda, M.; et al. Metformin Suppresses Azoxymethane-Induced Colorectal Aberrant Crypt Foci by Activating AMP-Activated Protein Kinase. Mol. Carcinog. 2010, 49, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Tomimoto, A.; Endo, H.; Sugiyama, M.; Fujisawa, T.; Hosono, K.; Takahashi, H.; Nakajima, N.; Nagashima, Y.; Wada, K.; Nakagama, H.; et al. Metformin Suppresses Intestinal Polyp Growth in ApcMin/+ Mice. Cancer Sci. 2008, 99, 2136–2141. [Google Scholar] [CrossRef]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Sakai, E.; Uchiyama, T.; Suzuki, K.; Iida, H.; Sakamoto, Y.; Yoneda, K.; et al. Metformin Suppresses Colorectal Aberrant Crypt Foci in a Short-Term Clinical Trial. Cancer Prev. Res. 2010, 3, 1077–1083. [Google Scholar] [CrossRef]
- Higurashi, T.; Arimoto, J.; Ashikari, K.; Takatsu, T.; Misawa, N.; Yoshihara, T.; Matsuura, T.; Fuyuki, A.; Ohkubo, H.; Nakajima, A. The Efficacy of Aspirin and Metformin Combination Therapy in Patients with Rectal Aberrant Crypt Foci: A Double-Blinded Randomized Controlled Trial. BMC Cancer 2020, 20, 1043. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, T.; Hosono, K.; Takahashi, H.; Komiya, Y.; Umezawa, S.; Sakai, E.; Uchiyama, T.; Taniguchi, L.; Hata, Y.; Uchiyama, S.; et al. Metformin for Chemoprevention of Metachronous Colorectal Adenoma or Polyps in Post-Polypectomy Patients without Diabetes: A Multicentre Double-Blind, Placebo-Controlled, Randomised Phase 3 Trial. Lancet Oncol. 2016, 17, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, C.H.; Eun, C.S.; Park, D.I.; Han, D.S. Metformin Use and the Risk of Colorectal Adenoma: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2017, 32, 957–965. [Google Scholar] [CrossRef]
- Cardel, M.; Jensen, S.M.; Pottegård, A.; Jørgensen, T.L.; Hallas, J. Long-Term Use of Metformin and Colorectal Cancer Risk in Type II Diabetics: A Population-Based Case-Control Study. Cancer Med. 2014, 3, 1458–1466. [Google Scholar] [CrossRef]
- Currie, C.J.; Poole, C.D.; Gale, E.A.M. The Influence of Glucose-Lowering Therapies on Cancer Risk in Type 2 Diabetes. Diabetologia 2009, 52, 1766–1777. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hsu, C.-C.; Wahlqvist, M.L.; Tsai, H.-N.; Chang, Y.-H.; Huang, Y.-C. Type 2 Diabetes Increases and Metformin Reduces Total, Colorectal, Liver and Pancreatic Cancer Incidences in Taiwanese: A Representative Population Prospective Cohort Study of 800,000 Individuals. BMC Cancer 2011, 11, 20. [Google Scholar] [CrossRef]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M.M. New Users of Metformin Are at Low Risk of Incident Cancer: A Cohort Study among People with Type 2 Diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Sehdev, A.; Shih, Y.-C.T.; Vekhter, B.; Bissonnette, M.B.; Olopade, O.I.; Polite, B.N. Metformin for Primary Colorectal Cancer Prevention in Patients with Diabetes: A Case-Control Study in a US Population. Cancer 2015, 121, 1071–1078. [Google Scholar] [CrossRef]
- Tseng, C.-H. Diabetes, Metformin Use, and Colon Cancer: A Population-Based Cohort Study in Taiwan. Eur. J. Endocrinol. 2012, 167, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Zheng, Z.-J.; Kan, H.; Song, Y.; Cui, W.; Zhao, G.; Kip, K.E. Reduced Risk of Colorectal Cancer with Metformin Therapy in Patients with Type 2 Diabetes: A Meta-Analysis. Diabetes Care 2011, 34, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.C.; Ferrara, A.; Achacoso, N.; Ehrlich, S.F.; Quesenberry, C.P.; Habel, L.A. A Cohort Study of Metformin and Colorectal Cancer Risk among Patients with Diabetes Mellitus. Cancer Epidemiol. Biomark. Prev. 2018, 27, 525–530. [Google Scholar] [CrossRef]
- Kowall, B.; Stang, A.; Rathmann, W.; Kostev, K. No Reduced Risk of Overall, Colorectal, Lung, Breast, and Prostate Cancer with Metformin Therapy in Diabetic Patients: Database Analyses from Germany and the UK. Pharmacoepidemiol. Drug. Saf. 2015, 24, 865–874. [Google Scholar] [CrossRef]
- Lin, C.-M.; Huang, H.-L.; Chu, F.-Y.; Fan, H.-C.; Chen, H.-A.; Chu, D.-M.; Wu, L.-W.; Wang, C.-C.; Chen, W.-L.; Lin, S.-H.; et al. Association between Gastroenterological Malignancy and Diabetes Mellitus and Anti-Diabetic Therapy: A Nationwide, Population-Based Cohort Study. PLoS ONE 2015, 10, e0125421. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Becker, C.; Meier, C.; Jick, S.S.; Meier, C.R. Use of Metformin Is Not Associated with a Decreased Risk of Colorectal Cancer: A Case-Control Analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 280–286. [Google Scholar] [CrossRef]
- Knapen, L.M.; Dittrich, S.T.A.M.; de Vries, F.; Starup-Linde, J.; Vestergaard, P.; Henry, R.M.A.; Stolk, L.M.L.; Neef, C.; Bazelier, M.T. Use of Biguanides and the Risk of Colorectal Cancer: A Register-Based Cohort Study. Curr. Drug. Saf. 2013, 8, 349–356. [Google Scholar] [CrossRef]
- Du, L.; Wang, M.; Kang, Y.; Li, B.; Guo, M.; Cheng, Z.; Bi, C. Prognostic Role of Metformin Intake in Diabetic Patients with Colorectal Cancer: An Updated Qualitative Evidence of Cohort Studies. Oncotarget 2017, 8, 26448–26459. [Google Scholar] [CrossRef]
- Takemoto, M.; Liao, J.K. Pleiotropic Effects of 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase Inhibitors. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Hentosh, P.; Yuh, S.H.; Elson, C.E.; Peffley, D.M. Sterol-Independent Regulation of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Tumor Cells. Mol. Carcinog. 2001, 32, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H. Cholesterol Inhibition, Cancer, and Chemotherapy. Lancet 1992, 339, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, N.; Mutoh, M.; Takasu, S.; Ueno, T.; Yamamoto, M.; Sugimura, T.; Wakabayashi, K. Inhibition of Intestinal Polyp Formation by Pitavastatin, a HMG-CoA Reductase Inhibitor. Cancer Prev. Res. 2011, 4, 445–453. [Google Scholar] [CrossRef]
- Suh, N.; Reddy, B.S.; DeCastro, A.; Paul, S.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Janakiram, N.B.; et al. Combination of Atorvastatin with Sulindac or Naproxen Profoundly Inhibits Colonic Adenocarcinomas by Suppressing the P65/β-Catenin/Cyclin D1 Signaling Pathway in Rats. Cancer Prev. Res. 2011, 4, 1895–1902. [Google Scholar] [CrossRef]
- Swamy, M.V.; Patlolla, J.M.R.; Steele, V.E.; Kopelovich, L.; Reddy, B.S.; Rao, C.V. Chemoprevention of Familial Adenomatous Polyposis by Low Doses of Atorvastatin and Celecoxib given Individually and in Combination to APCMin Mice. Cancer Res. 2006, 66, 7370–7377. [Google Scholar] [CrossRef]
- Wei, J.T.; Mott, L.A.; Baron, J.A.; Sandler, R.S.; Polyp Prevention Study Group. Reported Use of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors Was Not Associated with Reduced Recurrence of Colorectal Adenomas. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1026–1027. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Nazario, H.; Mahgoub, A.; Pandove, S.; Cipher, D.; Spechler, S.J. The Long-Term Use of Statins Is Associated with a Decreased Incidence of Adenomatous Colon Polyps. Digestion 2009, 79, 17–22. [Google Scholar] [CrossRef]
- Poynter, J.N.; Gruber, S.B.; Higgins, P.D.R.; Almog, R.; Bonner, J.D.; Rennert, H.S.; Low, M.; Greenson, J.K.; Rennert, G. Statins and the Risk of Colorectal Cancer. N. Engl. J. Med. 2005, 352, 2184–2192. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. Cholesterol and Recurrent Events Trial Investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Pedersen, T.R.; Berg, K.; Cook, T.J.; Faergeman, O.; Haghfelt, T.; Kjekshus, J.; Miettinen, T.; Musliner, T.A.; Olsson, A.G.; Pyörälä, K.; et al. Safety and Tolerability of Cholesterol Lowering with Simvastatin during 5 Years in the Scandinavian Simvastatin Survival Study. Arch. Intern. Med. 1996, 156, 2085–2092. [Google Scholar] [CrossRef]
- Browning, D.R.L.; Martin, R.M. Statins and Risk of Cancer: A Systematic Review and Metaanalysis. Int. J. Cancer 2007, 120, 833–843. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsavaris, N.; Sitaras, N.M. Statins and Cancer Risk: A Literature-Based Meta-Analysis and Meta-Regression Analysis of 35 Randomized Controlled Trials. J. Clin. Oncol. 2006, 24, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Kuoppala, J.; Lamminpää, A.; Pukkala, E. Statins and Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2008, 44, 2122–2132. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Emberson, J.R.; Kearney, P.M.; Blackwell, L.; Newman, C.; Reith, C.; Bhala, N.; Holland, L.; Peto, R.; Keech, A.; et al. Lack of Effect of Lowering LDL Cholesterol on Cancer: Meta-Analysis of Individual Data from 175,000 People in 27 Randomised Trials of Statin Therapy. PLoS ONE 2012, 7, e29849. [Google Scholar] [CrossRef]
- Lipkin, S.M.; Chao, E.C.; Moreno, V.; Rozek, L.S.; Rennert, H.; Pinchev, M.; Dizon, D.; Rennert, G.; Kopelovich, L.; Gruber, S.B. Genetic Variation in 3-Hydroxy-3-Methylglutaryl CoA Reductase Modifies the Chemopreventive Activity of Statins for Colorectal Cancer. Cancer Prev. Res. 2010, 3, 597–603. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty Acids from Fish: The Anti-Inflammatory Potential of Long-Chain Omega-3 Fatty Acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Wu, S.; Feng, B.; Li, K.; Zhu, X.; Liang, S.; Liu, X.; Han, S.; Wang, B.; Wu, K.; Miao, D.; et al. Fish Consumption and Colorectal Cancer Risk in Humans: A Systematic Review and Meta-Analysis. Am. J. Med. 2012, 125, 551–559.e5. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Zou, J.; Dong, J. Fish Consumption and Risk of Gastrointestinal Cancers: A Meta-Analysis of Cohort Studies. World J. Gastroenterol. 2014, 20, 15398–15412. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Toogood, G.J.; Hull, M.A. Omega-3 Polyunsaturated Fatty Acids for the Treatment and Prevention of Colorectal Cancer. Gut 2012, 61, 135–149. [Google Scholar] [CrossRef]
- Latham, P.; Lund, E.K.; Johnson, I.T. Dietary N-3 PUFA Increases the Apoptotic Response to 1,2-Dimethylhydrazine, Reduces Mitosis and Suppresses the Induction of Carcinogenesis in the Rat Colon. Carcinogenesis 1999, 20, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.G.; Lund, E.K.; Latham, P.; Pinder, A.C.; Johnson, I.T. Effect of Eicosapentaenoic Acid on the Proliferation and Incidence of Apoptosis in the Colorectal Cell Line HT29. Lipids 1999, 34, 1287–1295. [Google Scholar] [CrossRef]
- West, N.J.; Clark, S.K.; Phillips, R.K.S.; Hutchinson, J.M.; Leicester, R.J.; Belluzzi, A.; Hull, M.A. Eicosapentaenoic Acid Reduces Rectal Polyp Number and Size in Familial Adenomatous Polyposis. Gut 2010, 59, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Lampe, J.W.; Peters, U.; Vaughan, T.L.; White, E. Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer. Nutr. Cancer 2014, 66, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Ansary-Moghaddam, A.; Clifton, P.; Czernichow, S.; Parr, C.L.; Woodward, M. The Impact of Dietary and Lifestyle Risk Factors on Risk of Colorectal Cancer: A Quantitative Overview of the Epidemiological Evidence. Int. J. Cancer 2009, 125, 171–180. [Google Scholar] [CrossRef]
- Geelen, A.; Schouten, J.M.; Kamphuis, C.; Stam, B.E.; Burema, J.; Renkema, J.M.S.; Bakker, E.-J.; van’t Veer, P.; Kampman, E. Fish Consumption, n-3 Fatty Acids, and Colorectal Cancer: A Meta-Analysis of Prospective Cohort Studies. Am. J. Epidemiol. 2007, 166, 1116–1125. [Google Scholar] [CrossRef]
- Shen, X.-J.; Zhou, J.-D.; Dong, J.-Y.; Ding, W.-Q.; Wu, J.-C. Dietary Intake of N-3 Fatty Acids and Colorectal Cancer Risk: A Meta-Analysis of Data from 489 000 Individuals. Br. J. Nutr. 2012, 108, 1550–1556. [Google Scholar] [CrossRef]
- Choi, S.W.; Mason, J.B. Folate and Carcinogenesis: An Integrated Scheme. J. Nutr. 2000, 130, 129–132. [Google Scholar] [CrossRef]
- Biasco, G.; Di Marco, M.C. Folate and Prevention of Colorectal Cancer in Ulcerative Colitis. Eur. J. Cancer Prev. 2005, 14, 395–398. [Google Scholar] [CrossRef]
- Du, W.; Li, W.-Y.; Lu, R.; Fang, J.-Y. Folate and Fiber in the Prevention of Colorectal Cancer: Between Shadows and the Light. World J. Gastroenterol. 2010, 16, 921–926. [Google Scholar] [CrossRef]
- Kim, Y.-I. Folate and Colorectal Cancer: An Evidence-Based Critical Review. Mol. Nutr. Food Res. 2007, 51, 267–292. [Google Scholar] [CrossRef]
- Arber, N.; Levin, B. Chemoprevention of Colorectal Neoplasia: The Potential for Personalized Medicine. Gastroenterology 2008, 134, 1224–1237. [Google Scholar] [CrossRef]
- Lindzon, G.M.; Medline, A.; Sohn, K.-J.; Depeint, F.; Croxford, R.; Kim, Y.-I. Effect of Folic Acid Supplementation on the Progression of Colorectal Aberrant Crypt Foci. Carcinogenesis 2009, 30, 1536–1543. [Google Scholar] [CrossRef]
- Sanjoaquin, M.A.; Allen, N.; Couto, E.; Roddam, A.W.; Key, T.J. Folate Intake and Colorectal Cancer Risk: A Meta-Analytical Approach. Int. J. Cancer 2005, 113, 825–828. [Google Scholar] [CrossRef]
- Paspatis, G.A.; Kalafatis, E.; Oros, L.; Xourgias, V.; Koutsioumpa, P.; Karamanolis, D.G. Folate Status and Adenomatous Colonic Polyps. A Colonoscopically Controlled Study. Dis. Colon. Rectum 1995, 38, 64–67; discussion 67–68. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Sandler, R.S.; Haile, R.W.; Mandel, J.S.; Mott, L.A.; Greenberg, E.R. Folate Intake, Alcohol Consumption, Cigarette Smoking, and Risk of Colorectal Adenomas. J. Natl. Cancer Inst. 1998, 90, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Du, M.; Du, H.; Shu, Y.; Wang, M.; Zhu, L. Folic Acid Supplements and Colorectal Cancer Risk: Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2015, 5, 12044. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Stampfer, M.J.; Colditz, G.A.; Hunter, D.J.; Fuchs, C.; Rosner, B.A.; Speizer, F.E.; Willett, W.C. Multivitamin Use, Folate, and Colon Cancer in Women in the Nurses’ Health Study. Ann. Intern. Med. 1998, 129, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Jain, M.; Miller, A.B.; Howe, G.R.; Rohan, T.E. Dietary Intake of Folic Acid and Colorectal Cancer Risk in a Cohort of Women. Int. J. Cancer 2002, 97, 864–867. [Google Scholar] [CrossRef]
- Lashner, B.A.; Provencher, K.S.; Seidner, D.L.; Knesebeck, A.; Brzezinski, A. The Effect of Folic Acid Supplementation on the Risk for Cancer or Dysplasia in Ulcerative Colitis. Gastroenterology 1997, 112, 29–32. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Harpaz, N. Diagnosis and Management of Dysplasia in Patients with Inflammatory Bowel Diseases. Gastroenterology 2004, 126, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Ogino, S.; Fuchs, C.S. Folate and Vitamin B6 Intake and Risk of Colon Cancer in Relation to P53 Expression. Gastroenterology 2008, 135, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Taioli, E.; Garza, M.A.; Ahn, Y.O.; Bishop, D.T.; Bost, J.; Budai, B.; Chen, K.; Gemignani, F.; Keku, T.; Lima, C.S.P.; et al. Meta- and Pooled Analyses of the Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and Colorectal Cancer: A HuGE-GSEC Review. Am. J. Epidemiol. 2009, 170, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Hubner, R.A.; Houlston, R.S. MTHFR C677T and Colorectal Cancer Risk: A Meta-Analysis of 25 Populations. Int. J. Cancer 2007, 120, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Han, S.; Li, Y.; Mao, Y.; Xie, Y. Different Roles of MTHFR C677T and A1298C Polymorphisms in Colorectal Adenoma and Colorectal Cancer: A Meta-Analysis. J. Hum. Genet. 2007, 52, 73–85. [Google Scholar] [CrossRef]
- Wiseman, M. The Second World Cancer Research Fund/American Institute for Cancer Research Expert Report. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant Supplements for Preventing Gastrointestinal Cancers. Cochrane Database Syst. Rev. 2004, CD004183. [Google Scholar] [CrossRef]
- Papaioannou, D.; Cooper, K.L.; Carroll, C.; Hind, D.; Squires, H.; Tappenden, P.; Logan, R.F. Antioxidants in the Chemoprevention of Colorectal Cancer and Colorectal Adenomas in the General Population: A Systematic Review and Meta-Analysis. Colorectal Dis. 2011, 13, 1085–1099. [Google Scholar] [CrossRef]
- Pais, R.; Dumitraşcu, D.L. Do Antioxidants Prevent Colorectal Cancer? A Meta-Analysis. Rom. J. Intern. Med. 2013, 51, 152–163. [Google Scholar] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Spiegelman, D.; Hunter, D.J.; Albanes, D.; Bergkvist, L.; Buring, J.E.; Freudenheim, J.L.; Giovannucci, E.; Goldbohm, R.A.; Harnack, L.; et al. Intakes of Vitamins A, C, and E and Use of Multiple Vitamin Supplements and Risk of Colon Cancer: A Pooled Analysis of Prospective Cohort Studies. Cancer Causes Control 2010, 21, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Heine-Bröring, R.C.; Winkels, R.M.; Renkema, J.M.S.; Kragt, L.; van Orten-Luiten, A.-C.B.; Tigchelaar, E.F.; Chan, D.S.M.; Norat, T.; Kampman, E. Dietary Supplement Use and Colorectal Cancer Risk: A Systematic Review and Meta-Analyses of Prospective Cohort Studies. Int. J. Cancer 2015, 136, 2388–2401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Zhu, Z.; Zhang, J.; Chen, M.; Tang, P.; Li, K. Vitamin and Multiple-Vitamin Supplement Intake and Incidence of Colorectal Cancer: A Meta-Analysis of Cohort Studies. Med. Oncol. 2015, 32, 434. [Google Scholar] [CrossRef]
- Alkhenizan, A.; Hafez, K. The Role of Vitamin E in the Prevention of Cancer: A Meta-Analysis of Randomized Controlled Trials. Ann. Saudi Med. 2007, 27, 409–414. [Google Scholar] [CrossRef]
- Arain, M.A.; Abdul Qadeer, A. Systematic Review on “Vitamin E and Prevention of Colorectal Cancer”. Pak. J. Pharm. Sci. 2010, 23, 125–130. [Google Scholar]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-Carotene Supplementation and Cancer Risk: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef]
- Asano, T.K.; McLeod, R.S. Non Steroidal Anti-Inflammatory Drugs (NSAID) and Aspirin for Preventing Colorectal Adenomas and Carcinomas. Cochrane Database Syst. Rev. 2004, 2004, CD004079. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment with Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Newmark, H.L.; Lipkin, M. Calcium, Vitamin D, and Colon Cancer. Cancer Res. 1992, 52, 2067s–2070s. [Google Scholar] [PubMed]
- Tanaka, T.; Shinoda, T.; Yoshimi, N.; Niwa, K.; Iwata, H.; Mori, H. Inhibitory Effect of Magnesium Hydroxide on Methylazoxymethanol Acetate-Induced Large Bowel Carcinogenesis in Male F344 Rats. Carcinogenesis 1989, 10, 613–616. [Google Scholar] [CrossRef]
- Mori, H.; Morishita, Y.; Shinoda, T.; Tanaka, T. Preventive Effect of Magnesium Hydroxide on Carcinogen-Induced Large Bowel Carcinogenesis in Rats. Basic Life Sci. 1993, 61, 111–118. [Google Scholar] [CrossRef]
- Chen, G.-C.; Pang, Z.; Liu, Q.-F. Magnesium Intake and Risk of Colorectal Cancer: A Meta-Analysis of Prospective Studies. Eur. J. Clin. Nutr. 2012, 66, 1182–1186. [Google Scholar] [CrossRef]
- Wark, P.A.; Lau, R.; Norat, T.; Kampman, E. Magnesium Intake and Colorectal Tumor Risk: A Case-Control Study and Meta-Analysis. Am. J. Clin. Nutr. 2012, 96, 622–631. [Google Scholar] [CrossRef]
- Ko, H.J.; Youn, C.H.; Kim, H.M.; Cho, Y.J.; Lee, G.H.; Lee, W.K. Dietary Magnesium Intake and Risk of Cancer: A Meta-Analysis of Epidemiologic Studies. Nutr. Cancer 2014, 66, 915–923. [Google Scholar] [CrossRef]
- Newmark, H.L.; Wargovich, M.J.; Bruce, W.R. Colon Cancer and Dietary Fat, Phosphate, and Calcium: A Hypothesis. J. Natl. Cancer Inst. 1984, 72, 1323–1325. [Google Scholar]
- Lipkin, M.; Newmark, H. Effect of Added Dietary Calcium on Colonic Epithelial-Cell Proliferation in Subjects at High Risk for Familial Colonic Cancer. N. Engl. J. Med. 1985, 313, 1381–1384. [Google Scholar] [CrossRef]
- Van der Meer, R.; De Vries, H.T. Differential Binding of Glycine- and Taurine-Conjugated Bile Acids to Insoluble Calcium Phosphate. Biochem. J. 1985, 229, 265–268. [Google Scholar] [CrossRef]
- Wang, J.-L.; Lin, Y.-W.; Chen, H.-M.; Kong, X.; Xiong, H.; Shen, N.; Hong, J.; Fang, J.-Y. Calcium Prevents Tumorigenesis in a Mouse Model of Colorectal Cancer. PLoS ONE 2011, 6, e22566. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Beach, M.; Mandel, J.S.; van Stolk, R.U.; Haile, R.W.; Sandler, R.S.; Rothstein, R.; Summers, R.W.; Snover, D.C.; Beck, G.J.; et al. Calcium Supplements for the Prevention of Colorectal Adenomas. Calcium Polyp Prevention Study Group. N. Engl. J. Med. 1999, 340, 101–107. [Google Scholar] [CrossRef]
- Wallace, K.; Baron, J.A.; Cole, B.F.; Sandler, R.S.; Karagas, M.R.; Beach, M.A.; Haile, R.W.; Burke, C.A.; Pearson, L.H.; Mandel, J.S.; et al. Effect of Calcium Supplementation on the Risk of Large Bowel Polyps. J. Natl. Cancer Inst. 2004, 96, 921–925. [Google Scholar] [CrossRef]
- Grau, M.V.; Baron, J.A.; Sandler, R.S.; Wallace, K.; Haile, R.W.; Church, T.R.; Beck, G.J.; Summers, R.W.; Barry, E.L.; Cole, B.F.; et al. Prolonged Effect of Calcium Supplementation on Risk of Colorectal Adenomas in a Randomized Trial. J. Natl. Cancer Inst. 2007, 99, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bonithon-Kopp, C.; Kronborg, O.; Giacosa, A.; Räth, U.; Faivre, J. Calcium and Fibre Supplementation in Prevention of Colorectal Adenoma Recurrence: A Randomised Intervention Trial. Lancet 2000, 356, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.; Cooper, K.; Papaioannou, D.; Hind, D.; Pilgrim, H.; Tappenden, P. Supplemental Calcium in the Chemoprevention of Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Ther. 2010, 32, 789–803. [Google Scholar] [CrossRef]
- McCullough, M.L.; Robertson, A.S.; Rodriguez, C.; Jacobs, E.J.; Chao, A.; Carolyn, J.; Calle, E.E.; Willett, W.C.; Thun, M.J. Calcium, Vitamin D, Dairy Products, and Risk of Colorectal Cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control 2003, 14, 1–12. [Google Scholar] [CrossRef]
- Terry, P.; Baron, J.A.; Bergkvist, L.; Holmberg, L.; Wolk, A. Dietary Calcium and Vitamin D Intake and Risk of Colorectal Cancer: A Prospective Cohort Study in Women. Nutr. Cancer 2002, 43, 39–46. [Google Scholar] [CrossRef]
- Wu, K.; Willett, W.C.; Fuchs, C.S.; Colditz, G.A.; Giovannucci, E.L. Calcium Intake and Risk of Colon Cancer in Women and Men. J. Natl. Cancer Inst. 2002, 94, 437–446. [Google Scholar] [CrossRef]
- Zheng, W.; Anderson, K.E.; Kushi, L.H.; Sellers, T.A.; Greenstein, J.; Hong, C.P.; Cerhan, J.R.; Bostick, R.M.; Folsom, A.R. A Prospective Cohort Study of Intake of Calcium, Vitamin D, and Other Micronutrients in Relation to Incidence of Rectal Cancer among Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 1998, 7, 221–225. [Google Scholar]
- Wactawski-Wende, J.; Kotchen, J.M.; Anderson, G.L.; Assaf, A.R.; Brunner, R.L.; O’Sullivan, M.J.; Margolis, K.L.; Ockene, J.K.; Phillips, L.; Pottern, L.; et al. Calcium plus Vitamin D Supplementation and the Risk of Colorectal Cancer. N. Engl. J. Med. 2006, 354, 684–696. [Google Scholar] [CrossRef]
- Fedirko, V.; Bostick, R.M.; Flanders, W.D.; Long, Q.; Shaukat, A.; Rutherford, R.E.; Daniel, C.R.; Cohen, V.; Dash, C. Effects of Vitamin D and Calcium Supplementation on Markers of Apoptosis in Normal Colon Mucosa: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cancer Prev. Res. 2009, 2, 213–223. [Google Scholar] [CrossRef]
- Fedirko, V.; Bostick, R.M.; Flanders, W.D.; Long, Q.; Sidelnikov, E.; Shaukat, A.; Daniel, C.R.; Rutherford, R.E.; Woodard, J.J. Effects of Vitamin d and Calcium on Proliferation and Differentiation in Normal Colon Mucosa: A Randomized Clinical Trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Froicu, M.; Weaver, V.; Wynn, T.A.; McDowell, M.A.; Welsh, J.E.; Cantorna, M.T. A Crucial Role for the Vitamin D Receptor in Experimental Inflammatory Bowel Diseases. Mol. Endocrinol. 2003, 17, 2386–2392. [Google Scholar] [CrossRef]
- Froicu, M.; Zhu, Y.; Cantorna, M.T. Vitamin D Receptor Is Required to Control Gastrointestinal Immunity in IL-10 Knockout Mice. Immunology 2006, 117, 310–318. [Google Scholar] [CrossRef]
- Grau, M.V.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Beach, M.L.; Church, T.R.; Heber, D. Vitamin D, Calcium Supplementation, and Colorectal Adenomas: Results of a Randomized Trial. J. Natl. Cancer Inst. 2003, 95, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.M.; Helzlsouer, K.J.; Hollis, B.W.; Comstock, G.W. Colon Cancer and Serum Vitamin D Metabolite Levels 10-17 Years Prior to Diagnosis. Am. J. Epidemiol. 1995, 142, 608–611. [Google Scholar] [CrossRef]
- Feskanich, D.; Ma, J.; Fuchs, C.S.; Kirkner, G.J.; Hankinson, S.E.; Hollis, B.W.; Giovannucci, E.L. Plasma Vitamin D Metabolites and Risk of Colorectal Cancer in Women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1502–1508. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Dornschneider, G.; Izbicki, J.R.; Wilker, D.K.; Schweiberer, L. The Effects of Sex Steroids on Colon Carcinogenesis. Anticancer Drugs 1990, 1, 15–21. [Google Scholar] [CrossRef]
- Ma, J.; Pollak, M.N.; Giovannucci, E.; Chan, J.M.; Tao, Y.; Hennekens, C.H.; Stampfer, M.J. Prospective Study of Colorectal Cancer Risk in Men and Plasma Levels of Insulin-like Growth Factor (IGF)-I and IGF-Binding Protein-3. J. Natl. Cancer Inst. 1999, 91, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Witte, D.; Chirala, M.; Younes, A.; Li, Y.; Younes, M. Estrogen Receptor Beta Is Expressed in Human Colorectal Adenocarcinoma. Hum. Pathol. 2001, 32, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.A.; Terry, P.D.; Potter, J.D.; Bostick, R.M. Do Factors Related to Endogenous and Exogenous Estrogens Modify the Relationship between Obesity and Risk of Colorectal Adenomas in Women? Cancer Epidemiol. Biomark. Prev. 2007, 16, 676–683. [Google Scholar] [CrossRef]
- Jacobson, J.S.; Neugut, A.I.; Garbowski, G.C.; Ahsan, H.; Waye, J.D.; Treat, M.R.; Forde, K.A. Reproductive Risk Factors for Colorectal Adenomatous Polyps (New York City, NY, United States). Cancer Causes Control 1995, 6, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Peipins, L.A.; Newman, B.; Sandler, R.S. Reproductive History, Use of Exogenous Hormones, and Risk of Colorectal Adenomas. Cancer Epidemiol. Biomark. Prev. 1997, 6, 671–675. [Google Scholar]
- Potter, J.D.; Bostick, R.M.; Grandits, G.A.; Fosdick, L.; Elmer, P.; Wood, J.; Grambsch, P.; Louis, T.A. Hormone Replacement Therapy Is Associated with Lower Risk of Adenomatous Polyps of the Large Bowel: The Minnesota Cancer Prevention Research Unit Case-Control Study. Cancer Epidemiol. Biomark. Prev. 1996, 5, 779–784. [Google Scholar]
- Slattery, M.L.; Potter, J.D.; Curtin, K.; Edwards, S.; Ma, K.N.; Anderson, K.; Schaffer, D.; Samowitz, W.S. Estrogens Reduce and Withdrawal of Estrogens Increase Risk of Microsatellite Instability-Positive Colon Cancer. Cancer Res. 2001, 61, 126–130. [Google Scholar]
- Rennert, G.; Rennert, H.S.; Pinchev, M.; Lavie, O.; Gruber, S.B. Use of Hormone Replacement Therapy and the Risk of Colorectal Cancer. J. Clin. Oncol. 2009, 27, 4542–4547. [Google Scholar] [CrossRef]
- Prihartono, N.; Palmer, J.R.; Louik, C.; Shapiro, S.; Rosenberg, L. A Case-Control Study of Use of Postmenopausal Female Hormone Supplements in Relation to the Risk of Large Bowel Cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 443–447. [Google Scholar]
- Potter, J.D.; McMichael, A.J. Large Bowel Cancer in Women in Relation to Reproductive and Hormonal Factors: A Case-Control Study. J. Natl. Cancer Inst. 1983, 71, 703–709. [Google Scholar]
- Chlebowski, R.T.; Wactawski-Wende, J.; Ritenbaugh, C.; Hubbell, F.A.; Ascensao, J.; Rodabough, R.J.; Rosenberg, C.A.; Taylor, V.M.; Harris, R.; Chen, C.; et al. Estrogen plus Progestin and Colorectal Cancer in Postmenopausal Women. N. Engl. J. Med. 2004, 350, 991–1004. [Google Scholar] [CrossRef]
- Ritenbaugh, C.; Stanford, J.L.; Wu, L.; Shikany, J.M.; Schoen, R.E.; Stefanick, M.L.; Taylor, V.; Garland, C.; Frank, G.; Lane, D.; et al. Conjugated Equine Estrogens and Colorectal Cancer Incidence and Survival: The Women’s Health Initiative Randomized Clinical Trial. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and Benefits of Estrogen plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Zheng, Y.; Chia, V.M.; Morimoto, L.M.; Doria-Rose, V.P.; Templeton, A.; Thibodeau, S.N.; Potter, J.D. Estrogen plus Progestin Use, Microsatellite Instability, and the Risk of Colorectal Cancer in Women. Cancer Res. 2007, 67, 7534–7539. [Google Scholar] [CrossRef]
- Tannen, R.L.; Weiner, M.G.; Xie, D.; Barnhart, K. Estrogen Affects Post-Menopausal Women Differently than Estrogen plus Progestin Replacement Therapy. Hum. Reprod. 2007, 22, 1769–1777. [Google Scholar] [CrossRef]
- Clapper, M.L.; Chang, W.C.; Meropol, N.J. Chemoprevention of Colorectal Cancer. Curr. Opin. Oncol. 2001, 13, 307–313. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and Beverages and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies, an Update of the Evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is Dietary Fibre Truly Protective against Colon Cancer? A Systematic Review and Meta-Analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Trock, B.; Lanza, E.; Greenwald, P. Dietary Fiber, Vegetables, and Colon Cancer: Critical Review and Meta-Analyses of the Epidemiologic Evidence. J. Natl. Cancer Inst. 1990, 82, 650–661. [Google Scholar] [CrossRef]
- Haas, P.; Machado, M.J.; Anton, A.A.; Silva, A.S.S.; de Francisco, A. Effectiveness of Whole Grain Consumption in the Prevention of Colorectal Cancer: Meta-Analysis of Cohort Studies. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S6), 1–13. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary Fibre, Whole Grains, and Risk of Colorectal Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Lau, R.; Chan, D.S.M.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Nonlinear Reduction in Risk for Colorectal Cancer by Fruit and Vegetable Intake Based on Meta-Analysis of Prospective Studies. Gastroenterology 2011, 141, 106–118. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous Vegetables Intake and the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef]
- Kashino, I.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Nagata, C.; Inoue, M.; Tsugane, S.; et al. Vegetable Consumption and Colorectal Cancer Risk: An Evaluation Based on a Systematic Review and Meta-Analysis among the Japanese Population. Jpn. J. Clin. Oncol. 2015, 45, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, Y.; Qi, L.; Zhong, R.; Miao, X. Dietary Legume Consumption Reduces Risk of Colorectal Cancer: Evidence from a Meta-Analysis of Cohort Studies. Sci. Rep. 2015, 5, 8797. [Google Scholar] [CrossRef]
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and Cancer Risk in the Korean Population: A Meta- Analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8509–8519. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, A.; Grossman, M.; Yeung, K.; Sei, S.S.; Lipkin, S.; Kloor, M. Vaccines for Immunoprevention of DNA Mismatch Repair Deficient Cancers. J. Immunother. Cancer 2022, 10, e004416. [Google Scholar] [CrossRef]
- Jackson, K.; Samaddar, S.; Markiewicz, M.A.; Bansal, A. Vaccination-Based Immunoprevention of Colorectal Tumors. J. Clin. Gastroenterol. 2022, 57, 246–252. [Google Scholar] [CrossRef]
- Kloor, M.; Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.-R.; Al-Batran, S.-E.; Tariverdian, M.; Jäger, E.; von Knebel Doeberitz, M. A Frameshift Peptide Neoantigen-Based Vaccine for Mismatch Repair-Deficient Cancers: A Phase I/IIa Clinical Trial. Clin. Cancer Res. 2020, 26, 4503–4510. [Google Scholar] [CrossRef]
- Leoni, G.; D’Alise, A.M.; Cotugno, G.; Langone, F.; Garzia, I.; De Lucia, M.; Fichera, I.; Vitale, R.; Bignone, V.; Tucci, F.G.; et al. A Genetic Vaccine Encoding Shared Cancer Neoantigens to Treat Tumors with Microsatellite Instability. Cancer Res. 2020, 80, 3972–3982. [Google Scholar] [CrossRef]
- Nouscom, S.R.L. A Phase I/II, Multicenter, Open-Label Study of Nous-209 Genetic Vaccine for the Treatment of Microsatellite Unstable Solid Tumors. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04041310 (accessed on 17 April 2023).
- National Cancer Institute (NCI). A Phase Ib/II Clinical Trial of Nous-209 for Recurrent Neoantigen Immunogenicity and Cancer Immune Interception in Lynch Syndrome. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05078866 (accessed on 17 April 2023).
- Bautz, D.J.; Sherpa, A.T.; Threadgill, D.W. Prophylactic Vaccination Targeting ERBB3 Decreases Polyp Burden in a Mouse Model of Human Colorectal Cancer. Oncoimmunology 2017, 6, e1255395. [Google Scholar] [CrossRef]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 Vaccine for Individuals with Advanced Adenoma of the Colon: A Cancer Immunoprevention Feasibility Study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Schoen, R.E.; Boardman, L.A.; Cruz-Correa, M.; Bansal, A.; Kastenberg, D.; Hur, C.; Dzubinski, L.; Kaufman, S.F.; Rodriguez, L.M.; Richmond, E.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of MUC1 Peptide Vaccine for Prevention of Recurrent Colorectal Adenoma. Clin. Cancer Res. 2023, OF1–OF11. [Google Scholar] [CrossRef] [PubMed]
- LoGiudice, N.; Le, L.; Abuan, I.; Leizorek, Y.; Roberts, S.C. Alpha-Difluoromethylornithine, an Irreversible Inhibitor of Polyamine Biosynthesis, as a Therapeutic Strategy against Hyperproliferative and Infectious Diseases. Med. Sci. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, J.; Wilson, P.S.; Bull, A.W.; Nigro, N.D. Ornithine Decarboxylase Activity in the Rat and Human Colon. Cancer Res. 1984, 44, 3226–3230. [Google Scholar]
- Meyskens, F.L.; McLaren, C.E.; Pelot, D.; Fujikawa-Brooks, S.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; et al. Difluoromethylornithine plus Sulindac for the Prevention of Sporadic Colorectal Adenomas: A Randomized Placebo-Controlled, Double-Blind Trial. Cancer Prev. Res. 2008, 1, 32–38. [Google Scholar] [CrossRef]
- Burke, C.A.; Dekker, E.; Lynch, P.; Samadder, N.J.; Balaguer, F.; Hüneburg, R.; Burn, J.; Castells, A.; Gallinger, S.; Lim, R.; et al. Eflornithine plus Sulindac for Prevention of Progression in Familial Adenomatous Polyposis. N. Engl. J. Med. 2020, 383, 1028–1039. [Google Scholar] [CrossRef]
- Lynch, P.M.; Burke, C.A.; Phillips, R.; Morris, J.S.; Slack, R.; Wang, X.; Liu, J.; Patterson, S.; Sinicrope, F.A.; Rodriguez-Bigas, M.A.; et al. An International Randomised Trial of Celecoxib versus Celecoxib plus Difluoromethylornithine in Patients with Familial Adenomatous Polyposis. Gut 2016, 65, 286–295. [Google Scholar] [CrossRef]
- Saydjari, R.; Townsend, C.M.; Barranco, S.C.; Thompson, J.C. Effects of Cyclosporine and Alpha-Difluoromethylornithine on the Growth of Mouse Colon Cancer in Vitro. Life Sci. 1987, 40, 359–366. [Google Scholar] [CrossRef] [PubMed]
- McGarrity, T.J.; Peiffer, L.P. Selenium and Difluoromethylornithine Additively Inhibit DMH-Induced Distal Colon Tumor Formation in Rats Fed a Fiber-Free Diet. Carcinogenesis 1993, 14, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon Cancer and the Epidermal Growth Factor Receptor: Current Treatment Paradigms, the Importance of Diet, and the Role of Chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal. Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Janani, B.; Vijayakumar, M.; Priya, K.; Kim, J.H.; Prabakaran, D.S.; Shahid, M.; Al-Ghamdi, S.; Alsaidan, M.; Othman Bahakim, N.; Hassan Abdelzaher, M.; et al. EGFR-Based Targeted Therapy for Colorectal Cancer-Promises and Challenges. Vaccines 2022, 10, 499. [Google Scholar] [CrossRef]
- Wang, D.; Xia, D.; Dubois, R.N. The Crosstalk of PTGS2 and EGF Signaling Pathways in Colorectal Cancer. Cancers 2011, 3, 3894–3908. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Neklason, D.W.; Boucher, K.M.; Byrne, K.R.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Done, M.W.; Berry, T.; et al. Effect of Sulindac and Erlotinib vs Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 1266–1275. [Google Scholar] [CrossRef]
- Samadder, N.J.; Kuwada, S.K.; Boucher, K.M.; Byrne, K.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Westover, M.; Berry, T.; et al. Association of Sulindac and Erlotinib vs Placebo with Colorectal Neoplasia in Familial Adenomatous Polyposis: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 671–677. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 Promotes Tumour Incidence and Growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef]
- A Study of Guselkumab in Participants with Familial Adenomatous Polyposis—No Study Results Posted—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03649971 (accessed on 2 January 2023).
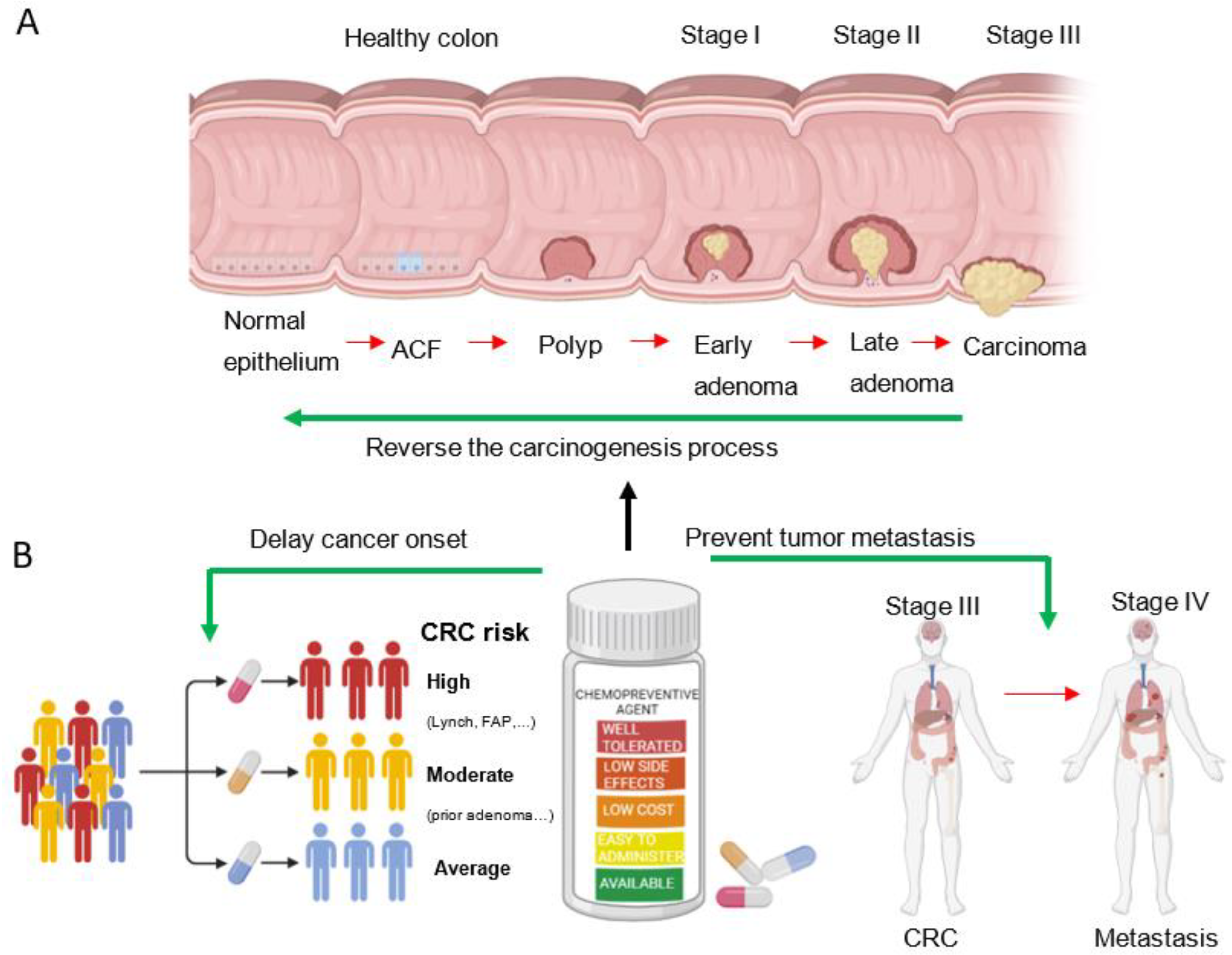
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepore Signorile, M.; Grossi, V.; Fasano, C.; Simone, C. Colorectal Cancer Chemoprevention: A Dream Coming True? Int. J. Mol. Sci. 2023, 24, 7597. https://doi.org/10.3390/ijms24087597
Lepore Signorile M, Grossi V, Fasano C, Simone C. Colorectal Cancer Chemoprevention: A Dream Coming True? International Journal of Molecular Sciences. 2023; 24(8):7597. https://doi.org/10.3390/ijms24087597
Chicago/Turabian StyleLepore Signorile, Martina, Valentina Grossi, Candida Fasano, and Cristiano Simone. 2023. "Colorectal Cancer Chemoprevention: A Dream Coming True?" International Journal of Molecular Sciences 24, no. 8: 7597. https://doi.org/10.3390/ijms24087597
APA StyleLepore Signorile, M., Grossi, V., Fasano, C., & Simone, C. (2023). Colorectal Cancer Chemoprevention: A Dream Coming True? International Journal of Molecular Sciences, 24(8), 7597. https://doi.org/10.3390/ijms24087597







