Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Article Eligibility: Inclusion and Exclusion Criteria
- Articles of type: case reports, case series, or secondary literature (reviews, meta-analysis, book chapters);
- Articles for which full text was impossible to source: conference abstracts and posters and unpublished data;
- Retracted articles;
- Patients under treatment for which pre-treatment data were unavailable;
- Patients suffering from illness for which pre-illness data were unavailable;
- Studies completed in silico, in vitro, or in vivo on nonhuman subjects.
2.2. Search Strategy
2.3. Data Collection
2.4. Quality Assessment
2.5. Data Analysis
3. Results
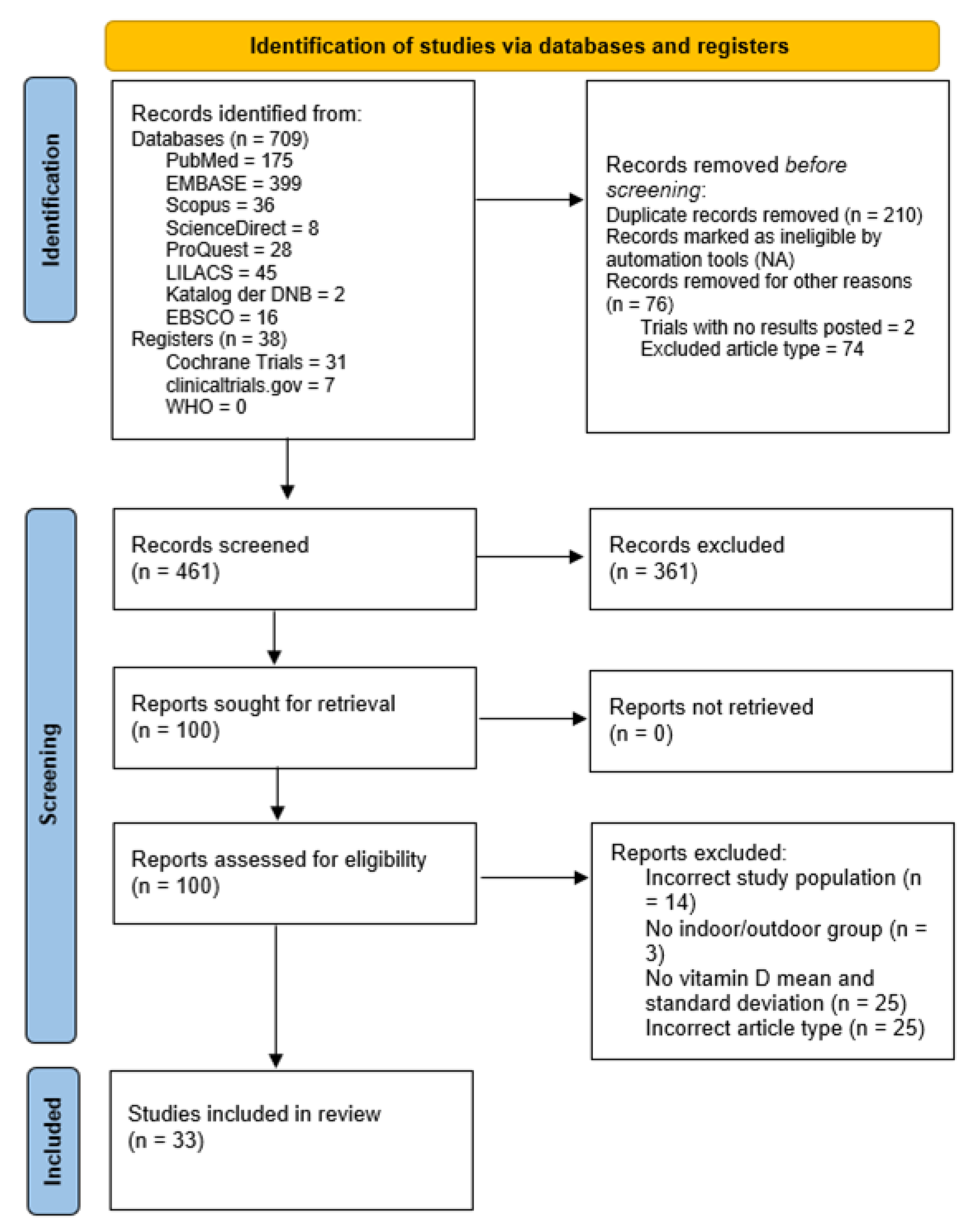
3.1. Study Identification and Selection
3.2. Quality Assessment and Risk-of-Bias Evaluation
3.3. Evaluation of Publication Bias

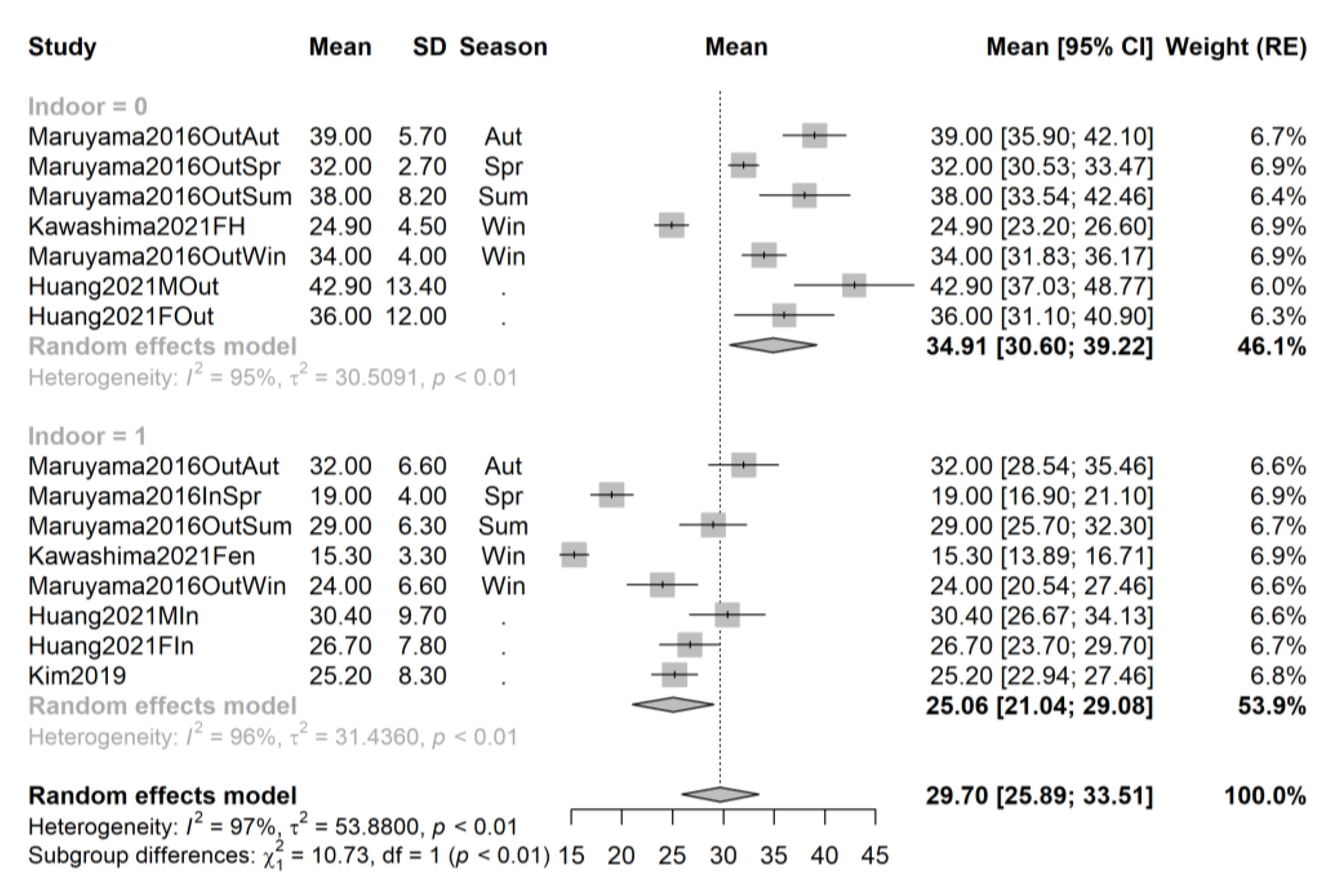
3.4. Pooling and Forest Plots
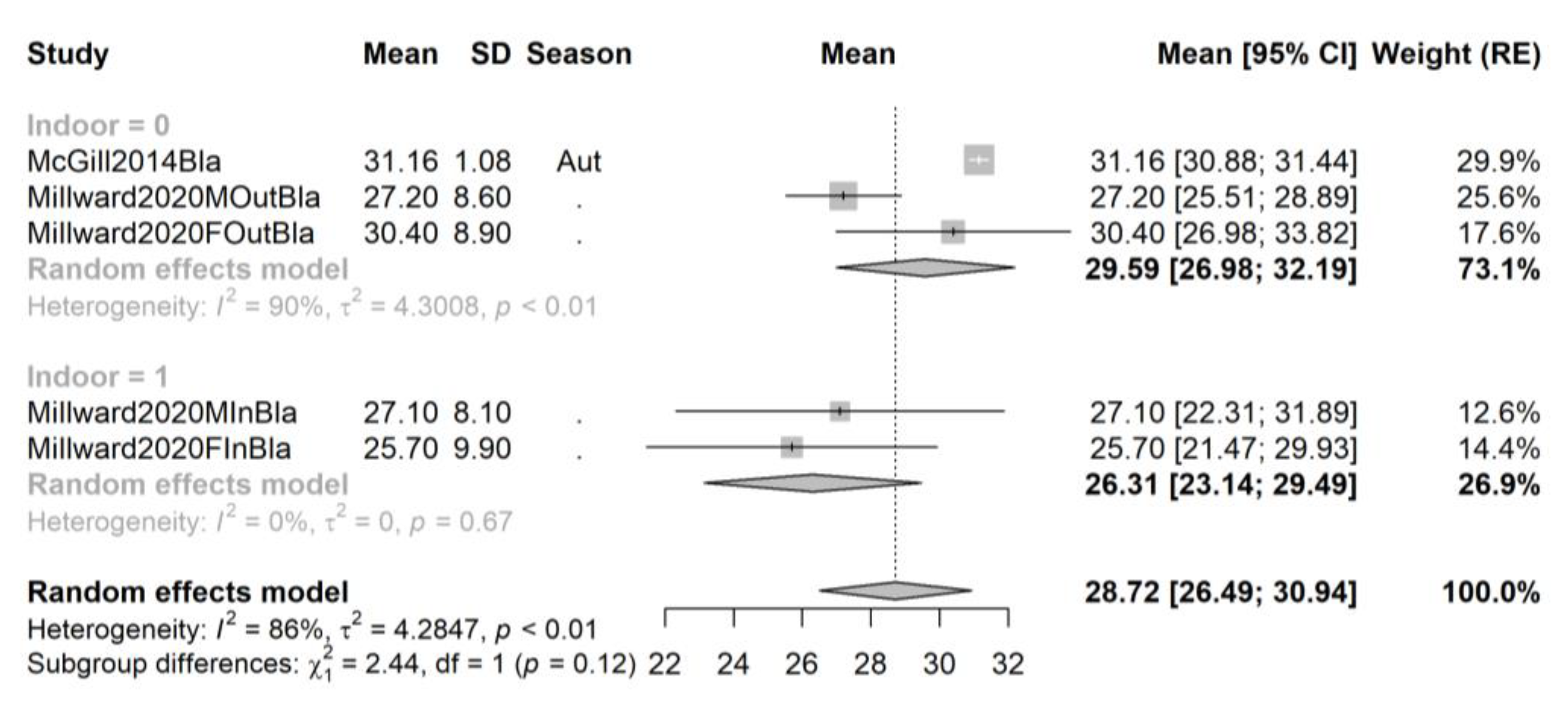
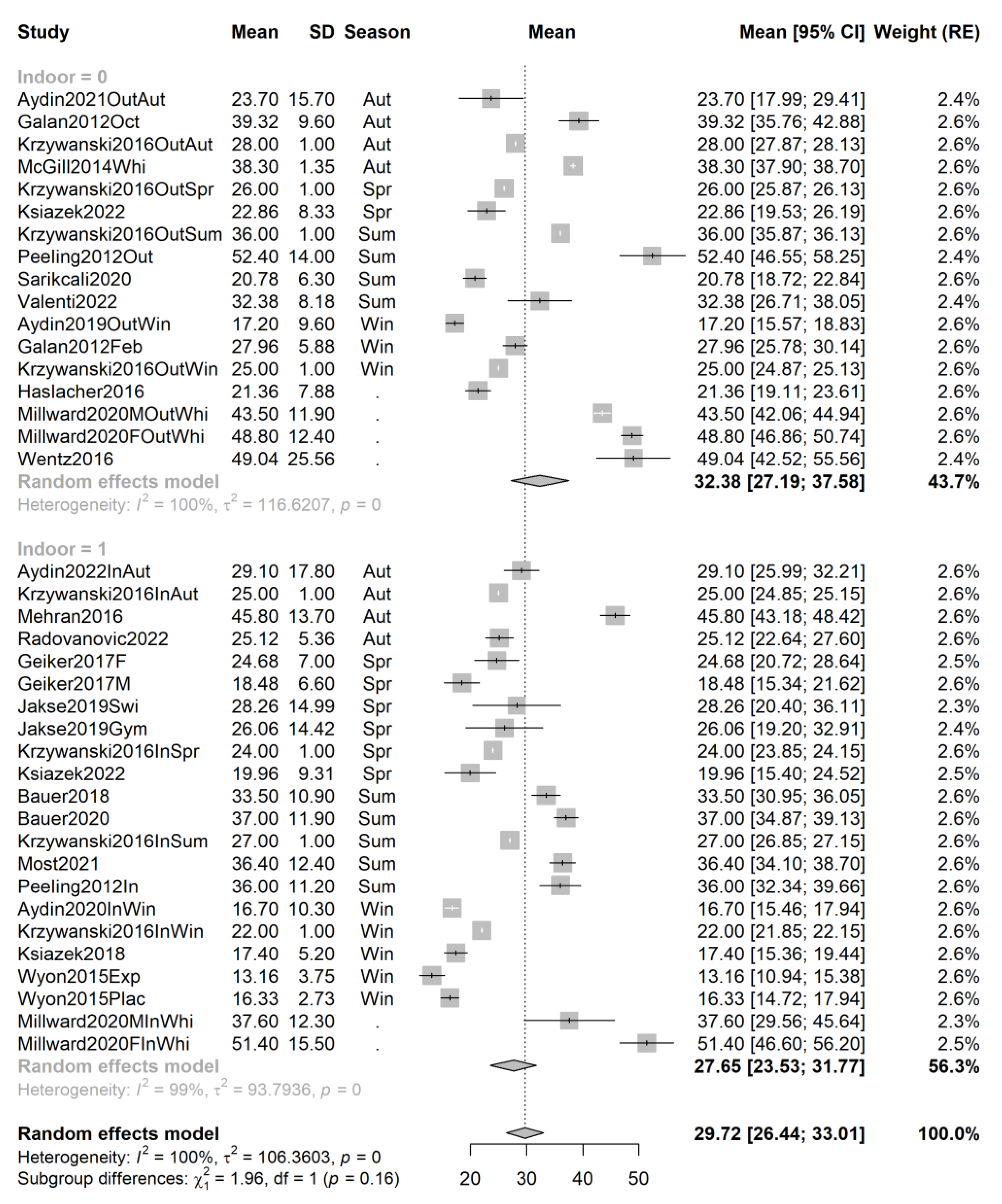
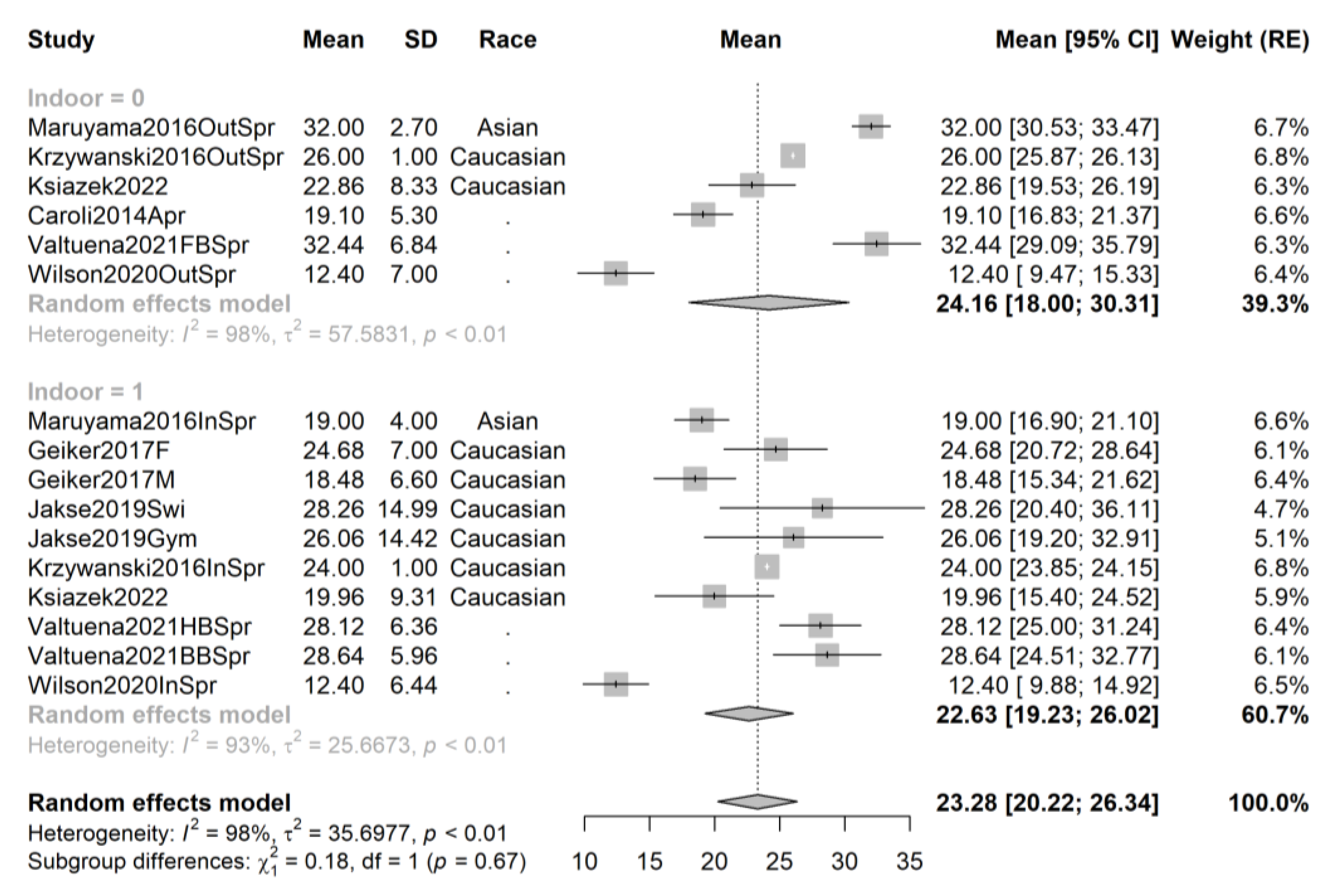
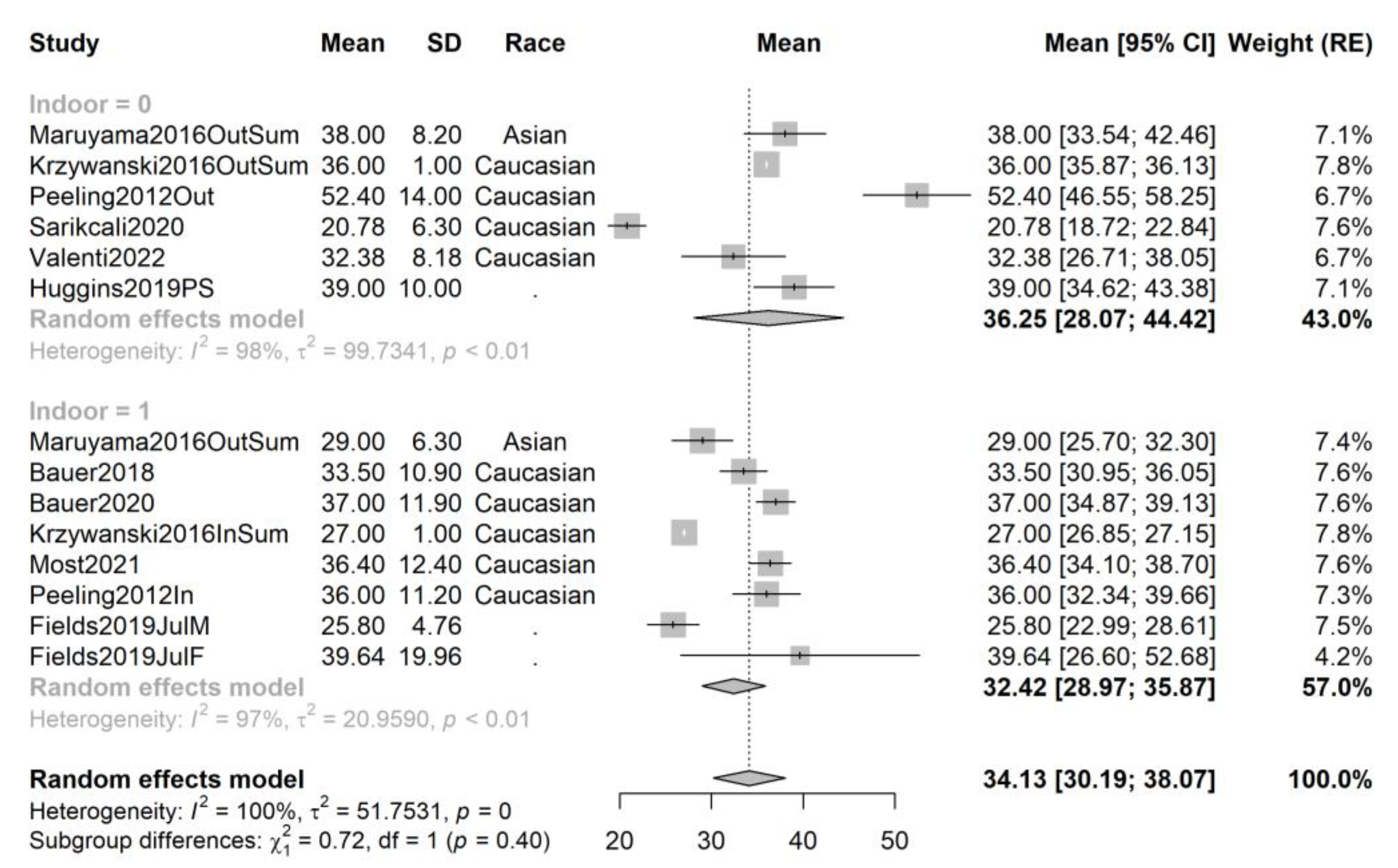
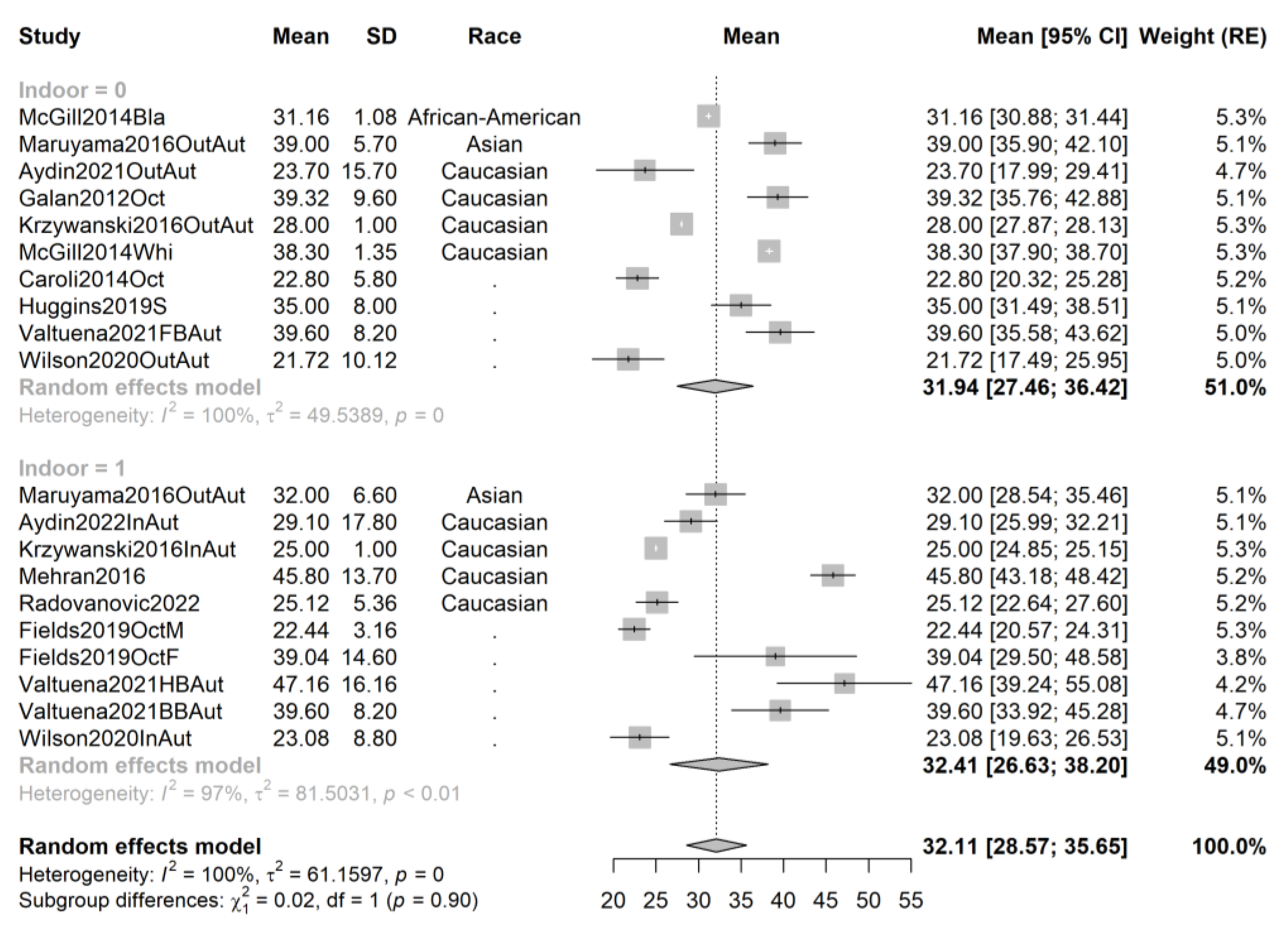

3.5. Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Rendic, S.P.; Peter Guengerich, F. Human cytochrome P450 enzymes 5–51 as targets of drugs and natural and environmental compounds: Mechanisms, induction, and inhibition—Toxic effects and benefits. Drug Metab. Rev. 2018, 50, 256–342. [Google Scholar] [CrossRef]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G.; Sboarina, A.; Vella, A. The Role of Vitamin D in the Immune System as a Pro-survival Molecule. Clin. Ther. 2017, 39, 894–916. [Google Scholar] [CrossRef]
- Wolf, S.T.; Kenney, W.L. The vitamin D-folate hypothesis in human vascular health. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R491–R501. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm.-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Saraff, V.; Shaw, N. Sunshine and vitamin D. Arch. Dis. Child. 2016, 101, 190–192. [Google Scholar] [CrossRef]
- Sowah, D.; Fan, X.; Dennett, L.; Hagtvedt, R.; Straube, S. Vitamin D levels and deficiency with different occupations: A systematic review. BMC Public Health 2017, 17, 51. [Google Scholar] [CrossRef]
- Taylor, P.N.; Davies, J.S. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef]
- Barsan, M.; Brata, A.M.; Ismaiel, A.; Dumitrascu, D.I.; Badulescu, A.-V.; Duse, T.A.; Dascalescu, S.; Popa, S.L.; Grad, S.; Muresan, L.; et al. The Pathogenesis of Cardiac Arrhythmias in Vitamin D Deficiency. Biomedicines 2022, 10, 1239. [Google Scholar] [CrossRef]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-De-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef]
- Seiler, N.; Tsiglopoulos, J.; Keem, M.; Das, S.; Waterdrinker, A. Prevalence of vitamin D deficiency among psychiatric inpatients: A systematic review. Int. J. Psychiatry Clin. Pract. 2022, 26, 330–336. [Google Scholar] [CrossRef]
- Saji Parel, N.; Krishna, P.V.; Gupta, A.; Uthayaseelan, K.; Uthayaseelan, K.; Kadari, M.; Subhan, M.; Kasire, S.P. Depression and Vitamin D: A Peculiar Relationship. Cureus 2022, 14, e24363. [Google Scholar] [CrossRef]
- Lawler, T.; Andersen, S.W. Serum 25-Hydroxyvitamin D and Cancer Risk: A Systematic Review of Mendelian Randomization Studies. Nutrients 2023, 15, 422. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef]
- Matta Reddy, A.; Iqbal, M.; Chopra, H.; Urmi, S.; Junapudi, S.; Bibi, S.; Gupta, S.K.; Pangi, V.N.; Singh, I.; Abdel-Daim, M.M. Pivotal role of vitamin D in mitochondrial health, cardiac function, and human reproduction. EXCLI J. 2022, 21, 967–990. [Google Scholar]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’Alicandro, G.; et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef]
- Harju, T.; Gray, B.; Mavroedi, A.; Farooq, A.; Reilly, J.J. Prevalence and novel risk factors for vitamin D insufficiency in elite athletes: Systematic review and meta-analysis. Eur. J. Nutr. 2022, 61, 3857–3871. [Google Scholar] [CrossRef] [PubMed]
- Ip, T.S.T.; Fu, S.C.; Ong, M.T.Y.; Yung, P.S.H. Vitamin D deficiency in athletes: Laboratory, clinical and field integration. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2022, 29, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Vitamin D, exercise, and immune health in athletes: A narrative review. Front. Immunol. 2022, 13, 954994. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.954994 (accessed on 20 December 2022). [CrossRef]
- Schwellnus, M.; Soligard, T.; Alonso, J.-M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ng, J.W.C.; Lee, J.K.W. Nutrition Recommendations for Table Tennis Players—A Narrative Review. Nutrients 2023, 15, 775. [Google Scholar] [CrossRef]
- Rojano-Ortega, D.; la Rosa, F.J.B. Effects of vitamin D supplementation on muscle function and recovery after exercise-induced muscle damage: A systematic review. J. Hum. Nutr. Diet. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 January 2023).
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 January 2023).
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Geissbühler, M.; Hincapié, C.A.; Aghlmandi, S.; Zwahlen, M.; Jüni, P.; da Costa, B.R. Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: A meta-epidemiological study. BMC Med. Res. Methodol. 2021, 21, 123. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 December 2022).
- RStudio: Integrated Development Environment for R.; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 1 December 2022).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Évid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Higgins, J.P.T. Meta-analysis and subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021; Available online: https://www.taylorfrancis.com/books/9781003107347 (accessed on 15 January 2023).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Aydın, C.G.; Dinçel, Y.M.; Arıkan, Y.; Taş, S.K.; Deniz, S. The effects of indoor and outdoor sports participation and seasonal changes on vitamin D levels in athletes. SAGE Open Med. 2019, 7, 2050312119837480. [Google Scholar] [CrossRef]
- Bauer, P.; Henni, S.; Dörr, O.; Bauer, T.; Hamm, C.W.; Most, A. High prevalence of vitamin D insufficiency in professional handball athletes. Physician Sportsmed. 2018, 47, 71–77. [Google Scholar] [CrossRef]
- Bauer, P.; Kraushaar, L.; Dörr, O.; Bauer, T.; Nef, H.; Hamm, C.W.; Most, A. Association of 25-hydroxy vitamin D level with the blood pressure response to a maximum exercise test among professional indoor athletes. Eur. J. Appl. Physiol. 2020, 120, 1931–1941. [Google Scholar] [CrossRef]
- Fields, J.B.; Gallo, S.; Worswick, J.M.; Busteed, D.R.; Jones, M.T. 25-Hydroxyvitamin D, Vitamin D Binding Protein, Bioavailable 25-Hydroxyvitamin D, and Body Composition in a Diverse Sample of Women Collegiate Indoor Athletes. J. Funct. Morphol. Kinesiol. 2020, 5, 32. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Hansen, M.; Jakobsen, J.; Kristensen, M.; Larsen, R.; Jørgensen, N.R.; Hansen, B.S.; Bügel, S. Vitamin D Status and Muscle Function Among Adolescent and Young Swimmers. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lum, D.; Haiyum, M.; Fairbairn, K.A. Vitamin D Status of Elite Athletes in Singapore and Its Associations with Muscle Function and Bone Health. J. Sci. Sport Exerc. 2021, 3, 385–393. [Google Scholar] [CrossRef]
- Jakse, B.; Sekulic, D.; Jakse, B.; Cuk, I.; Sajber, D. Bone health among indoor female athletes and associated factors; a cross-sectional study. Res. Sports Med. 2019, 28, 314–323. [Google Scholar] [CrossRef]
- Kawashima, I.; Hiraiwa, H.; Ishizuka, S.; Kawai, R.; Hoshino, Y.; Kusaka, Y.; Tsukahara, T. Comparison of vitamin D sufficiency between indoor and outdoor elite male collegiate athletes. Nagoya J. Med. Sci. 2021, 83, 219–226. [Google Scholar]
- Kim, D.K.; Park, G.; Kuo, L.-T.; Park, W.H. The Relationship between Vitamin D Status and Rotator Cuff Muscle Strength in Professional Volleyball Athletes. Nutrients 2019, 11, 2768. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Dziubek, W.; Pietraszewska, J.; Słowińska-Lisowska, M. Relationship between 25(OH)D levels and athletic performance in elite Polish judoists. Biol. Sport 2018, 35, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M.; Lombardi, G. Relationship Between Metabolites of Vitamin D, Free 25-(OH)D, and Physical Performance in Indoor and Outdoor Athletes. Front. Physiol. 2022, 13, 909086. [Google Scholar] [CrossRef] [PubMed]
- McGill, L.E. Serum Concentration Levels of 25(OH)D and Injury Reports in NCAA Division I Football Players. The University of Texas at Austin. Available online: https://repositories.lib.utexas.edu/handle/2152/26341 (accessed on 24 February 2023).
- Mehran, N.; Schulz, B.M.; Neri, B.R.; Robertson, W.J.; Limpisvasti, O. Prevalence of Vitamin D Insufficiency in Professional Hockey Players. Orthop. J. Sports Med. 2016, 4, 2325967116677512. [Google Scholar] [CrossRef] [PubMed]
- Most, A.; Dörr, O.; Nef, H.; Hamm, C.; Bauer, T.; Bauer, P. Influence of 25-Hydroxy-Vitamin D Insufficiency on Maximal Aerobic Power in Elite Indoor Athletes: A Cross-Sectional Study. Sports Med.-Open 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Fulton, S.K.; Binnie, M.; Goodman, C. Training Environment and Vitamin D Status in Athletes. Int. J. Sports Med. 2012, 34, 248–252. [Google Scholar] [CrossRef]
- Radovanović, D.; Stoičkov, V.; Pechanova, O.; Scanlan, A.T.; Jakovljević, V.; Stojanović, E. The Relationships Between 25-Hydroxyvitamin D and Echocardiographic Parameters in Female Basketball Players. Clin. J. Sport Med. 2022, 32, e492–e498. [Google Scholar] [CrossRef]
- Ricart, B.; Monteagudo, P.; Blasco-Lafarga, C. Hypovitaminosis D in Young Basketball Players: Association with Jumping and Hopping Performance Considering Gender. Int. J. Environ. Res. Public Health 2021, 18, 5446. [Google Scholar] [CrossRef]
- Sariakcali, B.; Ceylan, L.; Elioz, M. Evaluation of end-seasonal vitamin d, plasma lipid and other biochemical measurements in professional football players: The case of sivas province in turkey. Progr. Nutr. 2020, 22, e2020027. [Google Scholar] [CrossRef]
- Sghaier-Ayadi, A.; Feki, M.; Ayed, I.B.-B.; Abene, O.; Ben Fredj, M.; Kaabachi, N.; Chaouachi, A. Vitamin D status and determinants of deficiency in non-supplemented athletes during the winter months in Tunisia. Biol. Sport 2015, 32, 281–287. [Google Scholar] [CrossRef]
- Valtueña, J.; Dominguez, D.; Til, L.; González-Gross, M.; Drobnic, F. High prevalence of vitamin D insufficiency among elite Spanish athletes the importance of outdoor training adaptation. Nutr. Hosp. 2014, 30, 124–131. [Google Scholar] [PubMed]
- Wentz, L.M.; Liu, P.-Y.; Ilich, J.Z.; Haymes, E.M. Female Distance Runners Training in Southeastern United States Have Adequate Vitamin D Status. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Caroli, B.; Pasin, F.; Aloe, R.; Gnocchi, C.; Cas, A.D.; Galli, C.; Passeri, G. Characterization of skeletal parameters in a cohort of North Italian rugby players. J. Endocrinol. Investig. 2014, 37, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.B.; Payne, D.C.; Gallo, S.; Busteed, D.R.; Jones, M.T. Vitamin D Status Differs by Sex, Sport-Season, and Skin Pigmentation among Elite Collegiate Basketball Players. Sports 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Galan, F.; Ribas, J.; Sánchez-Martinez, P.M.; Calero, T.; Sánchez, A.B.; Muñoz, A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin. Nutr. 2012, 31, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Haslacher, H.; Nistler, S.; Batmyagmar, D.; Ponocny-Seliger, E.; Perkmann, T.; Scherzer, T.M.; Kundi, M.; Endler, G.; Ratzinger, F.; Pilger, A.; et al. Low Vitamin D Levels Do Not Predict Hyperglycemia in Elderly Endurance Athletes (but in Controls). PLoS ONE 2016, 11, e0157695. [Google Scholar] [CrossRef]
- Huggins, R.; Fortunati, A.R.; Curtis, R.M.; Looney, D.P.; West, C.A.; Lee, E.C.; Fragala, M.S.; Hall, M.L.; Casa, D.J. Monitoring Blood Biomarkers and Training Load Throughout a Collegiate Soccer Season. J. Strength Cond. Res. 2019, 33, 3065–3077. [Google Scholar] [CrossRef]
- Krzywanski, J.; Mikulski, T.; Krysztofiak, H.; Mlynczak, M.; Gaczynska, E.; Ziemba, A. Seasonal Vitamin D Status in Polish Elite Athletes in Relation to Sun Exposure and Oral Supplementation. PLoS ONE 2016, 11, e0164395. [Google Scholar] [CrossRef]
- Maruyama-Nagao, A.; Sakuraba, K.; Suzuki, Y. Seasonal variations in vitamin D status in indoor and outdoor female athletes. Biomed. Rep. 2016, 5, 113–117. [Google Scholar] [CrossRef]
- Millward, D.; Root, A.D.; Dubois, J.; Cohen, R.P.; Valdivia, L.; Helming, B.; Kokoskie, J.; Waterbrook, A.L.; Paul, S. Association of Serum Vitamin D Levels and Stress Fractures in Collegiate Athletes. Orthop. J. Sports Med. 2020, 8, 2325967120966967. [Google Scholar] [CrossRef]
- Wilson-Barnes, S.L.; Hunt, J.E.A.; Williams, E.L.; Allison, S.J.; Wild, J.J.; Wainwright, J.; Lanham-New, S.A.; Manders, R.J.F. Seasonal variation in vitamin D status, bone health and athletic performance in competitive university student athletes: A longitudinal study. J. Nutr. Sci. 2020, 9, e8. [Google Scholar] [CrossRef] [PubMed]
- Valtueña, J.; Aparicio-Ugarriza, R.; Medina, D.; Lizarraga, A.; Rodas, G.; González-Gross, M.; Drobnic, F. Vitamin D Status in Spanish Elite Team Sport Players. Nutrients 2021, 13, 1311. [Google Scholar] [CrossRef]
- Wyon, M.A.; Wolman, R.; Nevill, A.M.; Cloak, R.; Metsios, G.S.; Gould, D.; Ingham, A.; Koutedakis, Y. Acute Effects of Vitamin D3 Supplementation on Muscle Strength in Judoka Athletes: A Randomized Placebo-Controlled, Double-Blind Trial. Clin. J. Sport Med. 2016, 26, 279–284. [Google Scholar] [CrossRef]
- Valenti, M.T.; Braggio, M.; Minoia, A.; Dorelli, G.; Bertacco, J.; Bertoldo, F.; Cominacini, M.; De Simone, T.; Romanelli, M.G.; Bhandary, L.; et al. Effects of a 4400 km ultra-cycling non-competitive race and related training on body composition and circulating progenitors differentiation. J. Transl. Med. 2022, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology 2020, 162, bqaa218. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Pilarski, N.S.; Markland, A.D.; Marson, E.J.; Devall, A.; Hewison, M.; Morris, R.K.; Coomarasamy, A. Vitamin D and miscarriage: A systematic review and meta-analysis. Fertil. Steril. 2022, 118, 111–122. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. [Google Scholar] [CrossRef]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Jastrzębski, Z.; Hill, L.; Nikolaidis, P.T. Vitamin D and Stress Fractures in Sport: Preventive and Therapeutic Measures—A Narrative Review. Medicina 2021, 57, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Quan, M.; Cao, Z.-B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of Vitamin D Inadequacy in Athletes: A Systematic-Review and Meta-Analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.C.G.B.; Corrêa, M.P.; Holick, M.F.; Melo, E.V.; Lazaretti-Castro, M. Sun-induced production of vitamin D3 throughout 1 year in tropical and subtropical regions: Relationship with latitude, cloudiness, UV-B exposure and solar zenith angle. Photochem. Photobiol. Sci. 2021, 20, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Arantes, H.P.; Kulak, C.A.; Fernandes, C.E.; Zerbini, C.; Bandeira, F.; Barbosa, I.C.; Brenol, J.C.; Russo, L.A.; Borba, V.C.; Chiang, A.Y.; et al. Correlation between 25-hydroxyvitamin D levels and latitude in Brazilian postmenopausal women: From the Arzoxifene Generations Trial. Osteoporos. Int. 2013, 24, 2707–2712. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Lucas, R.M.; Harrison, S.L.; van der Mei, I.; Armstrong, B.K.; Whiteman, D.C.; Kricker, A.; Nowak, M.; Brodie, A.M.; Sun, J. The Contributions of Solar Ultraviolet Radiation Exposure and Other Determinants to Serum 25-Hydroxyvitamin D Concentrations in Australian Adults: The AusD Study. Am. J. Epidemiol. 2014, 179, 864–874. [Google Scholar] [CrossRef]
- Hagenau, T.; Vest, R.; Gissel, T.N.; Poulsen, C.S.; Erlandsen, M.; Mosekilde, L.; Vestergaard, P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos. Int. 2008, 20, 133–140. [Google Scholar] [CrossRef]
- Lucas, R.M.; Byrne, S.N.; Correale, J.; Ilschner, S.; Hart, P.H. Ultraviolet radiation, vitamin D and multiple sclerosis. Neurodegener. Dis. Manag. 2015, 5, 413–424. [Google Scholar] [CrossRef]
- Simpson, S., Jr.; Blizzard, L.; Otahal, P.; van der Mei, I.; Taylor, B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1132–1141. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, N.; Cheng, S.; Wang, Z.; Qin, Y. Gender Differences in Vitamin D Status in China. Med. Sci. Monit. 2019, 25, 7094–7099. [Google Scholar] [CrossRef] [PubMed]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Rieckmann, N.; Hintzpeter, B.; Mensink, G.B. Vitamin D status among adults in Germany—Results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015, 15, 641. [Google Scholar] [CrossRef]
- Minter, M.; Augustin, H.; van Odijk, J.; Vanfleteren, L.E.G.W. Gender Differences in Vitamin D Status and Determinants of Vitamin D Insufficiency in Patients with Chronic Obstructive Pulmonary Disease. Nutrients 2023, 15, 426. [Google Scholar] [CrossRef]
- Wilson, O.W.A.; Colinear, C.; Guthrie, D.; Bopp, M. Gender differences in college student physical activity, and campus recreational facility use, and comfort. J. Am. Coll. Health 2020, 70, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Lucock, M.; Chaplin, G.; Jablonski, N.G.; Veysey, M.; Scarlett, C.; Beckett, E. Distribution of variants in multiple vitamin D-related loci (DHCR7/NADSYN1, GC, CYP2R1, CYP11A1, CYP24A1, VDR, RXRα and RXRγ) vary between European, East-Asian and Sub-Saharan African-ancestry populations. Genes Nutr. 2020, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Göktaş, O.; Ersoy, C.; Ercan, I.; Can, F.E. Vitamin D status in the adult population of Bursa-Turkey. Eur. J. Gen. Pract. 2020, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Won, J.W.; Jung, S.K.; Jung, I.A.; Lee, Y. Seasonal Changes in Vitamin D Levels of Healthy Children in Mid-Latitude, Asian Urban Area. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 207–217. [Google Scholar] [CrossRef]
- Bleizgys, A.; Kurovskij, J. Vitamin D Levels of Out-Patients in Lithuania: Deficiency and Hypervitaminosis. Medicina 2018, 54, 25. [Google Scholar] [CrossRef] [PubMed]
- Neville, V.; Gant, N.; Folland, J.P. Thermoregulatory demands of Elite Professional America’s Cup Yacht Racing. Scand. J. Med. Sci. Sports 2010, 20, 475–484. [Google Scholar] [CrossRef]
- Murphy, R.J. Heat illness in the athlete. Am. J. Sports Med. 1984, 12, 258–261. [Google Scholar] [CrossRef]
- Guéritée, J.; House, J.R.; Redortier, B.; Tipton, M.J. The determinants of thermal comfort in cool water. Scand. J. Med. Sci. Sports 2015, 25, e459–e471. [Google Scholar] [CrossRef] [PubMed]
| Study | Reference | Type | Risk of Bias | Sample Size | Male (%) | Age (Mean ± SD Where Available) | Season | Indoor/Outdoor | Country (Latitude) | Sport Type |
|---|---|---|---|---|---|---|---|---|---|---|
| Aydin, 2019 | [40] | Cross-sectional | 7 of 10 | 555 | 0.4126 | 15.9 | Win–Aut | Both | Turkey (39° N) | Ballet, dance, defense sports, gymnastics, basketball, volleyball, athletics |
| Bauer, 2018 | [41] | Cross-sectional | 5 of 10 | 70 | 1 | 26.3 ± 4.9 | Sum | Indoor | Germany (50° N) | Handball |
| Bauer, 2020 | [42] | Cross-sectional | 5 of 10 | 120 | 1 | 25.8 ± 5.2 | Sum | Indoor | Germany (50° N) | Handball, ice hockey |
| Fields, 2020 | [43] | Cross-sectional | 5 of 10 | 36 | 0 | 19.4 ± 1.4 | Win | Indoor | USA (38.8° N) | Track and field, basketball, volleyball |
| Geiker, 2017 | [44] | Cross-sectional | 4 of 10 | 29 | 0.586 | 18 | Spr | Indoor | Denmark (56° N) | Swimming |
| Huang, 2021 | [45] | Cross-sectional | 5 of 10 | 95 | 0.4842 | 24.3 ± 4.3 | Multiple | Both | Singapore (1° N) | 15 sports |
| Jakse, 2019 | [46] | Cross-sectional | 4 of 10 | 31 | 0 | 16.6 ± 4.1 | Spr | Indoor | Slovenia (46° N) | Swimming, gymnastics |
| Kawashima, 2021 | [47] | Cross-sectional | 5 of 10 | 48 | 1 | 19.8 ± 0.9 | Win | Both | Japan (35° N) | Fencing, field hockey |
| Kim, 2019 | [48] | Cross-sectional | 4 of 10 | 52 | 1 | 23.8 ± 2.8 | NR | Indoor | Taiwan (NR) | Volleyball |
| Ksiazek, 2018 | [49] | Cross-sectional | 3 of 10 | 25 | 1 | 21.9 ± 9.8 | Win | Indoor | Poland (51° N) | Judo |
| Ksiazek, 2021 | [50] | Cross-sectional | 3 of 10 | 40 | 1 | 22.1 ± 3.4 | Spr | Both | Poland (51° N) | Judo, football |
| McGill, 2014 | [51] | Cross-sectional | 5 of 10 | 108 | NR | 20.0 ± 1.15 | Aut | Outdoor | USA (NR) | American football |
| Mehran, 2016 | [52] | Cross-sectional | 7 of 10 | 105 | 1 | 25.5 ± 4.4 | Aut | Indoor | USA (NR) | Ice hockey |
| Most, 2021 | [53] | Cross-sectional | 5 of 10 | 112 | 1 | 26.1 ± 5.2 | Sum | Indoor | Germany (50° N) | Handball, ice hockey |
| Peeling, 2012 | [54] | Cross-sectional | 6 of 10 | 72 | 0.597 | 16 ± 4 | Sum | Both | Australia (32° S) | Gymnastics, diving, sailing, field hockey, athletics, rowing, water polo, sprint cycling |
| Radovanovic, 2022 | [55] | Cross-sectional | 5 of 10 | 18 | 0 | 21.2 ± 3.9 | Aut | Indoor | Serbia (44° N) | Basketball |
| Ricart, 2021 | [56] | Cross-sectional | 5 of 10 | 27 | 0.481 | 15.8 ± 0.6 | Win | Indoor | Spain (39.5° N) | Basketball |
| Sariakcali, 2020 | [57] | Cross-sectional | 3 of 10 | 36 | 1 | 23.3 ± 3.5 | Sum | Outdoor | Turkey (40° N) | Football |
| Sghaier, 2015 | [58] | Cross-sectional | 5 of 10 | 150 | 0.616 | 18 ± 2 | Win | Both | Tunisia (mean 35° N) | Athletics, judo, karate, boxing, fencing |
| Valtuena, 2014 | [59] | Cross-sectional | 3 of 10 | 408 | 0.583 | 22.8 ± 8.4 | Multiple | Both | Spain (41.4° N) | 34 sports |
| Wentz, 2016 | [60] | Cross-sectional | 6 of 10 | 59 | 0 | 23.5 ± 4.9 | NR | Outdoor | USA (30.4° N) | Running |
| Caroli, 2014 | [61] | Cohort | 5 of 9 | 21 | 1 | 24.6 ± 4.3 | Spr–Aut | Outdoor | Italy (44.9° N) | Rugby |
| Fields, 2019 | [62] | Cohort | 6 of 9 | 20 | 0.55 | 19.9 ± 1.2 | Sum–Aut | Indoor | USA (38.8° N) | Basketball |
| Galan, 2012 | [63] | Cohort | 7 of 9 | 28 | 1 | 26.7 ± 3.6 | Aut–Win | Outdoor | Spain (37.4° N) | Football |
| Haslacher, 2016 | [64] | Cohort | 8 of 9 | 47 | 0.915 | 65 | NR | Outdoor | Austria (NR) | Marathoners, bicyclists |
| Huggins, 2019 | [65] | Cohort | 5 of 9 | 20 | 1 | 21 ± 1 | Sum–Aut | Outdoor | USA (NR) | Soccer |
| Krzywanski, 2016 | [66] | Cohort | 4 of 9 | 409 | 0.557 | 25.1 ± 0.5 | Spr–Sum–Win–Aut | Both | Poland (51.5° N) | Track and field, weightlifting, handball, volleyball |
| Maruyama, 2016 | [67] | Cohort | 7 of 9 | 27 | 0 | 20.6 ± 0.5 | Spr–Sum–Win–Aut | Both | Japan (35° N) | Soccer, basketball, volleyball |
| Millward, 2020 | [68] | Cohort | 9 of 9 | 802 | 0.62 | 18.7 ± 1.2 | Multiple | Both | USA (NR) | NCAA Division I student athletes |
| Wilson, 2020 | [69] | Cohort | 6 of 9 | 47 | 0.66 | 20.5 ± 1.7 | Spr–Aut | Both | UK (51.2° N) | 15 sports |
| Valtuena, 2021 | [70] | Cohort | 8 of 9 | 95 | 1 | 27.3 ± 4.6 | Spr–Aut–Win | Both | Spain (41.4° N) | Football, indoor football, basketball, handball, roller hockey |
| Wyon, 2015 | [71] | RCT | 6 of 9 | 22 | 1 | 27.5 ± 9 | Win | Indoor | UK (52.5° N) | Judo |
| Valenti, 2022 | [72] | Before and after | 7 of 12 | 8 | 1 | 47.5 ± 13.5 | Sum | Outdoor | Italy (NR) | Cycling |
| Variable (Coding) | Number of Included Studies | β Estimate | β Standard Error | β p-Value (df1, df2) | R2 (%) | |
|---|---|---|---|---|---|---|
| Type of sport * (indoor = 1) | 91 | 31.387 (<0.001) | −3.692 | 1.843 | 0.045 (1, 91) | 3.69 |
| Gender (female = 1) | 68 | 29.944 (<0.001) | 2.511 | 2.126 | 0.237 (1, 66) | 0.76 |
| Latitude * | 74 | 37.854 (<0.001) | −0.247 | 0.081 | 0.002 (1, 72) | 10.52 |
| Race (Caucasian = 1) | 54 | 29.773 (<0.001) | −0.064 | 2.962 | 0.983 (1, 52) | <0.01 |
| F-Test for Moderators | ||||||
|---|---|---|---|---|---|---|
| Coefficient | β Estimate | Standard Error | p-Value | F Statistic (df) | p-Value | R2 (%) |
| * | 32.067 | 1.645 | <0.001 | 28.605 (3) | <0.001 | 27.88 |
| * | −8.761 | 2.461 | <0.001 | |||
| 2.051 | 2.575 | 0.426 | ||||
| * | −8.001 | 2.266 | <0.001 | |||
| F-Test for Moderators | |||||||
|---|---|---|---|---|---|---|---|
| Coefficient | β Estimate | Standard Error | p-Value | F Statistic (df) | p-Value | R2 (%) | Sample Size |
| * | 46.470 | 5.852 | <0.001 | 32.339 (5) | <0.001 | 31.06 | 66 |
| Indoor | −1.262 | 1.807 | 0.485 | ||||
| Spring * | −5.837 | 2.529 | 0.021 | ||||
| Summer | 3.398 | 2.696 | 0.207 | ||||
| Winter * | −6.957 | 2.326 | 0.003 | ||||
| Latitude * | −0.353 | 0.131 | 0.007 | ||||
| F-Test for Moderators | |||||||
|---|---|---|---|---|---|---|---|
| Coefficient | β Estimate | Standard Error | p-Value | F Statistic (df) | p-Value | R2 (%) | Sample Size |
| * | 39.995 | 7.791 | <0.001 | 46.195 (5) | <0.001 | 52.98 | 40 |
| Indoor * | −4.446 | 1.915 | 0.020 | ||||
| Spring | −4.376 | 2.842 | 0.124 | ||||
| Summer | 4.804 | 2.695 | 0.075 | ||||
| Winter * | −8.586 | 2.586 | <0.001 | ||||
| Latitude | −0.183 | 0.170 | 0.283 | ||||
| Race (Asian = 1) | 0.672 | 2.924 | 0.818 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bârsan, M.; Chelaru, V.-F.; Râjnoveanu, A.-G.; Popa, Ș.L.; Socaciu, A.-I.; Bădulescu, A.-V. Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 7584. https://doi.org/10.3390/ijms24087584
Bârsan M, Chelaru V-F, Râjnoveanu A-G, Popa ȘL, Socaciu A-I, Bădulescu A-V. Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(8):7584. https://doi.org/10.3390/ijms24087584
Chicago/Turabian StyleBârsan, Maria, Vlad-Florin Chelaru, Armand-Gabriel Râjnoveanu, Ștefan Lucian Popa, Andreea-Iulia Socaciu, and Andrei-Vlad Bădulescu. 2023. "Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 8: 7584. https://doi.org/10.3390/ijms24087584
APA StyleBârsan, M., Chelaru, V.-F., Râjnoveanu, A.-G., Popa, Ș. L., Socaciu, A.-I., & Bădulescu, A.-V. (2023). Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(8), 7584. https://doi.org/10.3390/ijms24087584






