Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies
Abstract
1. Introduction
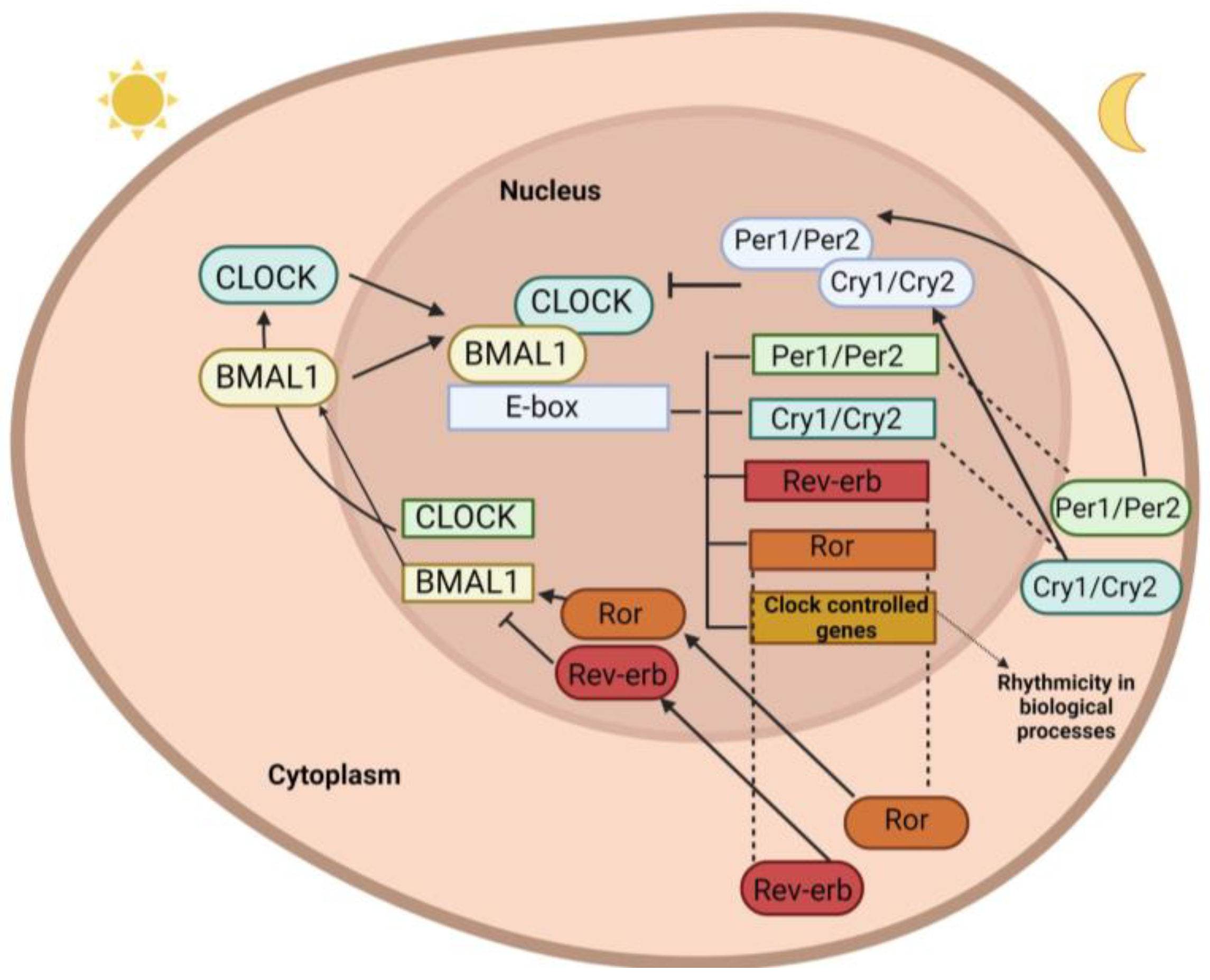
1.1. Clock Genes
1.2. Central Clock
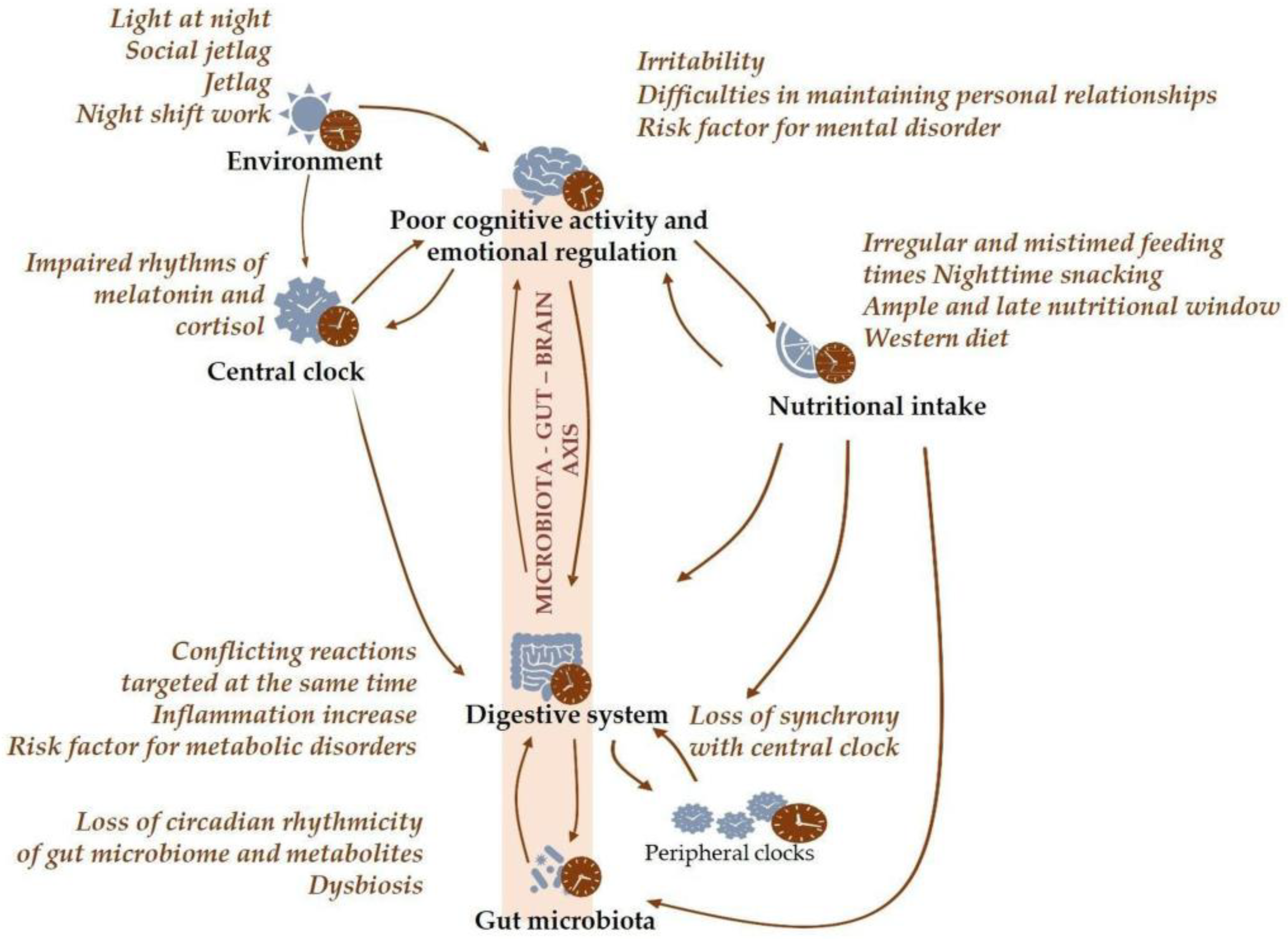
1.3. Chronodisruption
1.4. Chrono-Nutrition and Nutritional Psychiatry
1.5. Circadian Disruption and Brain Development and Function
1.6. Circadian Disruption in Mental Health Disorders
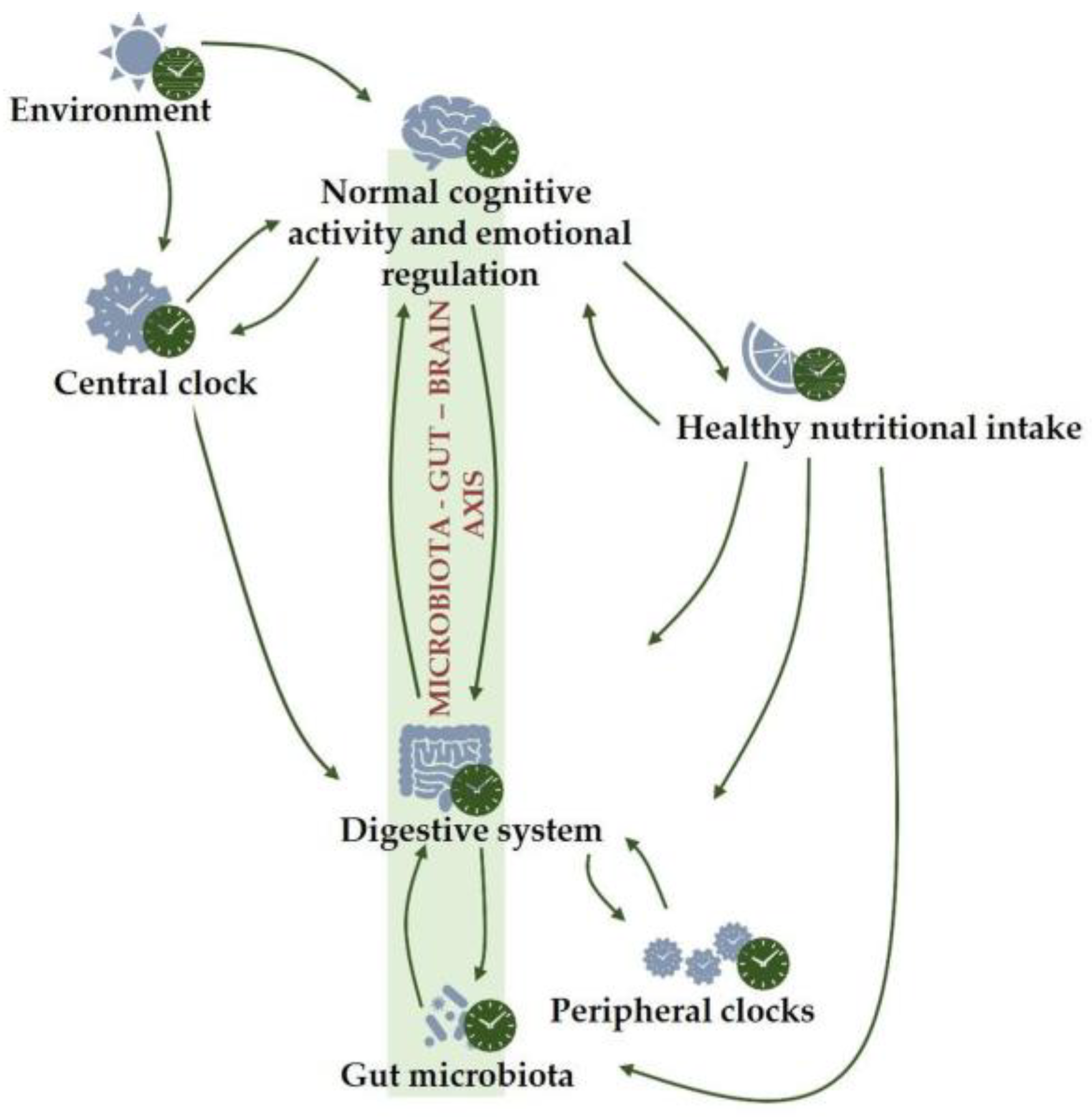
2. Diet, Gut Microbiota and Mental Health: The Microbiota–Gut–Brain Axis
2.1. Bidirectional Link between the Gut Microbiota and Circadian System
2.2. Timing the Microbes: Circadian Rhythmicity of the Gut Microbiome
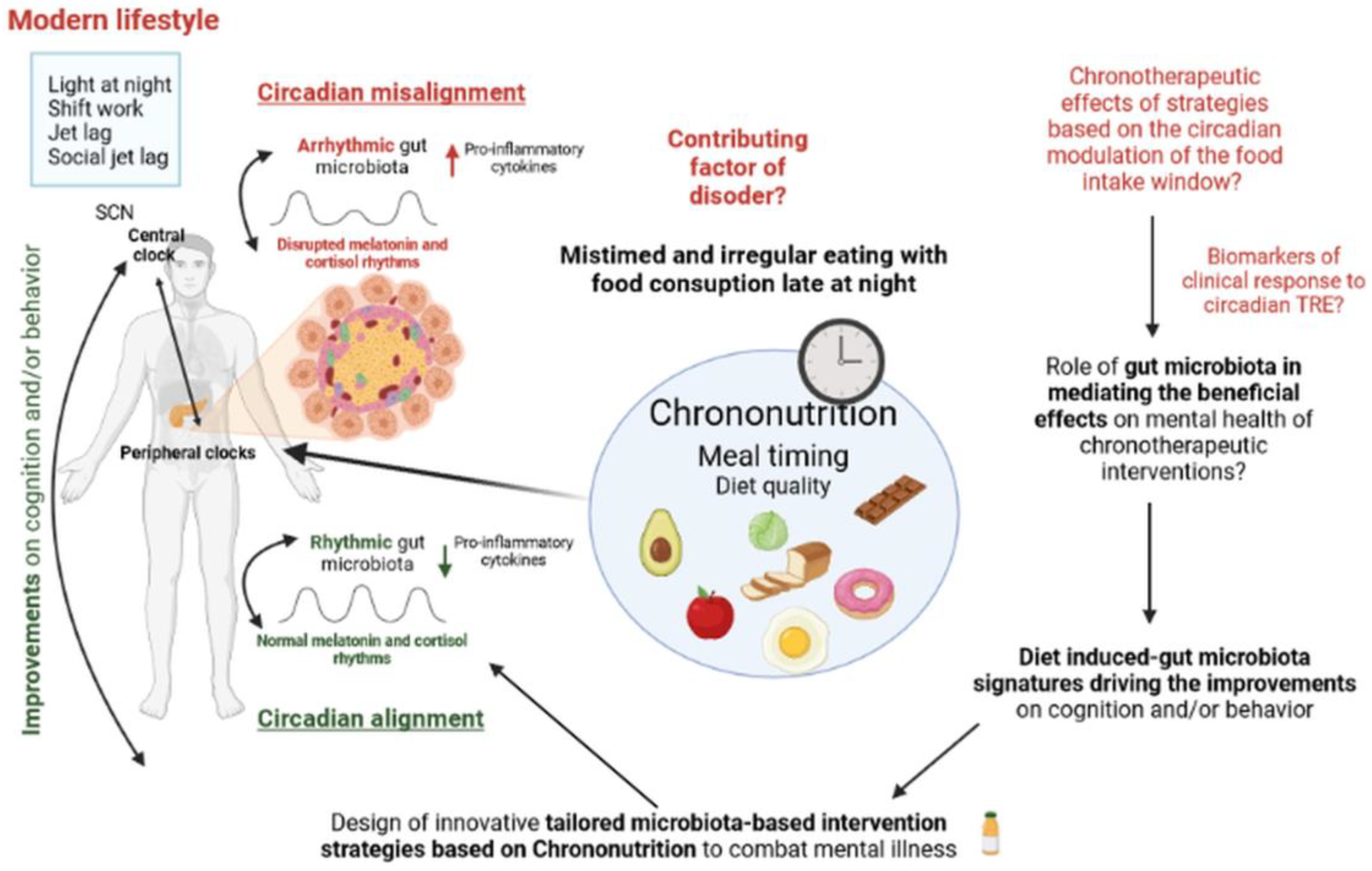
2.3. Irregular and Mistimed Eating Patterns as a Disruptive Factor in Mental Health
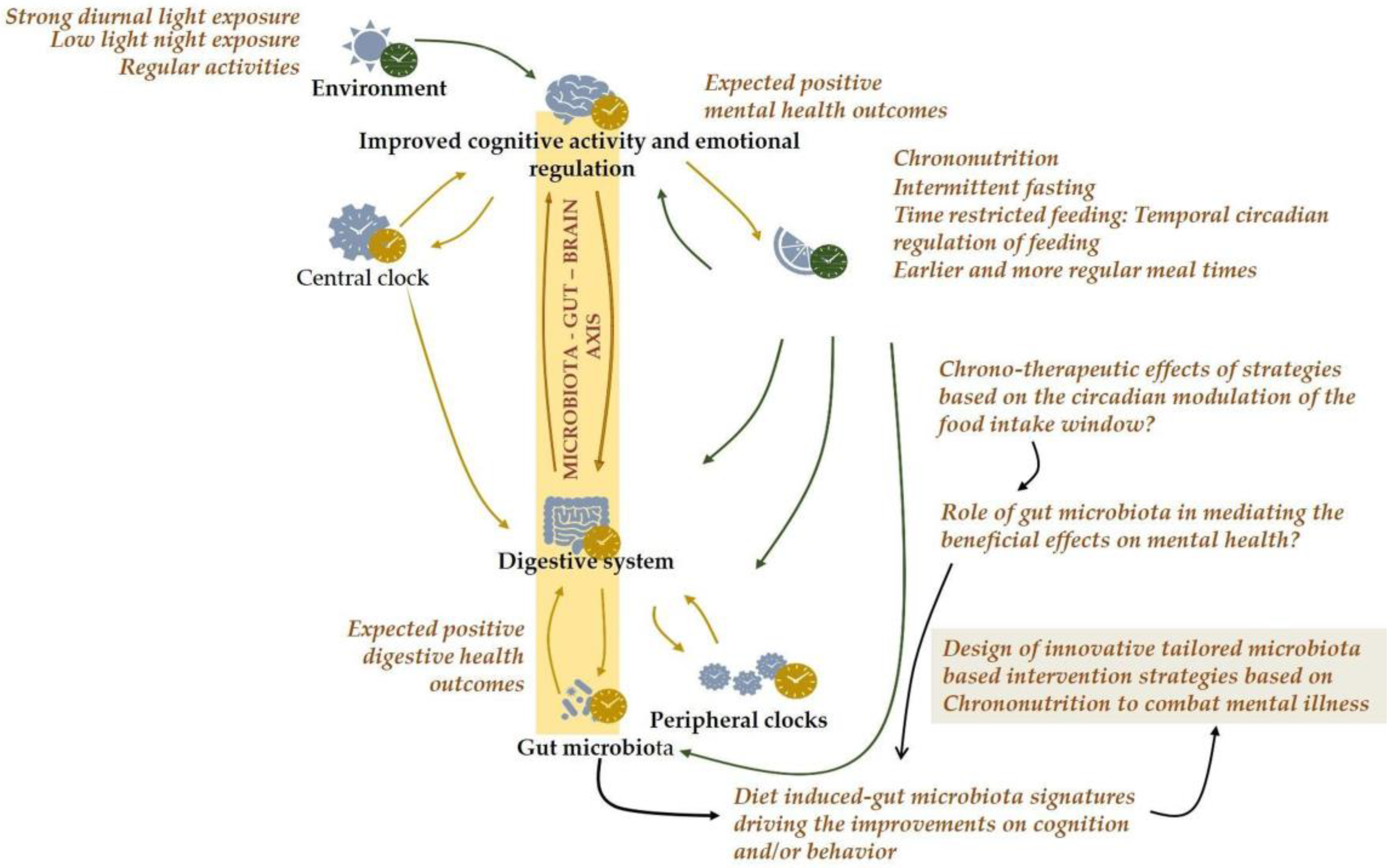
2.4. Temporal Circadian Regulation of Feeding as a Novel Therapeutic Approach for Targeting Mental Illness
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Singh, O.P. Comprehensive Mental Health Action Plan 2013–2030: We must rise to the challenge. Indian J. Psychiatry 2021, 63, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef]
- Alachkar, A.; Lee, J.; Asthana, K.; Monfared, R.V.; Chen, J.; Alhassen, S.; Samad, M.; Wood, M.; Mayer, E.A.; Baldi, P. The hidden link between circadian entropy and mental health disorders. Transl. Psychiatry 2022, 12, 281. [Google Scholar] [CrossRef]
- Walker, W.H., II; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Lane, J.M.; Qian, J.; Mignot, E.; Redline, S.; Scheer, F.A.J.L.; Saxena, R. Genetics of circadian rhythms and sleep in human health and disease. Nat. Rev. Genet. 2022, 24, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Kaur, S. Environmental light exposure and mealtime regularity: Implications for human health. Chronobiol. Int. 2022, 39, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.J. Lifestyle and Circadian Health: Where the Challenges Lie? Nutr. Metab. Insights 2019, 12, 1178638819869024. [Google Scholar] [CrossRef]
- Sollars, P.J.; Pickard, G.E. The Neurobiology of Circadian Rhythms. Psychiatr. Clin. N. Am. 2015, 38, 645–665. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Pazienza, V.; Vinciguerra, M. Clock Genes and Clock-Controlled Genes in the Regulation of Metabolic Rhythms. Chronobiol. Int. 2012, 29, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef]
- Pevet, P.; Challet, E. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. J. Physiol. 2011, 105, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Lack, L.; Bailey, M.; Lovato, N.; Wright, H. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat. Sci. Sleep 2009, 1, 1–8. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef]
- Feillet, C.A.; Albrecht, U.; Challet, E. “Feeding time” for the brain: A matter of clocks. J. Physiol. 2006, 100, 252–260. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Defining chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef]
- Rajaratnam, S.M.; Arendt, J. Health in a 24-h society. Lancet 2001, 358, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.; Vujovic, N.; Williams, J.S.; Scheer, F.A. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef]
- Paschos, G.K. Diurnal rhythms and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 333–338. [Google Scholar] [CrossRef]
- Parameswaran, G.; Ray, D.W. Sleep, circadian rhythms, and type 2 diabetes mellitus. Clin. Endocrinol. 2021, 96, 12–20. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, C.; Cao, Y.; Li, J.; Bi, F. Major roles of the circadian clock in cancer. Cancer Biol. Med. 2023, 20, 1–24. [Google Scholar] [CrossRef]
- Teichman, E.M.; O’riordan, K.J.; Gahan, C.G.; Dinan, T.G.; Cryan, J.F. When Rhythms Meet the Blues: Circadian Interactions with the Microbiota-Gut-Brain Axis. Cell Metab. 2020, 31, 448–471. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Zou, H.; Zhou, H.; Yan, R.; Yao, Z.; Lu, Q. Chronotype, circadian rhythm, and psychiatric disorders: Recent evidence and potential mechanisms. Front. Neurosci. 2022, 16, 811771. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Siddiqi, N.; Koyanagi, A.; Siskind, D.; Rosenbaum, S.; Galletly, C.; Allan, S.; Caneo, C.; Carney, R.; Carvalho, A.F.; et al. The Lancet Psychiatry Commission: A blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019, 6, 675–712. [Google Scholar] [CrossRef]
- Franzago, M.; Alessandrelli, E.; Notarangelo, S.; Stuppia, L.; Vitacolonna, E. Chrono-Nutrition: Circadian Rhythm and Personalized Nutrition. Int. J. Mol. Sci. 2023, 24, 2571. [Google Scholar] [CrossRef]
- Tahara, Y.; Makino, S.; Suiko, T.; Nagamori, Y.; Iwai, T.; Aono, M.; Shibata, S. Association between Irregular Meal Timing and the Mental Health of Japanese Workers. Nutrients 2021, 13, 2775. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Mogavero, M.P.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Time-restricted feeding is associated with mental health in elderly Italian adults. Chronobiol. Int. 2021, 38, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Vujovic, N.; Nguyen, H.; Rahman, N.; Heng, S.W.; Amira, S.; Scheer, F.A.J.L.; Chellappa, S.L. Daytime eating prevents mood vulnerability in night work. Proc. Natl. Acad. Sci. USA 2022, 119, e2206348119. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Time restricted feeding and mental health: A review of possible mechanisms on affective and cognitive disorders. Int. J. Food. Sci. Nutr. 2021, 72, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.; Forsyth, C.; Green, S.; Engen, P.; Keshavarzian, A. Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 131, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. Int. Rev. J. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Velarde, E.; Llorente, R.; Laviola, G. Disrupted Circadian Rhythm as a Common Player in Developmental Models of Neuropsychiatric Disorders. Curr. Top. Behav. Neurosci. 2016, 29, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Lee, E.J.; Choe, H.K.; Son, G.H.; Kim, K.; Chung, S. Programming effects of maternal stress on the circadian system of adult offspring. Exp. Mol. Med. 2020, 52, 473–484. [Google Scholar] [CrossRef]
- Smarr, B.L.; Grant, A.D.; Perez, L.; Zucker, I.; Kriegsfeld, L.J. Maternal and Early-Life Circadian Disruption Have Long-Lasting Negative Consequences on Offspring Development and Adult Behavior in Mice. Sci. Rep. 2017, 7, 3326. [Google Scholar] [CrossRef]
- Cissé, Y.M.; Russart, K.L.; Nelson, R.J. Parental Exposure to Dim Light at Night Prior to Mating Alters Offspring Adaptive Immunity. Sci. Rep. 2017, 7, 45497. [Google Scholar] [CrossRef]
- Cissé, Y.M.; Russart, K.L.; Nelson, R.J. Depressive-like behavior is elevated among offspring of parents exposed to dim light at night prior to mating. Psychoneuroendocrinology 2017, 83, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Vilches, N.; Spichiger, C.; Mendez, N.; Abarzua-Catalan, L.; Galdames, H.A.; Hazlerigg, D.G.; Richter, H.; Torres-Farfan, C. Gestational Chronodisruption Impairs Hippocampal Expression of NMDA Receptor Subunits Grin1b/Grin3a and Spatial Memory in the Adult Offspring. PLoS ONE 2014, 9, e91313. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Le Duc, D.; Roșca, A.E.; Zeca, V.; Chiţimuș, D.M.; Arsene, A.L.; Drăgoi, C.M.; Nicolae, A.C.; Zăgrean, L.; Schöneberg, T.; et al. Behavioral and molecular effects of prenatal continuous light exposure in the adult rat. Brain Res. 2016, 1650, 51–59. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Coogan, A.N.; McGowan, N.M. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. ADHD Atten. Deficit Hyperact. Disord. 2017, 9, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, S.; Çetin, F.H. The relationship between chronotype and obesity in children and adolescent with attention deficit hyperactivity disorder. Chronobiol. Int. 2019, 36, 1138–1147. [Google Scholar] [CrossRef]
- Van Andel, E.; Bijlenga, D.; Vogel, S.W.N.; Beekman, A.T.F.; Kooij, J.J.S. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: A randomized clinical trial. Chronobiol. Int. 2021, 38, 260–269. [Google Scholar] [CrossRef]
- Van Veen, M.M.; Kooij, J.S.; Boonstra, A.M.; Gordijn, M.C.; Van Someren, E.J. Delayed Circadian Rhythm in Adults with Attention-Deficit/Hyperactivity Disorder and Chronic Sleep-Onset Insomnia. Biol. Psychiatry 2010, 67, 1091–1096. [Google Scholar] [CrossRef]
- Carmassi, C.; Palagini, L.; Caruso, D.; Masci, I.; Nobili, L.; Vita, A.; Dell’osso, L. Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 2019, 10, 366. [Google Scholar] [CrossRef]
- Yang, Z.; Matsumoto, A.; Nakayama, K.; Jimbo, E.F.; Kojima, K.; Nagata, K.-I.; Iwamoto, S.; Yamagata, T. Circadian-relevant genes are highly polymorphic in autism spectrum disorder patients. Brain Dev. 2016, 38, 91–99. [Google Scholar] [CrossRef]
- Borniger, J.C.; Weil, Z.M.; Zhang, N.; Nelson, R.J. Dim Light at Night Does Not Disrupt Timing or Quality of Sleep in Mice. Chronobiol. Int. 2013, 30, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Torquati, L.; Mielke, G.I.; Brown, W.J.; Burton, N.W.; Kolbe-Alexander, T.L. Shift Work and Poor Mental Health: A Meta-Analysis of Longitudinal Studies. Am. J. Public Healthy 2019, 109, e13–e20. [Google Scholar] [CrossRef]
- Booker, L.A.; Sletten, T.; Alvaro, P.K.; Barnes, M.; Collins, A.; Chai-Coetzer, C.L.; Naqvi, A.; McMahon, M.; Lockley, S.W.; Rajaratnam, S.; et al. Exploring the associations between shift work disorder, depression, anxiety and sick leave taken amongst nurses. J. Sleep Res. 2020, 29, e12872. [Google Scholar] [CrossRef] [PubMed]
- McNeely, E.; Mordukhovich, I.; Tideman, S.; Gale, S.; Coull, B. Estimating the health consequences of flight attendant work: Comparing flight attendant health to the general population in a cross-sectional study. BMC Public Health 2018, 18, 346. [Google Scholar] [CrossRef]
- Levandovski, R.; Dantas, G.; Fernandes, L.C.; Caumo, W.; Torres, I.L.; Roenneberg, T.; Hidalgo, M.P.; Allebrandt, K.V. Depression Scores Associate With Chronotype and Social Jetlag in a Rural Population. Chronobiol. Int. 2011, 28, 771–778. [Google Scholar] [CrossRef]
- Lee, A.; Myung, S.-K.; Cho, J.J.; Jung, Y.-J.; Yoon, J.L.; Kim, M.Y. Night Shift Work and Risk of Depression: Meta-analysis of Observational Studies. J. Korean Med. Sci. 2017, 32, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Hong, S.-C. Prevalence of major depressive disorder in the general population of South Korea. J. Psychiatr. Res. 2006, 40, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Oenning, N.S.X.; Ziegelmann, P.K.; de Goulart, B.N.G.; Niedhammer, I. Occupational factors associated with major depressive disorder: A Brazilian population-based study. J. Affect. Disord. 2018, 240, 48–56. [Google Scholar] [CrossRef]
- Murcia, M.; Chastang, J.-F.; Niedhammer, I. Psychosocial work factors, major depressive and generalised anxiety disorders: Results from the French national SIP study. J. Affect. Disord. 2013, 146, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Knapen, S.E.; Der Lek, R.F.R.-V.; Antypa, N.; Meesters, Y.; Penninx, B.W.J.H.; Schoevers, R.A. Social jetlag and depression status: Results obtained from the Netherlands Study of Depression and Anxiety. Chronobiol. Int. 2018, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rusting, C.L.; Larsen, R.J. Diurnal patterns of unpleasant mood: Associations with neuroticism, depression, and anxiety. J. Pers. 1998, 66, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Vadnie, C.A.; McClung, C.A. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast. 2017, 2017, 1504507. [Google Scholar] [CrossRef]
- Emens, J.; Lewy, A.; Kinzie, J.M.; Arntz, D.; Rough, J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009, 168, 259–261. [Google Scholar] [CrossRef]
- Terman, J.S.; Terman, M.; Lo, E.-S.; Cooper, T.B. Circadian Time of Morning Light Administration and Therapeutic Response in Winter Depression. Arch. Gen. Psychiatry 2001, 58, 69–75. [Google Scholar] [CrossRef]
- Robillard, R.; Carpenter, J.S.; Feilds, K.-L.; Hermens, D.F.; White, D.; Naismith, S.L.; Bartlett, D.; Whitwell, B.; Southan, J.; Scott, E.M.; et al. Parallel Changes in Mood and Melatonin Rhythm Following an Adjunctive Multimodal Chronobiological Intervention With Agomelatine in People With Depression: A Proof of Concept Open Label Study. Front. Psychiatry 2018, 9, 624. [Google Scholar] [CrossRef]
- Landgraf, D.; Long, J.E.; Proulx, C.D.; Barandas, R.; Malinow, R.; Welsh, D.K. Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol. Psychiatry 2016, 80, 827–835. [Google Scholar] [CrossRef]
- Ben-Hamo, M.; Larson, T.A.; Duge, L.S.; Sikkema, C.; Wilkinson, C.W.; de la Iglesia, H.O.; González, M.M.C. Circadian Forced Desynchrony of the Master Clock Leads to Phenotypic Manifestation of Depression in Rats. Eneuro 2016, 3, ENEURO.0237-16.2016. [Google Scholar] [CrossRef]
- Arushanyan, B.; Popov, A.V. Influence of damage to the suprachiasmatic nuclei of the hypothalamus of rats on the dynamics of short-period fluctuations of normal and abnormal behavior. Neurosci. Behav. Physiol. 1995, 25, 290–295. [Google Scholar] [CrossRef]
- Tataroğlu, O.; Aksoy, A.; Yılmaz, A.; Canbeyli, R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004, 1001, 118–124. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Stoynova, T.; Ilieva, K.; Mitreva, R.; Atanasova, M. Agomelatine treatment corrects symptoms of depression and anxiety by restoring the disrupted melatonin circadian rhythms of rats exposed to chronic constant light. Pharmacol. Biochem. Behav. 2018, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- First, M.B. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, and Clinical Utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Le-Niculescu, H.; Patel, S.D.; Bhat, M.; Kuczenski, R.; Faraone, S.V.; Tsuang, M.T.; McMahon, F.; Schork, N.; Nurnberger, J.; Niculescu, A. Convergent functional genomics of genome-wide association data for bipolar disorder: Comprehensive identification of candidate genes, pathways and mechanisms. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150B, 155–181. [Google Scholar] [CrossRef]
- Katz, G.; Knobler, H.; Laibel, Z.; Strauss, Z.; Durst, R. Time zone change and major psychiatric morbidity: The results of a 6-year study in Jerusalem. Compr. Psychiatry 2002, 43, 37–40. [Google Scholar] [CrossRef]
- Malkoff-Schwartz, S.; Frank, E.; Anderson, B.P.; Hlastala, S.A.; Luther, J.F.; Sherrill, J.T.; Houck, P.R.; Kupfer, D.J. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol. Med. 2000, 30, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.K.; Kinrys, G. Treating Circadian Rhythm Disruption in Bipolar Disorder. Curr. Psychiatry Rep. 2019, 21, 14. [Google Scholar] [CrossRef]
- Sit, D.K.; McGowan, J.; Wiltrout, C.; Diler, R.S.; Dills, J.J.; Luther, J.; Yang, A.; Ciolino, J.D.; Seltman, H.; Wisniewski, S.R.; et al. Adjunctive Bright Light Therapy for Bipolar Depression: A Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2018, 175, 131–139. [Google Scholar] [CrossRef]
- Tapia-Osorio, A.; Salgado-Delgado, R.; Angeles-Castellanos, M.; Escobar, C. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav. Brain Res. 2013, 252, 1–9. [Google Scholar] [CrossRef]
- Borniger, J.C.; McHenry, Z.D.; Abi Salloum, B.A.A.; Nelson, R.J. Exposure to dim light at night during early development increases adult anxiety-like responses. Physiol. Behav. 2014, 133, 99–106. [Google Scholar] [CrossRef]
- Mansour, H.A.; Talkowski, M.E.; Wood, J.; Chowdari, K.V.; McClain, L.; Prasad, K.; Montrose, D.; Fagiolini, A.; Friedman, E.S.; Allen, M.H.; et al. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disord. 2009, 11, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Waters, F.; Sinclair, C.; Rock, D.; Jablensky, A.; Foster, R.G.; Wulff, K. Daily variations in sleep–wake patterns and severity of psychopathology: A pilot study in community-dwelling individuals with chronic schizophrenia. Psychiatry Res. 2011, 187, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Seney, M.L.; Cahill, K.; Enwright, J.F.; Logan, R.W.; Huo, Z.; Zong, W.; Tseng, G.; McClung, C.A. Diurnal rhythms in gene expression in the prefrontal cortex in schizophrenia. Nat. Commun. 2019, 10, 3355. [Google Scholar] [CrossRef]
- Ferrier, I.N.; Arendt, J.; Johnstone, E.C.; Crow, T.J. Reduced nocturnal melatonin secretion in chronic schizophrenia: Relationship to body weight. Clin. Endocrinol. 1982, 17, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Viganò, D.; Lissoni, P.; Rovelli, F.; Roselli, M.G.; Malugani, F.; Gavazzeni, C.; Conti, A.; Maestroni, G. A study of light/dark rhythm of melatonin in relation to cortisol and prolactin secretion in schizophrenia. Neuro Endocrinol. Lett. 2001, 22, 137–141. [Google Scholar]
- Kaneko, M.; Yokoyama, F.; Hoshino, Y.; Takahagi, K.; Murata, S.; Watanabe, M.; Kumashiro, H. Hypothalamic-Pituitary-Adrenal Axis Function in Chronic Schizophrenia: Association with Clinical Features. Neuropsychobiology 1992, 25, 1–7. [Google Scholar] [CrossRef]
- Mann, K.; Rossbach, W.; Müller, M.J.; Müller-Siecheneder, F.; Pott, T.; Linde, I.; Dittmann, R.W.; Hiemke, C. Nocturnal hormone profiles in patients with schizophrenia treated with olanzapine. Psychoneuroendocrinology 2006, 31, 256–264. [Google Scholar] [CrossRef]
- Oyewumi, L.K. Jet lag and relapse of schizoaffective psychosis despite maintenance clozapine treatment. Br. J. Psychiatry 1998, 173, 268. [Google Scholar] [CrossRef]
- Canevelli, M.; Bruno, G.; Vanacore, N.; de Lena, C.; Cesari, M. Are we really tackling the “evidence-based medicine issue” in Alzheimer’s disease? Eur. J. Intern. Med. 2016, 35, e29–e30. [Google Scholar] [CrossRef]
- Homolak, J.; Mudrovčić, M.; Vukić, B.; Toljan, K. Circadian Rhythm and Alzheimer’s Disease. Med. Sci. 2018, 6, 52. [Google Scholar] [CrossRef]
- Ahmad, F.; Sachdeva, P.; Sarkar, J.; Izhaar, R. Circadian dysfunction and Alzheimer’s disease–An updated review. Aging Med. 2022, 6, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; Parekh, P.K.; Kaplan, G.N.; Becker-Krail, D.D.; Williams, W.P., III; Yamaguchi, S.; Yoshino, J.; Shelton, M.A.; Zhu, X.; Zhang, H.; et al. NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Mol. Psychiatry 2019, 24, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Blackwell, T.; Cawthon, P.M.; Ancoli-Israel, S.; Stone, K.L.; Yaffe, K. Association of Circadian Abnormalities in Older Adults With an Increased Risk of Developing Parkinson Disease. JAMA Neurol. 2020, 77, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. More than a Gut Feeling: The Microbiota Regulates Neurodevelopment and Behavior. Neuropsychopharmacology 2015, 40, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional psychiatry: Towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Su, Y. New Insights into the Diurnal Rhythmicity of Gut Microbiota and Its Crosstalk with Host Circadian Rhythm. Animals 2022, 12, 1677. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Gombert, M. Circadian rhythms in the pathogenesis of gastrointestinal diseases. World J. Gastroenterol. 2018, 24, 4297–4303. [Google Scholar] [CrossRef]
- Gombert, M.; Carrasco-Luna, J.; Pin-Arboledas, G.; Codoñer-Franch, P. The connection of circadian rhythm to inflammatory bowel disease. Transl. Res. 2019, 206, 107–118. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef] [PubMed]
- Weger, B.D.; Gobet, C.; Yeung, J.; Martin, E.; Jimenez, S.; Betrisey, B.; Foata, F.; Berger, B.; Balvay, A.; Foussier, A.; et al. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab. 2019, 29, 362–382.e8. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in Intestinal Epithelium Is Orchestrated by the Circadian Clock and Microbiota Cues Transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, Y.; Li, Y.; Ye, C.; Ruhn, K.A.; Behrendt, C.L.; Olson, E.N.; Hooper, L.V. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 2019, 365, 1428–1434. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef]
- Govindarajan, K.; MacSharry, J.; Casey, P.G.; Shanahan, F.; Joyce, S.A.; Gahan, C.G.M. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS ONE 2016, 11, e0167319. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Fonken, L.K.; Nelson, R.J. Endocrine Effects of Circadian Disruption. Annu. Rev. Physiol. 2016, 78, 109–131. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef] [PubMed]
- Iesanu, M.I.; Zahiu, C.D.M.; Dogaru, I.-A.; Chitimus, D.M.; Pircalabioru, G.G.; Voiculescu, S.E.; Isac, S.; Galos, F.; Pavel, B.; O’mahony, S.M.; et al. Melatonin–Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions. Antioxidants 2022, 11, 2244. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.A.; Arguelles-Prieto, R.; Martinez-Madrid, M.J.; Reiter, R.; Hardeland, R.; Rol, M.A.; Madrid, J.A. Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. Int. J. Mol. Sci. 2014, 15, 23448–23500. [Google Scholar] [CrossRef]
- Paulose, J.K.; Wright, J.M.; Patel, A.G.; Cassone, V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE 2016, 11, e0146643. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.G.; Sourjik, V. Chemotaxis of Escherichia coli to major hormones and polyamines present in human gut. ISME J. 2018, 12, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Moya-Pérez, A.; Campillo, I.; la Paz, S.M.-D.; Cerrudo, V.; Perez-Villalba, A.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 Ameliorates Neuroendocrine Alterations Associated with an Exaggerated Stress Response and Anhedonia in Obese Mice. Mol. Neurobiol. 2018, 55, 5337–5352. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J.; et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl. Psychiatry 2018, 8, 187. [Google Scholar] [CrossRef]
- Herbert, J. Cortisol and depression: Three questions for psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef]
- Bellet, M.M.; Deriu, E.; Liu, J.Z.; Grimaldi, B.; Blaschitz, C.; Zeller, M.; Edwards, R.A.; Sahar, S.; Dandekar, S.; Baldi, P.; et al. Circadian clock regulates the host response to Salmonella. Proc. Natl. Acad. Sci. USA 2013, 110, 9897–9902. [Google Scholar] [CrossRef]
- Rosselot, A.E.; Hong, C.I.; Moore, S.R. Rhythm and bugs: Circadian clocks, gut microbiota, and enteric infections. Curr. Opin. Gastroenterol. 2016, 32, 7–11. [Google Scholar] [CrossRef]
- Zheng, D.; Ratiner, K.; Elinav, E. Circadian Influences of Diet on the Microbiome and Immunity. Trends Immunol. 2020, 41, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Mikocka-Walus, A.; Ford, A.C. Common mental disorders in irritable bowel syndrome: Pathophysiology, management, and considerations for future randomised controlled trials. Lancet Gastroenterol. Hepatol. 2021, 6, 401–410. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Collado, M.C.; Engen, P.A.; Bandín, C.; Cabrera-Rubio, R.; Voigt, R.M.; Green, S.J.; Naqib, A.; Keshavarzian, A.; Scheer, F.A.J.L.; Garaulet, M. Timing of food intake impacts daily rhythms of human salivary microbiota: A randomized, crossover study. FASEB J. 2018, 32, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Korecka, A.; Polizzi, A.; Lippi, Y.; Blum, Y.; Canlet, C.; Tremblay-Franco, M.; Gautier-Stein, A.; Burcelin, R.; Yen, Y.-C.; et al. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci. Rep. 2016, 6, 20127. [Google Scholar] [CrossRef]
- Sooriyaarachchi, P.; Jayawardena, R.; Pavey, T.; King, N.A. Shift work and the risk for metabolic syndrome among healthcare workers: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13489. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Qian, J.; Vujovic, N.; Morris, C.J.; Nedeltcheva, A.; Nguyen, H.; Rahman, N.; Heng, S.W.; Kelly, L.; Kerlin-Monteiro, K.; et al. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 2021, 7, eabg9910. [Google Scholar] [CrossRef]
- Martin, K.; Jackson, C.F.; Levy, R.G.; Cooper, P.N. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst. Rev. 2016, 2, CD001903. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef]
- Murray, A.J.; Knight, N.S.; Cole, M.A.; Cochlin, L.E.; Carter, E.; Tchabanenko, K.; Pichulik, T.; Gulston, M.K.; Atherton, H.J.; Schroeder, M.A.; et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016, 30, 4021–4032. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016, 48, 42–51. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Bernier, M.; Mattison, J.A.; Aon, M.A.; Kaiser, T.A.; Anson, R.M.; Ikeno, Y.; Anderson, R.M.; Ingram, D.K.; de Cabo, R. Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab. 2019, 29, 221–228.e3. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Malinowski, B.; Zalewska, K.; Węsierska, A.; Sokołowska, M.M.; Socha, M.; Liczner, G.; Pawlak-Osińska, K.; Wiciński, M. Intermittent Fasting in Cardiovascular Disorders—An Overview. Nutrients 2019, 11, 673. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Angoorani, P.; Ejtahed, H.-S.; Hasani-Ranjbar, S.; Siadat, S.D.; Soroush, A.R.; Larijani, B. Gut microbiota modulation as a possible mediating mechanism for fasting-induced alleviation of metabolic complications: A systematic review. Nutr. Metab. 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
| Diet Quality | Outcome |
|---|---|
| High-fat diet |
|
| High-fiber diets or short-chain fatty acids-containing diets |
|
| Mediterranean diet |
|
| Ketogenic diet |
|
| Meal timing (Chrononutrition) | Outcome |
| Time-restricted feeding |
|
| Intermittent fasting |
|
| Psychobiotic supplements | Outcome |
| Probiotics/Prebiotics/Synbiotics |
|
| Omega-3 fatty acids |
|
| Polyphenols |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codoñer-Franch, P.; Gombert, M.; Martínez-Raga, J.; Cenit, M.C. Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies. Int. J. Mol. Sci. 2023, 24, 7579. https://doi.org/10.3390/ijms24087579
Codoñer-Franch P, Gombert M, Martínez-Raga J, Cenit MC. Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies. International Journal of Molecular Sciences. 2023; 24(8):7579. https://doi.org/10.3390/ijms24087579
Chicago/Turabian StyleCodoñer-Franch, Pilar, Marie Gombert, José Martínez-Raga, and María Carmen Cenit. 2023. "Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies" International Journal of Molecular Sciences 24, no. 8: 7579. https://doi.org/10.3390/ijms24087579
APA StyleCodoñer-Franch, P., Gombert, M., Martínez-Raga, J., & Cenit, M. C. (2023). Circadian Disruption and Mental Health: The Chronotherapeutic Potential of Microbiome-Based and Dietary Strategies. International Journal of Molecular Sciences, 24(8), 7579. https://doi.org/10.3390/ijms24087579






