Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels
Abstract
1. Introduction
2. Results
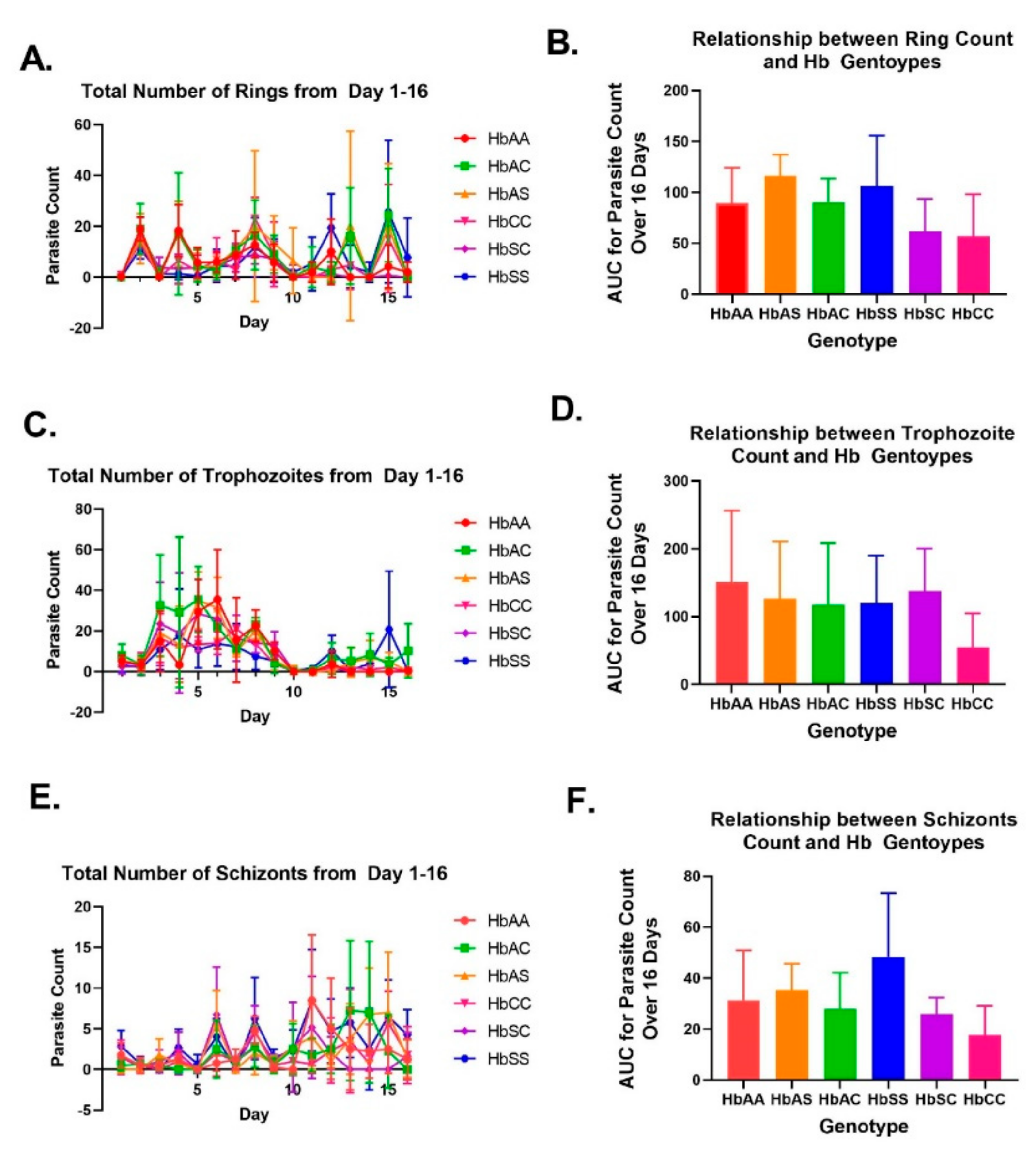
2.1. Variations in Overall Parasite Count Observed among Different Sickle Hb Genotypes
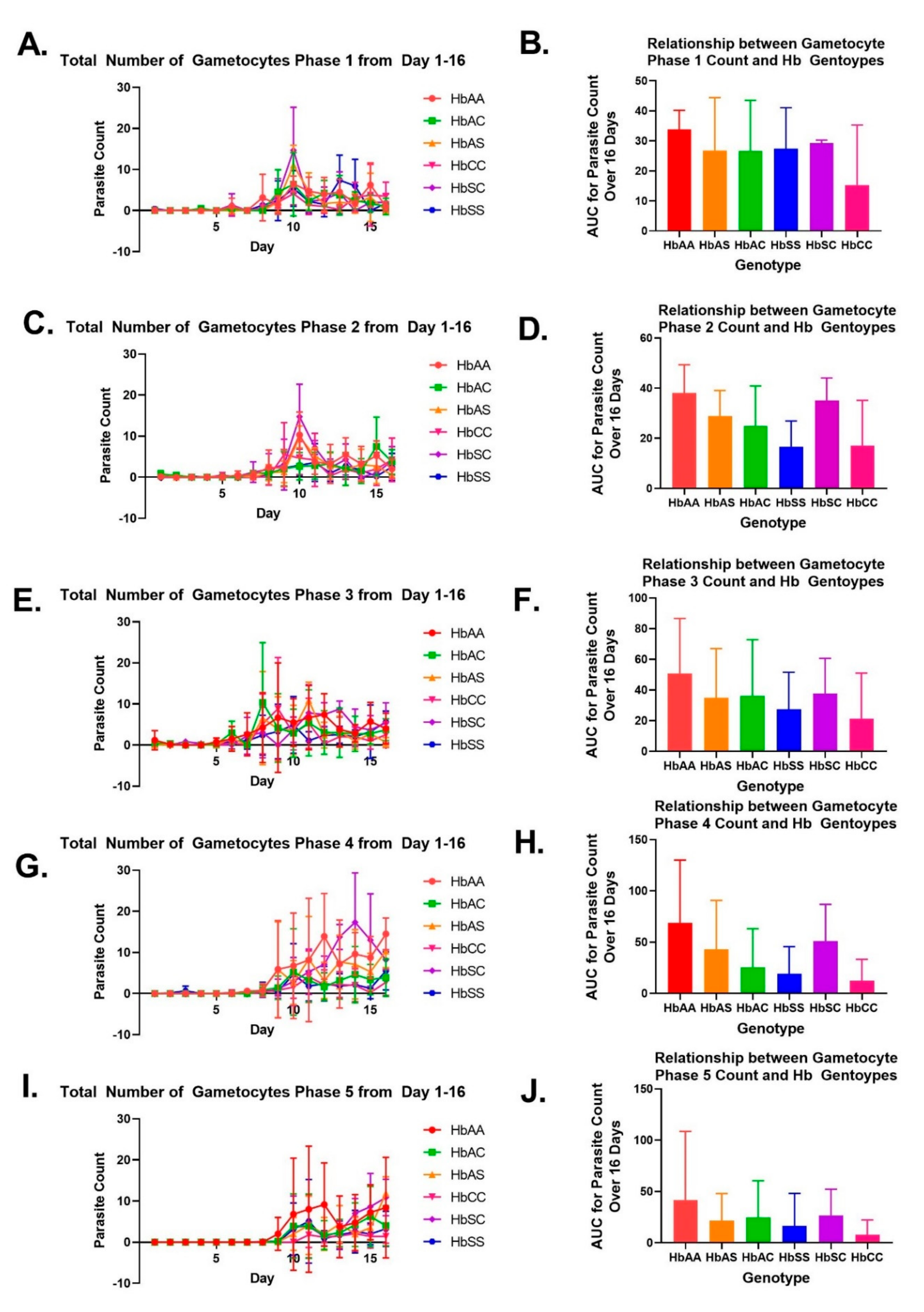
2.2. Variation in Parasite Stage Distribution among Different Sickle Hb Genotypes
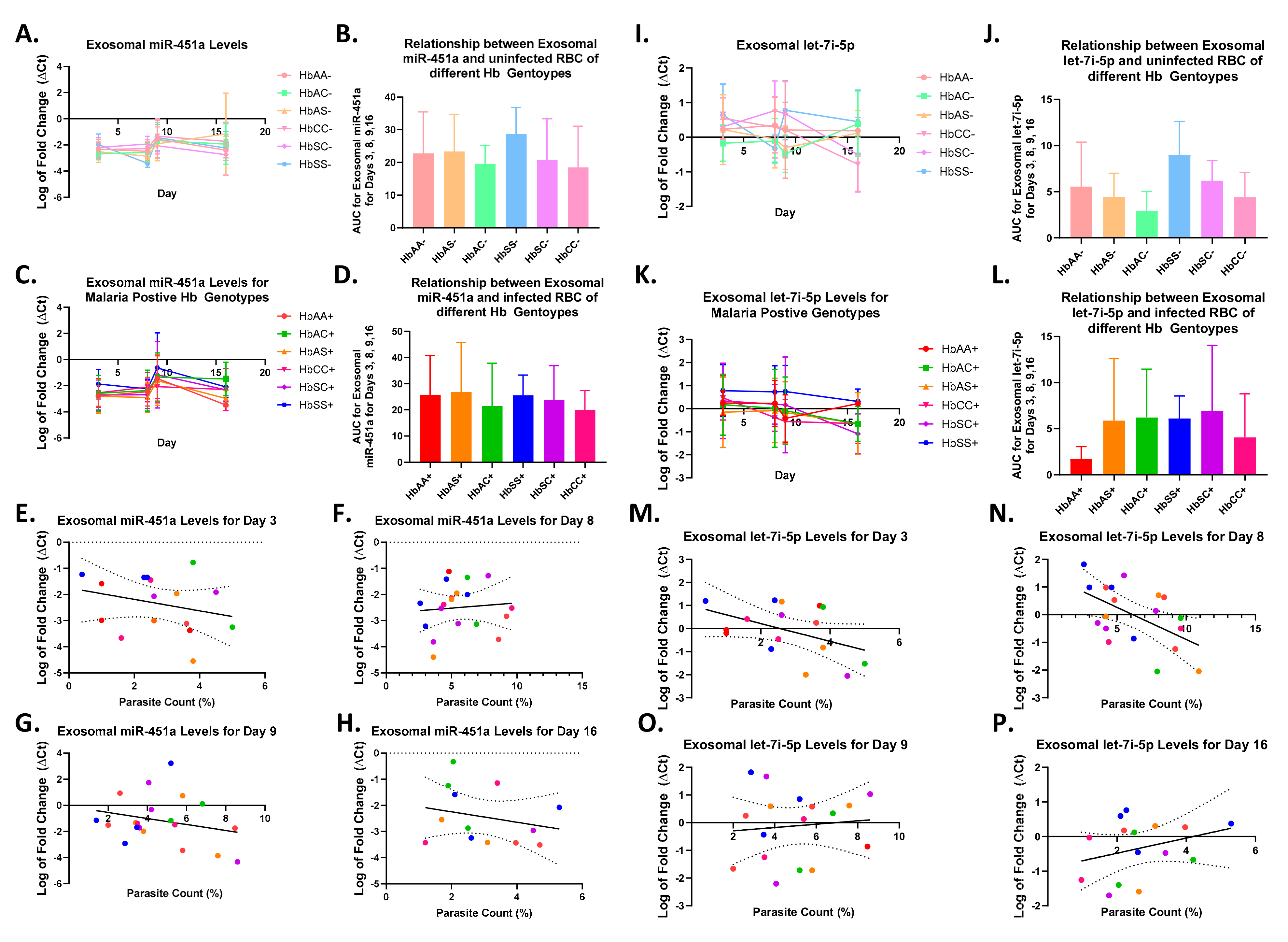
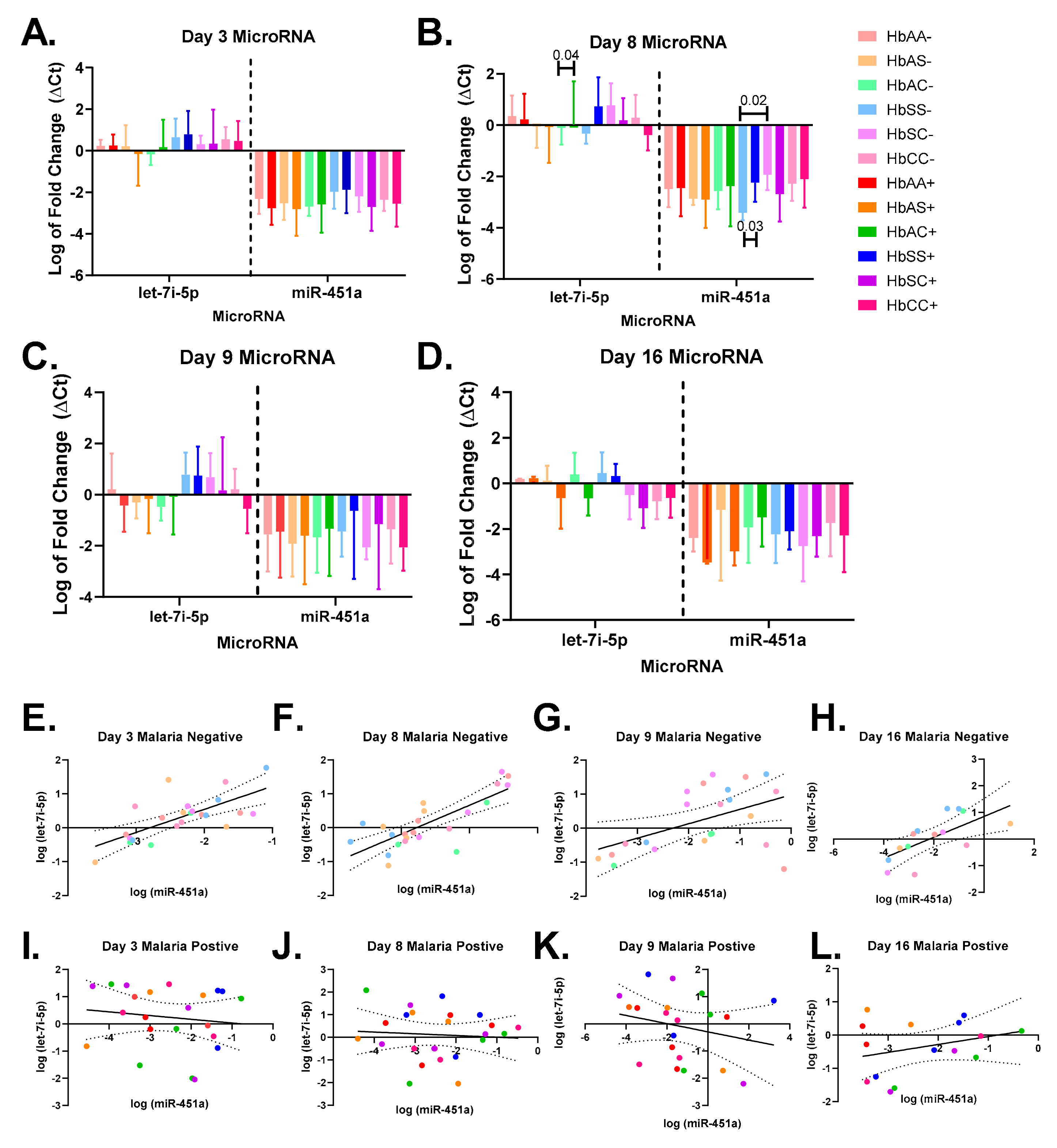
2.3. Alterations in Exosomal Levels of let-7i-5p and miR-451a Observed in P. falciparum-Infected Individuals with Different Sickle Hb Genotypes
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Laboratory Assessment of Blood Specimens
4.3. Culturing Pf 3D7
4.4. Exosomal miRNA Isolation and Quantification
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World malaria report 2022. In World Health Organization Global Malaria Programme Report; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Driss, A.; Hibbert, J.M.; Wilson, N.O.; Iqbal, S.A.; Adamkiewicz, T.V.; Stiles, J.K. Genetic polymorphisms linked to susceptibility to malaria. Malar. J. 2011, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.B.; Cox-Singh, J. Human infections with Plasmodium knowlesi--zoonotic malaria. Clin. Microbiol. Infect. 2015, 21, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Makani, J.; Komba, A.N.; Cox, S.E.; Oruo, J.; Mwamtemi, K.; Kitundu, J.; Magesa, P.; Rwezaula, S.; Meda, E.; Mgaya, J.; et al. Malaria in patients with sickle cell anemia: Burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood 2010, 115, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, M.; Terlouw, D.J.; Kolczak, M.S.; McElroy, P.D.; ter Kuile, F.O.; Kariuki, S.; Nahlen, B.L.; Lal, A.A.; Udhayakumar, V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 2002, 359, 1311–1312. [Google Scholar] [CrossRef]
- Lamptey, H.; Ofori, M.F.; Adu, B.; Kusi, K.A.; Dickson, E.K.; Quakyi, I.; Alifrangis, M. Association between alpha-thalassaemia trait, Plasmodium falciparum asexual parasites and gametocyte carriage in a malaria endemic area in Southern Ghana. BMC Res. Notes 2019, 12, 134. [Google Scholar] [CrossRef]
- Driss, A.; Asare, K.O.; Hibbert, J.M.; Gee, B.E.; Adamkiewicz, T.V.; Stiles, J.K. Sickle Cell Disease in the Post Genomic Era: A Monogenic Disease with a Polygenic Phenotype. Genom. Insights 2009, 2009, 23–48. [Google Scholar] [CrossRef]
- Quinn, C.T.; Rogers, Z.R.; Buchanan, G.R. Survival of children with sickle cell disease. Blood 2004, 103, 4023–4027. [Google Scholar] [CrossRef]
- LaMonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; Nicchitta, C.V.; et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef]
- Harp, K.O.; Botchway, F.; Dei-Adomakoh, Y.; Wilson, M.D.; Hood, J.L.; Adjei, A.A.; Stiles, J.K.; Driss, A. Hemoglobin Genotypes Modulate Inflammatory Response to Plasmodium Infection. Front. Immunol. 2020, 11, 593546. [Google Scholar] [CrossRef]
- Harp, K.O.; Botchway, F.; Dei-Adomakoh, Y.; Wilson, M.D.; Mubasher, M.; Adjei, A.A.; Thompson, W.E.; Stiles, J.K.; Driss, A. Analysis of clinical presentation, hematological factors, self-reported bed net usage, and malaria burden in sickle cell disease patients. EClinicalMedicine 2021, 39, 101045. [Google Scholar] [CrossRef]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Chamnanchanunt, S.; Fucharoen, S.; Umemura, T. Circulating microRNAs in malaria infection: Bench to bedside. Malar. J. 2017, 16, 334. [Google Scholar] [CrossRef]
- Cyrus, C. The Role of miRNAs as Therapeutic Tools in Sickle Cell Disease. Medicina 2021, 57, 1106. [Google Scholar] [CrossRef]
- Oxendine Harp, K.; Bashi, A.; Botchway, F.; Dei-Adomakoh, Y.; Iqbal, S.A.; Wilson, M.D.; Adjei, A.A.; Stiles, J.K.; Driss, A. MicroRNAs miR-451a and Let-7i-5p Profiles in Circulating Exosomes Vary among Individuals with Different Sickle Hemoglobin Genotypes and Malaria. J. Clin. Med. 2022, 11, 500. [Google Scholar] [CrossRef]
- Biassi, T.P.; Guerra-Shinohara, E.M.; Moretti, P.N.S.; de Freitas Dutra, V.; Cabanas-Pedro, A.C.; Mecabo, G.; Colleoni, G.W.B.; Figueiredo, M.S. miRNA profile and disease severity in patients with sickle cell anemia. Ann. Hematol. 2022, 101, 27–34. [Google Scholar] [CrossRef]
- Matsumura, T.; Sugimachi, K.; Iinuma, H.; Takahashi, Y.; Kurashige, J.; Sawada, G.; Ueda, M.; Uchi, R.; Ueo, H.; Takano, Y.; et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br. J. Cancer 2015, 113, 275–281. [Google Scholar] [CrossRef]
- Guo, W.; Gao, Y.; Li, N.; Shao, F.; Wang, C.; Wang, P.; Yang, Z.; Li, R.; He, J. Exosomes: New players in cancer (Review). Oncol Rep. 2017, 38, 665–675. [Google Scholar] [CrossRef]
- Li, R.; Chibbar, R.; Xiang, J. Novel EXO-T vaccine using polyclonal CD4+ T cells armed with HER2-specific exosomes for HER2-positive breast cancer. Onco Targets Ther. 2018, 11, 7089–7093. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Ramezani, M.; Jalalian, S.A.; Abnous, K.; Taghdisi, S.M. Exosomes, new biomarkers in early cancer detection. Anal. Biochem. 2019, 571, 1–13. [Google Scholar] [CrossRef]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- De Jong, R.M.; Tebeje, S.K.; Meerstein-Kessel, L.; Tadesse, F.G.; Jore, M.M.; Stone, W.; Bousema, T. Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites. Immunol. Rev. 2020, 293, 190–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Wang, Y.; Telen, M.J.; Chi, J.T. The genomic analysis of erythrocyte microRNA expression in sickle cell diseases. PLoS ONE 2008, 3, e2360. [Google Scholar] [CrossRef] [PubMed]
- Chamnanchanunt, S.; Kuroki, C.; Desakorn, V.; Enomoto, M.; Thanachartwet, V.; Sahassananda, D.; Sattabongkot, J.; Jenwithisuk, R.; Fucharoen, S.; Svasti, S.; et al. Downregulation of plasma miR-451 and miR-16 in Plasmodium vivax infection. Exp. Parasitol 2015, 155, 19–25. [Google Scholar] [CrossRef]
- Kucuk, U.; Eyuboglu, M.; Kucuk, H.O.; Degirmencioglu, G. Importance of using proper post hoc test with ANOVA. Int. J. Cardiol. 2016, 209, 346. [Google Scholar] [CrossRef]
- Carter, R.; Mendis, K.N. Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev. 2002, 15, 564–594. [Google Scholar] [CrossRef]
- Modiano, D.; Luoni, G.; Sirima, B.S.; Simpore, J.; Verra, F.; Konate, A.; Rastrelli, E.; Olivieri, A.; Calissano, C.; Paganotti, G.M.; et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 2001, 414, 305–308. [Google Scholar] [CrossRef]
- Agarwal, A.; Guindo, A.; Cissoko, Y.; Taylor, J.G.; Coulibaly, D.; Kone, A.; Kayentao, K.; Djimde, A.; Plowe, C.V.; Doumbo, O.; et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood 2000, 96, 2358–2363. [Google Scholar] [CrossRef]
- Thompson, G.R. Significance of haemoglobins S and C in Ghana. Br. Med. J. 1962, 1, 682–685. [Google Scholar] [CrossRef]
- Ringelhann, B.; Hathorn, M.K.; Jilly, P.; Grant, F.; Parniczky, G. A new look at the protection of hemoglobin AS and AC genotypes against plasmodium falciparum infection: A census tract approach. Am. J. Hum. Genet. 1976, 28, 270–279. [Google Scholar]
- Fairhurst, R.M.; Fujioka, H.; Hayton, K.; Collins, K.F.; Wellems, T.E. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: Implications for the malaria protective effect of the homozygous state. Blood 2003, 101, 3309–3315. [Google Scholar] [CrossRef]
- White, N.J. Malaria parasite clearance. Malar. J. 2017, 16, 88. [Google Scholar] [CrossRef]
- Da Guarda, C.C.; Yahouedehou, S.; Santiago, R.P.; Neres, J.; Fernandes, C.F.L.; Aleluia, M.M.; Figueiredo, C.V.B.; Fiuza, L.M.; Carvalho, S.P.; Oliveira, R.M.; et al. Sickle cell disease: A distinction of two most frequent genotypes (HbSS and HbSC). PLoS ONE 2020, 15, e0228399. [Google Scholar] [CrossRef]
- Bowers, A.S.; Pepple, D.J.; Reid, H.L. Oxygen delivery index in subjects with normal haemoglobin (HbAA), sickle cell trait (HbAS) and homozygous sickle cell disease (HbSS). Clin. Hemorheol. Microcirc. 2008, 40, 303–309. [Google Scholar] [CrossRef]
- Archer, N.M.; Petersen, N.; Clark, M.A.; Buckee, C.O.; Childs, L.M.; Duraisingh, M.T. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, 7350–7355. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Tzounakas, V.L.; Dzieciatkowska, M.; Arvaniti, V.Z.; Papageorgiou, E.G.; Papassideri, I.S.; Stamoulis, K.; D’Alessandro, A.; Kriebardis, A.G.; Antonelou, M.H. Innate Variability in Physiological and Omics Aspects of the Beta Thalassemia Trait-Specific Donor Variation Effects. Front. Physiol. 2022, 13, 907444. [Google Scholar] [CrossRef]
- Cyrklaff, M.; Srismith, S.; Nyboer, B.; Burda, K.; Hoffmann, A.; Lasitschka, F.; Adjalley, S.; Bisseye, C.; Simpore, J.; Mueller, A.K.; et al. Oxidative insult can induce malaria-protective trait of sickle and fetal erythrocytes. Nat. Commun. 2016, 7, 13401. [Google Scholar] [CrossRef]
- Destro-Bisol, G.; D’Aloja, E.; Spedini, G.; Scatena, R.; Giardina, B.; Pascali, V. Brief communication: Resistance to Falciparum malaria in alpha-thalassemia, oxidative stress, and hemoglobin oxidation. Am. J. Phys. Anthropol. 1999, 109, 269–273. [Google Scholar]
- Driss, A.; Wilson, N.O.; Mason, K.; Hyacinth, H.I.; Hibbert, J.M.; Serjeant, G.R.; Stiles, J.K. Elevated IL-1α and CXCL10 Serum Levels Occur in Patients with Homozygous Sickle Cell Disease and a History of Acute Splenic Sequestration. Dis. Markers 2012, 32, 295–300. [Google Scholar] [CrossRef]
- Iqbal, S.A.; Botchway, F.; Badu, K.; Wilson, N.O.; Dei-Adomakoh, Y.; Dickinson-Copeland, C.M.; Chinbuah, H.; Adjei, A.A.; Wilson, M.; Stiles, J.K.; et al. Hematological Differences among Malaria Patients in Rural and Urban Ghana. J. Trop. Pediatr. 2016, 62, 477–486. [Google Scholar] [CrossRef]
- Utter, C.; Serrano, A.E.; Glod, J.W.; Leibowitz, M.J. Association of Plasmodium falciparum with Human Endothelial Cells in vitro. Yale J. Biol. Med. 2017, 90, 183–193. [Google Scholar] [PubMed]
- WHO; FIND; CDC. Methods Manual for Product Testing of Malaria Rapid Diagnostic Tests, Manual of Standard Operating Procedures for Assessment of Malaria Rapid Diagnostic Tests within the Product Testing Programme of the World Health Organization At the US Centers for Disease Control and Prevention, Atlanta. In Malaria Methods Manual—SOP, 7th ed.; Foundation for Innovative New Diagnostics (FIND): Geneva, Switzerland; WHO Global Malaria Programme (GMP): Geneva, Switzerland; National Center for Global Health, Division of Malaria and Parasitic Diseases, United States Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2018. [Google Scholar]
- Fitri, L.E.; Sardjono, T.W.; Simamora, D.; Sumarno, R.P.; Setyawati, S.K. High dose of N-acetylcysteine increase H2O2 and MDA levels and decrease GSH level of HUVECs exposed with malaria serum. Trop. Biomed. 2011, 28, 7–15. [Google Scholar] [PubMed]
- Harp, D.; Driss, A.; Mehrabi, S.; Chowdhury, I.; Xu, W.; Liu, D.; Garcia-Barrio, M.; Taylor, R.N.; Gold, B.; Jefferson, S.; et al. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016, 365, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Parasite Phase | Sickle Hb Genotype | R2, p-Value | Equation |
|---|---|---|---|
| Overall | HbAA | 0.007, 0.51 | Y = −0.04306*X + 4.645 |
| HbAC | 0.0004, 0.9 | Y = 0.01109*X + 4.156 | |
| HbAS | 0.07, 0.7 | Y = −0.1243*X + 5.144 | |
| HbSC | 4.053 × 10−5, 0.96 | Y = −0.002809*X + 3.956 | |
| HbSS | 0.08, 0.3 | Y = 0.1234*X + 1.997 | |
| HbCC | 0.04, 0.22 | Y = −0.08116*X + 3.263 | |
| Rings | HbAA | 0.08, 0.02 | Y = −0.4903*X + 9.943 |
| HbAC | 0.0001, 0.9 | Y = 0.02736*X + 7.332 | |
| HbAS | 0.0003, 0.9 | Y = −0.06652*X + 9.395 | |
| HbSC | 0.19, 0.0007 | Y = −0.5460*X + 8.440 | |
| HbSS | 0.06, 0.06 | Y = 0.6375*X + 1.517 | |
| HbCC | 0.003, 0.72 | Y = −0.1128*X + 6.341 | |
| Trophozoites | HbAA | 0.12, 0.005 | Y = −1.089*X + 18.59 |
| HbAC | 0.14, 0.009 | Y = −1.148*X + 21.47 | |
| HbAS | 0.19, 0.001 | Y = −1.274*X + 21.20 | |
| HbSC | 0.16, 0.0002 | Y = −1.150*X + 18.90 | |
| HbSS | 0.005, 0.57 | Y = −0.1838*X + 8.988 | |
| HbCC | 0.31, 0.0001 | Y = −0.8259*X + 13.23 | |
| Schizonts | HbAA | 0.04, 0.09 | Y = 0.1672*X + 0.6515 |
| HbAC | 0.06, 0.1 | Y = 0.2371*X − 0.005741 | |
| HbAS | 0.13, 0.01 | Y = 0.3098*X − 0.2333 | |
| HbSC | 0.0004, 0.88 | Y = −0.01609*X + 2.068 | |
| HbSS | 0.13, 0.005 | Y = 0.3023*X + 0.7634 | |
| HbCC | 0.07, 0.08 | Y = 0.1743*X + 0.09902 | |
| Phase-One Gametocytes | HbAA | 0.2, 0.0002 | Y = 0.3219*X − 0.5794 |
| HbAC | 0.05, 0.12 | Y = 0.1844*X + 0.4737 | |
| HbAS | 0.06, 0.08 | Y = 0.2160*X − 0.01239 | |
| HbSC | 0.06, 0.06 | Y = 0.2687*X − 0.0385 | |
| HbSS | 0.12, 0.006 | Y = 0.2808*X − 0.5905 | |
| HbCC | 0.14, 0.01 | Y = 0.2417*X − 0.7511 | |
| Phase-Two Gametocytes | HbAA | 0.19, 0.0004 | Y = 0.3639*X − 0.6327 |
| HbAC | 0.17, 0.004 | Y = 0.3254*X − 0.9284 | |
| HbAS | 0.09, 0.03 | Y = 0.2320*X + 0.003216 | |
| HbSC | 0.09, <0.0001 | Y = 0.2067*X − 0.2117 | |
| HbSS | 0.1, 0.01 | Y = 0.1711*X − 0.3202 | |
| HbCC | 0.08, 0.07 | Y = 0.2067*X − 0.2117 | |
| Phase-Three Gametocytes | HbAA | 0.1, 0.008 | Y = 0.3840*X + 0.08443 |
| HbAC | 0.03, 0.24 | Y = 0.2023*X + 0.9809 | |
| HbAS | 0.03, 0.2 | Y = 0.1946*X + 0.7020 | |
| HbSC | 0.28, <0.0001 | Y = 0.4977*X − 1.247 | |
| HbSS | 0.14, 0.004 | Y = 0.2872*X − 0.5419 | |
| HbCC | 0.03, 0.31 | Y = 0.1289*X + 0.7532 | |
| Phase-Four Gametocytes | HbAA | 0.31, <0.0001 | Y = 0.9743*X − 3.516 |
| HbAC | 0.1, 0.03 | Y = 0.3262*X − 0.9221 | |
| HbAS | 0.24, 0.0002 | Y = 0.6697*X − 2.554 | |
| HbSC | 0.45, <0.0001 | Y = 0.9901*X − 4.328 | |
| HbSS | 0.13, 0.005 | Y = 0.2512*X − 0.7567 | |
| HbCC | 0.09, 0.05 | Y = 0.1706*X − 0.3764 | |
| Phase-Five Gametocytes | HbAA | 0.16, 0.001 | Y = 0.6156*X − 2.219 |
| HbAC | 0.14, 0.009 | Y = 0.3842*X − 1.539 | |
| HbAS | 0.3, <0.0001 | Y = 0.4824*X − 2.425 | |
| HbSC | 0.34, <0.0001 | Y = 0.5963*X − 2.738 | |
| HbSS | 0.09, 0.02 | Y = 0.2421*X − 0.8741 | |
| HbCC | 0.11, 0.03 | Y = 0.1417*X − 0.5574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oxendine Harp, K.; Bashi, A.; Botchway, F.; Addo-Gyan, D.; Tetteh-Tsifoanya, M.; Lamptey, A.; Djameh, G.; Iqbal, S.A.; Lekpor, C.; Banerjee, S.; et al. Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels. Int. J. Mol. Sci. 2023, 24, 7546. https://doi.org/10.3390/ijms24087546
Oxendine Harp K, Bashi A, Botchway F, Addo-Gyan D, Tetteh-Tsifoanya M, Lamptey A, Djameh G, Iqbal SA, Lekpor C, Banerjee S, et al. Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels. International Journal of Molecular Sciences. 2023; 24(8):7546. https://doi.org/10.3390/ijms24087546
Chicago/Turabian StyleOxendine Harp, Keri, Alaijah Bashi, Felix Botchway, Daniel Addo-Gyan, Mark Tetteh-Tsifoanya, Amanda Lamptey, Georgina Djameh, Shareen A. Iqbal, Cecilia Lekpor, Saswati Banerjee, and et al. 2023. "Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels" International Journal of Molecular Sciences 24, no. 8: 7546. https://doi.org/10.3390/ijms24087546
APA StyleOxendine Harp, K., Bashi, A., Botchway, F., Addo-Gyan, D., Tetteh-Tsifoanya, M., Lamptey, A., Djameh, G., Iqbal, S. A., Lekpor, C., Banerjee, S., Wilson, M. D., Dei-Adomakoh, Y., Adjei, A. A., Stiles, J. K., & Driss, A. (2023). Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels. International Journal of Molecular Sciences, 24(8), 7546. https://doi.org/10.3390/ijms24087546








