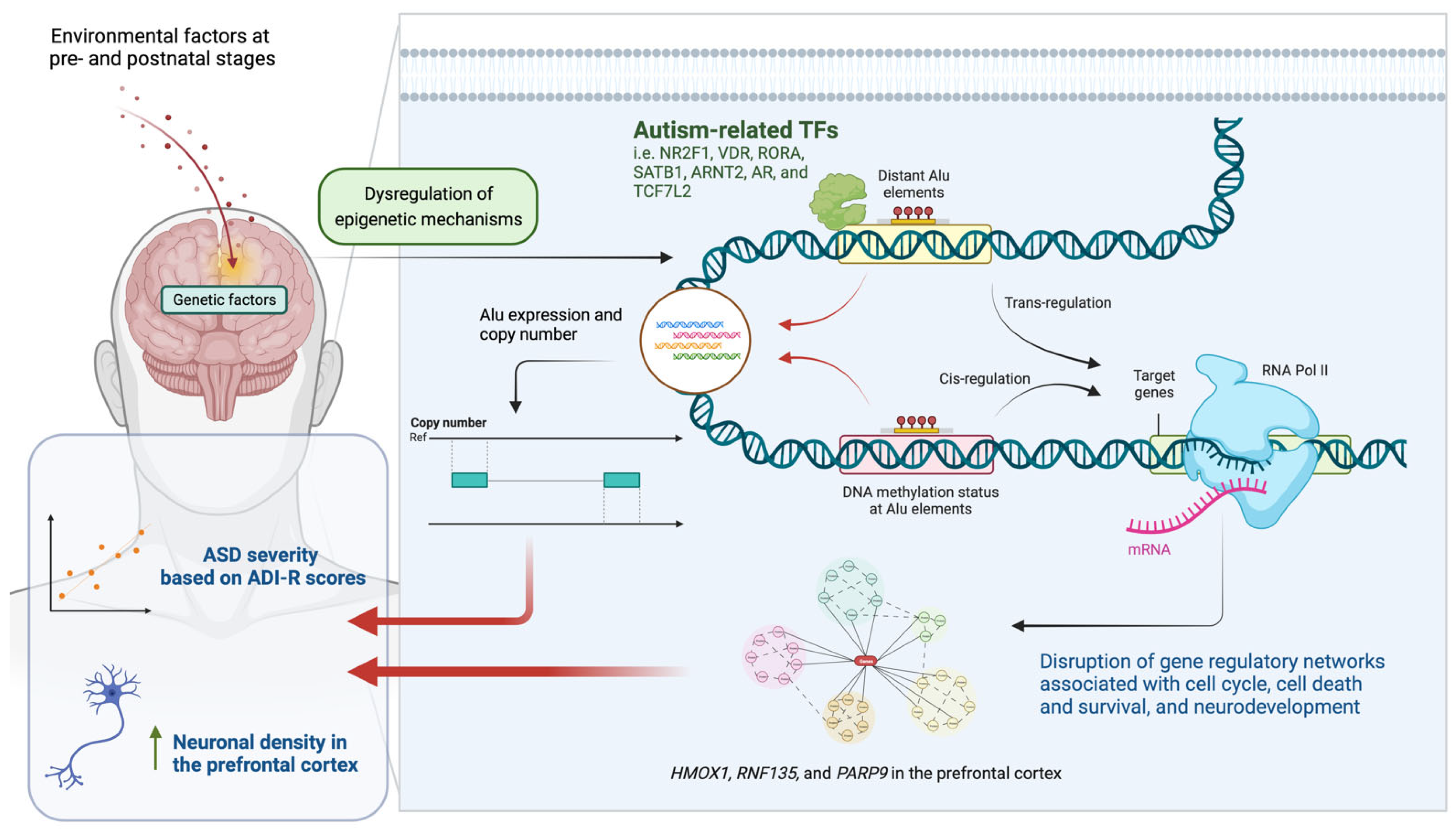
Epigenetic Gene-Regulatory Loci in Alu Elements Associated with Autism Susceptibility in the Prefrontal Cortex of ASD
Abstract
1. Introduction
2. Results
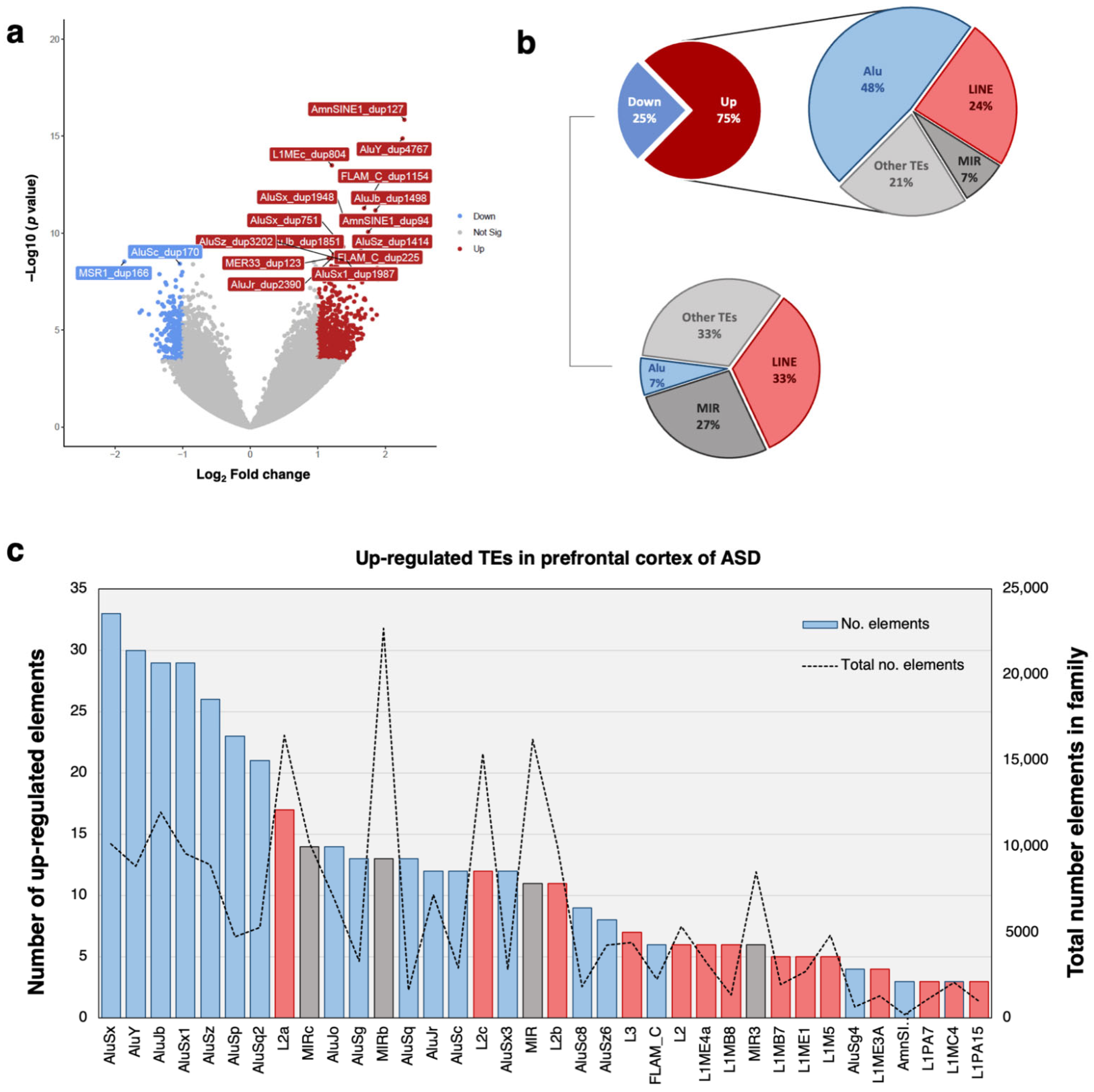
2.1. Transposable Element Profiling in the Prefrontal Cortex of ASD
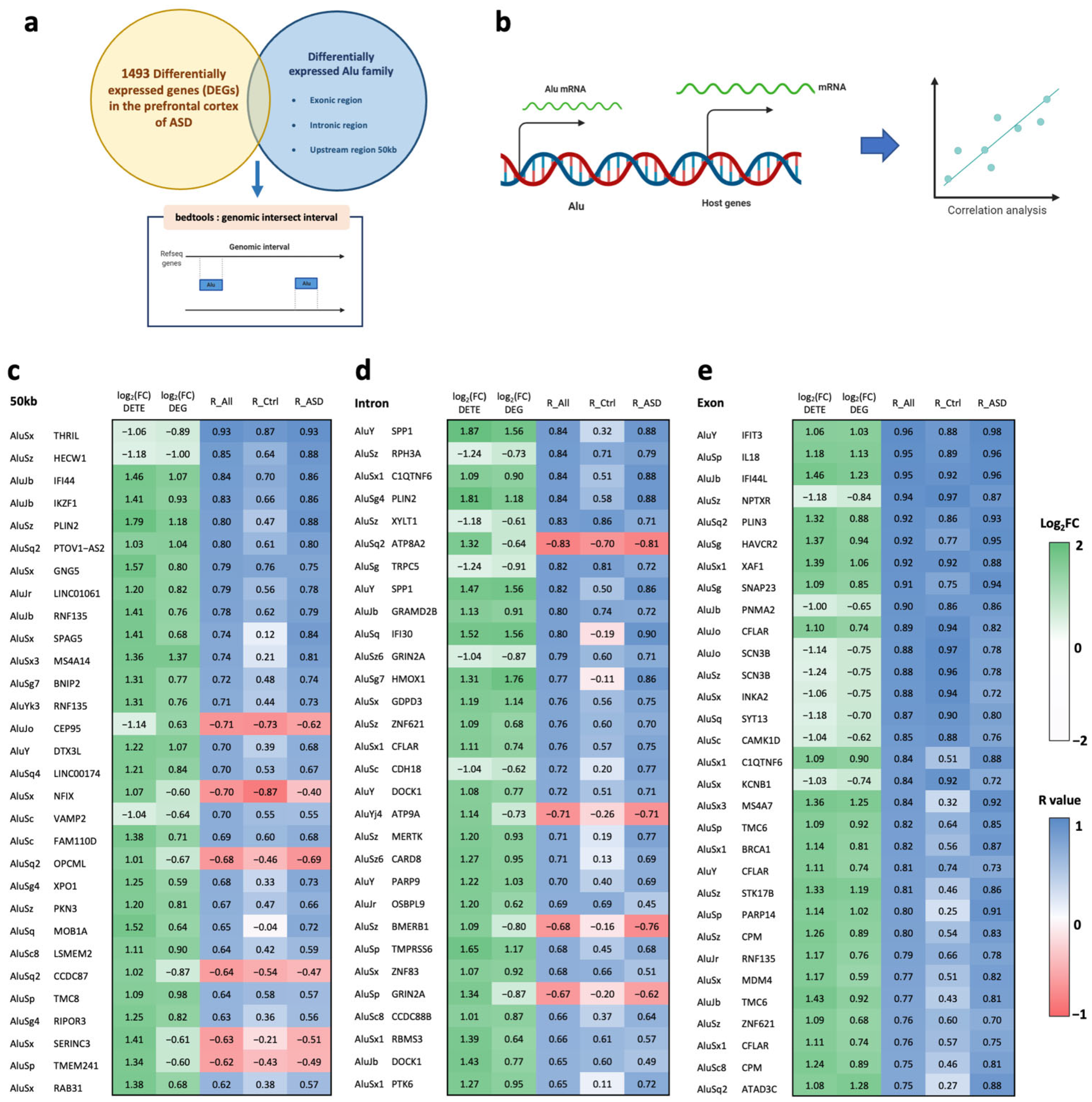
2.2. Correlations between Alu Expression and Differentially Expressed Genes in the Prefrontal Cortex of ASD Individuals in Cis- and Trans-Regulatory Manners
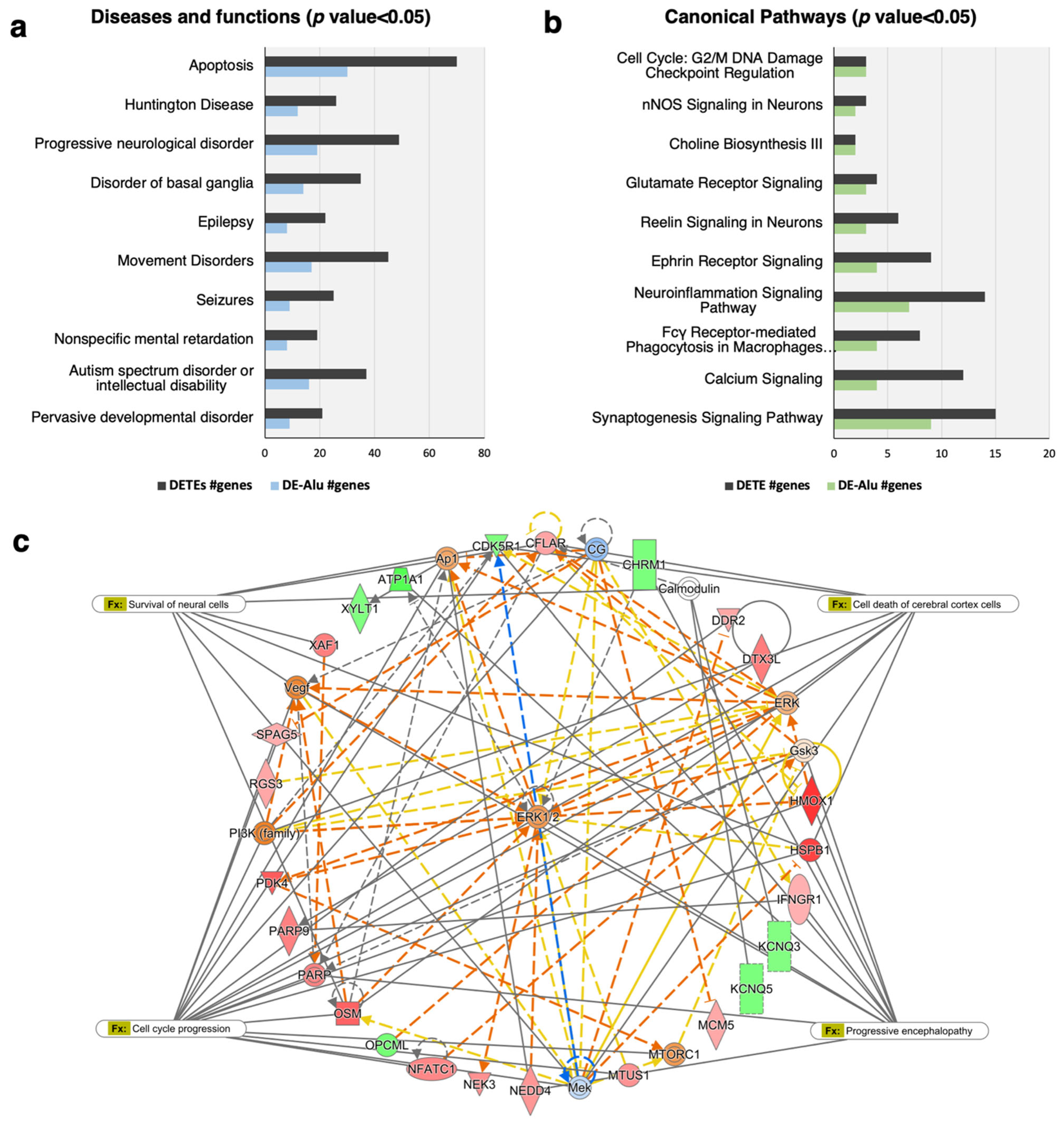
2.3. The Alu-Mediated Gene Regulatory Network in the Prefrontal Cortex of ASD Individuals Is Associated with ASD and the Cell Death and Survival of Neural Cells
2.4. Consensus Sequence and Transcription Factor Binding Sites at DE-Alu Elements
2.5. DNA Methylation of Alu Is Altered in the Prefrontal Cortex of ASD Individuals
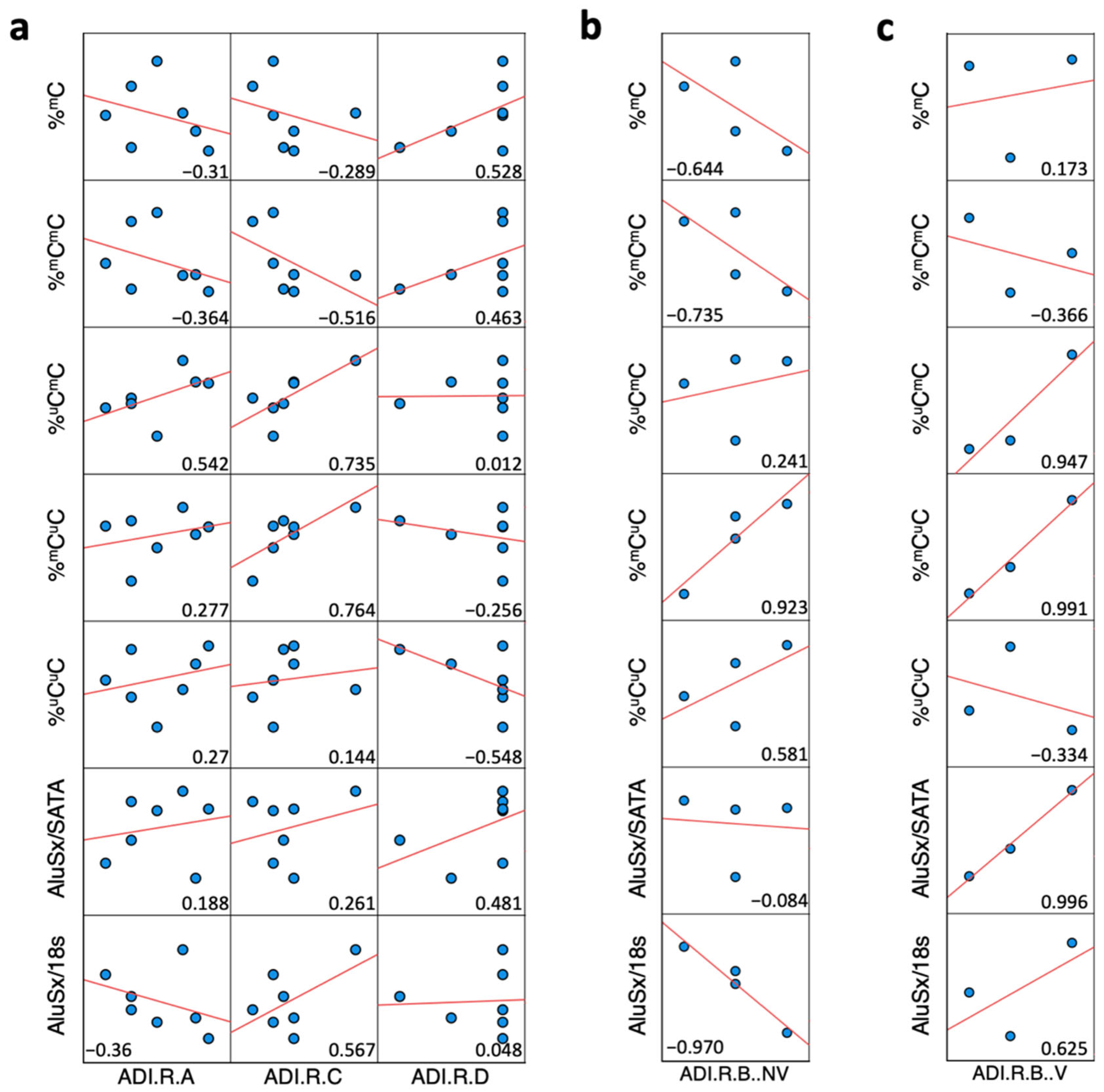
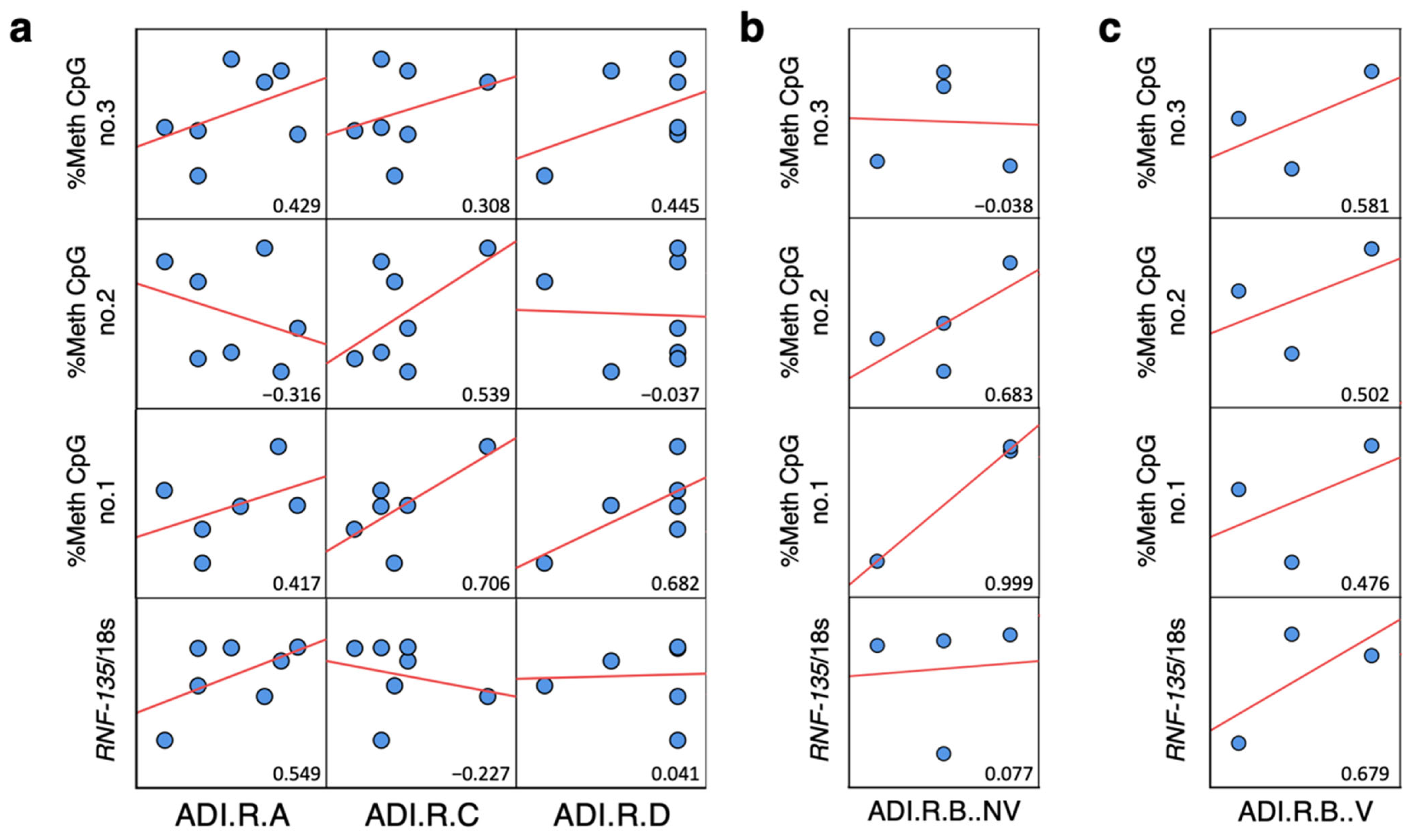
2.6. Correlation Analysis between ADI-R Score and DNA Methylation and Expression of Alu Elements in ASD Brain Tissues
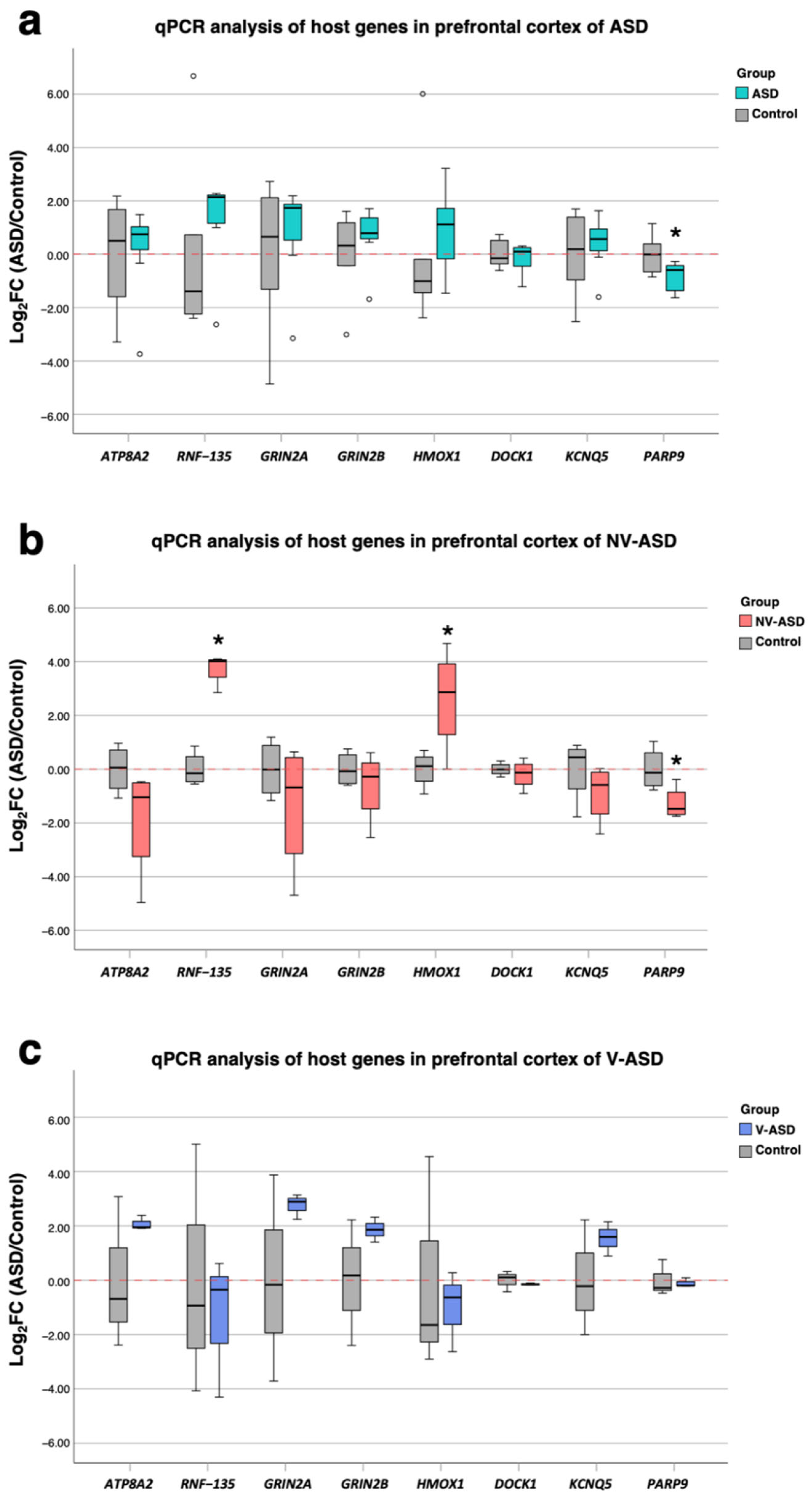
2.7. Confirmation of Expression Levels of DEGs Associated with Alu Elements in Postmortem Brain Tissues
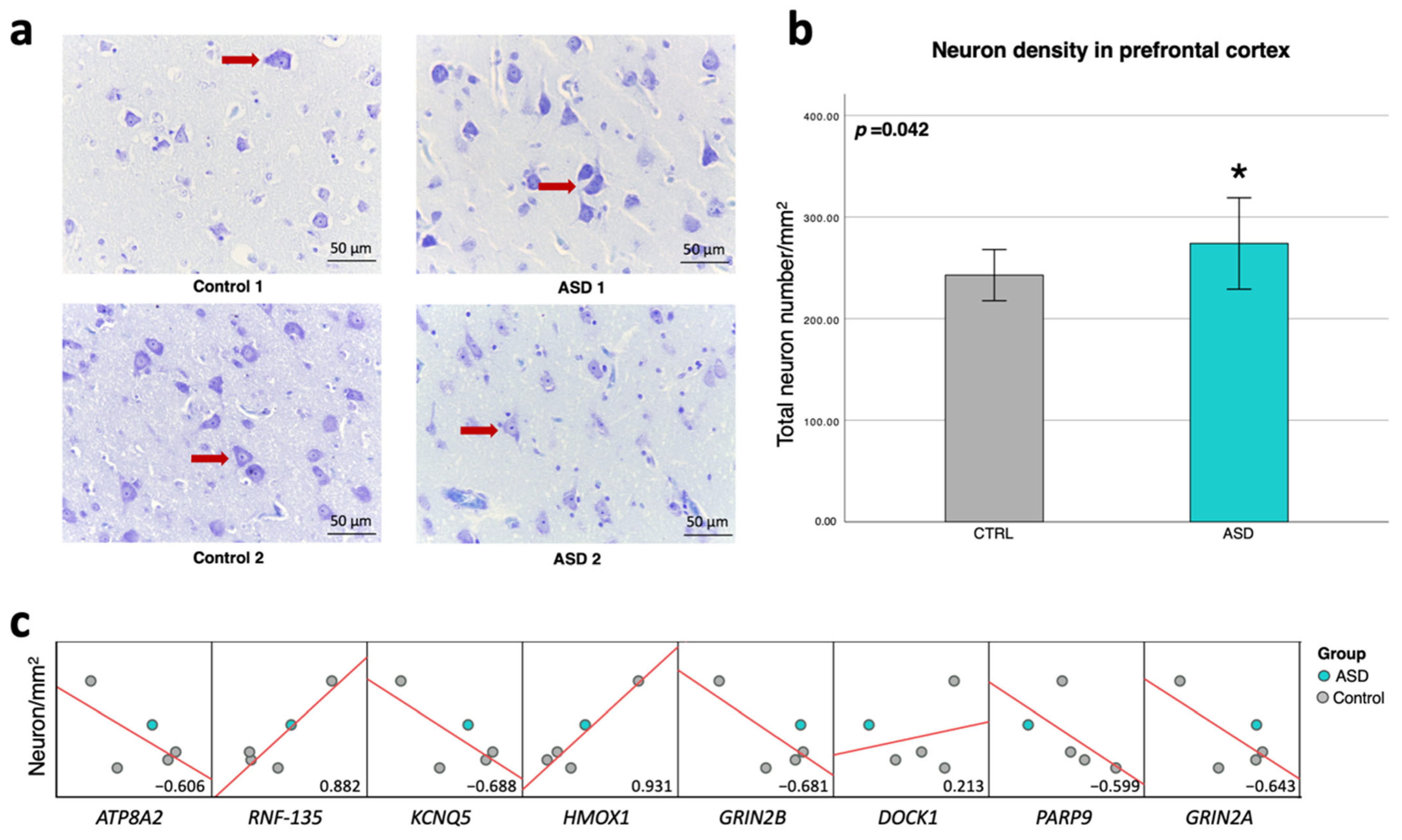
2.8. RNF135 and HMOX1 Genes Are Related to Neuron Number in the Prefrontal Cortex
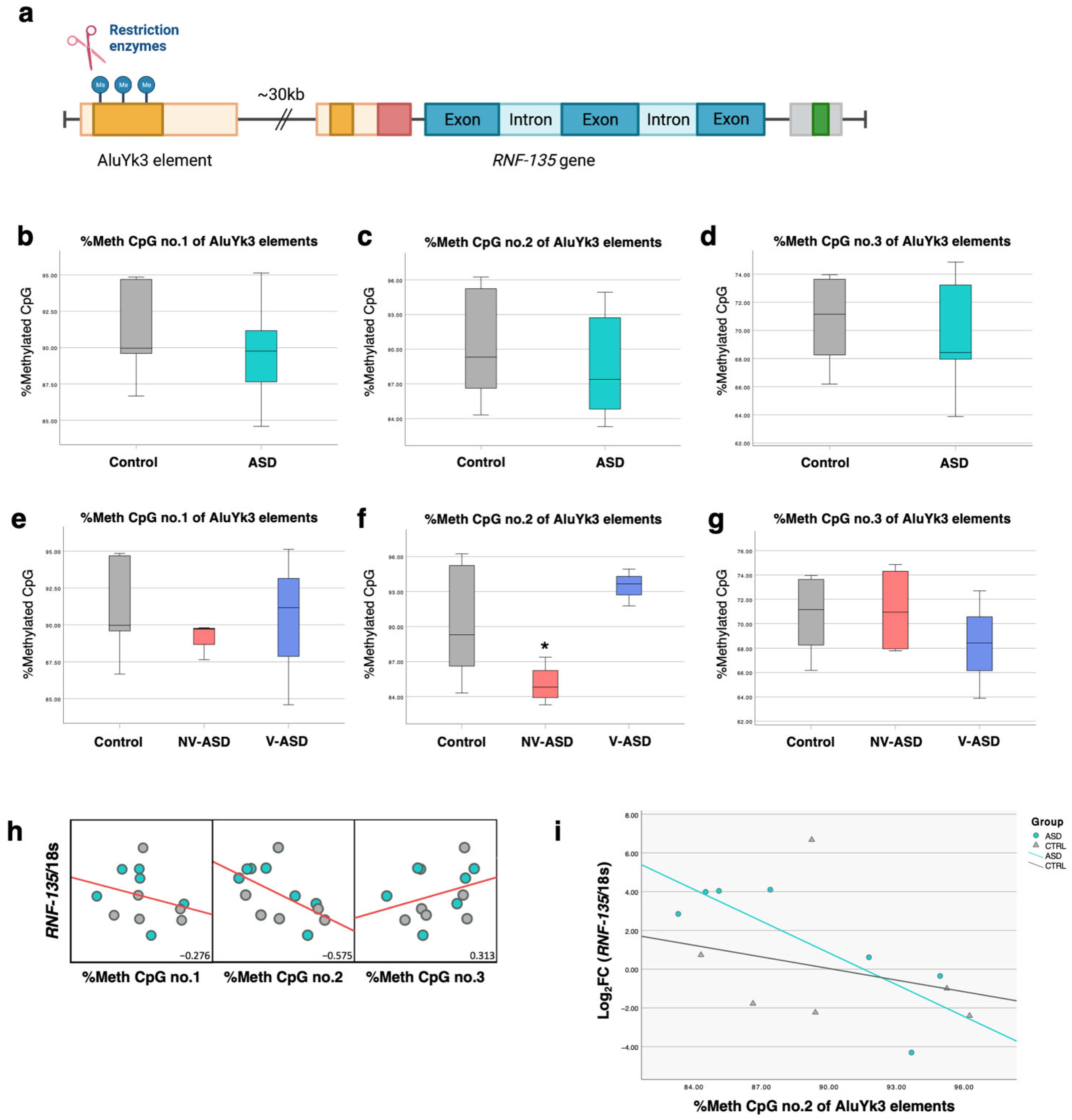
2.9. Locus-Specific DNA Methylation of AluYk3 Located in the Upstream Region of the RNF-135 Gene
3. Discussion
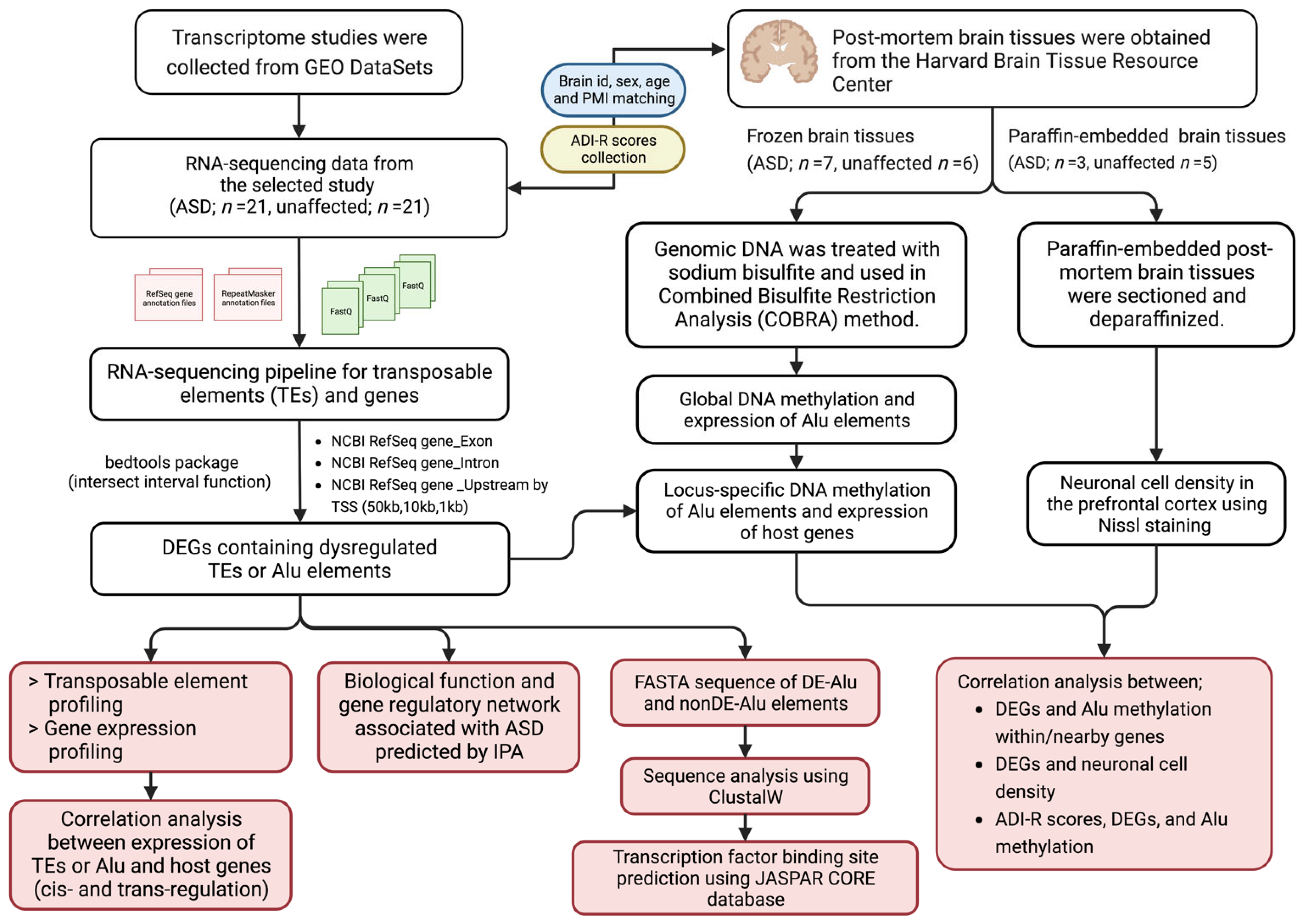
4. Materials and Methods
4.1. Data Collection
4.2. RNA-Seq Data Reanalysis for Transposable Element Profiling in the Prefrontal Cortex of ASD Individuals
4.3. Identification of Genomic Loci of Differentially Expressed TEs Located Within/near Protein-Coding Genes
4.4. Cis- and Trans-Regulation of DETEs and the Expression Level of Nearby and Distant Genes
4.5. Identification of Biological Functions, Pathways, and Autism Candidate Genes of Differentially Expressed Genes Associated with TEs
4.6. Multiple Sequence Alignment Analysis and Transcription Factor Binding Site Prediction of Alu Elements
4.7. DNA Extraction from Postmortem Brain Tissues
4.8. Combined Bisulfite Restriction Analysis (COBRA) for Alu Methylation Level and Patterns
4.9. Quantitative RT–PCR Analysis
4.10. Nissl Staining and Quantification of Neuron Number
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.; Fitzgerald, R.; Furnier, S.; Hughes, M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Schaefer, G.B.; Mendelsohn, N.J. Genetics evaluation for the etiologic diagnosis of autism spectrum disorders. Genet. Med. 2008, 10, 4–12. [Google Scholar] [CrossRef]
- Bolte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Kanlayaprasit, S.; Thongkorn, S.; Panjabud, P.; Jindatip, D.; Hu, V.W.; Kikkawa, T.; Osumi, N.; Sarachana, T. Autism-Related Transcription Factors Underlying the Sex-Specific Effects of Prenatal Bisphenol A Exposure on Transcriptome-Interactome Profiles in the Offspring Prefrontal Cortex. Int. J. Mol. Sci. 2021, 22, 13201. [Google Scholar] [CrossRef]
- Stamou, M.; Streifel, K.M.; Goines, P.E.; Lein, P.J. Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol. Teratol. 2013, 36, 3–16. [Google Scholar] [CrossRef]
- Thongkorn, S.; Kanlayaprasit, S.; Jindatip, D.; Tencomnao, T.; Hu, V.W.; Sarachana, T. Sex Differences in the Effects of Prenatal Bisphenol A Exposure on Genes Associated with Autism Spectrum Disorder in the Hippocampus. Sci. Rep. 2019, 9, 3038. [Google Scholar] [CrossRef]
- Thongkorn, S.; Kanlayaprasit, S.; Panjabud, P.; Saeliw, T.; Jantheang, T.; Kasitipradit, K.; Sarobol, S.; Jindatip, D.; Hu, V.W.; Tencomnao, T.; et al. Sex differences in the effects of prenatal bisphenol A exposure on autism-related genes and their relationships with the hippocampus functions. Sci. Rep. 2021, 11, 1241. [Google Scholar] [CrossRef]
- Tordjman, S.; Somogyi, E.; Coulon, N.; Kermarrec, S.; Cohen, D.; Bronsard, G.; Bonnot, O.; Weismann-Arcache, C.; Botbol, M.; Lauth, B.; et al. Gene x Environment interactions in autism spectrum disorders: Role of epigenetic mechanisms. Front. Psychiatry 2014, 5, 53. [Google Scholar] [CrossRef]
- Thongkorn, S.; Kanlayaprasit, S.; Kasitipradit, K.; Lertpeerapan, P.; Panjabud, P.; Hu, V.W.; Jindatip, D.; Sarachana, T. Investigation of autism-related transcription factors underlying sex differences in the effects of bisphenol A on transcriptome profiles and synaptogenesis in the offspring hippocampus. Biol. Sex. Differ. 2023, 14, 8. [Google Scholar] [CrossRef]
- Pichitpunpong, C.; Thongkorn, S.; Kanlayaprasit, S.; Yuwattana, W.; Plaingam, W.; Sangsuthum, S.; Aizat, W.M.; Baharum, S.N.; Tencomnao, T.; Hu, V.W.; et al. Phenotypic subgrouping and multi-omics analyses reveal reduced diazepam-binding inhibitor (DBI) protein levels in autism spectrum disorder with severe language impairment. PLoS ONE 2019, 14, e0214198. [Google Scholar] [CrossRef]
- Tremblay, M.W.; Jiang, Y.H. DNA Methylation and Susceptibility to Autism Spectrum Disorder. Annu. Rev. Med. 2019, 70, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ellis, S.E.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef] [PubMed]
- Ladd-Acosta, C.; Hansen, K.D.; Briem, E.; Fallin, M.D.; Kaufmann, W.E.; Feinberg, A.P. Common DNA methylation alterations in multiple brain regions in autism. Mol. Psychiatry 2014, 19, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Nardone, S.; Sams, D.S.; Zito, A.; Reuveni, E.; Elliott, E. Dysregulation of Cortical Neuron DNA Methylation Profile in Autism Spectrum Disorder. Cereb. Cortex. 2017, 27, 5739–5754. [Google Scholar] [CrossRef]
- Voineagu, I.; Wang, X.; Johnston, P.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011, 474, 380–384. [Google Scholar] [CrossRef]
- Garcia-Perez, J.L.; Widmann, T.J.; Adams, I.R. The impact of transposable elements on mammalian development. Development 2016, 143, 4101–4114. [Google Scholar] [CrossRef]
- Deininger, P.L.; Moran, J.V.; Batzer, M.A.; Kazazian, H.H., Jr. Mobile elements and mammalian genome evolution. Curr. Opin. Genet. Dev. 2003, 13, 651–658. [Google Scholar] [CrossRef]
- Chu, W.M.; Liu, W.M.; Schmid, C.W. RNA polymerase III promoter and terminator elements affect Alu RNA expression. Nucleic Acids Res. 1995, 23, 1750–1757. [Google Scholar] [CrossRef]
- Saeliw, T.; Tangsuwansri, C.; Thongkorn, S.; Chonchaiya, W.; Suphapeetiporn, K.; Mutirangura, A.; Tencomnao, T.; Hu, V.W.; Sarachana, T. Integrated genome-wide Alu methylation and transcriptome profiling analyses reveal novel epigenetic regulatory networks associated with autism spectrum disorder. Mol. Autism. 2018, 9, 27. [Google Scholar] [CrossRef]
- Tangsuwansri, C.; Saeliw, T.; Thongkorn, S.; Chonchaiya, W.; Suphapeetiporn, K.; Mutirangura, A.; Tencomnao, T.; Hu, V.W.; Sarachana, T. Investigation of epigenetic regulatory networks associated with autism spectrum disorder (ASD) by integrated global LINE-1 methylation and gene expression profiling analyses. PLoS ONE 2018, 13, e0201071. [Google Scholar] [CrossRef]
- Saeliw, T.; Permpoon, T.; Iadsee, N.; Tencomnao, T.; Hu, V.W.; Sarachana, T.; Green, D.; Sae-Lee, C. LINE-1 and Alu methylation signatures in autism spectrum disorder and their associations with the expression of autism-related genes. Sci. Rep. 2022, 12, 13970. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, M.V.; de la Torre-Aguilar, M.J.; Morales-Ruiz, T.; Gómez-Fernández, A.; Flores-Rojas, K.; Gil-Campos, M.; Martin-Borreguero, P.; Ariza, R.R.; Roldán-Arjona, T.; Perez-Navero, J.L. Analysis of Global and Local DNA Methylation Patterns in Blood Samples of Patients With Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 685310. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Hirsch, J.; Eyal, E.; Knisbacher, B.A.; Roth, J.; Cesarkas, K.; Dor, C.; Farage-Barhom, S.; Kunik, V.; Simon, A.J.; Gal, M.; et al. Whole-genome sequencing reveals principles of brain retrotransposition in neurodevelopmental disorders. Cell Res. 2018, 28, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Shpyleva, S.; Melnyk, S.; Pavliv, O.; Pogribny, I.; Jill James, S. Overexpression of LINE-1 Retrotransposons in Autism Brain. Mol. Neurobiol. 2018, 55, 1740–1749. [Google Scholar] [CrossRef]
- Su, M.; Han, D.; Boyd-Kirkup, J.; Yu, X.; Han, J.J. Evolution of Alu elements toward enhancers. Cell Rep. 2014, 7, 376–385. [Google Scholar] [CrossRef]
- Bai, X.; Li, F.; Zhang, Z. A hypothetical model of trans-acting R-loops-mediated promoter-enhancer interactions by Alu elements. J. Genet. Genom. 2021, 48, 1007–1019. [Google Scholar] [CrossRef]
- Zhang, X.O.; Gingeras, T.R.; Weng, Z. Genome-wide analysis of polymerase III-transcribed Alu elements suggests cell-type-specific enhancer function. Genome Res. 2019, 29, 1402–1414. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar]
- Larsen, P.A.; Hunnicutt, K.E.; Larsen, R.J.; Yoder, A.D.; Saunders, A.M. Warning SINEs: Alu elements, evolution of the human brain, and the spectrum of neurological disease. Chromosome Res. 2018, 26, 93–111. [Google Scholar] [CrossRef]
- Linker, S.B.; Marchetto, M.C.; Narvaiza, I.; Denli, A.M.; Gage, F.H. Examining non-LTR retrotransposons in the context of the evolving primate brain. BMC Biol. 2017, 15, 68. [Google Scholar] [CrossRef]
- Ottesen, E.W.; Seo, J.; Singh, N.N.; Singh, R.N. A Multilayered Control of the Human Survival Motor Neuron Gene Expression by Alu Elements. Front. Microbiol. 2017, 8, 2252. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Kugel, H.; Redlich, R.; Grotegerd, D.; Bürger, C.; Bürkner, P.-C.; Opel, N.; Dohm, K.; Zaremba, D.; Meinert, S.; et al. Association of Serotonin Transporter Gene AluJb Methylation with Major Depression, Amygdala Responsiveness, 5-HTTLPR/rs25531 Polymorphism, and Stress. Neuropsychopharmacology 2018, 43, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Hsieh, T.-J.; Kuo, M.-C.; Tsai, M.-L.; Tsai, K.-L.; Chen, C.-H.; Yang, Y.-H. Functional regulation of Alu element of human angiotensin-converting enzyme gene in neuron cells. Neurobiol. Aging 2013, 34, 1921.e1–1921.e7. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Smit, A.; Boissinot, S. Molecular evolution and tempo of amplification of human LINE-1 retrotransposons since the origin of primates. Genome Res. 2006, 16, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Batzer, M.A.; Deininger, P.L. Alu repeats and human genomic diversity. Nat. Rev. Genet. 2002, 3, 370–379. [Google Scholar] [CrossRef]
- Tsirigos, A.; Rigoutsos, I. Alu and b1 repeats have been selectively retained in the upstream and intronic regions of genes of specific functional classes. PLoS Comput. Biol. 2009, 5, e1000610. [Google Scholar] [CrossRef]
- Polak, P.; Domany, E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genom. 2006, 7, 133. [Google Scholar] [CrossRef]
- Ferrari, R.; de Llobet Cucalon, L.I.; Di Vona, C.; Le Dilly, F.; Vidal, E.; Lioutas, A.; Oliete, J.Q.; Jochem, L.; Cutts, E.; Dieci, G.; et al. TFIIIC Binding to Alu Elements Controls Gene Expression via Chromatin Looping and Histone Acetylation. Mol. Cell 2020, 77, 475–487.e11. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Sahin, M. Autism and the synapse: Emerging mechanisms and mechanism-based therapies. Curr. Opin. Neurol. 2015, 28, 91–102. [Google Scholar] [CrossRef]
- Careaga, M.; Van de Water, J.; Ashwood, P. Immune dysfunction in autism: A pathway to treatment. Neurotherapeutics 2010, 7, 283–292. [Google Scholar] [CrossRef]
- Hu, V.W.; Sarachana, T.; Sherrard, R.M.; Kocher, K.M. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Knickmeyer, R.C.; Belmonte, M.K. Sex differences in the brain: Implications for explaining autism. Science 2005, 310, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Chiang, V.S.; DeRosa, H.; Park, J.H.; Hunter, R.G. The Role of Transposable Elements in Sexual Development. Front. Behav. Neurosci. 2022, 16, 923732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X. The Correlation Between Vitamin D Receptor (VDR) Gene Polymorphisms and Autism: A Meta-analysis. J. Mol. Neurosci. 2020, 70, 260–268. [Google Scholar] [CrossRef]
- Saad, K.; Abdel-Rahman, A.A.; Elserogy, Y.M.; Al-Atram, A.A.; Cannell, J.J.; Bjorklund, G.; Abdel-Reheim, M.K.; Othman, H.A.K.; El-Houfey, A.A.; El-Aziz, N.H.R.A.; et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr. Neurosci. 2016, 19, 346–351. [Google Scholar] [CrossRef]
- Macova, L.; Bicikova, M.; Ostatnikova, D.; Hill, M.; Starka, L. Vitamin D, neurosteroids and autism. Physiol. Res. 2017, 66 (Suppl. 3), S333–S340. [Google Scholar] [CrossRef]
- Lowe, C.B.; Bejerano, G.; Haussler, D. Thousands of human mobile element fragments undergo strong purifying selection near developmental genes. Proc. Natl. Acad. Sci. USA 2007, 104, 8005–8010. [Google Scholar] [CrossRef]
- Goodier, J.L. Restricting retrotransposons: A review. Mob. DNA 2016, 7, 16. [Google Scholar] [CrossRef]
- Bollati, V.; Baccarelli, A.; Hou, L.; Bonzini, M.; Fustinoni, S.; Cavallo, D.; Byun, H.-M.; Jiang, J.; Marinelli, B.; Pesatori, A.C.; et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007, 67, 876–880. [Google Scholar] [CrossRef]
- Breton, C.V.; Byun, H.M.; Wenten, M.; Pan, F.; Yang, A.; Gilliland, F.D. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 2009, 180, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.M.; Motta, V.; Panni, T.; Bertazzi, P.A.; Apostoli, P.; Hou, L.; Baccarelli, A.A. Evolutionary age of repetitive element subfamilies and sensitivity of DNA methylation to airborne pollutants. Part Fibre Toxicol. 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Lapp, H.E.; Hunter, R.G. Early life exposures, neurodevelopmental disorders, and transposable elements. Neurobiol. Stress 2019, 11, 100174. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Yang, L. ALUternative Regulation for Gene Expression. Trends Cell Biol. 2017, 27, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Z.; Zhang, J.; Li, Z.; Lin, X.; Li, J.; Wang, S.; Yang, C.; Wu, Q.; Ye, A.Y.; Wang, M.; et al. Targeted resequencing of 358 candidate genes for autism spectrum disorder in a Chinese cohort reveals diagnostic potential and genotype-phenotype correlations. Hum. Mutat. 2019, 40, 801–815. [Google Scholar] [CrossRef]
- Tastet, J.; Decalonne, L.; Marouillat, S.; Malvy, J.; Thepault, R.A.; Toutain, A.; Paubel, A.; Tabagh, R.; Bénédetti, H.; Laumonnier, F.; et al. Mutation screening of the ubiquitin ligase gene RNF135 in French patients with autism. Psychiatr. Genet. 2015, 25, 263–267. [Google Scholar] [CrossRef]
- Ferrari, L.; Scuvera, G.; Tucci, A.; Bianchessi, D.; Rusconi, F.; Menni, F.; Battaglioli, E.; Milani, D.; Riva, P. Identification of an atypical microdeletion generating the RNF135-SUZ12 chimeric gene and causing a position effect in an NF1 patient with overgrowth. Hum. Genet. 2017, 136, 1329–1339. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Liu, Y.; Yao, Y.; Lv, X.; Dong, B.; Li, J.; Ren, S.; Yao, Y.; Xu, Y. RNF135, RING finger protein, promotes the proliferation of human glioblastoma cells in vivo and in vitro via the ERK pathway. Sci. Rep. 2016, 6, 20642. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Piotrowski, A.; Callens, T.; Fu, C.; Wimmer, K.; Claes, K.B.; Messiaen, L. Decoding NF1 Intragenic Copy-Number Variations. Am. J. Hum. Genet. 2015, 97, 238–249. [Google Scholar] [CrossRef]
- Dixon, M.L.; Christoff, K. The lateral prefrontal cortex and complex value-based learning and decision making. Neurosci. Biobehav. Rev. 2014, 45, 9–18. [Google Scholar] [CrossRef]
- Dumontheil, I.; Burgess, P.W.; Blakemore, S.J. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev. Med. Child. Neurol. 2008, 50, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.A. Hierarchical processing in the prefrontal cortex in a variety of cognitive domains. Front. Syst. Neurosci. 2014, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Mouton, P.R.; Calhoun, M.E.; Semendeferi, K.; Ahrens-Barbeau, C.; Hallet, M.J.; Barnes, C.C.; Pierce, K. Neuron number and size in prefrontal cortex of children with autism. JAMA 2011, 306, 2001–2010. [Google Scholar] [CrossRef]
- Falcone, C.; Mevises, N.Y.; Hong, T.; Dufour, B.; Chen, X.; Noctor, S.C.; Cerdeño, V.M. Neuronal and glial cell number is altered in a cortical layer-specific manner in autism. Autism 2021, 25, 2238–2253. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Camacho, J.; Combs, Z.; Ariza, J.; Lechpammer, M.; Noctor, S.C.; Martínez-Cerdeño, V. Preliminary findings suggest the number and volume of supragranular and infragranular pyramidal neurons are similar in the anterior superior temporal area of control subjects and subjects with autism. Neurosci. Lett. 2015, 589, 98–103. [Google Scholar] [CrossRef]
- Oblak, A.L.; Rosene, D.L.; Kemper, T.L.; Bauman, M.L.; Blatt, G.J. Altered posterior cingulate cortical cyctoarchitecture, but normal density of neurons and interneurons in the posterior cingulate cortex and fusiform gyrus in autism. Autism Res. 2011, 4, 200–211. [Google Scholar] [CrossRef]
- Liu, X.; Han, D.; Somel, M.; Jiang, X.; Hu, H.; Guijarro, P.; Zhang, N.; Mitchell, A.; Halene, T.; Ely, J.J.; et al. Disruption of an Evolutionarily Novel Synaptic Expression Pattern in Autism. PLoS Biol. 2016, 14, e1002558. [Google Scholar] [CrossRef]
- Wright, C.; Shin, J.H.; Rajpurohit, A.; Deep-Soboslay, A.; Collado-Torres, L.; Brandon, N.J.; Hyde, T.M.; Kleinman, J.E.; Jaffe, A.E.; Cross, A.J.; et al. Altered expression of histamine signaling genes in autism spectrum disorder. Transl Psychiatry 2017, 7, e1126. [Google Scholar] [CrossRef]
- Irimia, M.; Weatheritt, R.J.; Ellis, J.D.; Parikshak, N.N.; Gonatopoulos-Pournatzis, T.; Babor, M.; Quesnel-Vallieres, M.; Tapial, J.; Raj, B.; O’Hanlon, D.; et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 2014, 159, 1511–1523. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Teissandier, A.; Servant, N.; Barillot, E.; Bourc’his, D. Tools and best practices for retrotransposon analysis using high-throughput sequencing data. Mobile DNA 2019, 10, 52. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Navarro Gonzalez, J.; Zweig, A.S.; Speir, M.L.; Schmelter, D.; Rosenbloom, K.R.; Raney, B.J.; Powell, C.C.; Nassar, L.R.; Maulding, N.D.; Lee, C.M.; et al. The UCSC Genome Browser database: 2021 update. Nucleic Acids Res. 2021, 49, D1046–D1057. [Google Scholar] [CrossRef]
- Abrahams, B.S.; Arking, D.E.; Campbell, D.B.; Mefford, H.C.; Morrow, E.M.; Weiss, L.A.; Menashe, I.; Wadkins, T.; Banerjee-Basu, S.; Packer, A. SFARI Gene 2.0: A community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism. 2013, 4, 36. [Google Scholar] [CrossRef]
- Oliveros, J.C.; Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 23 February 2023).
- Bodenhofer, U.; Bonatesta, E.; Horejs-Kainrath, C.; Hochreiter, S. msa: An R package for multiple sequence alignment. Bioinformatics 2015, 31, 3997–3999. [Google Scholar] [CrossRef]
- Wagih, O. ggseqlogo: A versatile R package for drawing sequence logos. Bioinformatics 2017, 33, 3645–3647. [Google Scholar] [CrossRef] [PubMed]
- Fornes, O.; Castro-Mondragon, J.A.; Khan, A.; van der Lee, R.; Zhang, X.; Richmond, P.A.; Modi, B.P.; Correard, S.; Gheorghe, M.; Baranašić, D.; et al. JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020, 48, D87–D92. [Google Scholar] [CrossRef] [PubMed]
- Coufal, N.G.; Garcia-Perez, J.L.; Peng, G.E.; Yeo, G.W.; Mu, Y.; Lovci, M.T.; Morell, M.; O’Shea, K.S.; Moran, J.V.; Gage, F.H. L1 retrotransposition in human neural progenitor cells. Nature 2009, 460, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.; Andreola, F.; Sciamanna, I.; Sinibaldi-Vallebona, P.; Serafino, A.; Spadafora, C. Increased expression and copy number amplification of LINE-1 and SINE B1 retrotransposable elements in murine mammary carcinoma progression. Oncotarget 2013, 4, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cabezas, M.A.; John, Y.J.; Barbas, H.; Zikopoulos, B. Distinction of Neurons, Glia and Endothelial Cells in the Cerebral Cortex: An Algorithm Based on Cytological Features. Front. Neuroanat. 2016, 10, 107. [Google Scholar] [CrossRef]



| Genomic Feature | DETEs Position | No. of DETEs Located in DEGs | No. of Significantly Correlating Loci | ||
|---|---|---|---|---|---|
| All | Control | ASD | |||
| All transposable elements | |||||
| All features | 18,045 | 1587 | 1109 | 390 | 534 |
| 50 kb upstream region | 8199 | 507 | 293 | 63 | 119 |
| 10 kb upstream region | 1893 | 120 | 82 | 19 | 26 |
| 1 kb upstream region | 236 | 9 | 7 | 1 | 3 |
| Intronic region | 7232 | 744 | 522 | 136 | 195 |
| Exonic region | 485 | 207 | 205 | 171 | 191 |
| Alu elements | |||||
| All features | - | 659 | 417 | 86 | 161 |
| 50 kb upstream region | - | 328 | 181 | 33 | 67 |
| Intronic region | - | 291 | 197 | 27 | 58 |
| Exonic region | - | 40 | 39 | 26 | 36 |
| Trans-Regulation | DETEs-DEGs | Correlation Probability | ASD | Control | ||
|---|---|---|---|---|---|---|
| Significant Probability | DETE Elements | Significant Probability | DETE Elements | |||
| Alu elements | No. correlation loci | 491,198 | 40,599 | 316 | 7141 | 196 |
| No. DEGs | 1493 | 1465 | - | 1239 | - | |
| Matrix ID | Symbol | Relative Score | Sequence ID | Start | End | Strand | Predicted Sequence |
|---|---|---|---|---|---|---|---|
| MA1535.1 | NR2C1 | 0.98463 | DEAluS | 8 | 16 | + | TGAGGTCAG |
| MA1535.1 | NR2C1 | 0.81979 | DEAluS | 16 | 24 | + | GGAGTTCAA |
| MA0018.2 | CREB1 | 0.9342 | DEAluS | 8 | 15 | + | TGAGGTCA |
| MA0018.2 | CREB1 | 0.86424 | DEAluS | 8 | 15 | − | TGACCTCA |
| MA0018.4 | CREB1 | 0.85236 | DEAluS | 5 | 17 | + | ACTTGAGGTCAGG |
| MA0018.3 | CREB1 | 0.84877 | DEAluS | 6 | 17 | + | CTTGAGGTCAGG |
| MA0018.3 | CREB1 | 0.84877 | DEAluS | 6 | 17 | − | CCTGACCTCAAG |
| MA0684.2 | RUNX3 | 0.92281 | DEAluS | 5 | 16 | − | CTGACCTCAAGT |
| MA0684.1 | RUNX3 | 0.80775 | DEAluS | 6 | 15 | − | TGACCTCAAG |
| MA1534.1 | NR1I3 | 0.92153 | DEAluS | 16 | 24 | − | TTGAACTCC |
| MA1534.1 | NR1I3 | 0.85571 | DEAluS | 8 | 16 | − | CTGACCTCA |
| MA0017.2 | NR2F1 | 0.91535 | DEAluS | 7 | 19 | + | TTGAGGTCAGGAG |
| MA0693.2 | VDR | 0.90919 | DEAluS | 16 | 23 | + | GGAGTTCA |
| MA0693.2 | VDR | 0.89944 | DEAluS | 8 | 15 | + | TGAGGTCA |
| MA0160.1 | NR4A2 | 0.90185 | DEAluS | 9 | 16 | + | GAGGTCAG |
| MA0160.1 | NR4A2 | 0.82039 | DEAluS | 23 | 30 | + | AAGACCAG |
| MA0160.2 | NR4A2 | 0.80399 | DEAluS | 6 | 15 | + | CTTGAGGTCA |
| MA0672.1 | NKX2-3 | 0.89714 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA1111.1 | NR2F2 | 0.87947 | DEAluS | 7 | 17 | + | TTGAGGTCAGG |
| MA1587.1 | ZNF135 | 0.87829 | DEAluS | 13 | 26 | − | TCTTGAACTCCTGA |
| MA1531.1 | NR1D1 | 0.87418 | DEAluS | 10 | 24 | + | AGGTCAGGAGTTCAA |
| MA0909.1 | HOXD13 | 0.86899 | DEAluS | 58 | 67 | + | CTACTAAAAA |
| MA0093.2 | USF1 | 0.8659 | DEAluS | 1 | 11 | − | CTCAAGTGATT |
| MA0093.1 | USF1 | 0.82827 | DEAluS | 3 | 9 | − | CAAGTGA |
| MA0093.1 | USF1 | 0.8266 | DEAluS | 4 | 10 | + | CACTTGA |
| MA0093.2 | USF1 | 0.82641 | DEAluS | 11 | 21 | − | AACTCCTGACC |
| MA1532.1 | NR1D2 | 0.86489 | DEAluS | 10 | 24 | + | AGGTCAGGAGTTCAA |
| MA1532.2 | NR1D2 | 0.84991 | DEAluS | 9 | 23 | + | GAGGTCAGGAGTTCA |
| MA0871.1 | TFEC | 0.86383 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA0871.1 | TFEC | 0.85006 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0871.2 | TFEC | 0.8011 | DEAluS | 1 | 11 | − | CTCAAGTGATT |
| MA0154.2 | EBF1 | 0.8628 | DEAluS | 3 | 13 | − | ACCTCAAGTGA |
| MA0154.2 | EBF1 | 0.84908 | DEAluS | 2 | 12 | + | ATCACTTGAGG |
| MA0154.1 | EBF1 | 0.84704 | DEAluS | 3 | 12 | − | CCTCAAGTGA |
| MA1632.1 | ATF2 | 0.86072 | DEAluS | 5 | 17 | + | ACTTGAGGTCAGG |
| MA0901.1 | HOXB13 | 0.85918 | DEAluS | 58 | 67 | + | CTACTAAAAA |
| MA0158.1 | HOXA5 | 0.85801 | DEAluS | 4 | 11 | − | CTCAAGTG |
| MA0158.1 | HOXA5 | 0.82169 | DEAluS | 14 | 21 | + | CAGGAGTT |
| MA0650.1 | HOXA13 | 0.85681 | DEAluS | 58 | 67 | + | CTACTAAAAA |
| MA1649.1 | ZBTB12 | 0.85649 | DEAluS | 17 | 27 | − | GTCTTGAACTC |
| MA0058.1 | MAX | 0.85576 | DEAluS | 1 | 10 | + | AATCACTTGA |
| MA0831.2 | TFE3 | 0.85542 | DEAluS | 2 | 9 | − | CAAGTGAT |
| MA0831.1 | TFE3 | 0.83464 | DEAluS | 12 | 21 | − | AACTCCTGAC |
| MA0831.2 | TFE3 | 0.83003 | DEAluS | 12 | 19 | − | CTCCTGAC |
| MA0831.3 | TFE3 | 0.81998 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0831.3 | TFE3 | 0.81998 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA0511.2 | RUNX2 | 0.85286 | DEAluS | 7 | 15 | − | TGACCTCAA |
| MA0899.1 | HOXA10 | 0.85 | DEAluS | 57 | 67 | + | TCTACTAAAAA |
| MA0071.1 | RORA | 0.84916 | DEAluS | 6 | 15 | + | CTTGAGGTCA |
| MA0828.1 | SREBF2 | 0.84851 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0828.1 | SREBF2 | 0.83605 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA0828.1 | SREBF2 | 0.81121 | DEAluS | 12 | 21 | − | AACTCCTGAC |
| MA0036.1 | GATA2 | 0.84608 | DEAluS | 1 | 5 | − | TGATT |
| MA0464.2 | BHLHE40 | 0.84575 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0464.2 | BHLHE40 | 0.82176 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA0745.1 | SNAI2 | 0.84549 | DEAluS | 3 | 11 | − | CTCAAGTGA |
| MA0745.1 | SNAI2 | 0.8117 | DEAluS | 24 | 32 | − | GGCTGGTCT |
| MA0636.1 | BHLHE41 | 0.84511 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA0636.1 | BHLHE41 | 0.83972 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0729.1 | RARA | 0.83842 | DEAluS | 9 | 26 | + | GAGGTCAGGAGTTCAAGA |
| MA1112.1 | NR4A1 | 0.83437 | DEAluS | 7 | 16 | + | TTGAGGTCAG |
| MA1902.1 | NFkb | 0.83413 | DEAluS | 37 | 58 | + | CAACATGGTGAAACCCCGTCTC |
| MA0913.2 | HOXD9 | 0.8321 | DEAluS | 58 | 67 | + | CTACTAAAAA |
| MA0122.2 | NKX3-2 | 0.83136 | DEAluS | 2 | 10 | + | ATCACTTGA |
| MA1655.1 | ZNF341 | 0.8311 | DEAluS | 23 | 34 | + | AAGACCAGCCTG |
| MA0833.2 | ATF4 | 0.83022 | DEAluS | 39 | 52 | + | ACATGGTGAAACCC |
| MA1656.1 | ZNF449 | 0.82761 | DEAluS | 21 | 34 | + | TCAAGACCAGCCTG |
| MA1963.1 | SATB1 | 0.82693 | DEAluS | 55 | 67 | + | TCTCTACTAAAAA |
| MA1464.1 | ARNT2 | 0.82595 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA1558.1 | SNAI1 | 0.82506 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA1558.1 | SNAI1 | 0.80459 | DEAluS | 12 | 21 | + | GTCAGGAGTT |
| MA0100.3 | MYB | 0.8245 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA1505.1 | HOXC8 | 0.82307 | DEAluS | 3 | 10 | − | TCAAGTGA |
| MA0664.1 | MLXIPL | 0.8215 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0664.1 | MLXIPL | 0.80617 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA0002.1 | RUNX1 | 0.82024 | DEAluS | 5 | 15 | + | ACTTGAGGTCA |
| MA0842.1 | NRL | 0.81706 | DEAluS | 12 | 22 | − | GAACTCCTGAC |
| MA0663.1 | MLX | 0.81579 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0525.1 | TP63 | 0.81575 | DEAluS | 19 | 38 | + | GTTCAAGACCAGCCTGGCCA |
| MA0007.2 | AR | 0.81533 | DEAluS | 23 | 37 | + | AAGACCAGCCTGGCC |
| MA0063.2 | NKX2-5 | 0.81522 | DEAluS | 2 | 12 | + | ATCACTTGAGG |
| MA0063.2 | NKX2-5 | 0.80188 | DEAluS | 57 | 67 | + | TCTACTAAAAA |
| MA1563.1 | SOX18 | 0.81158 | DEAluS | 39 | 46 | − | CACCATGT |
| MA1496.1 | HOXA4 | 0.81143 | DEAluS | 12 | 19 | + | GTCAGGAG |
| MA1496.1 | HOXA4 | 0.8107 | DEAluS | 41 | 48 | + | ATGGTGAA |
| MA1151.1 | RORC | 0.81043 | DEAluS | 4 | 15 | + | CACTTGAGGTCA |
| MA0692.1 | TFEB | 0.80964 | DEAluS | 2 | 11 | − | CTCAAGTGAT |
| MA0692.1 | TFEB | 0.80394 | DEAluS | 12 | 21 | − | AACTCCTGAC |
| MA0692.1 | TFEB | 0.80321 | DEAluS | 2 | 11 | + | ATCACTTGAG |
| MA1570.1 | TFAP4 | 0.80899 | DEAluS | 36 | 45 | − | ACCATGTTGG |
| MA1570.1 | TFAP4 | 0.80622 | DEAluS | 36 | 45 | + | CCAACATGGT |
| MA0763.1 | ETV3 | 0.80785 | DEAluS | 4 | 13 | − | ACCTCAAGTG |
| MA1498.1 | HOXA7 | 0.80731 | DEAluS | 20 | 27 | − | GTCTTGAA |
| MA1540.1 | NR5A1 | 0.80706 | DEAluS | 6 | 16 | + | CTTGAGGTCAG |
| MA1540.1 | NR5A1 | 0.8025 | DEAluS | 20 | 30 | + | TTCAAGACCAG |
| MA0492.1 | JUND | 0.80596 | DEAluS | 3 | 17 | + | TCACTTGAGGTCAGG |
| MA0523.1 | TCF7L2 | 0.805 | DEAluS | 16 | 29 | + | GGAGTTCAAGACCA |
| MA0819.2 | CLOCK | 0.8047 | DEAluS | 37 | 46 | − | CACCATGTTG |
| MA0522.2 | TCF3 | 0.8032 | DEAluS | 12 | 21 | − | AACTCCTGAC |
| MA0820.1 | FIGLA | 0.80267 | DEAluS | 12 | 21 | − | AACTCCTGAC |
| MA0488.1 | JUN | 0.80233 | DEAluS | 4 | 16 | + | CACTTGAGGTCAG |
| MA1549.1 | POU6F1 | 0.80132 | DEAluS | 12 | 21 | + | GTCAGGAGTT |
| MA1541.1 | NR6A1 | 0.8013 | DEAluS | 14 | 30 | + | CAGGAGTTCAAGACCAG |
| MA0827.1 | OLIG3 | 0.80016 | DEAluS | 36 | 45 | + | CCAACATGGT |
| AluSx Methylation | %mC | %mCmC | %uCmC | %mCuC | %uCuC |
|---|---|---|---|---|---|
| ASD | 38.837 | 26.496 | 21.128 | 19.748 | 32.629 |
| Ctrl | 39.304 | 27.634 | 20.843 | 19.254 | 32.270 |
| p value (Effect size, Cohen’s d) | 0.201 (−0.756) | 0.104 (−0.985) | 0.037 (1.324) | 0.133 (0.904) | 0.348 (0.546) |
| NV-ASD | 38.986 | 26.873 | 21.105 | 19.502 | 32.519 |
| Ctrl | 39.165 | 27.282 | 20.951 | 19.396 | 32.370 |
| p value (Effect size, Cohen’s d) | 0.726 (0.042) | 0.661 (−0.185) | 0.373 (0.807) | 0.809 (0.270) | 0.789 (−0.209) |
| V-ASD | 38.638 | 25.992 | 21.158 | 20.075 | 32.775 |
| Ctrl | 39.797 | 28.642 | 20.696 | 18.849 | 31.812 |
| p value (Effect size, Cohen’s d) | 0.019 (−3.131) | 0.004 (−4.826) | 0.023 (2.925) | 0.031 (2.666) | 0.086 (1.853) |
| DEGs | log2(FC) DEGs | p-adj DEGs | DETEs | log2(FC) DETEs | p-adj DETEs | Type | R_All (BH-p) | R_Ctrl (BH-p) | R_ASD (BH-p) |
|---|---|---|---|---|---|---|---|---|---|
| ATP8A2 | −0.64 | 2.82 × 10−3 | AluSq2 | 1.32 | 1.00 × 10−4 | Intron | −0.83 (3.27 × 10−10) | −0.70 (1.60 × 10−3) | −0.81 (5.37 × 10−05) |
| ATP8A2 | −0.64 | 2.82 × 10−3 | AluSg7 | 1.03 | 7.11 × 10−3 | Intron | −0.57 (3.83 × 10−4) | 0.11 (7.28 × 10−1) | −0.62 (8.92 × 10−3) |
| ATP8A2 | −0.64 | 2.82 × 10−3 | AluJb | 1.24 | 1.55 × 10−3 | Intron | −0.48 (4.57 × 10−3) | −0.22 (4.73 × 10−1) | −0.32 (2.60 × 10−1) |
| HMOX1 | 1.76 | 3.07 × 10−6 | AluSg7 | 1.31 | 3.69 × 10−3 | Intron | 0.77 (3.53 × 10−8) | −0.11 (7.25 × 10−1) | 0.86 (5.23 × 10−6) |
| PARP9 | 1.03 | 1.26 × 10−3 | AluY | 1.22 | 6.30 × 10−3 | Intron | 0.70 (1.96 × 10−6) | 0.40 (1.34 × 10−1) | 0.69 (2.26 × 10−3) |
| GRIN2A | −0.87 | 6.51 × 10−4 | AluSz6 | −1.04 | 1.65 × 10−3 | Intron | 0.79 (1.32 × 10−8) | 0.60 (1.24 × 10−2) | 0.71 (1.44 × 10−3) |
| GRIN2A | −0.87 | 6.51 × 10−4 | AluSp | 1.34 | 5.76 × 10−4 | Intron | −0.67 (1.15 × 10−05) | −0.20 (5.00 × 10−1) | −0.62 (8.64 × 10−3) |
| GRIN2B | −0.76 | 3.73 × 10−3 | AluSg4 | 1.81 | 4.59 × 10−4 | Intron + 50 kb | −0.50 (2.67 × 10−3) | −0.11 (7.33 × 10−1) | −0.62 (8.84 × 10−3) |
| GRIN2B | −0.76 | 3.73 × 10−3 | AluSx1 | 1.32 | 5.91 × 10−3 | Intron + 50 kb | −0.45 (8.49 × 10−3) | −0.26 (3.67 × 10−1) | −0.34 (2.27 × 10−1) |
| DOCK1 | 0.77 | 7.08 × 10−4 | AluY | 1.08 | 5.41 × 10−3 | Intron | 0.72 (8.93 × 10−7) | 0.51 (4.50 × 10−2) | 0.71 (1.50 × 10−3) |
| RNF135 | 0.76 | 8.96 × 10−4 | AluJr | 1.17 | 3.68 × 10−3 | exon | 0.79 (1.08 × 10−8) | 0.66 (4.08 × 10−3) | 0.78 (1.92 × 10−4) |
| RNF135 | 0.76 | 8.96 × 10−4 | AluJb | 1.41 | 2.37 × 10−3 | 50 kb | 0.78 (2.48 × 10−8) | 0.62 (8.84 × 10−3) | 0.79 (1.19 × 10−4) |
| RNF135 | 0.76 | 8.96 × 10−4 | AluYk3 | 1.31 | 4.24 × 10−3 | 50 kb | 0.71 (1.71 × 10−6) | 0.44 (9.05 × 10−2) | 0.73 (7.43 × 10−4) |
| KCNQ5 | −0.63 | 4.90 × 10−3 | AluSc | 1.48 | 5.65 × 10−3 | Intron + 50 kb | −0.59 (2.07 × 10−4) | 0.21 (4.88 × 10−1) | −0.66 (4.15 × 10−3) |
| KCNQ5 | −0.63 | 4.90 × 10−3 | AluSz | 1.15 | 3.13 × 10−3 | Intron + 50 kb | −0.41 (2.01 × 10−2) | −0.08 (8.13 × 10−1) | −0.31 (2.70 × 10−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeliw, T.; Kanlayaprasit, S.; Thongkorn, S.; Songsritaya, K.; Sanannam, B.; Sae-Lee, C.; Jindatip, D.; Hu, V.W.; Sarachana, T. Epigenetic Gene-Regulatory Loci in Alu Elements Associated with Autism Susceptibility in the Prefrontal Cortex of ASD. Int. J. Mol. Sci. 2023, 24, 7518. https://doi.org/10.3390/ijms24087518
Saeliw T, Kanlayaprasit S, Thongkorn S, Songsritaya K, Sanannam B, Sae-Lee C, Jindatip D, Hu VW, Sarachana T. Epigenetic Gene-Regulatory Loci in Alu Elements Associated with Autism Susceptibility in the Prefrontal Cortex of ASD. International Journal of Molecular Sciences. 2023; 24(8):7518. https://doi.org/10.3390/ijms24087518
Chicago/Turabian StyleSaeliw, Thanit, Songphon Kanlayaprasit, Surangrat Thongkorn, Kwanjira Songsritaya, Bumpenporn Sanannam, Chanachai Sae-Lee, Depicha Jindatip, Valerie W. Hu, and Tewarit Sarachana. 2023. "Epigenetic Gene-Regulatory Loci in Alu Elements Associated with Autism Susceptibility in the Prefrontal Cortex of ASD" International Journal of Molecular Sciences 24, no. 8: 7518. https://doi.org/10.3390/ijms24087518
APA StyleSaeliw, T., Kanlayaprasit, S., Thongkorn, S., Songsritaya, K., Sanannam, B., Sae-Lee, C., Jindatip, D., Hu, V. W., & Sarachana, T. (2023). Epigenetic Gene-Regulatory Loci in Alu Elements Associated with Autism Susceptibility in the Prefrontal Cortex of ASD. International Journal of Molecular Sciences, 24(8), 7518. https://doi.org/10.3390/ijms24087518







