Evaluation of Some Safety Parameters of Dual Histamine H3 and Sigma-2 Receptor Ligands with Anti-Obesity Potential
Abstract
1. Introduction
2. Results
2.1. The Effect of KSK-61, KSK-63, KSK-73, and KSK-74 on Locomotor Activity in Mice
2.2. The Effect of KSK-61, KSK-63, KSK-73, and KSK-74 on Motor Coordination in the Chimney Test
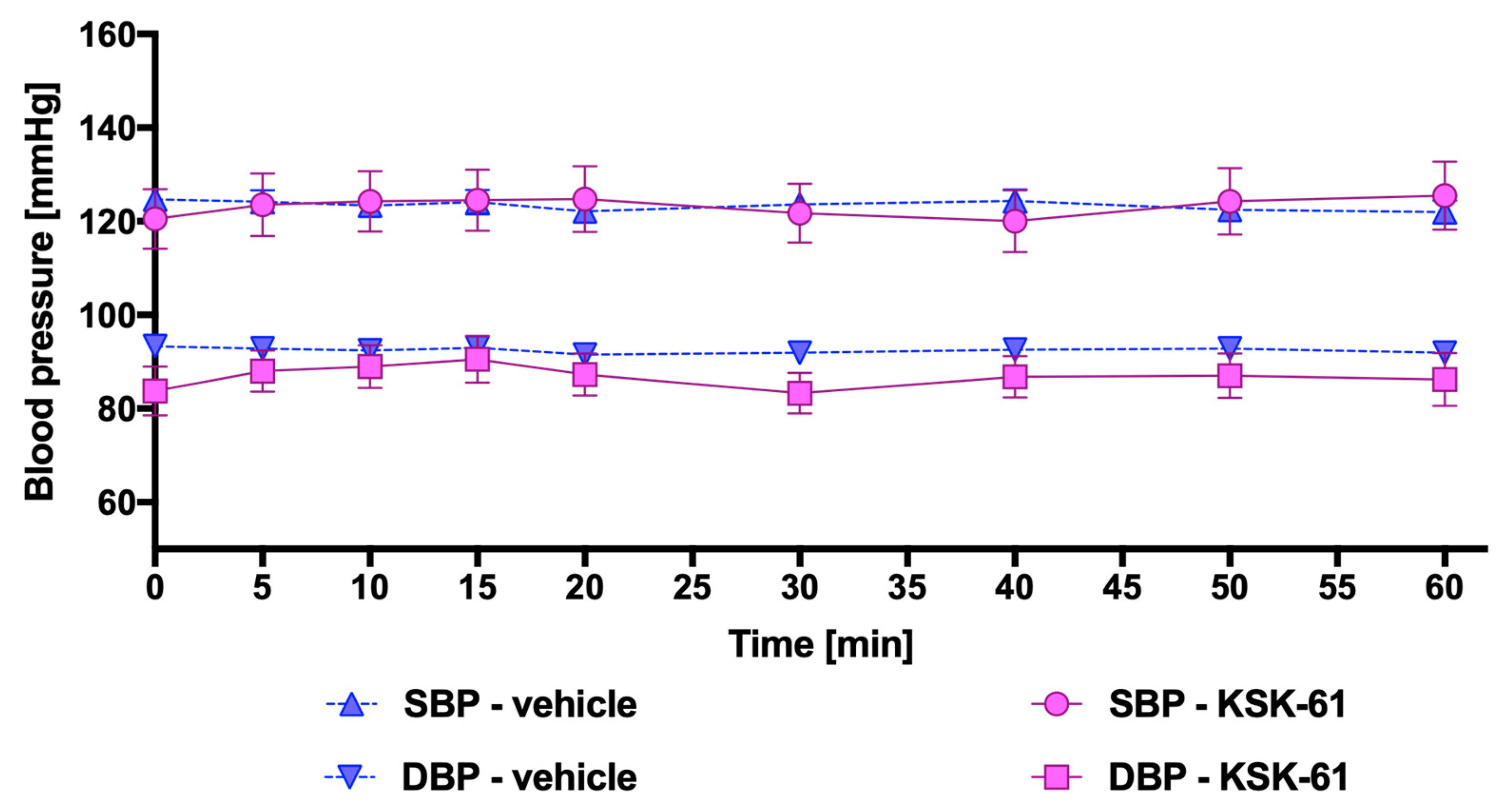
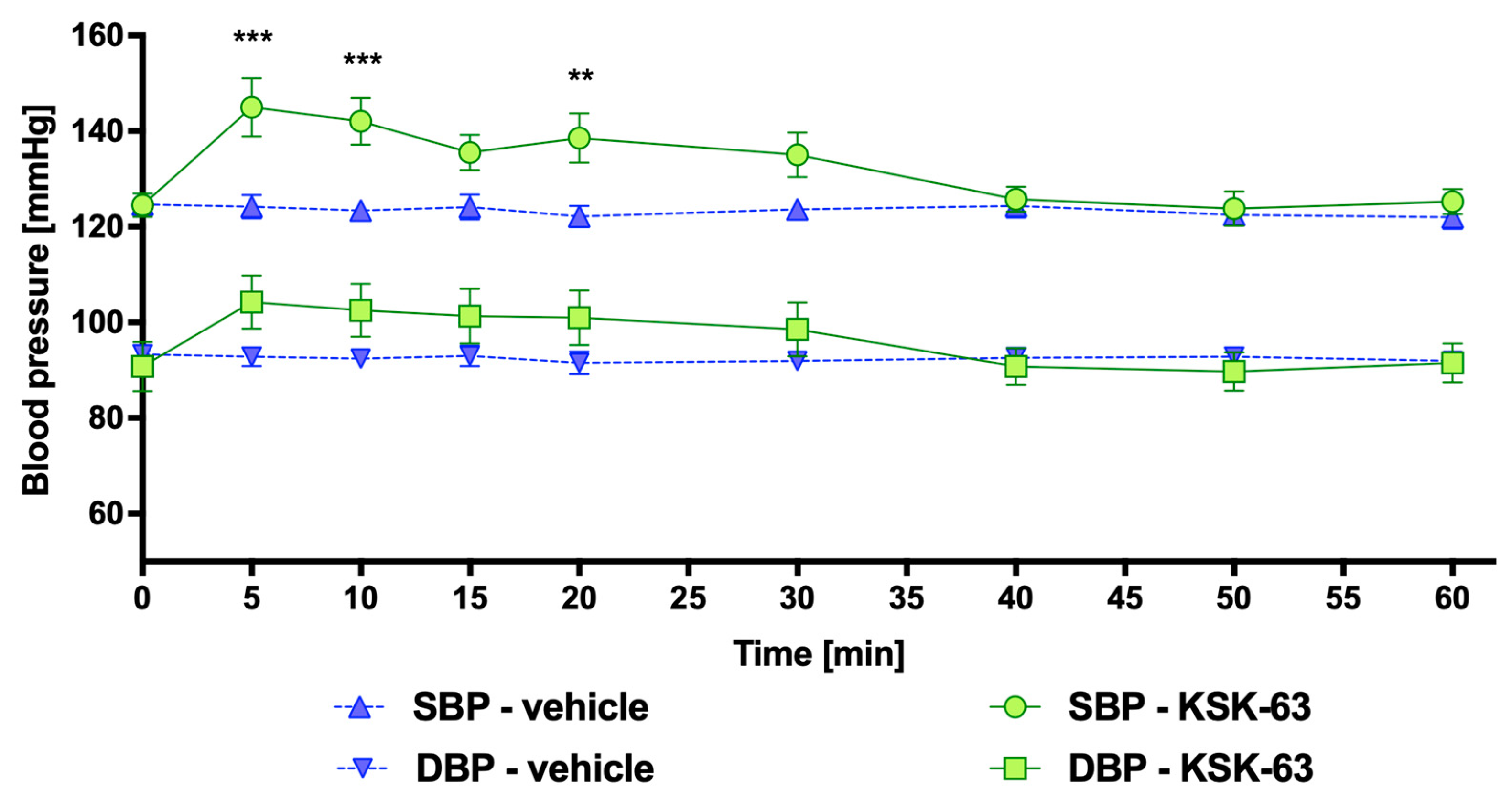
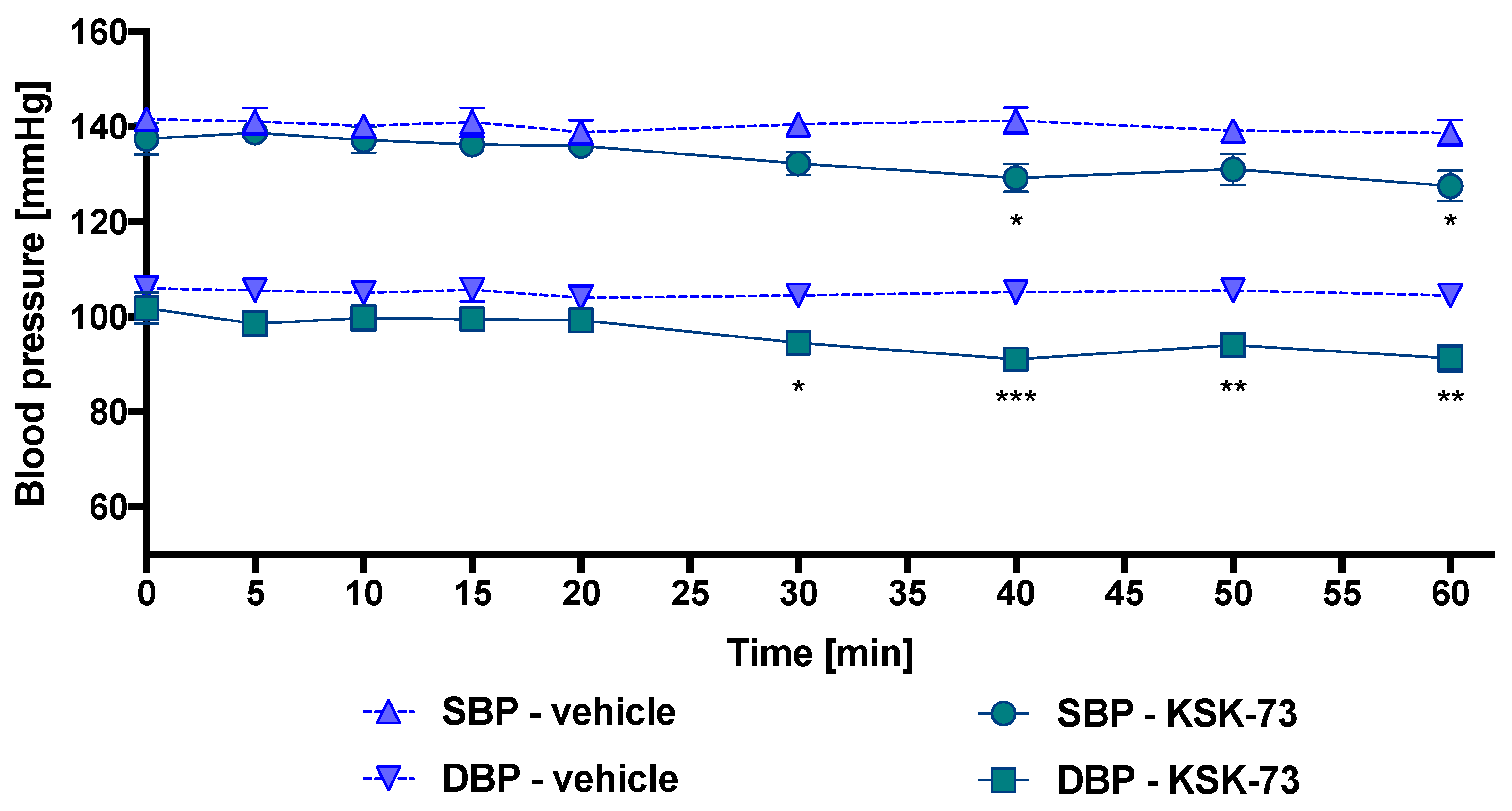
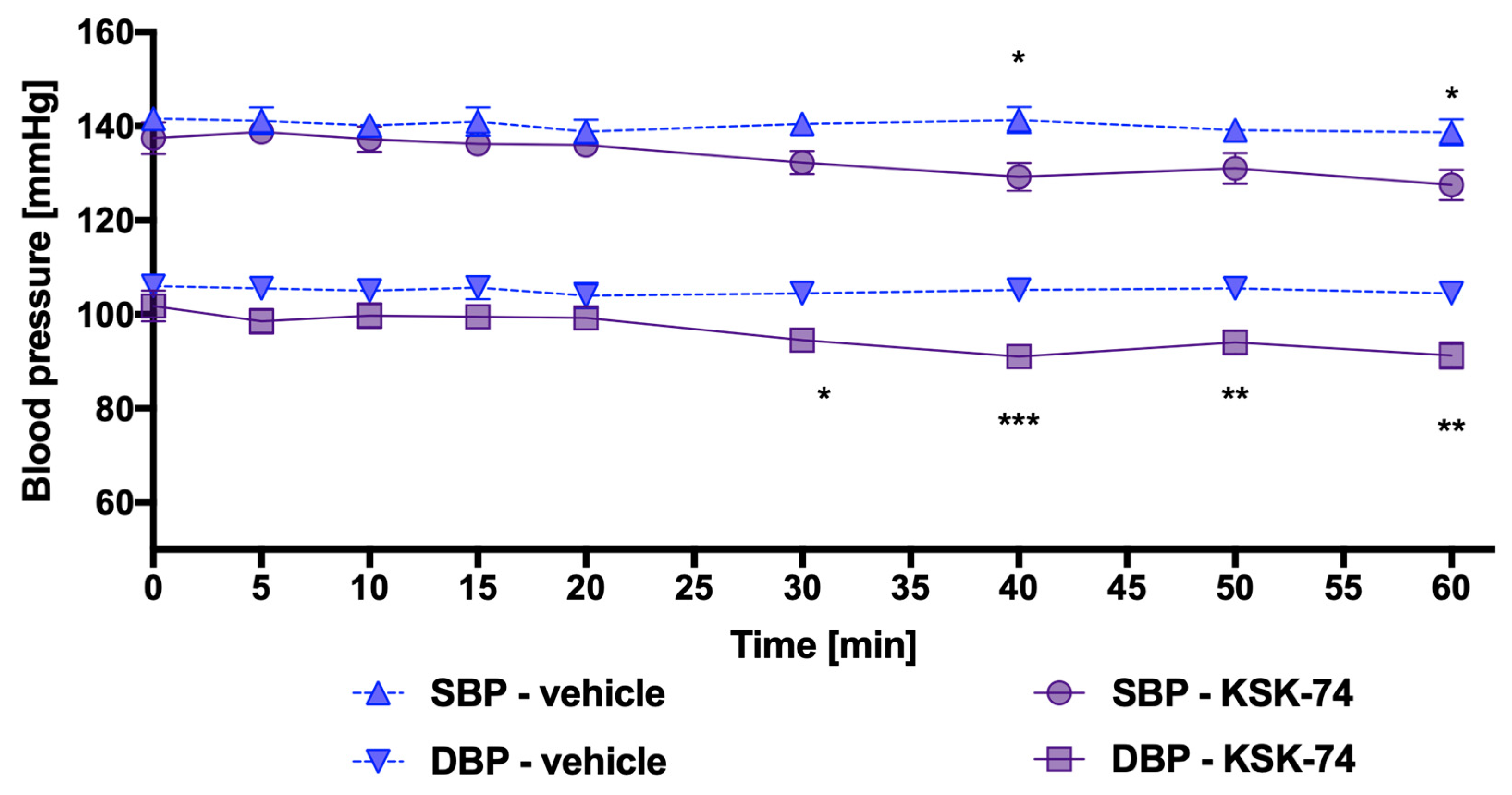
2.3. Influence of KSK-61, KSK-63, KSK-73, and KSK-74 on Blood Pressure in Normotensive Rats
2.4. Potency to Block the Human Ether-A-Go-Go-Related Gene (hERG)
2.5. Effects of KSK-61, KSK-63, KSK-73, and KSK-74 on Heart Rate and Electrocardiogram (ECG) Intervals
2.6. Effects of 30-Day Intraperitoneal Administration of KSK-61, KSK-63, KSK-73, or KSK-74 on Plasma Levels of AlaT (Alanine Aminotransferase), AspaT (Aspartate Aminotransferase), gGT (Gamma-Glutamyl Transpeptidases), and ALP (Alkaline Phosphatase) of Rats Fed a Palatable Diet
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. In Vivo Studies
4.3.1. The Effect of KSK-61, KSK-63, KSK-73, and KSK-74 on Locomotor Activity
4.3.2. The Effect of KSK-61, KSK-63, KSK-73, and KSK-74 on the Motor Coordination in the Chimney Test
4.3.3. Influence of KSK-61, KSK-63, KSK-73, and KSK-74 on Blood Pressure in Rats
4.3.4. The Effect of KSK-61, KSK-63, KSK-73, and KSK-74 on Normal Electrocardiogram in Rats
4.4. Biochemical Assays
4.5. In Vitro Electrophysiological Studies
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef]
- Arrang, J.M.; Garbarg, M.; Schwartz, J.C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983, 302, 832–837. [Google Scholar] [CrossRef]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Dinsa, G.D.; Goryakin, Y.; Fumagalli, E.; Suhrcke, M. Obesity and socioeconomic status in developing countries: A systemic review. Obes. Rev. 2012, 13, 1067–1079. [Google Scholar] [CrossRef][Green Version]
- Flanagan, E.W.; Beyl, R.A.; Fearnbach, S.N.; Altazan, A.D.; Martin, C.K.; Redman, L.M. The impact of COVID-19 Stay-At-Home Orders on Health Behaviors in Adults. Obesity 2021, 29, 438–445. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef]
- Kheniser, K.; Saxon, D.R.; Kashyp, S.R. Long-term weight loss strategies for obesity. J. Clin. Endocrinol. Metab. 2021, 106, 1854–1866. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef]
- Ioannides-Demos, L.L.; Proietto, J.; McNeil, J.J. Pharmacotherapy for obesity. Drugs 2005, 65, 1391–1418. [Google Scholar] [CrossRef]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,3-Dinitrophenol (DNP): A Weight Loss Agent with Significant Acute Toxicity and Risk of Death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef][Green Version]
- Hsu, Y.W.; Chu, D.C.; Ku, P.W.; Liou, T.H.; Chou, P. Pharmacotherapy for Obesity: Past, Present and Future. J. Exp. Clin. Med. 2010, 2, 118–123. [Google Scholar] [CrossRef]
- Torp-Pedersen, C.; Caterson, I.; Coutinho, W.; Finer, N.; Van Gaal, L.; Maggioni, A.P.; Sharma, A.; Brisco, W.; Deaton, R.; Shepherd, G.; et al. Cardiovascular responses to weight management and sibutramine in high-risk subjects: An analysis from the SCOUT trial. Eur. Heart J. 2007, 28, 2915–2923. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X.; Aronne, L.J.; Heshmati, H.M.; Devin, J.; Rosenstock, J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: A randomized controlled trial. JAMA 2006, 295, 761–775. [Google Scholar] [CrossRef][Green Version]
- Cignarella, A.; Busetto, L.; Vettor, R. Pharmacotherapy of obesity: An update. Pharmacol. Res. 2021, 169, 105649. [Google Scholar] [CrossRef]
- Belviq. Prescribing Information; Eisai Inc.: Woodcliff Lake, NJ, USA, 2012; p. 07677. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf (accessed on 14 October 2021).
- Bohula, E.A.; Wiviott, S.D.; McGuire, D.K.; Inzucchi, S.E.; Kuder, J.; Im, K.; Fanola, C.L.; Qamar, A.; Brown, C.; Budaj, A.; et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N. Engl. J. Med. 2018, 379, 1107–1117. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Requests the Withdrawal of the Weight-Loss Drug Belviq, Belviq XR (Lorcaserin) from the Market; FDA Drug Safety Communication: Silver Spring, MD, USA, 13 February 2020. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market (accessed on 18 February 2023).
- Chapman, K.L.; Holzgrefe, H.; Black, L.E.; Brown, M.; Chellman, G.; Coperman, C.; Couch, J.; Creton, J.; Gehen, S.; Hoberman, A.; et al. Pharmaceutical toxicology: Designing studies to reduce animal use, while maximizing human translation. Regul. Toxicol. Pharmacol. 2013, 66, 88–103. [Google Scholar] [CrossRef][Green Version]
- Mika, K.; Szafarz, M.; Bednarski, M.; Kuder, K.; Szczepańska, K.; Pociecha, K.; Pomierny, B.; Kieć-Kononowicz, K.; Sapa, J.; Kotańska, M. Metabolic benefits of novel histamine H3 receptor ligands in the model of excessive eating: The importance of intrinsic activity and pharmacokinetic properties. Biomed. Pharm. 2021, 142, 111952. [Google Scholar] [CrossRef]
- Mika, K.; Szafarz, M.; Bednarski, M.; Latacz, G.; Sudoł, S.; Handzlik, J.; Pociecha, K.; Knutelska, J.; Nicosia, N.; Szczepańska, K.; et al. Histamine H3 Receptor Ligands-KSK-59 and KSK-73-Reduce Body Weight Gain in a Rat Model of Excessive Eating. Pharmaceuticals 2021, 14, 1080. [Google Scholar] [CrossRef]
- Mika, K.; Szafarz, M.; Zadrożna, M.; Nowak, B.; Bednarski, M.; Szczepańska, K.; Pociecha, K.; Kubacka, M.; Nicosia, N.; Juda, I.; et al. KSK-74: Dual Histamine H3 and Sigma-2 Receptor Ligand with Anti-Obesity Potential. Int. J. Mol. Sci. 2022, 23, 7011. [Google Scholar] [CrossRef]
- Szczepańska, K.; Pockes, S.; Podlewska, K.; Horing, C.; Mika, K.; Latacz, G.; Bednarski, M.; Siwek, A.; Karcz, T.; Nagl, M.; et al. Structural modifications in the distal, regulatory region of histamine H3 receptor antagonists leading to the identification of a potent anti-obesity agent. Eur. J. Med. Chem. 2021, 213, 113041. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, K.; Podlewska, S.; Dichiara, M.; Gentile, D.; Patamia, V.; Rosier, N.; Monnich, D.; Contero, M.C.R.; Karcz, T.; Łażewska, D.; et al. Structural and Molecular Insight into Piperazine and Piperidine Derivatives as Histamine H3 and Sigma-1 Receptor Antagonists with Promising Antinociceptive Properties. ACS Chem. Neurosci. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- EMA. Assessment Report Wakix. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002616/WC500204749.pdf (accessed on 18 February 2023).
- Lynch, J.J., 3rd; Castagné, V.; Moser, P.C.; Mittelstadt, S.W. Comparison of methods for the assessment of locomotor activity in rodent safety pharmacology studies. J. Pharmacol. Toxicol. Methods 2011, 64, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.J., 3rd; Mittelstadt, S.W. Can locomotor screening be utilized as a first tiered approach for pre-clinical CNS/neurobehavioral safety testing? J. Pharmacol. Toxicol. Methods 2009, 60, 232. [Google Scholar] [CrossRef]
- Mohsen, A.; Yoshikawa, T.; Miura, Y.; Nakamura, T.; Naganuma, F.; Shibuya, K.; Iida, T.; Harada, R.; Okamura, N.; Watanabe, T.; et al. Mechanism of the histamine H(3) receptor-mediated increase in exploratory locomotor activity and anxiety-like behaviours in mice. Neuropharmacology 2014, 81, 188–194. [Google Scholar] [CrossRef]
- Wilson, L.L.; Alleyne, A.R.; Eans, S.O.; Cirino, T.J.; Stacy, H.M.; Mottinelli, M.; Intagliata, S.; McCurdy, C.R.; McLaughlin, J.P. Characterization of CM-398, a Novel Selective Sigma-2 Receptor Ligand, as a Potential Therapeutic for Neuropathic Pain. Molecules 2022, 27, 3617. [Google Scholar] [CrossRef]
- Lever, J.R.; Miller, D.K.; Green, C.L.; Fergason-Cantrell, E.A.; Watkinson, L.D.; Carmack, T.L.; Fan, K.H.; Lever, S.Z. A selective sigma-2 receptor ligand antagonizes cocaine-induced hyperlocomotion in mice. Synapse 2014, 68, 73–84. [Google Scholar] [CrossRef]
- Bowdle, T.A.; Even, A.; Shen, D.D.; Swardstrom, M. Methadone for the induction of anesthesia: Plasma histamine concentration, arterial blood pressure, and heart rate. Anesth. Analg. 2004, 98, 1692–1697. [Google Scholar] [CrossRef]
- Lorenz, W. Histamine release in man. Agents Actions 1975, 5, 402–416. [Google Scholar] [CrossRef]
- Raschi, E.; Vasina, V.; Poluzzi, E.; De Ponti, F. The hERG K+ channel: Target and antitarget strategies in drug development. Pharmacol. Res. 2008, 57, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. Drugs, hERG and sudden death. Cell Calcium 2004, 35, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Levoin, N.; Labeeuw, O.; Calmels, T.; Poupardin-Olivier, O.; Berrebi-Bertrand, I.; Lecomte, J.M.; Schwartz, J.C.; Capet, M. Novel and highly potent histamine H3 receptor ligands. Part 1: Withdrawing of hERG activity. Bioorg. Med. Chem. Lett. 2011, 21, 5378–5383. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Boone, L.; Meyer, D.; Cusick, P.; Ennulat, D.; Provencher Bolliger, A.; Everds, N.; Meador, V.; Elliott, G.; Honor, D.; Bounous, D.; et al. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Path. 2005, 34, 182–188. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Chalasani, N. Laboratory Tests in Liver Disease. In Practical Hepatic Pathology: A Diagnostic Approach; Saunders: Philadelphia, PA, USA, 2011; pp. 55–62. [Google Scholar]
- Lee, D.H.; Ha, M.H.; Kim, J.H.; Christiani, D.C.; Gross, M.D.; Steffes, M.; Blomhoff, R.; Jacobs, D.R., Jr. Gamma-glutamyltransferase and diabetes--a 4 year follow-up study. Diabetologia 2003, 46, 359–364. [Google Scholar] [CrossRef][Green Version]
- Cho, N.H.; Jang, H.C.; Choi, S.H.; Kim, H.R.; Lee, H.K.; Chan, J.C.N.; Soo, L. Abnormal liver function test predicts type 2 diabetes: A community-based prospective study. Diabetes Care 2007, 30, 2566–2568. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Wang, J.; Han, X.; Hu, H.; Wang, F.; Yu, C.; Yuan, J.; Yao, P.; Li, X.; Yang, K.; et al. Serum alanine transaminase levels predict type 2 diabetes risk among a middle-aged and elderly Chinese population. Ann. Hepatol. 2019, 18, 298–303. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jung, S.; Kim, K.N. Considering serum alanine aminotransferase and gamma-glutamyltransferase levels together strengthen the prediction of impaired fasting glucose risk: A cross-sectional and longitudinal study. Sci. Rep. 2021, 11, 3333. [Google Scholar] [CrossRef]
- Szczepańska, K.; Karcz, T.; Siwek, A.; Kuder, K.J.; Latacz, G.; Bednarski, M.; Szafarz, M.; Hagenow, S.; Lubelska, A.; Olejarz-Maciej, A.; et al. Structural modifications and in vitro pharmacological evaluation of 4-pyridyl-piperazine derivatives as an active and selective histamine H3 receptor ligands. Bioorg. Chem. 2019, 91, 103071. [Google Scholar] [CrossRef]
- Dudek, M.; Kuder, K.; Kołaczkowski, M.; Olczyk, A.; Żmudzka, E.; Rak, A.; Bednarski, M.; Pytka, K.; Sapa, J.; Kieć-Kononowicz, K. H3 histamine receptor antagonist pitolisant reverses some subchronic disturbances induced by olanzapine in mice. Metab. Brain Dis. 2016, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boissier, J.R.; Tardy, J.; Divierres, J.C. A new simple method to explore the tranquilizing action: The chimney test (French). Med. Exp. 1960, 3, 81–84. [Google Scholar]
- Kotańska, M.; Kulig, K.; Marcinkowska, M.; Bednarski, M.; Malawska, K.; Zaręba, P. Metabolic benefits of 1-(3-(4-(o-tolyl) piperazin-1-yl) propyl) pyrrolidin-2-one: A non-selective α-adrenoceptor antagonist. J. Endocrinol. Investig. 2018, 41, 609–619. [Google Scholar] [CrossRef][Green Version]
- De Clerck, F.; Van de Water, A.; D’Aubioul, J.; Lu, H.R.; Van Rossem, K.; Hermans, A.; Van Ammel, K. In vivo measurement of QT prolongation, dispersion and arrhythmogenesis: Application to the preclinical cardiovascular safety pharmacology of a new chemicalentity. Fundam. Clin. Pharmacol. 2002, 16, 125–140. [Google Scholar] [CrossRef] [PubMed]
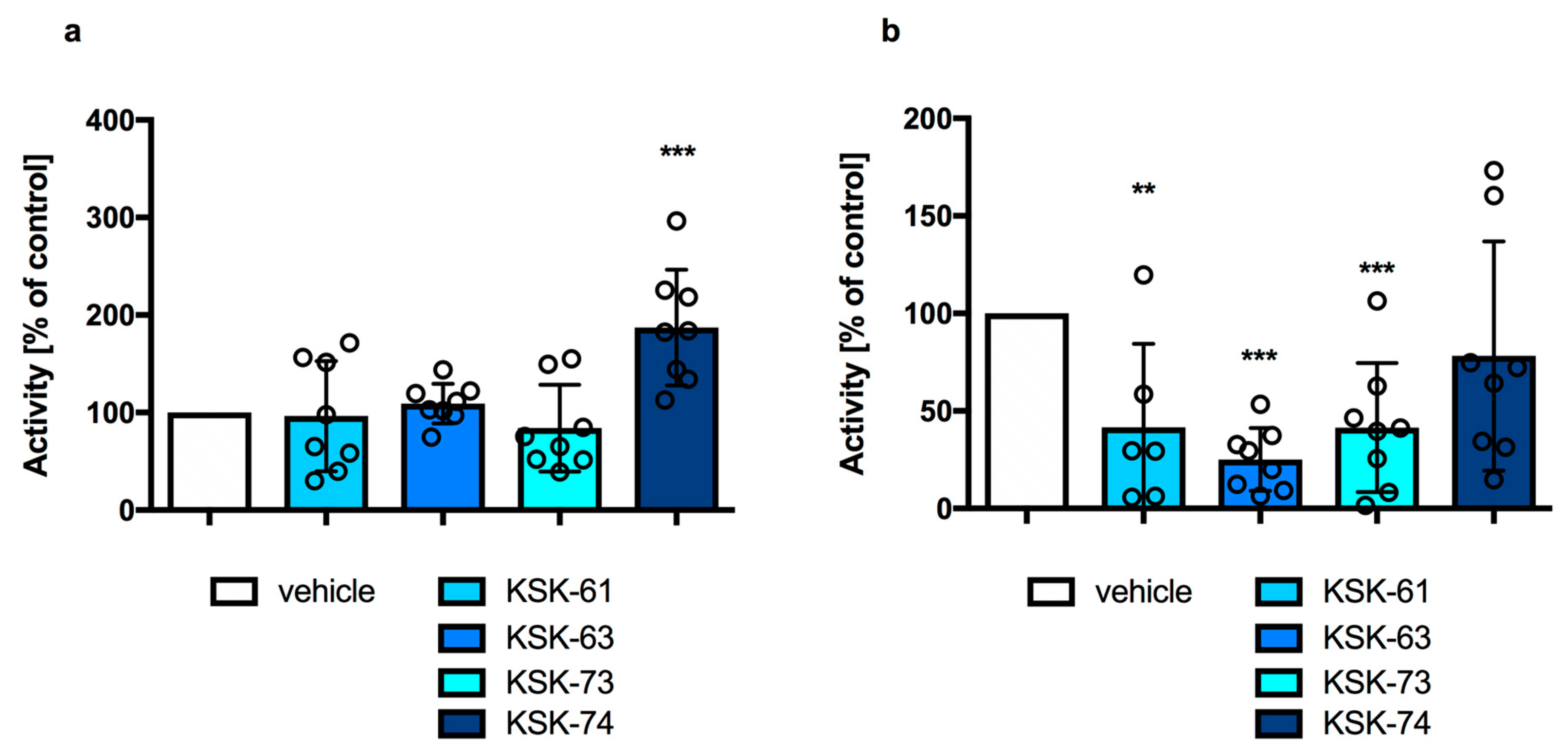


| Compound (mg/kg) | n | % of Mice Impaired |
|---|---|---|
| vehicle | 8 | 0% |
| KSK-61 (10) | 8 | 25% |
| KSK-61 (20) | 8 | 12.5% |
| KSK-61 (30) | 8 | 25% |
| KSK-63 (10) | 8 | 0% |
| KSK-63 (20) | 8 | 0% |
| KSK-63 (30) | 8 | 0% |
| KSK-73 (10) | 8 | 12.5% |
| KSK-73 (20) | 8 | 0% |
| KSK-73 (30) | 8 | 0% |
| KSK-74 (10) | 8 | 0% |
| KSK-74 (20) | 8 | 12.5% |
| KSK-74 (30) | 8 | 12.5% |
| Compound | Mean IC50 ± SEM (µM) |
|---|---|
| KSK-61 | 2.657 ± 0.217 |
| KSK-63 | 0.570 ± 0.050 |
| KSK-73 | 2.067 ± 0.402 |
| KSK-74 | 3.397 ± 0.289 |
| Verapamil | 0.525 ± 0.052 |
| Parameter | Time (min) | |||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | ||
| KSK-61 (10 mg/kg b.w.) | Heart rate (beats/min) | 373.2 ± 20.3 | 369.5 ± 9.5 | 380.2 ± 30.5 | 349.5 ± 21.3 | 360.4 ± 20.3 |
| PQ (ms) | 53 ± 1.6 | 52.8 ± 1.5 | 54.5 ± 3.5 | 53.3 ± 1.8 | 53 ± 1.6 | |
| QRS (ms) | 20.8 ± 0.8 | 21.5 ± 0.5 | 21.8 ±1.1 | 21.8 ± 0.8 | 21.8 ± 0.4 | |
| QT (ms) | 56.3 ± 4.1 | 56.3 ± 2.2 | 58.8 ± 2.2 | 57.5 ± 4.3 | 55 ± 5.0 | |
| KSK-63 (10 mg/kg b.w.) | Heart rate (beats/min) | 393.8 ± 34.8 | 362.8 ± 38.0 | 365.2 ± 34.8 | 344.3 ± 30.8 | 344.3 ± 30.8 |
| PQ (ms) | 57.5 ± 2.5 | 55.0 ± 5.0 | 58.75 ± 1.3 | 55.0 ± 5.0 | 62.5 ± 2.1 | |
| QRS (ms) | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20 ± 0.0 | 20.0 ± 0.0 | |
| QT (ms) | 63.3 ± 5.1 | 64.4 ± 2.6 | 65.3 ± 4.7 | 62.1 ± 1.6 | 65.2 ± 3.6 | |
| KSK-73 (10 mg/kg b.w.) | Heart rate (beats/min) | 370.0 ± 23.9 | 375.5 ± 23.9 | 377.1 ± 27.3 | 395.8 ± 33.3 | 377.0 ± 26.4 |
| PQ (ms) | 56.3 ± 2.2 | 51.3 ± 2.2 | 51.3 ± 2.2 | 52.5 ± 2.5 | 53.8 ± 2.2 | |
| QRS (ms) | 22.7 ± 5.4 | 22.7 ± 5.4 | 22.7 ± 5.4 | 20.0 ± 0.0 | 20.0 ± 0.0 | |
| QT (ms) | 61.3 ± 2.2 | 63.8 ± 4.1 | 60.0 ± 7.1 | 58.8 ± 2.2 | 63.8 ± 6.5 | |
| KSK-74 (10 mg/kg b.w.) | Heart rate (beats/min) | 381.8 ± 19.9 | 356.3 ± 18.9 | 355.3 ± 32.2 | 364.6 ± 18.1 | 370.8 ± 23.9 |
| PQ (ms) | 56.3 ± 4.1 | 53.8 ± 4.1 | 56.3 ± 2.2 | 57.5 ± 2.5 | 55.0 ± 5.0 | |
| QRS (ms) | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | |
| QT (ms) | 62.5 ± 4.3 | 60.0 ± 5.0 | 61.3 ± 6.5 | 65.0 ± 6.1 | 62.5 ± 8.3 | |
| Symbol | Structure | H3R Ki (nM) | σ1R Ki (nM) | σ2R Ki (nM) | H3/σ1 | H3/σ2 | σ1/σ2 |
|---|---|---|---|---|---|---|---|
| KSK-61 | 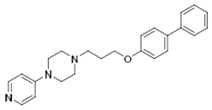 | 21.1 | 638.26 | 108.14 | 0.03 | 0.20 | 5.90 |
| KSK-63 | 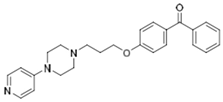 | 3.12 | 726.11 | 29.24 | 0.004 | 0.11 | 24.83 |
| KSK-73 | 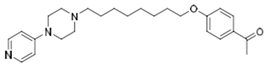 | 40.5 | 408.30 | 59.70 | 0.1 | 0.68 | 6.84 |
| KSK-74 | 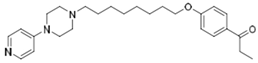 | 38.9 | 274.16 | 65.92 | 0.14 | 0.59 | 4.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mika, K.; Szafarz, M.; Bednarski, M.; Siwek, A.; Szczepańska, K.; Kieć-Kononowicz, K.; Kotańska, M. Evaluation of Some Safety Parameters of Dual Histamine H3 and Sigma-2 Receptor Ligands with Anti-Obesity Potential. Int. J. Mol. Sci. 2023, 24, 7499. https://doi.org/10.3390/ijms24087499
Mika K, Szafarz M, Bednarski M, Siwek A, Szczepańska K, Kieć-Kononowicz K, Kotańska M. Evaluation of Some Safety Parameters of Dual Histamine H3 and Sigma-2 Receptor Ligands with Anti-Obesity Potential. International Journal of Molecular Sciences. 2023; 24(8):7499. https://doi.org/10.3390/ijms24087499
Chicago/Turabian StyleMika, Kamil, Małgorzata Szafarz, Marek Bednarski, Agata Siwek, Katarzyna Szczepańska, Katarzyna Kieć-Kononowicz, and Magdalena Kotańska. 2023. "Evaluation of Some Safety Parameters of Dual Histamine H3 and Sigma-2 Receptor Ligands with Anti-Obesity Potential" International Journal of Molecular Sciences 24, no. 8: 7499. https://doi.org/10.3390/ijms24087499
APA StyleMika, K., Szafarz, M., Bednarski, M., Siwek, A., Szczepańska, K., Kieć-Kononowicz, K., & Kotańska, M. (2023). Evaluation of Some Safety Parameters of Dual Histamine H3 and Sigma-2 Receptor Ligands with Anti-Obesity Potential. International Journal of Molecular Sciences, 24(8), 7499. https://doi.org/10.3390/ijms24087499





