Association of Circadian Clock Gene Expression with Pediatric/Adolescent Asthma and Its Comorbidities
Abstract
1. Introduction
2. Results
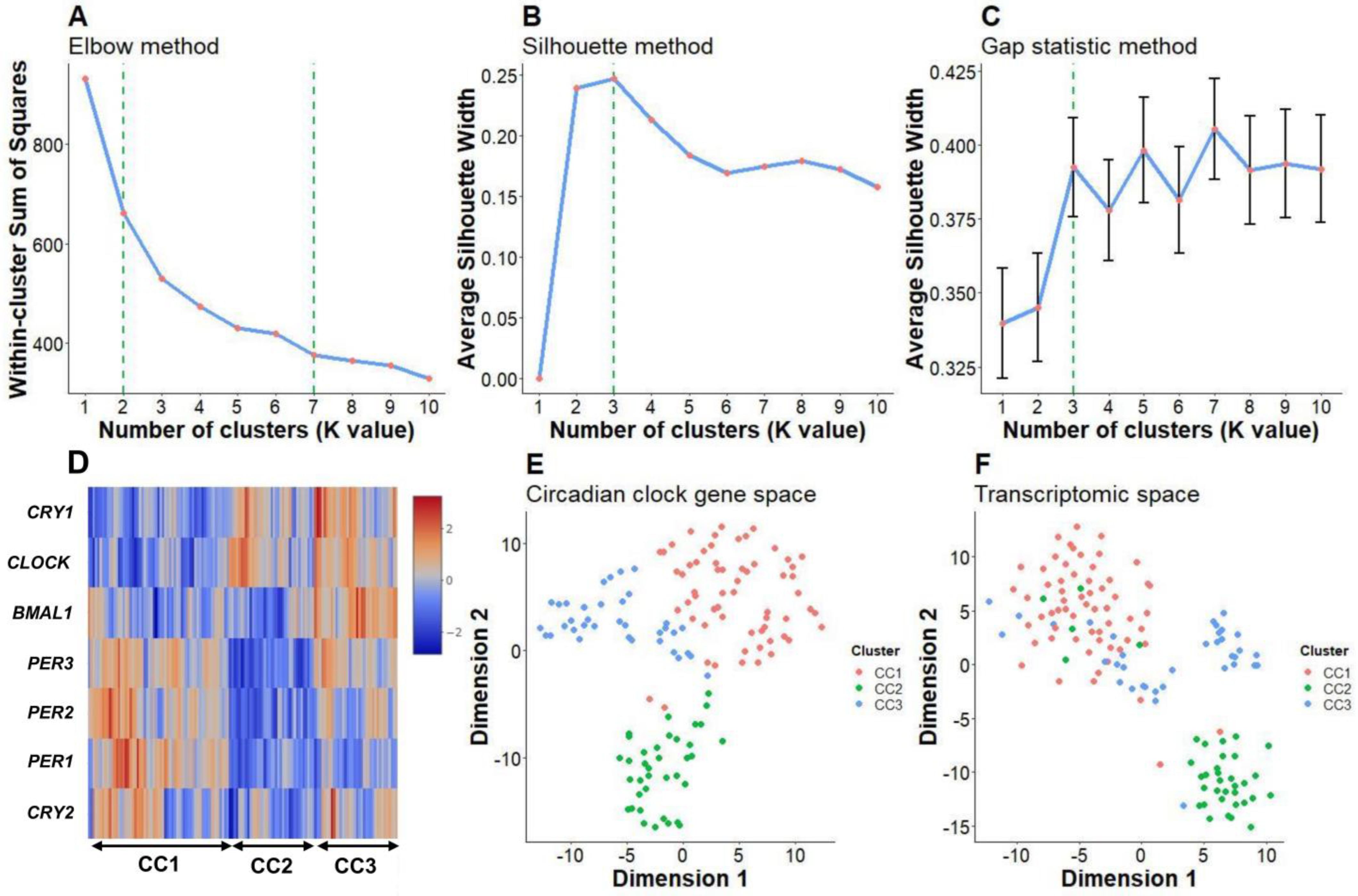
2.1. Clustering Analysis Showed Three Distinct Core Circadian Clock Gene Expression Patterns in Patients with Pediatric/Adolescent Asthma
2.2. Core Circadian Clock Gene Expression Patterns May Be Related to Biological Differences in Pediatric/Adolescent Asthma
2.3. CC2 and CC3 Subtypes Were Characterized by a Low Frequency of Allergic Rhinitis and Atopic Dermatitis, Respectively
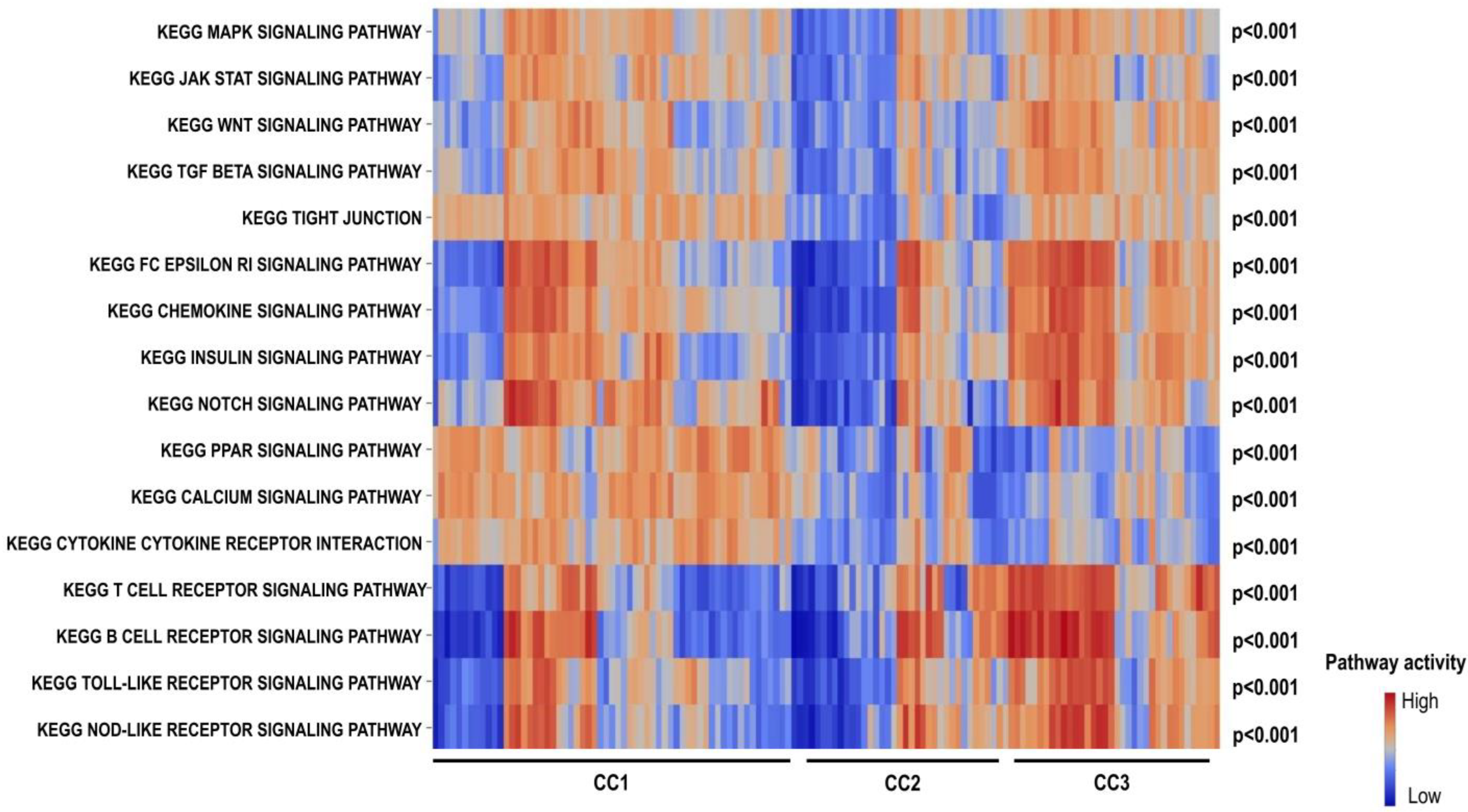
2.4. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis Revealed Lower Activation of the FcεRI Receptor Signaling Pathway in CC2 and Lower Activation of Cytokine–Cytokine Receptor Interaction Pathways in CC3
3. Discussion
4. Materials and Methods
4.1. Data Processing
4.2. Clustering Analysis
4.3. Pathway Analysis
4.4. Analysis Platform
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Aymerich, J.; Benet, M.; Saeys, Y.; Pinart, M.; Basagana, X.; Smit, H.A.; Siroux, V.; Just, J.; Momas, I.; Ranciere, F.; et al. Phenotyping asthma, rhinitis and eczema in MeDALL population-based birth cohorts: An allergic comorbidity cluster. Allergy 2015, 70, 973–984. [Google Scholar] [CrossRef]
- Pullerits, T.; Ronmark, E.P.; Ekerljung, L.; Palmqvist, M.A.; Arvidsson, M.; Mincheva, R.; Backman, H.; Kankaanranta, H.; Ilmarinen, P.; Radinger, M.; et al. The triad of current asthma, rhinitis and eczema is uncommon among adults: Prevalence, sensitization profiles, and risk factors. Respir. Med. 2021, 176, 106250. [Google Scholar] [CrossRef]
- Scheer, F.; Hilton, M.F.; Evoniuk, H.L.; Shiels, S.A.; Malhotra, A.; Sugarbaker, R.; Ayers, R.T.; Israel, E.; Massaro, A.F.; Shea, S.A. The endogenous circadian system worsens asthma at night independent of sleep and other daily behavioral or environmental cycles. Proc. Natl. Acad. Sci. USA 2021, 118, e2018486118. [Google Scholar] [CrossRef] [PubMed]
- Durrington, H.J.; Krakowiak, K.; Meijer, P.; Begley, N.; Maidstone, R.; Goosey, L.; Gibbs, J.E.; Blaikley, J.F.; Gregory, L.G.; Lloyd, C.M.; et al. Circadian asthma airway responses are gated by REV-ERBalpha. Eur. Respir. J. 2020, 56, 1902407. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, O.; Luni, C.; Li, Y.; Angiolillo, S.; Qin, W.; Panariello, F.; Cacchiarelli, D.; Takahashi, J.S.; Elvassore, N. Synchronization between peripheral circadian clock and feeding-fasting cycles in microfluidic device sustains oscillatory pattern of transcriptome. Nat. Commun. 2021, 12, 6185. [Google Scholar] [CrossRef] [PubMed]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909.e20. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Lemonnier, N.; Melen, E.; Jiang, Y.; Joly, S.; Menard, C.; Aguilar, D.; Acosta-Perez, E.; Bergstrom, A.; Boutaoui, N.; Bustamante, M.; et al. A novel whole blood gene expression signature for asthma, dermatitis, and rhinitis multimorbidity in children and adolescents. Allergy 2020, 75, 3248–3260. [Google Scholar] [CrossRef]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J. Clin. Cell. Immunol. 2014, 5, 202. [Google Scholar] [CrossRef]
- Hatzler, L.; Panetta, V.; Lau, S.; Wagner, P.; Bergmann, R.L.; Illi, S.; Bergmann, K.E.; Keil, T.; Hofmaier, S.; Rohrbach, A.; et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J. Allergy Clin. Immunol. 2012, 130, 894–901.e895. [Google Scholar] [CrossRef]
- Scadding, G.K.; Smith, P.K.; Blaiss, M.; Roberts, G.; Hellings, P.W.; Gevaert, P.; Mc Donald, M.; Sih, T.; Halken, S.; Zieglmayer, P.U.; et al. Allergic Rhinitis in Childhood and the New EUFOREA Algorithm. Front. Allergy 2021, 2, 706589. [Google Scholar] [CrossRef] [PubMed]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev. Clin. Immunol. 2021, 17, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chinnappan, M.; Prestwood, C.A.; Edwards, M.; Artami, M.; Thompson, B.M.; Eckert, K.M.; Vale, G.; Zouboulis, C.C.; McDonald, J.G.; et al. Interleukins 4 and 13 drive lipid abnormalities in skin cells through regulation of sex steroid hormone synthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2100749118. [Google Scholar] [CrossRef]
- Jansen, R.; Batista, S.; Brooks, A.I.; Tischfield, J.A.; Willemsen, G.; van Grootheest, G.; Hottenga, J.J.; Milaneschi, Y.; Mbarek, H.; Madar, V.; et al. Sex differences in the human peripheral blood transcriptome. BMC Genom. 2014, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.J.; Joehanes, R.; Pilling, L.C.; Schurmann, C.; Conneely, K.N.; Powell, J.; Reinmaa, E.; Sutphin, G.L.; Zhernakova, A.; Schramm, K.; et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 2015, 6, 8570. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hopp, L.; Arakelyan, A.; Kirsten, H.; Engel, C.; Wirkner, K.; Krohn, K.; Burkhardt, R.; Thiery, J.; Loeffler, M.; et al. The Human Blood Transcriptome in a Large Population Cohort and Its Relation to Aging and Health. Front. Big Data 2020, 3, 548873. [Google Scholar] [CrossRef] [PubMed]
- Homuth, G.; Wahl, S.; Muller, C.; Schurmann, C.; Mader, U.; Blankenberg, S.; Carstensen, M.; Dorr, M.; Endlich, K.; Englbrecht, C.; et al. Extensive alterations of the whole-blood transcriptome are associated with body mass index: Results of an mRNA profiling study involving two large population-based cohorts. BMC Med. Genom. 2015, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Loizides-Mangold, U.; Chanon, S.; Gobet, C.; Hulo, N.; Isenegger, L.; Weger, B.D.; Migliavacca, E.; Charpagne, A.; Betts, J.A.; et al. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife 2018, 7, e34114. [Google Scholar] [CrossRef]
- Krakowiak, K.; Downton, P.; Blaikley, J.; Durrington, H. L1 Blood immune cells clock mechanism is altered in asthma, with a time-of-day dependent response to steroids in vitro. Thorax 2021, 76, A230. [Google Scholar] [CrossRef]
- Krakowiak, K.; Maidstone, R.; Downton, P.; Blaikley, J.; Durrington, H. Expression Of Clock Gene PER3 Is Altered In Asthma. Am. J. Respir. Crit. Care Med. 2022, 2022, A1259. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, Y.C.; Wang, T.N.; Fang, W.F.; Chang, Y.C.; Chen, Y.M.; Chen, I.Y.; Lin, M.C.; Yang, M.Y. Disrupted Expression of Circadian Clock Genes in Patients with Bronchial Asthma. J. Asthma Allergy 2021, 14, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Long, D.M.; Giebultowicz, J.M. Age-Related Changes in the Expression of the Circadian Clock Protein PERIOD in Drosophila Glial Cells. Front. Physiol. 2017, 8, 1131. [Google Scholar] [CrossRef]
- Adler, P.; Chiang, C.K.; Mayne, J.; Ning, Z.; Zhang, X.; Xu, B.; Cheng, H.M.; Figeys, D. Aging Disrupts the Circadian Patterns of Protein Expression in the Murine Hippocampus. Front. Aging Neurosci. 2019, 11, 368. [Google Scholar] [CrossRef]
- Chen, C.Y.; Logan, R.W.; Ma, T.; Lewis, D.A.; Tseng, G.C.; Sibille, E.; McClung, C.A. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2016, 113, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Timmons, G.A.; Carroll, R.G.; O’Siorain, J.R.; Cervantes-Silva, M.P.; Fagan, L.E.; Cox, S.L.; Palsson-McDermott, E.; Finlay, D.K.; Vincent, E.E.; Jones, N.; et al. The Circadian Clock Protein BMAL1 Acts as a Metabolic Sensor In Macrophages to Control the Production of Pro IL-1beta. Front. Immunol. 2021, 12, 700431. [Google Scholar] [CrossRef]
- Hemmers, S.; Rudensky, A.Y. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep. 2015, 11, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Nobis, C.C.; Dubeau Laramee, G.; Kervezee, L.; Maurice De Sousa, D.; Labrecque, N.; Cermakian, N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc. Natl. Acad. Sci. USA 2019, 116, 20077–20086. [Google Scholar] [CrossRef]
- Kawauchi, T.; Ishimaru, K.; Nakamura, Y.; Nakano, N.; Hara, M.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Clock-dependent temporal regulation of IL-33/ST2-mediated mast cell response. Allergol. Int. 2017, 66, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Harama, D.; Shimokawa, N.; Hara, M.; Suzuki, R.; Tahara, Y.; Ishimaru, K.; Katoh, R.; Okumura, K.; Ogawa, H.; et al. Circadian clock gene Period2 regulates a time-of-day-dependent variation in cutaneous anaphylactic reaction. J. Allergy Clin. Immunol. 2011, 127, 1038–1045.e1–3. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Nakano, N.; Ishimaru, K.; Hara, M.; Ikegami, T.; Tahara, Y.; Katoh, R.; Ogawa, H.; Okumura, K.; Shibata, S.; et al. Circadian regulation of allergic reactions by the mast cell clock in mice. J. Allergy Clin. Immunol. 2014, 133, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef] [PubMed]
- Langwinski, W.; Sobkowiak, P.; Narozna, B.; Wojsyk-Banaszak, I.; Dmitrzak-Weglarz, M.; Stachowiak, Z.; Nowakowska, J.; Breborowicz, A.; Szczepankiewicz, A. Association of circadian clock TIMELESS variants and expression with asthma risk in children. Clin. Respir. J. 2020, 14, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.; Xie, W.; Agapov, E.; Brown, S.; Steinberg, D.; Tidwell, R.; Sajol, G.; Schutz, R.; Weaver, R.; Yu, H.; et al. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol. 2018, 11, 97–111. [Google Scholar] [CrossRef]
- Haldar, P.; Carsin, A.E.; Debnath, S.; Maity, S.G.; Annesi-Maesano, I.; Garcia-Aymerich, J.; Bandyopadhayay, A.; Moitra, S.; Kogevinas, M.; Moitra, S. Individual circadian preference (chronotype) is associated with asthma and allergic symptoms among adolescents. ERJ Open Res. 2020, 6, 00226-2020. [Google Scholar] [CrossRef]
- Reiter, J.; Ramagopal, M.; Gileles-Hillel, A.; Forno, E. Sleep disorders in children with asthma. Pediatr. Pulmonol. 2022, 57, 1851–1859. [Google Scholar] [CrossRef]
- Yurtsever, T.; Schilling, T.M.; Kolsch, M.; Turner, J.D.; Meyer, J.; Schachinger, H.; Schote, A.B. The acute and temporary modulation of PERIOD genes by hydrocortisone in healthy subjects. Chronobiol. Int. 2016, 33, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
| Asthma | ||||
|---|---|---|---|---|
| Control (n = 209) | CC1 (n = 61) | CC2 (n = 37) | CC3 (n = 36) | |
| Age | 4.6 (3.8–18.1) | 16.8 (3.9–18.0) | 4.0 ** (0.1–17.0) | 16.8 (4.5–17.8) |
| Gender | ||||
| Female | 106 (50.7%) | 28 (45.9%) | 14 (37.8%) | 15 (41.7%) |
| Male | 103 (49.3%) | 33 (54.1%) | 23 (62.2%) | 21 (58.3%) |
| Comorbidities | ||||
| AR | 17 (27.9%) | 3 (8.1%) ** | 16 (44.4%) | |
| AD | 14 (23.0%) | 16 (43.2%) | 2 (5.6%) ** | |
| AR & AD | 11 (18.0%) | 1 (2.7%) | 4 (11.1%) | |
| No | 19 (31.1%) | 17 (46%) | 14 (38.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.Q.V.; Le, M.-K.; Nguyen, T.-A.; Kondo, T.; Nakao, A. Association of Circadian Clock Gene Expression with Pediatric/Adolescent Asthma and Its Comorbidities. Int. J. Mol. Sci. 2023, 24, 7477. https://doi.org/10.3390/ijms24087477
Tran NQV, Le M-K, Nguyen T-A, Kondo T, Nakao A. Association of Circadian Clock Gene Expression with Pediatric/Adolescent Asthma and Its Comorbidities. International Journal of Molecular Sciences. 2023; 24(8):7477. https://doi.org/10.3390/ijms24087477
Chicago/Turabian StyleTran, Nguyen Quoc Vuong, Minh-Khang Le, Thuy-An Nguyen, Tetsuo Kondo, and Atsuhito Nakao. 2023. "Association of Circadian Clock Gene Expression with Pediatric/Adolescent Asthma and Its Comorbidities" International Journal of Molecular Sciences 24, no. 8: 7477. https://doi.org/10.3390/ijms24087477
APA StyleTran, N. Q. V., Le, M.-K., Nguyen, T.-A., Kondo, T., & Nakao, A. (2023). Association of Circadian Clock Gene Expression with Pediatric/Adolescent Asthma and Its Comorbidities. International Journal of Molecular Sciences, 24(8), 7477. https://doi.org/10.3390/ijms24087477





