Blood Platelets in Infection: The Multiple Roles of the Platelet Signalling Machinery
Abstract
1. Introduction
2. Platelets in Infection
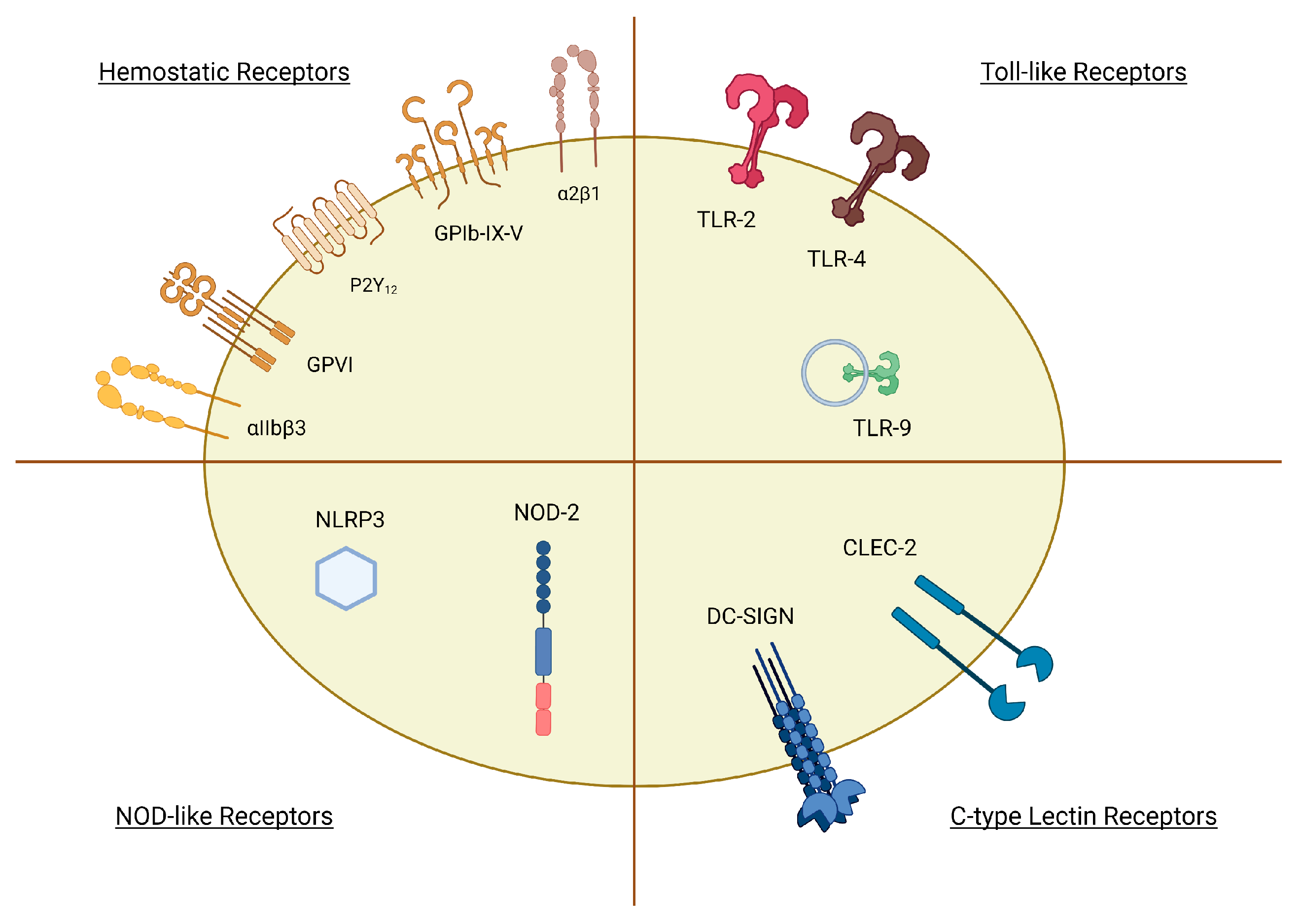
3. Platelet Receptors in the Response to Infection
3.1. Pattern Recognition Receptors (PRRs)
3.1.1. TLRs
3.1.2. CLRs
3.1.3. NLRs
3.2. Haemostatic Receptors
4. Shedding of Platelet Surface Proteins in Infection
4.1. sCD40L
4.2. sTLT-1
4.3. sP-Selectin
4.4. sGPIbα, sGPVI, and sCLEC2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rohlfing, A.K.; Rath, D.; Geisler, T.; Gawaz, M. Platelets and COVID-19. Hamostaseologie 2021, 41, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets contribute to disease severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2021, 32, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levi, M.; Levy, J.H. Intracellular communication and immunothrombosis in sepsis. J. Thromb. Haemost. 2022, 20, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Youssefian, T.; Drouin, A.; Massé, J.M.; Guichard, J.; Cramer, E.M. Host defense role of platelets: Engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 2002, 99, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Ahmad, Z.; Rosenberger, G.; Fan, S.; Nicolai, L.; Busch, B.; Yavuz, G.; Luckner, M.; Ishikawa-Ankerhold, H.; Hennel, R.; et al. Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell 2017, 171, 1368–1382.e1323. [Google Scholar] [CrossRef]
- Verschoor, A.; Neuenhahn, M.; Navarini, A.A.; Graef, P.; Plaumann, A.; Seidlmeier, A.; Nieswandt, B.; Massberg, S.; Zinkernagel, R.M.; Hengartner, H.; et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat. Immunol. 2011, 12, 1194–1201. [Google Scholar] [CrossRef]
- White, J.G. Platelets are covercytes, not phagocytes: Uptake of bacteria involves channels of the open canalicular system. Platelets 2005, 16, 121–131. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Kho, S.; Barber, B.E.; Johar, E.; Andries, B.; Poespoprodjo, J.R.; Kenangalem, E.; Piera, K.A.; Ehmann, A.; Price, R.N.; William, T.; et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood 2018, 132, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Schwertz, H.; Hottz, E.D.; Rowley, J.W.; Manne, B.K.; Washington, A.V.; Hunter-Mellado, R.; Tolley, N.D.; Christensen, M.; Eustes, A.S.; et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood 2019, 133, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef]
- Kim, S.J.; Jenne, C.N. Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin. Immunol. 2016, 28, 546–554. [Google Scholar] [CrossRef]
- McDonald, B.; Davis, R.P.; Kim, S.-J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef]
- Gerdes, N.; Zhu, L.; Ersoy, M.; Hermansson, A.; Hjemdahl, P.; Hu, H.; Hansson, G.K.; Li, N. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. Thromb. Haemost. 2011, 106, 353–362. [Google Scholar] [CrossRef]
- Liu, C.Y.; Battaglia, M.; Lee, S.H.; Sun, Q.H.; Aster, R.H.; Visentin, G.P. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25− (nonregulatory) T cells. J. Immunol. 2005, 174, 2680–2686. [Google Scholar] [CrossRef]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L. Platelet-mediated modulation of adaptive immunity: A communication link between innate and adaptive immune compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Elzey, B.D.; Ratliff, T.L.; Sowa, J.M.; Crist, S.A. Platelet CD40L at the interface of adaptive immunity. Thromb. Res. 2011, 127, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Schiefelbein, K.; Lipsky, S.; Leunig, A.; Hoffknecht, M.; Pekayvaz, K.; Raude, B.; Marx, C.; Ehrlich, A.; Pircher, J.; et al. Vascular surveillance by haptotactic blood platelets in inflammation and infection. Nat. Commun. 2020, 11, 5778. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Johnson, R.C.; Rayburn, H.; Hynes, R.O.; Wagner, D.D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 1993, 74, 541–554. [Google Scholar] [CrossRef]
- Martins, P.; van Gils, J.M.; Mol, A.; Hordijk, P.L.; Zwaginga, J.J. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of β(1) and β(2) integrins. J. Leukoc. Biol. 2006, 79, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Massberg, S. Patrolling the vascular borders: Platelets in immunity to infection and cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef]
- Wong, C.H.; Jenne, C.N.; Petri, B.; Chrobok, N.L.; Kubes, P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 2013, 14, 785–792. [Google Scholar] [CrossRef]
- Parker, Z.F.; Rux, A.H.; Riblett, A.M.; Lee, F.H.; Rauova, L.; Cines, D.B.; Poncz, M.; Sachais, B.S.; Doms, R.W. Platelet Factor 4 Inhibits and Enhances HIV-1 Infection in a Concentration-Dependent Manner by Modulating Viral Attachment. AIDS Res. Hum. Retrovir. 2016, 32, 705–717. [Google Scholar] [CrossRef]
- Palankar, R.; Kohler, T.P.; Krauel, K.; Wesche, J.; Hammerschmidt, S.; Greinacher, A. Platelets kill bacteria by bridging innate and adaptive immunity via platelet factor 4 and FcγRIIA. J. Thromb. Haemost. 2018, 16, 1187–1197. [Google Scholar] [CrossRef]
- McMorran, B.J.; Wieczorski, L.; Drysdale, K.E.; Chan, J.A.; Huang, H.M.; Smith, C.; Mitiku, C.; Beeson, J.G.; Burgio, G.; Foote, S.J. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 2012, 338, 1348–1351. [Google Scholar] [CrossRef]
- Cella, M.; Scheidegger, D.; Palmer-Lehmann, K.; Lane, P.; Lanzavecchia, A.; Alber, G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: TT help via APC activation. J. Exp. Med. 1996, 184, 747–752. [Google Scholar] [CrossRef]
- Czapiga, M.; Kirk, A.D.; Lekstrom-Himes, J. Platelets deliver costimulatory signals to antigen-presenting cells: A potential bridge between injury and immune activation. Exp. Hematol. 2004, 32, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Wuescher, L.M.; Worth, R.G. Platelets Enhance Dendritic Cell Responses against Staphylococcus aureus through CD40-CD40L. Infect. Immun. 2018, 86, e00186-18. [Google Scholar] [CrossRef] [PubMed]
- Haribhai, D.; Luo, X.; Chen, J.; Jia, S.; Shi, L.; Schroeder, J.A.; Weiler, H.; Aster, R.H.; Hessner, M.J.; Hu, J.; et al. TGF-β1 along with other platelet contents augments Treg cells to suppress anti-FVIII immune responses in hemophilia A mice. Blood Adv. 2016, 1, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.K.; Brashler, J.R.; Stout, B.K.; Johnson, H.G.; Sanders, M.E. Platelet-derived growth factor can activate purified primate, phorbol myristate acetate-primed eosinophils. Int. Arch. Allergy Appl. Immunol. 1991, 94, 167–168. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kisucka, J.; Brill, A.; Walsh, M.T.; Scheiflinger, F.; Wagner, D.D. ADAMTS13: A new link between thrombosis and inflammation. J. Exp. Med. 2008, 205, 2065–2074. [Google Scholar] [CrossRef]
- Denis, C.V.; André, P.; Saffaripour, S.; Wagner, D.D. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 4072–4077. [Google Scholar] [CrossRef]
- Kasuda, S.; Matsui, H.; Ono, S.; Matsunari, Y.; Nishio, K.; Shima, M.; Hatake, K.; Sugimoto, M. Relevant role of von Willebrand factor in neutrophil recruitment in a mouse sepsis model involving cecal ligation and puncture. Haematologica 2016, 101, e52–e54. [Google Scholar] [CrossRef]
- D’Apuzzo, M.; Rolink, A.; Loetscher, M.; Hoxie, J.A.; Clark-Lewis, I.; Melchers, F.; Baggiolini, M.; Moser, B. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur. J. Immunol. 1997, 27, 1788–1793. [Google Scholar] [CrossRef]
- Sozzani, S.; Luini, W.; Borsatti, A.; Polentarutti, N.; Zhou, D.; Piemonti, L.; D’Amico, G.; Power, C.A.; Wells, T.; Gobbi, M. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. 1997, 159, 1993–2000. [Google Scholar] [CrossRef]
- Kantele, J.M.; Kurk, S.; Jutila, M.A. Effects of continuous exposure to stromal cell-derived factor-1α on T cell rolling and tight adhesion to monolayers of activated endothelial cells. J. Immunol. 2000, 164, 5035–5040. [Google Scholar] [CrossRef]
- Vanderstocken, G.; Bondue, B.; Horckmans, M.; Di Pietrantonio, L.; Robaye, B.; Boeynaems, J.-M.; Communi, D. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. J. Immunol. 2010, 185, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Dürk, T.; Panther, E.; Müller, T.; Sorichter, S.; Ferrari, D.; Pizzirani, C.; Di Virgilio, F.; Myrtek, D.; Norgauer, J.; Idzko, M. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int. Immunol. 2005, 17, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Iken, K.; Chheng, S.; Fargin, A.; Goulet, A.-C.; Kouassi, E. Serotonin Upregulates Mitogen-Stimulated B Lymphocyte Proliferation through 5-HT1AReceptors. Cell. Immunol. 1995, 163, 1–9. [Google Scholar] [CrossRef]
- McMorran, B.J.; Marshall, V.M.; de Graaf, C.; Drysdale, K.E.; Shabbar, M.; Smyth, G.K.; Corbin, J.E.; Alexander, W.S.; Foote, S.J. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 2009, 323, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Avellaneda, D.; Mosso-Pani, M.A.; Sánchez-Torres, L.E.; Castro-Mussot, M.E.; Corona-de la Peña, N.A.; Salazar, M.I. Dengue Virus Induces the Release of sCD40L and Changes in Levels of Membranal CD42b and CD40L Molecules in Human Platelets. Viruses 2018, 10, 357. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Wagner, D.D.; Frenette, P.S. The vessel wall and its interactions. Blood 2008, 111, 5271–5281. [Google Scholar] [CrossRef]
- Min, Y.; Hao, L.; Liu, X.; Tan, S.; Song, H.; Ni, H.; Sheng, Z.; Jooss, N.; Liu, X.; Malmström, R.E.; et al. Platelets fine-tune effector responses of naïve CD4+ T cells via platelet factor 4-regulated transforming growth factor β signaling. Cell. Mol. Life Sci. 2022, 79, 247. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Poon, M.C.; Han, Z.; Gu, D.; Xu, M.; Jia, H.; Yang, R.; Han, Z.C. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2007, 78, 139–143. [Google Scholar] [CrossRef]
- Yu, J.; Heck, S.; Patel, V.; Levan, J.; Yu, Y.; Bussel, J.B.; Yazdanbakhsh, K. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood J. Am. Soc. Hematol. 2008, 112, 1325–1328. [Google Scholar] [CrossRef]
- Nanki, T.; Lipsky, P.E. Cutting edge: Stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J. Immunol. 2000, 164, 5010–5014. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.J.; Stier, P.; Ernst, T.; Wong, G.G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol. Cell. Biol. 1989, 9, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Smith, M.; Hasley, R.B.; Stephany, D.; Harned, A.; Nagashima, K.; Abdullah, S.; Pittaluga, S.; Imamichi, T.; Qin, J. Activated platelet–T-cell conjugates in peripheral blood of patients with HIV infection: Coupling coagulation/inflammation and T cells. AIDS 2015, 29, 1297. [Google Scholar] [CrossRef]
- Rondina, M.T.; Brewster, B.; Grissom, C.K.; Zimmerman, G.A.; Kastendieck, D.H.; Harris, E.S.; Weyrich, A.S. In vivo platelet activation in critically ill patients with primary 2009 influenza A (H1N1). Chest 2012, 141, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Medeiros-de-Moraes, I.M.; Vieira-de-Abreu, A.; de Assis, E.F.; Vals-de-Souza, R.; Castro-Faria-Neto, H.C.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J. Immunol. 2014, 193, 1864–1872. [Google Scholar] [CrossRef]
- Rondina, M.T.; Carlisle, M.; Fraughton, T.; Brown, S.M.; Miller III, R.R.; Harris, E.S.; Weyrich, A.S.; Zimmerman, G.A.; Supiano, M.A.; Grissom, C.K. Platelet–monocyte aggregate formation and mortality risk in older patients with severe sepsis and septic shock. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 225–231. [Google Scholar] [CrossRef]
- Hottz, E.D.; Quirino-Teixeira, A.C.; Merij, L.B.; Pinheiro, M.B.M.; Rozini, S.V.; Bozza, F.A.; Bozza, P.T. Platelet–leukocyte interactions in the pathogenesis of viral infections. Platelets 2022, 33, 200–207. [Google Scholar] [CrossRef]
- Assinger, A.; Kral, J.B.; Yaiw, K.C.; Schrottmaier, W.C.; Kurzejamska, E.; Wang, Y.; Mohammad, A.A.; Religa, P.; Rahbar, A.; Schabbauer, G.; et al. Human cytomegalovirus-platelet interaction triggers toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 801–809. [Google Scholar] [CrossRef]
- Venkata, C.; Kashyap, R.; Farmer, J.C.; Afessa, B. Thrombocytopenia in adult patients with sepsis: Incidence, risk factors, and its association with clinical outcome. J. Intensive Care 2013, 1, 9. [Google Scholar] [CrossRef]
- Raadsen, M.; Du Toit, J.; Langerak, T.; van Bussel, B.; van Gorp, E.; Goeijenbier, M. Thrombocytopenia in Virus Infections. J. Clin. Med. 2021, 10, 877. [Google Scholar] [CrossRef]
- Claushuis, T.A.; van Vught, L.A.; Scicluna, B.P.; Wiewel, M.A.; Klein Klouwenberg, P.M.; Hoogendijk, A.J.; Ong, D.S.; Cremer, O.L.; Horn, J.; Franitza, M. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood J. Am. Soc. Hematol. 2016, 127, 3062–3072. [Google Scholar] [CrossRef] [PubMed]
- Amison, R.T.; O’Shaughnessy, B.G.; Arnold, S.; Cleary, S.J.; Nandi, M.; Pitchford, S.C.; Bragonzi, A.; Page, C.P. Platelet Depletion Impairs Host Defense to Pulmonary Infection with Pseudomonas aeruginosa in Mice. Am. J. Respir. Cell Mol. Biol. 2018, 58, 331–340. [Google Scholar] [CrossRef] [PubMed]
- de Stoppelaar, S.F.; van’t Veer, C.; Claushuis, T.A.; Albersen, B.J.; Roelofs, J.J.; van der Poll, T. Thrombocytopenia impairs host defense in gram-negative pneumonia–derived sepsis in mice. Blood J. Am. Soc. Hematol. 2014, 124, 3781–3790. [Google Scholar] [CrossRef] [PubMed]
- De Stoppelaar, S.; Van’t Veer, C.; Roelofs, J.; Claushuis, T.; De Boer, O.; Tanck, M.; Hoogendijk, A.; Van Der Poll, T. Platelet and endothelial cell P-selectin are required for host defense against Klebsiella pneumoniae-induced pneumosepsis. J. Thromb. Haemost. 2015, 13, 1128–1138. [Google Scholar] [CrossRef]
- Portier, I.; Campbell, R.A. Role of Platelets in Detection and Regulation of Infection. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 70–78. [Google Scholar] [CrossRef]
- Chaipan, C.; Soilleux, E.J.; Simpson, P.; Hofmann, H.; Gramberg, T.; Marzi, A.; Geier, M.; Stewart, E.A.; Eisemann, J.; Steinkasserer, A.; et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J. Virol. 2006, 80, 8951–8960. [Google Scholar] [CrossRef]
- Sung, P.S.; Huang, T.F.; Hsieh, S.L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef]
- Delierneux, C.; Donis, N.; Servais, L.; Wéra, O.; Lecut, C.; Vandereyken, M.; Musumeci, L.; Rahmouni, S.; Schneider, J.; Eble, J.A.; et al. Targeting of C-type lectin-like receptor 2 or P2Y12 for the prevention of platelet activation by immunotherapeutic CpG oligodeoxynucleotides. J. Thromb. Haemost. 2017, 15, 983–997. [Google Scholar] [CrossRef]
- Simon, A.Y.; Sutherland, M.R.; Pryzdial, E.L. Dengue virus binding and replication by platelets. Blood 2015, 126, 378–385. [Google Scholar] [CrossRef]
- Assinger, A.; Laky, M.; Schabbauer, G.; Hirschl, A.M.; Buchberger, E.; Binder, B.R.; Volf, I. Efficient phagocytosis of periodontopathogens by neutrophils requires plasma factors, platelets and TLR2. J. Thromb. Haemost. 2011, 9, 799–809. [Google Scholar] [CrossRef]
- Niklaus, M.; Klingler, P.; Weber, K.; Koessler, A.; Kuhn, S.; Boeck, M.; Kobsar, A.; Koessler, J. Platelet Toll-Like-Receptor-2 and -4 Mediate Different Immune-Related Responses to Bacterial Ligands. TH Open 2022, 6, e156–e167. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, A.L.; Svensson, M.; Mörgelin, M.; Svanborg, C.; Tarr, P.I.; Mooney, J.C.; Watkins, S.L.; Johnson, R.; Karpman, D. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood 2006, 108, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, X.; Li, Y.; Wang, X.; Tan, S.; Chen, F. LPS enhances platelets aggregation via TLR4, which is related to mitochondria damage caused by intracellular ROS, but not extracellular ROS. Cell. Immunol. 2018, 328, 86–92. [Google Scholar] [CrossRef]
- Thon, J.N.; Peters, C.G.; Machlus, K.R.; Aslam, R.; Rowley, J.; Macleod, H.; Devine, M.T.; Fuchs, T.A.; Weyrich, A.S.; Semple, J.W.; et al. T granules in human platelets function in TLR9 organization and signaling. J. Cell Biol. 2012, 198, 561–574. [Google Scholar] [CrossRef]
- Lien, T.S.; Chan, H.; Sun, D.S.; Wu, J.C.; Lin, Y.Y.; Lin, G.L.; Chang, H.H. Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice. Front. Immunol. 2021, 12, 616394. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Hu, L.; Zhai, L.; Xue, R.; Ye, J.; Chen, L.; Cheng, G.; Mruk, J.; Kunapuli, S.P. Nucleotide-binding oligomerization domain 2 receptor is expressed in platelets and enhances platelet activation and thrombosis. Circulation 2015, 131, 1160–1170. [Google Scholar] [CrossRef]
- Zahn, A.; Jennings, N.; Ouwehand, W.H.; Allain, J.P. Hepatitis C virus interacts with human platelet glycoprotein VI. J. Gen. Virol. 2006, 87, 2243–2251. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; de Vos, A.F.; Nieswandt, B.; Boon, L.; Roelofs, J.J.T.H.; de Boer, O.J.; van ’t Veer, C.; van der Poll, T. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood 2018, 131, 864–876. [Google Scholar] [CrossRef]
- Hu, H.; Armstrong, P.C.; Khalil, E.; Chen, Y.C.; Straub, A.; Li, M.; Soosairajah, J.; Hagemeyer, C.E.; Bassler, N.; Huang, D.; et al. GPVI and GPIbα mediate staphylococcal superantigen-like protein 5 (SSL5) induced platelet activation and direct toward glycans as potential inhibitors. PLoS ONE 2011, 6, e19190. [Google Scholar] [CrossRef]
- Flierl, U.; Nero, T.L.; Lim, B.; Arthur, J.F.; Yao, Y.; Jung, S.M.; Gitz, E.; Pollitt, A.Y.; Zaldivia, M.T.; Jandrot-Perrus, M.; et al. Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators. J. Exp. Med. 2015, 212, 129–137. [Google Scholar] [CrossRef] [PubMed]
- de Haas, C.J.; Weeterings, C.; Vughs, M.M.; de Groot, P.G.; Van Strijp, J.A.; Lisman, T. Staphylococcal superantigen-like 5 activates platelets and supports platelet adhesion under flow conditions, which involves glycoprotein Ibalpha and alpha IIb beta 3. J. Thromb. Haemost. 2009, 7, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.; Wu, H.; Kerrigan, S.W.; Meade, G.; Cox, D.; Ian Douglas, C.W. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br. J. Haematol. 2005, 129, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bensing, B.A.; López, J.A.; Sullam, P.M. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect. Immun. 2004, 72, 6528–6537. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, M.; van Schooten, C.J.; Kerrigan, S.W.; Emsley, J.; Silverman, G.J.; Cox, D.; Lenting, P.J.; Foster, T.J. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. Febs J. 2006, 273, 4831–4841. [Google Scholar] [CrossRef]
- Byrne, M.F.; Kerrigan, S.W.; Corcoran, P.A.; Atherton, J.C.; Murray, F.E.; Fitzgerald, D.J.; Cox, D.M. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology 2003, 124, 1846–1854. [Google Scholar] [CrossRef]
- Gavrilovskaya, I.N.; Brown, E.J.; Ginsberg, M.H.; Mackow, E.R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 1999, 73, 3951–3959. [Google Scholar] [CrossRef]
- Gupalo, E.; Kuk, C.; Qadura, M.; Buriachkovskaia, L.; Othman, M. Platelet–adenovirus vs. inert particles interaction: Effect on aggregation and the role of platelet membrane receptors. Platelets 2013, 24, 383–391. [Google Scholar] [CrossRef]
- Brennan, M.P.; Loughman, A.; Devocelle, M.; Arasu, S.; Chubb, A.J.; Foster, T.J.; Cox, D. Elucidating the role of Staphylococcus epidermidis serine–aspartate repeat protein G in platelet activation. J. Thromb. Haemost. 2009, 7, 1364–1372. [Google Scholar] [CrossRef]
- Petersen, H.J.; Keane, C.; Jenkinson, H.F.; Vickerman, M.M.; Jesionowski, A.; Waterhouse, J.C.; Cox, D.; Kerrigan, S.W. Human platelets recognize a novel surface protein, PadA, on Streptococcus gordonii through a unique interaction involving fibrinogen receptor GPIIbIIIa. Infect. Immun. 2010, 78, 413–422. [Google Scholar] [CrossRef]
- Miajlovic, H.; Zapotoczna, M.; Geoghegan, J.A.; Kerrigan, S.W.; Speziale, P.; Foster, T.J. Direct interaction of iron-regulated surface determinant IsdB of Staphylococcus aureus with the GPIIb/IIIa receptor on platelets. Microbiology 2010, 156, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R.; Loughman, A.; Keane, F.; Brennan, M.; Knobel, M.; Higgins, J.; Visai, L.; Speziale, P.; Cox, D.; Foster, T.J. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol. Microbiol. 2006, 59, 212–230. [Google Scholar] [CrossRef]
- O´Brien, L.; Kerrigan, S.W.; Kaw, G.; Hogan, M.; Penadés, J.; Litt, D.; Fitzgerald, D.J.; Foster, T.J.; Cox, D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: Roles for the clumping factors ClfA and ClfB, the serine–aspartate repeat protein SdrE and protein A. Mol. Microbiol. 2002, 44, 1033–1044. [Google Scholar] [CrossRef]
- Coulson, B.S.; Londrigan, S.L.; Lee, D.J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA 1997, 94, 5389–5394. [Google Scholar] [CrossRef] [PubMed]
- Arman, M.; Krauel, K. Human platelet IgG Fc receptor FcγRIIA in immunity and thrombosis. J. Thromb. Haemost. 2015, 13, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Paré, G.; Rousseau, M.; Cloutier, N.; Dubuc, I.; Lévesque, T.; Borgeat, P.; Flamand, L. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood 2014, 123, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cavaillon, J.-M.; Cognasse, F. Bench-to-bedside review: Platelets and active immune functions—New clues for immunopathology? Crit. Care 2013, 17, 236. [Google Scholar] [CrossRef]
- Hally, K.; Fauteux-Daniel, S.; Hamzeh-Cognasse, H.; Larsen, P.; Cognasse, F. Revisiting Platelets and Toll-Like Receptors (TLRs): At the Interface of Vascular Immunity and Thrombosis. Int. J. Mol. Sci. 2020, 21, 6150. [Google Scholar] [CrossRef]
- Ebermeyer, T.; Cognasse, F.; Berthelot, P.; Mismetti, P.; Garraud, O.; Hamzeh-Cognasse, H. Platelet Innate Immune Receptors and TLRs: A Double-Edged Sword. Int. J. Mol. Sci. 2021, 22, 7894. [Google Scholar] [CrossRef]
- Galgano, L.; Guidetti, G.F.; Torti, M.; Canobbio, I. The Controversial Role of LPS in Platelet Activation In Vitro. Int. J. Mol. Sci. 2022, 23, 10900. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K. Platelets and cancer-associated thrombosis: Focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood 2019, 134, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Gabriel, G.; von Köckritz-Blickwede, M. Detrimental Role of Neutrophil Extracellular Traps during Dengue Virus Infection. Trends Immunol. 2020, 41, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Bozza, F.A.; Bozza, P.T. Platelets in Immune Response to Virus and Immunopathology of Viral Infections. Front. Med. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-de-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013, 122, 3405–3414. [Google Scholar] [CrossRef]
- Qiao, J.; Wu, X.; Luo, Q.; Wei, G.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Zi, J.; Ju, W.; et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica 2018, 103, 1568–1576. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Baik, C.H.; Travis, O.K.; White, D.L.; Young, C.M.; Austin Pierce, W.; Shields, C.A.; Poudel, B.; Williams, J.M. NLRP3 inflammasome activation in platelets in response to sepsis. Physiol. Rep. 2019, 7, e14073. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Travis, O.K.; Tramel, R.W.; Borges-Rodriguez, M.; Baik, C.H.; Greer, M.; Giachelli, C.A.; Tardo, G.A.; Williams, J.M. NLRP3 inflammasome inhibition attenuates sepsis-induced platelet activation and prevents multi-organ injury in cecal-ligation puncture. PLoS ONE 2020, 15, e0234039. [Google Scholar] [CrossRef]
- Vogel, S.; Kamimura, S.; Arora, T.; Smith, M.L.; Almeida, L.E.F.; Combs, C.A.; Thein, S.L.; Quezado, Z.M.N. NLRP3 inflammasome and bruton tyrosine kinase inhibition interferes with upregulated platelet aggregation and in vitro thrombus formation in sickle cell mice. Biochem. Biophys. Res. Commun. 2021, 555, 196–201. [Google Scholar] [CrossRef]
- Gomes de Azevedo-Quintanilha, I.; Campos, M.M.; Teixeira Monteiro, A.P.; Dantas do Nascimento, A.; Calheiros, A.S.; Oliveira, D.M.; Dias, S.S.G.; Soares, V.C.; Santos, J.D.C.; Tavares, I.; et al. Increased platelet activation and platelet-inflammasome engagement during chikungunya infection. Front. Immunol. 2022, 13, 958820. [Google Scholar] [CrossRef]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018, 11, 49–60. [Google Scholar] [CrossRef]
- Zhong, H.; Waresi, M.; Zhang, W.; Han, L.; Zhao, Y.; Chen, Y.; Zhou, P.; Chang, L.; Pan, G.; Wu, B.; et al. NOD2-mediated P2Y12 upregulation increases platelet activation and thrombosis in sepsis. Biochem. Pharmacol. 2021, 194, 114822. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.J.; Manukjan, G.; Pflug, A.; Winter, N.; Weigel, M.; Nagler, N.; Kredel, M.; Lâm, T.T.; Nieswandt, B.; Weismann, D.; et al. Acquired platelet GPVI receptor dysfunction in critically ill patients with sepsis. Blood 2021, 137, 3105–3115. [Google Scholar] [CrossRef]
- Bondu, V.; Wu, C.; Cao, W.; Simons, P.C.; Gillette, J.; Zhu, J.; Erb, L.; Zhang, X.F.; Buranda, T. Low-affinity binding in cis to P2Y(2)R mediates force-dependent integrin activation during hantavirus infection. Mol. Biol. Cell 2017, 28, 2887–2903. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Pawankar, R.; Suzuki, K.; Nakahata, T.; Furukawa, S.; Okumura, K.; Ra, C. Functional Expression of the High Affinity Receptor for IgE (FcεRI) in Human Platelets and Its’ Intracellular Expression in Human Megakaryocytes. Blood 1999, 93, 2543–2551. [Google Scholar] [CrossRef]
- Da’dara, A.A.; Skelly, P.J. Schistosomes versus platelets. Thromb. Res. 2014, 134, 1176–1181. [Google Scholar] [CrossRef]
- Qian, K.; Xie, F.; Gibson, A.W.; Edberg, J.C.; Kimberly, R.P.; Wu, J. Functional expression of IgA receptor FcalphaRI on human platelets. J. Leukoc. Biol. 2008, 84, 1492–1500. [Google Scholar] [CrossRef]
- Fong, K.P.; Barry, C.; Tran, A.N.; Traxler, E.A.; Wannemacher, K.M.; Tang, H.Y.; Speicher, K.D.; Blair, I.A.; Speicher, D.W.; Grosser, T.; et al. Deciphering the human platelet sheddome. Blood 2011, 117, e15–e26. [Google Scholar] [CrossRef]
- Montague, S.J.; Andrews, R.K.; Gardiner, E.E. Mechanisms of receptor shedding in platelets. Blood 2018, 132, 2535–2545. [Google Scholar] [CrossRef]
- Amelirad, A.; Shamsasenjan, K.; Akbarzadehlaleh, P.; Pashoutan Sarvar, D. Signaling Pathways of Receptors Involved in Platelet Activation and Shedding of These Receptors in Stored Platelets. Adv. Pharm. Bull. 2019, 9, 38–47. [Google Scholar] [CrossRef]
- Berndt, M.C.; Karunakaran, D.; Gardiner, E.E.; Andrews, R.K. Programmed autologous cleavage of platelet receptors. J. Thromb. Haemost. 2007, 5 (Suppl. S1), 212–219. [Google Scholar] [CrossRef]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Rahman, M.; Zhang, S.; Chew, M.; Syk, I.; Jeppsson, B.; Thorlacius, H. Platelet shedding of CD40L is regulated by matrix metalloproteinase-9 in abdominal sepsis. J. Thromb. Haemost. 2013, 11, 1385–1398. [Google Scholar] [CrossRef]
- Danese, S.; Katz, J.A.; Saibeni, S.; Papa, A.; Gasbarrini, A.; Vecchi, M.; Fiocchi, C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut 2003, 52, 1435–1441. [Google Scholar] [CrossRef]
- Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int. J. Mol. Sci. 2014, 15, 22342–22364. [Google Scholar] [CrossRef]
- Bustamante, A.E.; Jaime-Pérez, J.C.; Cordero-Pérez, P.; Galindo-Rodríguez, G.; Muñoz-Espinosa, L.E.; Villarreal-Villarreal, C.D.; Mercado-Longoria, R. A High Level of Soluble CD40L Is Associated with P. aeruginosa Infection in Patients with Cystic Fibrosis. PLoS ONE 2016, 11, e0168819. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Barreto, A.S.; Bomfim, L.G.; Leite, T.R.; Dos Santos, P.L.; de Almeida, R.P.; da Silva, Â.M.; Duthie, M.S.; Reed, S.G.; de Moura, T.R.; et al. Soluble CD40 Ligand in Sera of Subjects Exposed to Leishmania infantum Infection Reduces the Parasite Load in Macrophages. PLoS ONE 2015, 10, e0141265. [Google Scholar] [CrossRef]
- Inwald, D.P.; Faust, S.N.; Lister, P.; Peters, M.J.; Levin, M.; Heyderman, R.; Klein, N.J. Platelet and soluble CD40L in meningococcal sepsis. Intensive Care Med. 2006, 32, 1432–1437. [Google Scholar] [CrossRef]
- Davidson, D.C.; Hirschman, M.P.; Spinelli, S.L.; Morrell, C.N.; Schifitto, G.; Phipps, R.P.; Maggirwar, S.B. Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals. J. Immunol. 2011, 186, 584–591. [Google Scholar] [CrossRef]
- Assinger, A.; Laky, M.; Badrnya, S.; Esfandeyari, A.; Volf, I. Periodontopathogens induce expression of CD40L on human platelets via TLR2 and TLR4. Thromb. Res. 2012, 130, e73–e78. [Google Scholar] [CrossRef]
- Rahman, M.; Zhang, S.; Chew, M.; Ersson, A.; Jeppsson, B.; Thorlacius, H. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann. Surg. 2009, 250, 783–790. [Google Scholar] [CrossRef]
- Hwaiz, R.; Rahman, M.; Zhang, E.; Thorlacius, H. Rac1 regulates platelet shedding of CD40L in abdominal sepsis. Lab. Investig. 2014, 94, 1054–1063. [Google Scholar] [CrossRef]
- Jin, R.; Yu, S.; Song, Z.; Zhu, X.; Wang, C.; Yan, J.; Wu, F.; Nanda, A.; Granger, D.N.; Li, G. Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integrin Mac-1 and protein kinase C zeda (PKCζ) in neutrophils: Implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS ONE 2013, 8, e64631. [Google Scholar] [CrossRef]
- Nannizzi-Alaimo, L.; Alves, V.L.; Phillips, D.R. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation 2003, 107, 1123–1128. [Google Scholar] [CrossRef]
- Graff, J.; Harder, S.; Wahl, O.; Scheuermann, E.H.; Gossmann, J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: Effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin. Pharmacol. Ther. 2005, 78, 468–476. [Google Scholar] [CrossRef]
- Davidson, D.C.; Jackson, J.W.; Maggirwar, S.B. Targeting platelet-derived soluble CD40 ligand: A new treatment strategy for HIV-associated neuroinflammation? J. Neuroinflamm. 2013, 10, 144. [Google Scholar] [CrossRef]
- Washington, A.V.; Gibot, S.; Acevedo, I.; Gattis, J.; Quigley, L.; Feltz, R.; De La Mota, A.; Schubert, R.L.; Gomez-Rodriguez, J.; Cheng, J.; et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J. Clin. Investig. 2009, 119, 1489–1501. [Google Scholar] [CrossRef]
- Gattis, J.L.; Washington, A.V.; Chisholm, M.M.; Quigley, L.; Szyk, A.; McVicar, D.W.; Lubkowski, J. The structure of the extracellular domain of triggering receptor expressed on myeloid cells like transcript-1 and evidence for a naturally occurring soluble fragment. J. Biol. Chem. 2006, 281, 13396–13403. [Google Scholar] [CrossRef]
- Ferrer-Acosta, Y.; González, M.; Fernández, M.; Valance, W.A. Emerging Roles for Platelets in Inflammation and Disease. J. Infect. Dis. Ther. 2014, 2, 149. [Google Scholar] [CrossRef]
- Blann, A.D.; Nadar, S.K.; Lip, G.Y. The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart J. 2003, 24, 2166–2179. [Google Scholar] [CrossRef]
- Bodary, P.F.; Homeister, J.W.; Vargas, F.B.; Wickenheiser, K.J.; Cudney, S.S.; Bahrou, K.L.; Ohman, M.; Rabbani, A.B.; Eitzman, D.T. Generation of soluble P- and E-selectins in vivo is dependent on expression of P-selectin glycoprotein ligand-1. J. Thromb. Haemost. 2007, 5, 599–603. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Kemperman, H.; Roest, M.; Kesecioglu, J.; de Lange, D.W. Soluble P-selectin as a Biomarker for Infection and Survival in Patients With a Systemic Inflammatory Response Syndrome on the Intensive Care Unit. Biomark. Insights 2017, 12, 1177271916684823. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Buchberger, E.; Laky, M.; Esfandeyari, A.; Brostjan, C.; Volf, I. Periodontopathogens induce soluble P-selectin release by endothelial cells and platelets. Thromb. Res. 2011, 127, e20–e26. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Karunakaran, D.; Shen, Y.; Arthur, J.F.; Andrews, R.K.; Berndt, M.C. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J. Thromb. Haemost. 2007, 5, 1530–1537. [Google Scholar] [CrossRef]
- Bergmeier, W.; Piffath, C.L.; Cheng, G.; Dole, V.S.; Zhang, Y.; von Andrian, U.H.; Wagner, D.D. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ. Res. 2004, 95, 677–683. [Google Scholar] [CrossRef]
- Cheng, H.; Yan, R.; Li, S.; Yuan, Y.; Liu, J.; Ruan, C.; Dai, K. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibalpha shedding. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2128–H2135. [Google Scholar] [CrossRef]
- de Mast, Q.; de Groot, P.G.; van Heerde, W.L.; Roestenberg, M.; van Velzen, J.F.; Verbruggen, B.; Roest, M.; McCall, M.; Nieman, A.E.; Westendorp, J.; et al. Thrombocytopenia in early malaria is associated with GP1b shedding in absence of systemic platelet activation and consumptive coagulopathy. Br. J. Haematol. 2010, 151, 495–503. [Google Scholar] [CrossRef]
- Bridges, D.J.; Bunn, J.; van Mourik, J.A.; Grau, G.; Preston, R.J.; Molyneux, M.; Combes, V.; O’Donnell, J.S.; de Laat, B.; Craig, A. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 2010, 115, 1472–1474. [Google Scholar] [CrossRef]
- Yamashita, Y.; Naitoh, K.; Wada, H.; Ikejiri, M.; Mastumoto, T.; Ohishi, K.; Hosaka, Y.; Nishikawa, M.; Katayama, N. Elevated plasma levels of soluble platelet glycoprotein VI (GPVI) in patients with thrombotic microangiopathy. Thromb. Res. 2014, 133, 440–444. [Google Scholar] [CrossRef]
- Montague, S.J.; Delierneux, C.; Lecut, C.; Layios, N.; Dinsdale, R.J.; Lee, C.S.; Poulter, N.S.; Andrews, R.K.; Hampson, P.; Wearn, C.M.; et al. Soluble GPVI is elevated in injured patients: Shedding is mediated by fibrin activation of GPVI. Blood Adv. 2018, 2, 240–251. [Google Scholar] [CrossRef]
- Tanaka, K.; Tanaka, M.; Watanabe, N.; Ito, M.; Pastan, I.; Koizumi, M.; Matsusaka, T. C-type lectin-like receptor (CLEC)-2, the ligand of podoplanin, induces morphological changes in podocytes. Sci. Rep. 2022, 12, 22356. [Google Scholar] [CrossRef]
- Ishikura, H.; Irie, Y.; Kawamura, M.; Hoshino, K.; Nakamura, Y.; Mizunuma, M.; Maruyama, J.; Nakashio, M.; Suzuki-Inoue, K.; Kitamura, T. Early recognition of sepsis-induced coagulopathy using the C2PAC index: A ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets 2022, 33, 935–944. [Google Scholar] [CrossRef] [PubMed]
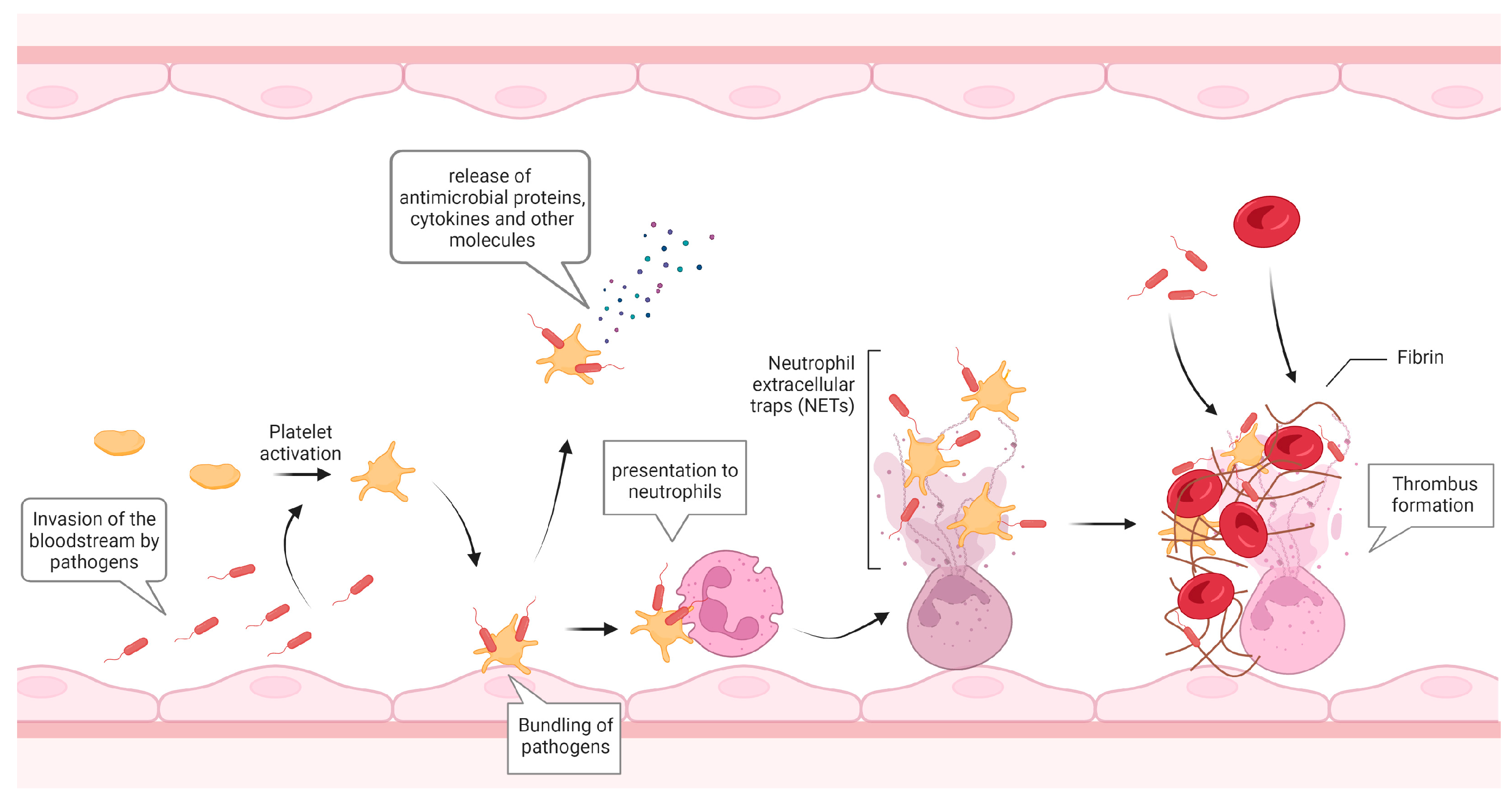
| Direct Platelet-Mediated Responses | References |
|---|---|
| Immunothrombosis | [4,5] |
| Pathogen trapping | [6,7,8,9] |
| Release of antimicrobial effectors | [10,11,12] |
| Expression of antiviral molecule | [13] |
| Indirect Platelet-Mediated Responses | References |
| Neutrophil activation and NETosis | [14,15,16,17] |
| Shuttling of blood-borne bacteria to CD8α+ dendritic cells | [8] |
| CD4+ T-cell differentiation | [18,19] |
| Induction of Ig isotype switching | [20,21] |
| Release of pro-inflammatory molecules | See Table 2 |
| Leukocyte recruitment | [8,10,14,22,23,24] |
| Molecules | Effect |
|---|---|
| PF4 | First-line defence against invading pathogens [28]. Intraerythrocytic parasite killing [11,29] and activation of neutrophils [14,15,16,17]. |
| Soluble CD40 Ligand | APC maturation and activation, production of interferon-γ by T cells, and differentiation of naïve T cells into effector cells [30,31]. Stimulation of dendritic cells [32]. Regulation of B-cell isotype switching and CD8 T-cell responses. |
| TGF-β1 | Conversion of conventional CD4+ T cells into induced regulatory T cells [33] |
| PDGF | Attraction of monocytes to the site of the vascular injury and production of superoxide anions from eosinophils [34]. |
| VWF | Increase of inflammation and neutrophils extravasation [35,36,37]. |
| SDF-1 | Potent chemoattractant of monocytes, T and pre-B lymphocytes [38], and dendritic cells [39]. Effect on T-cell rolling and tight adhesion to activated endothelial cells [40]. |
| ADP | Increase of antigen endocytosis and processing [41]. |
| Serotonin | Stimulation of monocytes [42] and lymphocytes [43]. |
| P-selectin | Recruitment and activation of both innate and adaptive immune responses. |
| Receptor | Pathogens/PAMPs |
|---|---|
| PRRs | |
| CLRs | |
| - CLEC-2 | HIV [66], DV [67], CpG ODN [68] |
| - DC-SIGN | HIV [66], DV [69] |
| TLRs | |
| - TLR2 | Periodontopathogens [70], HCMV [58], Pam3CSK4 [71] |
| - TLR4 | LPS [72,73,74] |
| - TLR9 | CpG ODN [75] |
| NLRs | |
| - NLRP3 | DV-induced ROS products [76] |
| - NOD2 | MDP [77] |
| Haemostatic Receptors | |
| GPVI | HCV [78], SSL5 [79,80], CpG ODN [81] |
| GPIb | SSL5 [82], SrpA [83], GspB, Hsa [84]. Protein A (SpA) [85], H. Pylori [86] |
| Integrin αIIbβ3 | Hantavirus [87], Adenovirus [88], SSL5 [82], SdrG [89], PadA [90], IsdB [91], FnBPA, FnBPB [92], ClfA, ClfB [93] |
| Integrin α2β1 | Rotavirus [94] |
| FcγRIIA | IgG-opsonized cells [95], IAV (H5N1) [96], FnBPA, FnBPB [92] |
| P2Y12 | CpG ODN [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivigno, S.M.G.; Guidetti, G.F.; Barbieri, S.S.; Zarà, M. Blood Platelets in Infection: The Multiple Roles of the Platelet Signalling Machinery. Int. J. Mol. Sci. 2023, 24, 7462. https://doi.org/10.3390/ijms24087462
Trivigno SMG, Guidetti GF, Barbieri SS, Zarà M. Blood Platelets in Infection: The Multiple Roles of the Platelet Signalling Machinery. International Journal of Molecular Sciences. 2023; 24(8):7462. https://doi.org/10.3390/ijms24087462
Chicago/Turabian StyleTrivigno, Silvia M. G., Gianni Francesco Guidetti, Silvia Stella Barbieri, and Marta Zarà. 2023. "Blood Platelets in Infection: The Multiple Roles of the Platelet Signalling Machinery" International Journal of Molecular Sciences 24, no. 8: 7462. https://doi.org/10.3390/ijms24087462
APA StyleTrivigno, S. M. G., Guidetti, G. F., Barbieri, S. S., & Zarà, M. (2023). Blood Platelets in Infection: The Multiple Roles of the Platelet Signalling Machinery. International Journal of Molecular Sciences, 24(8), 7462. https://doi.org/10.3390/ijms24087462







