Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith
Abstract
1. Introduction
2. Results and Discussion
2.1. Silica-Based Xerogel Monolith
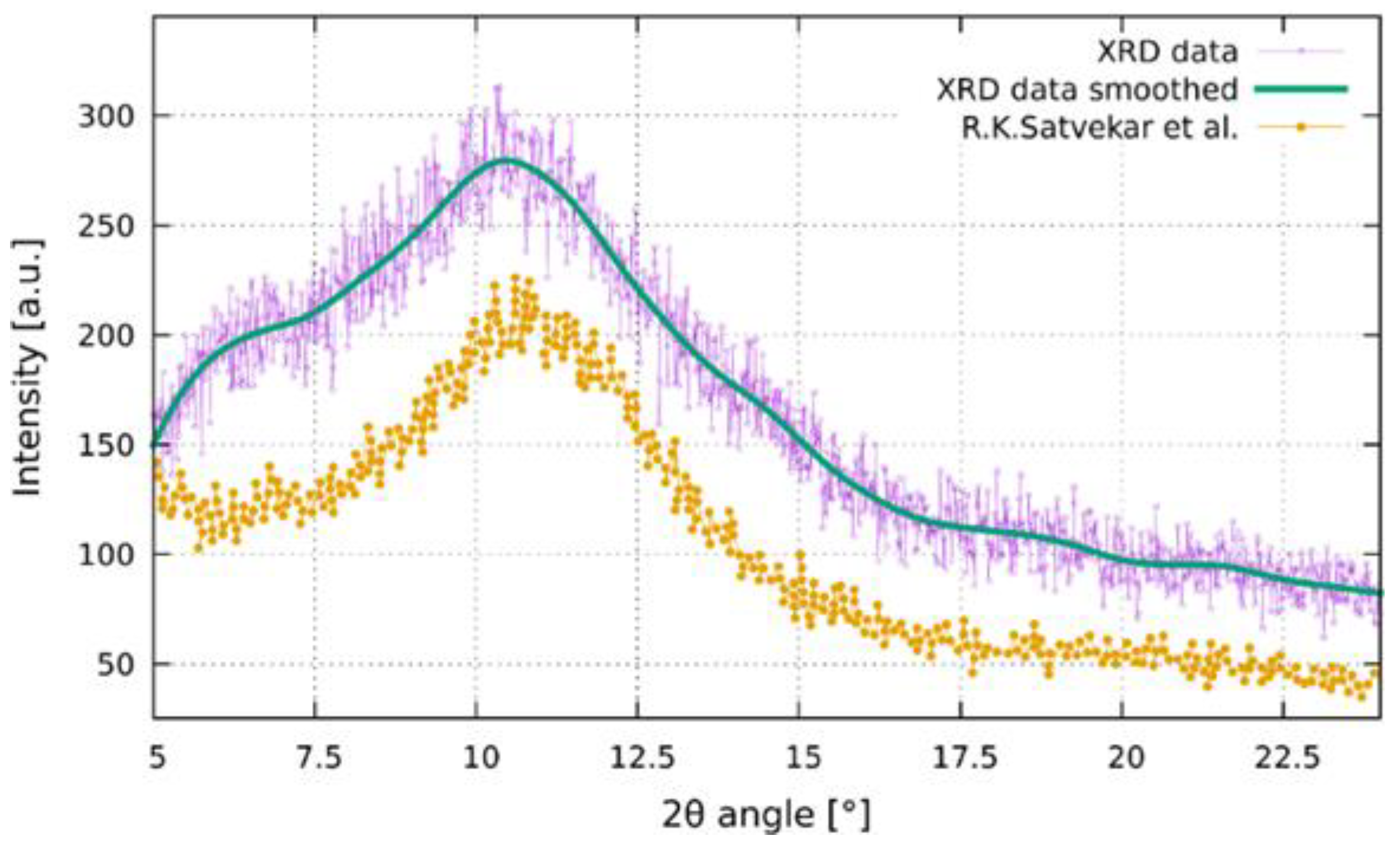
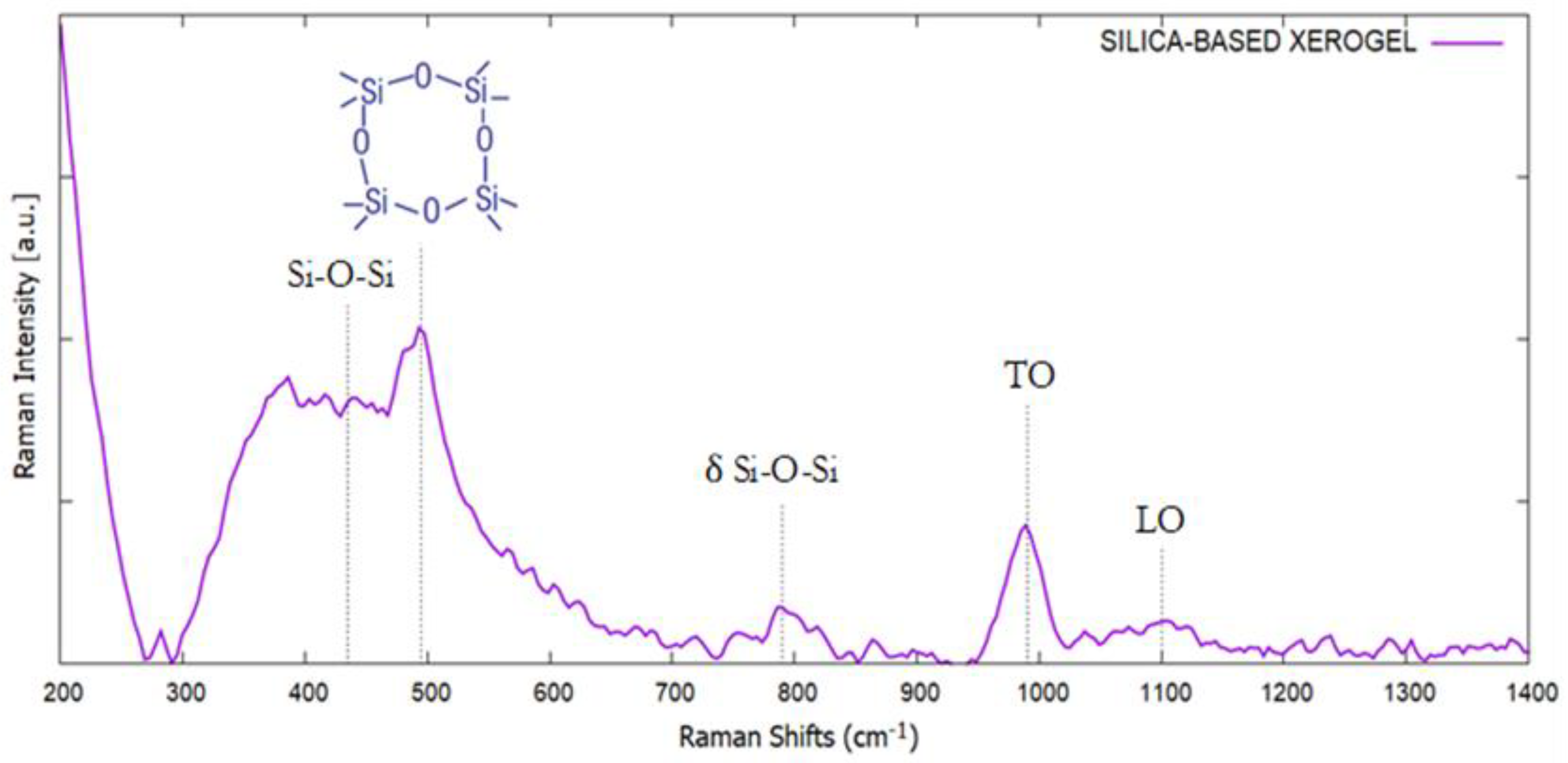
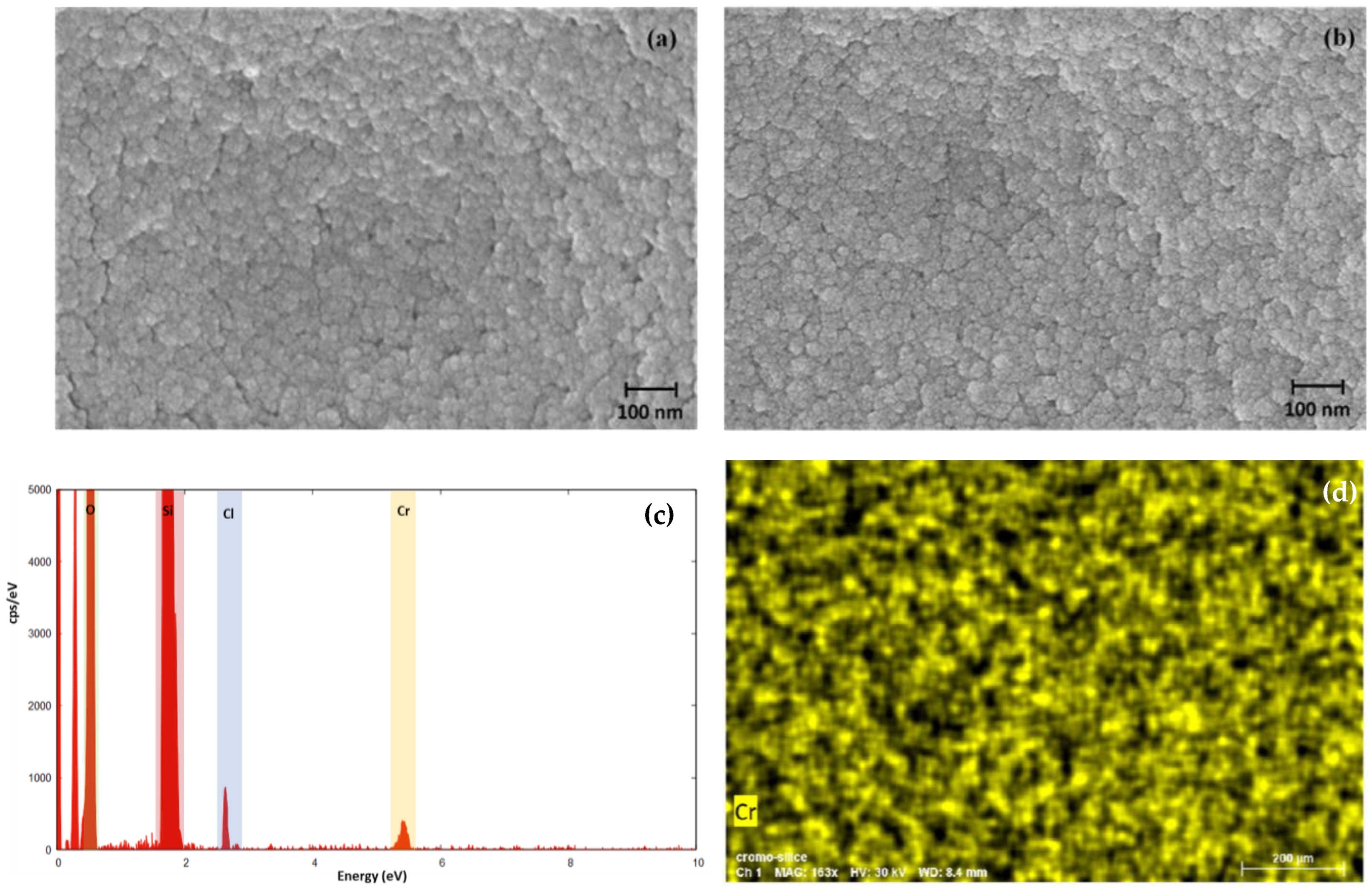
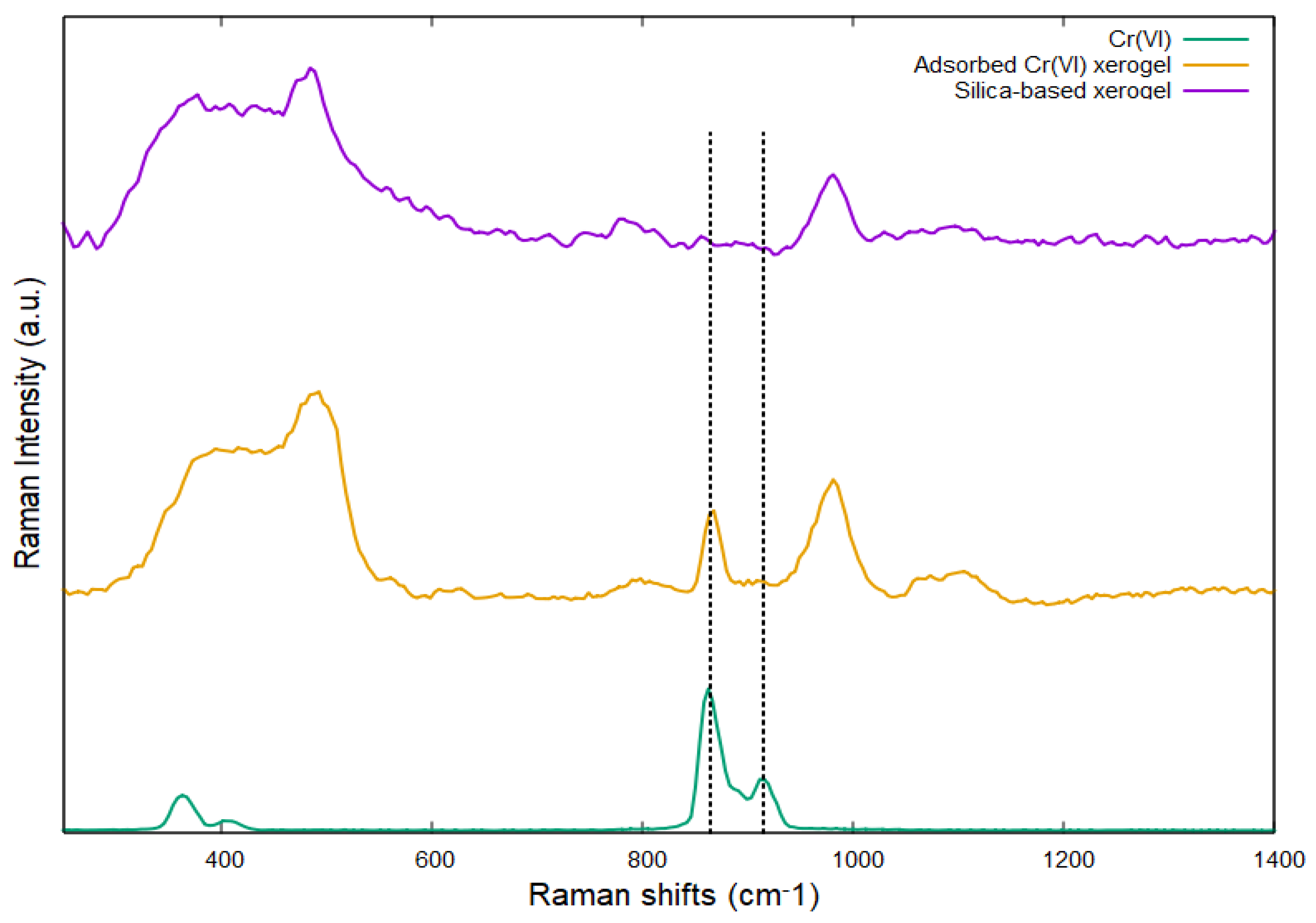
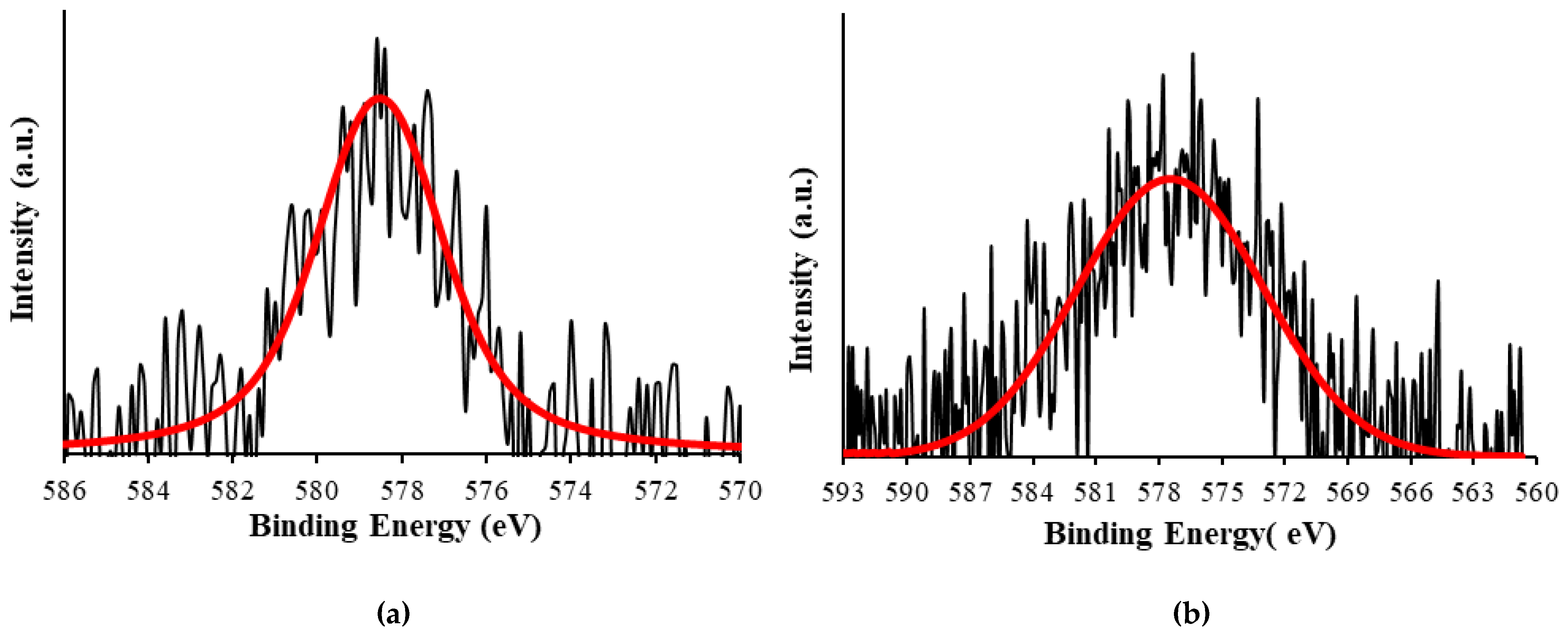
2.2. Silica-Based Xerogel Characterization
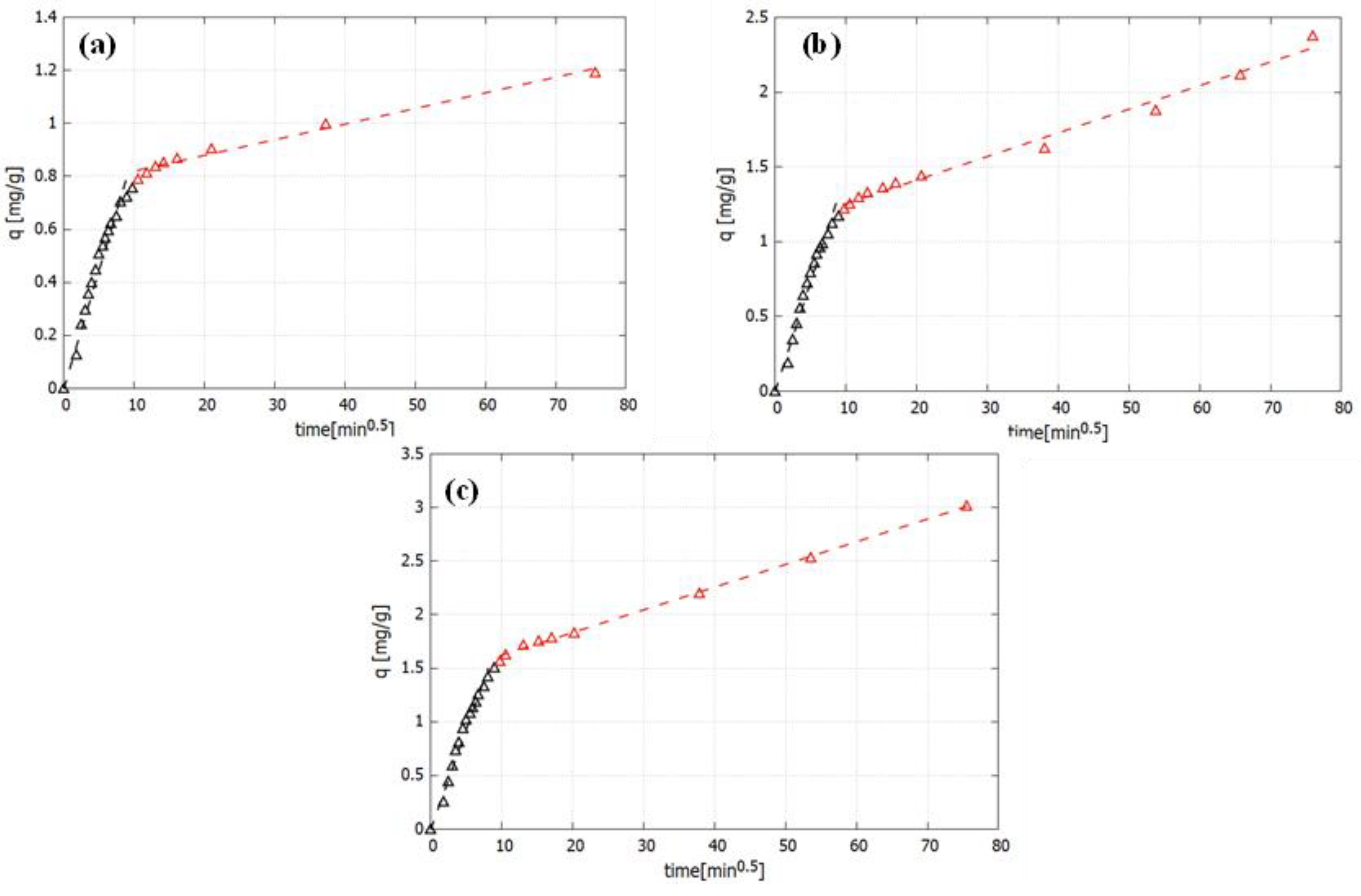
2.3. Kinetic Studies
2.4. Absorption Equilibrium Study
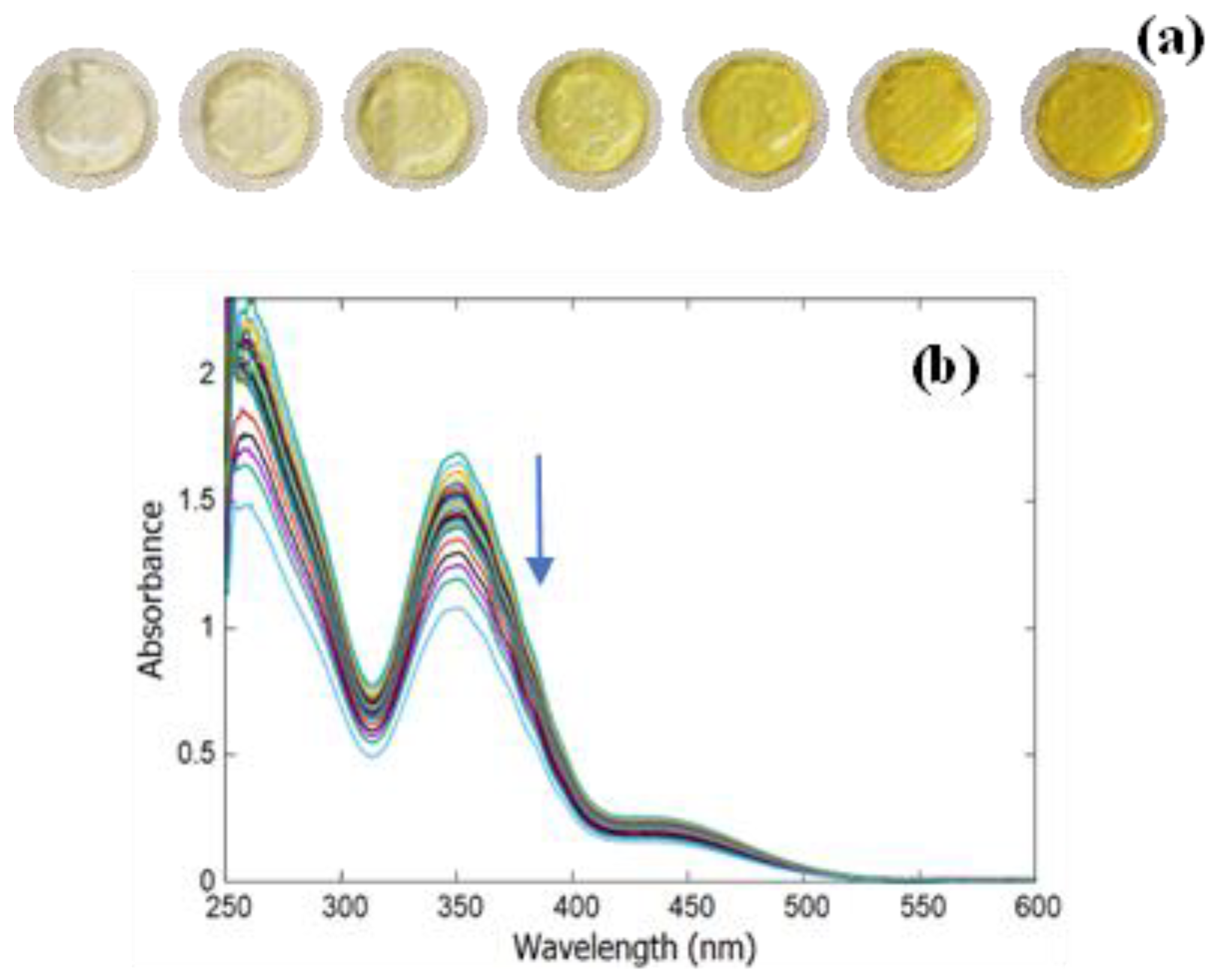
2.5. Material Regeneration
3. Materials and Methods
3.1. Silica-Based Xerogel Preparation
3.2. Xerogel Characterization
3.3. Chromium Absorption Test and Kinetics Studies
3.4. Material Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vodyanitskii, Y.N. Standards for the contents of heavy metals in soils of some states. Ann. Agrar. Sci. 2016, 14, 257–263. [Google Scholar] [CrossRef]
- Dinker, M.K.; Kulkarni, P.S. Recent Advances in Silica-Based Materials for the Removal of Hexavalent Chromium: A Review. J. Chem. Eng. Data 2015, 60, 2521–2540. [Google Scholar] [CrossRef]
- Guan, X.Y.; Fan, H.J.; Yan, S.X.; Chang, J.M. Chromium(VI) concurrent detoxification and immobilization by gallate: Kinetics, equilibrium, thermodynamics, and mechanism studies. J. Environ. Chem. Eng. 2017, 5, 5762–5769. [Google Scholar] [CrossRef]
- Chebeir, M.; Chen, G.D.; Liu, H.Z. Emerging investigators series: Frontier review: Occurrence and speciation of chromium in drinking water distribution systems. Environ. Sci.-Water Res. Technol. 2016, 2, 906–914. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974; pp. 257–260. [Google Scholar]
- Njoya, O.; Zhao, S.; Qu, Y.; Shen, J.; Wang, B.; Shi, H.; Chen, Z. Performance and potential mechanism of Cr(VI) reduction and subsequent Cr(III) precipitation using sodium borohydride driven by oxalate. J. Environ. Manag. 2020, 275, 111165. [Google Scholar] [CrossRef]
- Marzouk, I.; Dammak, L.; Chaabane, L.; Hamrouni, B. Optimization of Chromium (Vi) Removal by Donnan Dialysis. Am. J. Anal. Chem. 2013, 4, 8. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, J.; Ou, X.; Liu, X.; Song, Y.; Tian, C.; Rong, W.; Shi, Z.; Dang, Z.; Lin, Z. Effective Extraction of Cr(VI) from Hazardous Gypsum Sludge via Controlling the Phase Transformation and Chromium Species. Environ. Sci. Technol. 2018, 52, 13336–13342. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, Y.; Lei, X.; Hu, B.; Chen, H.; Yu, G. Study on highly efficient Cr(VI) removal from wastewater by sinusoidal alternating current coagulation. J. Environ. Manag. 2019, 249, 109322. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Lei, X.; Lu, C.; Liu, J.; Zhang, G.; Zhang, B.; Zhang, Q. A magnetic ion exchange resin with high efficiency of removing Cr (VI). Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125279. [Google Scholar] [CrossRef]
- Mitra, S.; Sarkar, A.; Sen, S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Busetty, S. Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 1367–1397. [Google Scholar]
- Mondal, N.K.; Chakraborty, S. Adsorption of Cr(VI) from aqueous solution on graphene oxide (GO) prepared from graphite: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Miglio, V.; Zaccone, C.; Vittoni, C.; Braschi, I.; Buscaroli, E.; Golemme, G.; Marchese, L.; Bisio, C. Silica Monolith for the Removal of Pollutants from Gas and Aqueous Phases. Molecules 2021, 26, 1316. [Google Scholar] [CrossRef]
- Shimizu, T.; Kanamori, K.; Nakanishi, K. Silicone-Based Organic–Inorganic Hybrid Aerogels and Xerogels. Chem.—A Eur. J. 2017, 23, 5176–5187. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. Engl. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Bryans, T.R.; Brawner, V.L.; Quitevis, E.L. Microstructure and Porosity of Silica Xerogel Monoliths Prepared by the Fast Sol-Gel Method. J. Sol-Gel Sci. Technol. 2000, 17, 211–217. [Google Scholar] [CrossRef]
- Lenza RF, S.; Nunes EH, M.; Vasconcelos DC, L.; Vasconcelos, W.L. Preparation of sol–gel silica samples modified with drying control chemical additives. J. Non-Cryst. Solids 2015, 423–424, 35–40. [Google Scholar] [CrossRef]
- Einarsrud, M.-A.; Haereid, S.; Wittwer, V. Some thermal and optical properties of a new transparent silica xerogel material with low density. Sol. Energy Mater. Sol. Cells 1993, 31, 341–347. [Google Scholar] [CrossRef]
- Wright, J.D.; Sommerdijk, N.A.J.M. Sol-Gel Materials Chemistry and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2001. [Google Scholar]
- Satvekar, R.K.; Phadatare, M.R.; Patil, R.N.; Tiwale, B.M.; Pawar, S.H. Influence of Silane Content on the Optical Properties of Sol Gel Derived Spin Coated Silica Thin Films. Int. J. Basic Appl. Sci. 2012, 1, 9. [Google Scholar] [CrossRef][Green Version]
- Saavedra, R.; León, M.; Martin, P.; Jiménez-Rey, D.; Vila, R.; Girard, S.; Boukenter, A.; Ouerdane, Y. Raman measurements in silica glasses irradiated with energetic ions. Fundam. Appl. Silica Adv. Dielectr. (Sio2014) 2014, 1624, 118–124. [Google Scholar] [CrossRef]
- Heili, M.; Poumellec, B.; Burov, E.; Gonnet, C.; Le Losq, C.; Neuville, D.R.; Lancry, M. The dependence of Raman defect bands in silica glasses on densification revisited. J. Mater. Sci. 2016, 51, 1667. [Google Scholar] [CrossRef]
- Varkentina, N.; Dussauze, M.; Royon, A.; Ramme, M.; Petit, Y.; Canioni, L. High repetition rate femtosecond laser irradiation of fused silica studied by Raman spectroscopy. Opt. Mater. Express 2016, 6, 79–90. [Google Scholar] [CrossRef]
- Fan, H.; Hartshorn, C.; Buchheit, T.; Tallant, D.; Assink, R.; Simpson, R.; Kissel, D.J.; Lacks, D.J.; Torquato, S.; Brinker, C.J. Modulus-density scaling behaviour and framework architecture of nanoporous self-assembled silicas. Nat. Mater. 2007, 6, 418–423. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; D’Amato, C.A.; Zannotti, M. Kinetic Model for Simultaneous Adsorption/Photodegradation Process of Alizarin Red S in Water Solution by Nano-TiO2 under Visible Light. Catalysts 2016, 6, 84. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Kajjumba, G.W.E.S.; Öngen, A.; Özcan, H.K.; Aydin, S. Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Bilgili, M.S.; Varank, G.; Sekman, E.; Top, S.; Özçimen, D.; Yazıcı, R. Modeling 4-Chlorophenol Removal from Aqueous Solutions by Granular Activated Carbon. Environ. Model. Assess. 2012, 17, 289–300. [Google Scholar] [CrossRef]
- Zhang, Z.; Liba, D.; Alvarado, L.; Chen, A. Separation and recovery of Cr(III) and Cr(VI) using electrodeionization as an efficient approach. Sep. Purif. Technol. 2014, 137, 86–93. [Google Scholar] [CrossRef]
- Sanchez-Hachair, A.; Hofmann, A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1,5-diphenylcarbazide colorimetry. Comptes Rendus Chim. 2018, 21, 890–896. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A.; Ferraro, S.; Cespi, M.; Bonacucina, G.; Minicucci, M.; Di Cicco, A. Exfoliation of graphite into graphene in aqueous solution: An application as graphene/TiO2 nanocomposite to improve visible light photocatalytic activity. RSC Adv. 2016, 6, 93048–93055. [Google Scholar] [CrossRef]
- Lowe, B.M.; Skylaris, C.-K.; Green, N.G. Acid-base dissociation mechanisms and energetics at the silica–water interface: Anactivationless process. J. Colloid Interface Sci. 2015, 451, 231–244. [Google Scholar] [CrossRef]
- Karthik, C.; Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Arulselvi, P.I. Biosorption and biotransformation of Cr(VI) by novel Cellulosimicrobium funkei strain AR6. J. Taiwan Inst. Chem. Eng. 2017, 70, 282–290. [Google Scholar] [CrossRef]
- Zarghampour, F.; Yamini, Y.; Baharfar, M.; Javadian, G.; Faraji, M. On-chip electromembrane extraction followed by sensitive digital image-based colorimetry for determination of trace amounts of Cr(vi). Anal. Methods 2020, 12, 483–490. [Google Scholar] [CrossRef]
- Bandara, P.C.; Peña-Bahamonde, J.; Rodrigues, D.F. Redox mechanisms of conversion of Cr(VI) to Cr(III) by graphene oxide-polymer composite. Sci. Rep. 2020, 10, 9237. [Google Scholar] [CrossRef]



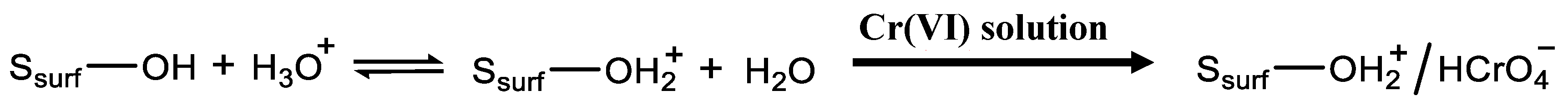


| Cr(VI) (mg/L). | qexp (mg/g) | Pseudo-First Order | Pseudo-Second Order | Elovich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg/g) | R2 | k2 (min−1) | qe (mg/g) | R2 | qe (mg/g) | α (mg/g min) | β (g/mg) | R2 | ||
| 36.53 | 1.1903 | 0.0137 | 1.1903 | 0.9170 | 0.0146 | 1.1784 | 0.9980 | 1.3616 | 0.1605 | 6.3694 | 0.9603 |
| 55.41 | 2.3751 | 0.0102 | 2.3751 | 0.9078 | 0.0066 | 2.3493 | 0.9873 | 2.2835 | 0.3030 | 3.8610 | 0.9755 |
| 73.78 | 3.0160 | 0.0991 | 3.0160 | 0.8830 | 0.0034 | 2.9656 | 0.9895 | 2.9769 | 0.3804 | 3.0321 | 0.9824 |
| Cr(VI) (mg/L) | Intraparticle Diffusion | |||||
|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | |||||
| kd1 (mg/g min0.5) | Duration (min) | R2 | kd2 (mg/g min0.5) | Duration (min) | R2 | |
| 36.53 | 0.0891 | 90 | 0.9712 | 0.0069 | ---- | 0.9879 |
| 55.41 | 0.1462 | 80 | 0.9734 | 0.0158 | ---- | 0.987 |
| 73.78 | 0.1745 | 80 | 0.9762 | 0.0212 | ---- | 0.9978 |
| Cr(VI) (mg/L) | Qe (mg/g) |
|---|---|
| 36.53 | 1.1903 |
| 55.41 | 2.3751 |
| 73.78 | 3.0160 |
| 103.21 | 5.4302 |
| 151.45 | 6.4223 |
| 211.84 | 9.0002 |
| 269.20 | 10.8608 |
| Freundlich Isotherm Model | |
|---|---|
| 1/n | 1.0742 |
| lnKF | 3.5333 |
| KF | 0.0292 |
| R2 | 0.9812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannotti, M.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith. Int. J. Mol. Sci. 2023, 24, 7430. https://doi.org/10.3390/ijms24087430
Zannotti M, Rossi A, Minicucci M, Ferraro S, Petetta L, Giovannetti R. Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith. International Journal of Molecular Sciences. 2023; 24(8):7430. https://doi.org/10.3390/ijms24087430
Chicago/Turabian StyleZannotti, Marco, Andrea Rossi, Marco Minicucci, Stefano Ferraro, Laura Petetta, and Rita Giovannetti. 2023. "Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith" International Journal of Molecular Sciences 24, no. 8: 7430. https://doi.org/10.3390/ijms24087430
APA StyleZannotti, M., Rossi, A., Minicucci, M., Ferraro, S., Petetta, L., & Giovannetti, R. (2023). Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith. International Journal of Molecular Sciences, 24(8), 7430. https://doi.org/10.3390/ijms24087430








