Scandium-44: Diagnostic Feasibility in Tumor-Related Angiogenesis
Abstract
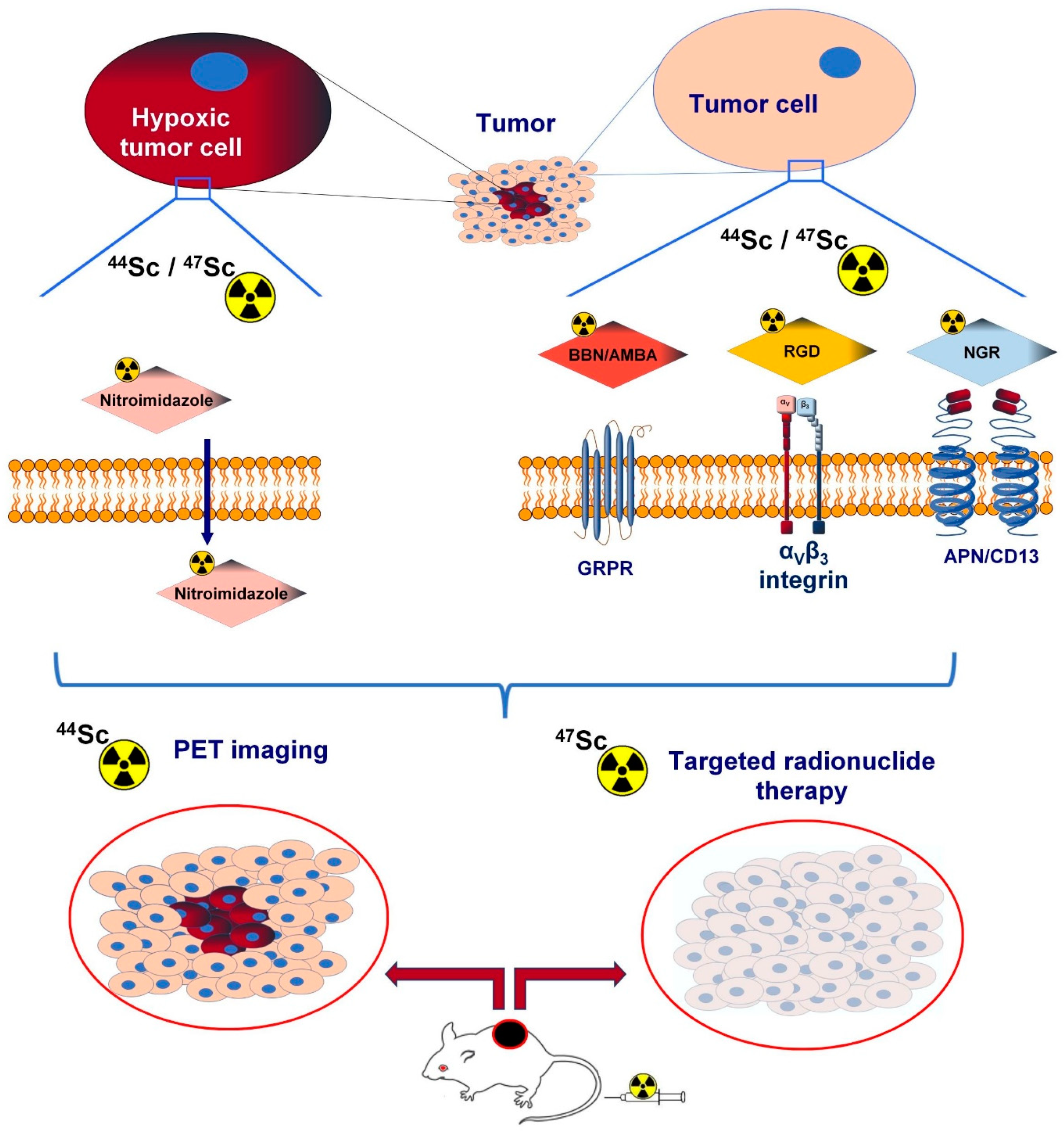
1. Introduction
1.1. Tumour-Related Angiogenesis
1.2. Scandium-44 (44Sc)
1.3. PET Radioisotopes Other Than Scandium-44 (44Sc)
2. Integrin avb3
3. Bombesin- and Gastrin-Releasing Peptide Receptor (GRPR)
4. Hypoxia-Associated 2-Nitroimidazole (NI) Derivatives
5. Vascular Endothelial Growth Factor Receptor (VEGFR)
6. Aminopeptidase N (APN/CD13)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, B. Mechanistic insights on the inhibition of tumor angiogenesis. J. Mol. Med. 2001, 78, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis. In Cancer Medicine, 5th ed.; Holland, J.F., Frei, E., III, Bast, R.C., Jr., Eds.; B.C. Decker: Hamilton, ON, Canada, 2000; pp. 132–152. [Google Scholar]
- Folkman, J. Angiogenesis. In Harrison’s Principles of Internal Medicine, 15th ed.; Braunwald, E., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Jameson, J.L., et al., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 517–530. [Google Scholar]
- Whitelock, J.M.; Murdoch, A.D.; Iozzo, R.V.; Underwood, P.A. The Degradation of Human Endothelial Cell-derived Perlecan and Release of Bound Basic Fibroblast Growth Factor by Stromelysin, Collagenase, Plasmin, and Heparanases. J. Biol. Chem. 1996, 271, 10079–10086. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Asabella, A.N.; Altini, C.; Ferrari, C.; Rubini, G.; Di Palo, A. Multimodality Imaging in Tumor Angiogenesis: Present Status and Perspectives. Int. J. Mol. Sci. 2017, 18, 1864. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Bergsland, E.K. Update on Clinical Trials Targeting Vascular Endothelial Growth Factor in Cancer. Am. J. Health Pharm. 2004, 61, S12–S20. [Google Scholar] [CrossRef]
- Siemann, D.W.; Chaplin, D.J.; Horsman, M. Vascular-targeting therapies for treatment of malignant disease. Cancer 2004, 100, 2491–2499. [Google Scholar] [CrossRef]
- Stacy, M.R.; Maxfield, M.W.; Sinusas, A.J. Targeted molecular imaging of angiogenesis in PET and SPECT: A review. Yale J. Biol. Med. 2012, 85, 75–86. [Google Scholar]
- Huclier-Markai, S.; Alliot, C.; Kerdjoudj, R.; Mougin-Degraef, M.; Chouin, N.; Haddad, F. Promising Scandium Radionuclides for Nuclear Medicine: A Review on the Production and Chemistry up to In Vivo Proofs of Concept. Cancer Biotherapy Radiopharm. 2018, 33, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.A.; Koller, A.J.; Chen, Z.; Ahn, S.H.; Loveless, C.S.; Cingoranelli, S.J.; Yang, Y.; Cirri, A.; Johnson, C.J.; Lapi, S.E.; et al. Homologous Structural, Chemical, and Biological Behavior of Sc and Lu Complexes of the Picaga Bifunctional Chelator: Toward Development of Matched Theranostic Pairs for Radiopharmaceutical Applications. Bioconjugate Chem. 2021, 32, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Snow, M.S.; Foley, A.; Ward, J.L.; Kinlaw, M.T.; Stoner, J.; Carney, K.P. High purity 47Sc production using high-energy photons and natural vanadium targets. Appl. Radiat. Isot. 2021, 178, 109934. [Google Scholar] [CrossRef] [PubMed]
- Türler, A. Matched Pair Theranostics. Chimia 2019, 73, 947–949. [Google Scholar] [CrossRef]
- Hernandez, R.; Valdovinos, H.F.; Yang, Y.; Chakravarty, R.; Hong, H.; Barnhart, T.E.; Cai, W. 44Sc: An Attractive Isotope for Peptide-Based PET Imaging. Mol. Pharm. 2014, 11, 2954–2961. [Google Scholar] [CrossRef]
- Severin, G.; Engle, J.W.; Valdovinos, H.; Barnhart, T.; Nickles, R. Cyclotron produced 44gSc from natural calcium. Appl. Radiat. Isot. 2012, 70, 1526–1530. [Google Scholar] [CrossRef]
- Valdovinos, H.; Hernandez, R.; Barnhart, T.; Graves, S.; Cai, W.; Nickles, R. Separation of cyclotron-produced 44Sc from a natural calcium target using a dipentyl pentylphosphonate functionalized extraction resin. Appl. Radiat. Isot. 2015, 95, 23–29. [Google Scholar] [CrossRef]
- van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751. [Google Scholar] [CrossRef]
- Norman, E.B.; Browne, E.; Chan, Y.D.; Goldman, I.D.; Larimer, R.-M.; Lesko, K.T.; Nelson, M.; Wietfeldt, F.E.; Zlimen, I. Half-life of44Ti. Phys. Rev. C 1998, 57, 2010–2016. [Google Scholar] [CrossRef]
- Roesch, F. Scandium-44: Benefits of a long-lived PET radionuclide available from the (44)Ti/(44)Sc generator system. Curr. Radiopharm. 2012, 5, 187–201. [Google Scholar] [CrossRef]
- Welch, M.J.; McCarthy, T.J. The potential role of generator-produced radiopharmaceuticals in clinical PET. J. Nucl. Med. 2000, 41, 315–317. [Google Scholar] [PubMed]
- Filosofov, D.V.; Loktionova, N.S.; Rösch, F. A 44Ti/44Sc radionuclide generator for potential application of 44Sc-based PET-radiopharmaceuticals. Radiochim. Acta 2010, 98, 149–156. [Google Scholar] [CrossRef]
- García-Toraño, E.; Peyres, V.; Roteta, M.; Sánchez-Cabezudo, A.; Romero, E.; Ortega, A.M. Standardisation and precise determination of the half-life of 44 Sc. Appl. Radiat. Isot. 2016, 109, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI. Radiopharm. Chem. 2021, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, M.; Cussonneau, J.-P.; Matulewicz, T.; Haddad, F. Radionuclide candidates for β+γ coincidence PET: An overview. Appl. Radiat. Isot. 2020, 155, 108898. [Google Scholar] [CrossRef]
- Ferguson, S.; Jans, H.-S.; Wuest, M.; Riauka, T.; Wuest, F. Comparison of scandium-44 g with other PET radionuclides in pre-clinical PET phantom imaging. EJNMMI Phys. 2019, 6, 23. [Google Scholar] [CrossRef]
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Rösch, F.; Baum, R.P. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: On the way to THERANOSTICS. Dalton Trans. 2011, 40, 6104–6111. [Google Scholar] [CrossRef]
- Eppard, E.; de la Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gärtner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical Translation and First In-Human Use of [44Sc]Sc-PSMA-617 for PET Imaging of Metastasized Castrate-Resistant Prostate Cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef]
- Koumarianou, E.; Loktionova, N.; Fellner, M.; Roesch, F.; Thews, O.; Pawlak, D.; Archimandritis, S.; Mikolajczak, R. 44Sc-DOTA-BN[2-14]NH2 in comparison to 68Ga-DOTA-BN[2-14]NH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? Appl. Radiat. Isot. 2012, 70, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Eppard, E. Pre-Therapeutic Dosimetry Employing Scandium-44 for Radiolabeling PSMA-617. In Prostatectomy; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Khawar, A.M.; Eppard, E.; Sinnes, J.P.D.; Roesch, F.; Ahmadzadehfar, H.M.; Kürpig, S.; Meisenheimer, M.M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. [44Sc]Sc-PSMA-617 Biodistribution and Dosimetry in Patients With Metastatic Castration-Resistant Prostate Carcinoma. Clin. Nucl. Med. 2018, 43, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benešová, M.; Schmid, R.M.; Türler, A.; Schibli, R.; van der Meulen, N.P.; Müller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—Preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Deilami-Nezhad, L.; Moghaddam-Banaem, L.; Sadeghi, M. Development of bone seeker radiopharmaceuticals by Scandium-47 and estimation of human absorbed dose. Appl. Radiat. Isot. 2017, 129, 108–116. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Bilewicz, A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J. Inorg. Biochem. 2011, 105, 313–320. [Google Scholar] [CrossRef]
- Viola-Villegas, N.; Doyle, R.P. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid (H4DOTA): Structural overview and analyses on structure–stability relationships. Co-ord. Chem. Rev. 2009, 253, 1906–1925. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with 44Sc-Labeled Cetuximab Fab Fragment. Bioconjugate Chem. 2014, 25, 2197–2204. [Google Scholar] [CrossRef]
- Nagy, G.; Dénes, N.; Kis, A.; Szabó, J.P.; Berényi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsényi, G. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific 68Ga- and 44Sc-labeled DOTA-NAPamide in melanoma imaging. Eur. J. Pharm. Sci. 2017, 106, 336–344. [Google Scholar] [CrossRef]
- Mausner, L.F.; Joshi, V.; Kolsky, K.L. Evaluation of chelating agents for radioimmunotherapy with scandium-47. J. Nucl. Med. 1995, 36. [Google Scholar]
- Kolsky, K.; Joshi, V.; Mausner, L.; Srivastava, S. Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy. Appl. Radiat. Isot. 1998, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.V.M.; Gnesin, S.; Strobel, K.; Pérez, M.D.S.; Roos, J.E.; Müller, C.; van der Meulen, N.P. Fifty Shades of Scandium: Comparative Study of PET Capabilities Using Sc-43 and Sc-44 with Respect to Conventional Clinical Radionuclides. Diagnostics 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; van der Meulen, N.P.; Grubmüller, B.; Klette, I.; Kulkarni, H.R.; Türler, A.; Schibli, R.; Baum, R.P.; Dash, A.; Chakraborty, S.; et al. First-in-Human PET/CT Imaging of Metastatic Neuroendocrine Neoplasms with Cyclotron-Produced 44Sc-DOTATOC: A Proof-of-Concept Study. Cancer Biotherapy Radiopharm. 2017, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.M. Positron emission tomography (PET) imaging with (18)F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2011, 2, 55–76. [Google Scholar] [PubMed]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 1–17. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Richter, S.; Wuest, F. 18F-Labeled Peptides: The Future Is Bright. Molecules 2014, 19, 20536–20556. [Google Scholar] [CrossRef]
- Asti, M.; De Pietri, G.; Fraternali, A.; Grassi, E.; Sghedoni, R.; Fioroni, F.; Roesch, F.; Versari, A.; Salvo, D. Validation of 68Ge/68Ga generator processing by chemical purification for routine clinical application of 68Ga-DOTATOC. Nucl. Med. Biol. 2008, 35, 721–724. [Google Scholar] [CrossRef]
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 53–63. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Reber, J.; Fischer, C.; Zhernosekov, K.; Türler, A.; Schibli, R. Promises of Cyclotron-Produced 44Sc as a Diagnostic Match for Trivalent β−-Emitters: In Vitro and In Vivo Study of a 44Sc-DOTA-Folate Conjugate. J. Nucl. Med. 2013, 54, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.; de Blois, E.; Chan, H.S.; Konijnenberg, M.; Kwekkeboom, D.J.; Krenning, E.P. 68Ga-labeled DOTA-Peptides and 68Ga-labeled Radiopharmaceuticals for Positron Emission Tomography: Current Status of Research, Clinical Applications, and Future Perspectives. Semin. Nucl. Med. 2011, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-Octreotide PET in Neuroendocrine Tumors: Comparison with Somatostatin Receptor Scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Roesch, F.; Riss, P.J. The Renaissance of the 68Ge/68Ga Radionuclide Generator Initiates New Developments in 68Ga Radiopharmaceutical Chemistry. Curr. Top. Med. Chem. 2010, 10, 1633–1668. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Janecka, A. Synthesis of Target-Specific Radiolabeled Peptides for Diagnostic Imaging. Bioconjugate Chem. 2003, 14, 3–17. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical and Clinical Research. Cancer Biotherapy Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- Holland, J.P.; Ferdani, R.; Anderson, C.J.; Lewis, J.S. Copper-64 Radiopharmaceuticals for Oncologic Imaging. PET Clin. 2009, 4, 49–67. [Google Scholar] [CrossRef]
- Walczak, R.; Krajewski, S.; Szkliniarz, K.; Sitarz, M.; Abbas, K.; Choiński, J.; Jakubowski, A.; Jastrzębski, J.; Majkowska, A.; Simonelli, F.; et al. Cyclotron production of 43Sc for PET imaging. EJNMMI Phys. 2015, 2, 33. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Cai, W. PET Tracers Based on Zirconium-89. Curr. Radiopharm. 2011, 4, 131–139. [Google Scholar] [CrossRef]
- de Ruijter, L.K.; Hooiveld-Noeken, J.S.; Giesen, D.; Hooge, M.N.L.-D.; Kok, I.C.; Brouwers, A.H.; Elias, S.G.; Nguyen, M.T.; Lu, H.; Gietema, J.A.; et al. First-in-Human Study of the Biodistribution and Pharmacokinetics of 89Zr-CX-072, a Novel Immunopet Tracer Based on an Anti–PD-L1 Probody. Clin. Cancer Res. 2021, 27, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Mulgaonkar, A.; Elias, R.; Woolford, L.; Guan, B.; Nham, K.; Kapur, P.; Christie, A.; Tcheuyap, V.T.; Singla, N.; Bowman, I.A.; et al. ImmunoPET Imaging with 89Zr-Labeled Atezolizumab Enables In Vivo Evaluation of PD-L1 in Tumorgraft Models of Renal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and Characterization of Clinical-Grade 89Zr-Trastuzumab for HER2/neu ImmunoPET Imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef]
- Nagengast, W.B.; De Vries, E.G.; Hospers, G.A.; Mulder, N.H.; De Jong, J.R.; Hollema, H.; Brouwers, A.H.; Van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In Vivo VEGF Imaging with Radiolabeled Bevacizumab in a Human Ovarian Tumor Xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Perk, L.R.; Visser, G.W.M.; Vosjan, M.J.W.D.; Walsum, M.S.-V.; Tijink, B.M.; Leemans, C.R.; van Dongen, G.A.M.S. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 2005, 46, 1898–1906. [Google Scholar]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2012, 40, 3–14. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.M.; Boellaard, R.; Boerman, O.C.; Van Eerd, J.; Snow, G.B.; A Lammertsma, A.; van Dongen, G.A.M.S. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J. Nucl. Med. 2003, 44, 1663–1670. [Google Scholar]
- Zeglis, B.M.; Houghton, J.L.; Evans, M.J.; Viola, N.; Lewis, J.S. Underscoring the Influence of Inorganic Chemistry on Nuclear Imaging with Radiometals. Inorg. Chem. 2014, 53, 1880–1899. [Google Scholar] [CrossRef]
- Alzimami, K.S.; Ma, A.K. Effective dose to staff members in a positron emission tomography/CT facility using zirconium-89. Br. J. Radiol. 2013, 86, 20130318. [Google Scholar] [CrossRef]
- Vugts, D.J.; van Dongen, G.A. 89Zr-labeled compounds for PET imaging guided personalized therapy. Drug Discov. Today Technol. 2011, 8, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Kálmán-Szabó, I.; Szabó, J.P.; Arató, V.; Dénes, N.; Opposits, G.; Jószai, I.; Kertész, I.; Képes, Z.; Fekete, A.; Szikra, D.; et al. PET Probes for Preclinical Imaging of GRPR-Positive Prostate Cancer: Comparative Preclinical Study of [68Ga]Ga-NODAGA-AMBA and [44Sc]Sc-NODAGA-AMBA. Int. J. Mol. Sci. 2022, 23, 10061. [Google Scholar] [CrossRef] [PubMed]
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; van der Meulen, N.P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI. Radiopharm. Chem. 2017, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Szikra, D.; Trencsényi, G.; Fekete, A.; Garai, I.; Giani, A.M.; Negri, R.; Masciocchi, N.; Maiocchi, A.; Uggeri, F.; et al. AAZTA: An Ideal Chelating Agent for the Development of 44 Sc PET Imaging Agents. Angew. Chem. Int. Ed. 2017, 56, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Szücs, D.; Csupász, T.; Szabó, J.P.; Kis, A.; Gyuricza, B.; Arató, V.; Forgács, V.; Vágner, A.; Nagy, G.; Garai, I.; et al. Synthesis, Physicochemical, Labeling and In Vivo Characterization of 44Sc-Labeled DO3AM-NI as a Hypoxia-Sensitive PET Probe. Pharmaceuticals 2022, 15, 666. [Google Scholar] [CrossRef]
- Masłowska, K.; Redkiewicz, P.; Halik, P.K.; Witkowska, E.; Tymecka, D.; Walczak, R.; Choiński, J.; Misicka, A.; Gniazdowska, E. Scandium-44 Radiolabeled Peptide and Peptidomimetic Conjugates Targeting Neuropilin-1 Co-Receptor as Potential Tools for Cancer Diagnosis and Anti-Angiogenic Therapy. Biomedicines 2023, 11, 564. [Google Scholar] [CrossRef]
- Wilder, R.L. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 2002, 61 (Suppl. S2), 96–99. [Google Scholar] [CrossRef]
- Eble, J.A.; Haier, J.A.E.A.J. Integrins in Cancer Treatment. Curr. Cancer Drug Targets 2006, 6, 89–105. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Chen, X. Integrin αvβ3-targeted cancer therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef]
- Kumar, C.C. Integrin αvβ3 as a Therapeutic Target for Blocking Tumor-Induced Angiogenesis. Curr. Drug Targets 2003, 4, 123–131. [Google Scholar] [CrossRef]
- A Schwartz, M. Integrin signaling revisited. Trends Cell Biol. 2001, 11, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Glaser, M.; Morrison, M.; Solbakken, M.; Arukwe, J.; Karlsen, H.; Wiggen, U.; Champion, S.; Kindberg, G.M.; Cuthbertson, A. Radiosynthesis and Biodistribution of Cyclic RGD Peptides Conjugated with Novel [18F]Fluorinated Aldehyde-Containing Prosthetic Groups. Bioconjugate Chem. 2008, 19, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Weber, W.A.; Beer, A.J.; Vabuliene, E.; Reim, D.; Sarbia, M.; Becker, K.-F.; Goebel, M.; Hein, R.; Wester, H.-J.; et al. Noninvasive Visualization of the Activated αvβ3 Integrin in Cancer Patients by Positron Emission Tomography and [18F]Galacto-RGD. PLoS Med. 2005, 2, e70. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Walsh, J.; Walsh, J.; Chen, G.; Gangadharmath, U.; Kasi, D.; Scott, P.; Haka, M.; Collier, T.; Padgett, H. Synthesis of an 18F-labeled RGD peptide for imaging αvβ3 integrin expression in vivo. J. Nucl. Med. 2009, 50, 1939. [Google Scholar]
- Lang, L.; Li, W.; Guo, N.; Ma, Y.; Zhu, L.; Kiesewetter, D.O.; Shen, B.; Niu, G.; Chen, X. Comparison Study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET Imaging of U87MG Tumors in Mice. Bioconjugate Chem. 2011, 22, 2415–2422. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Chen, K.; Yan, Y.; Watzlowik, P.; Wester, H.-J.; Chin, F.T.; Chen, X. 18F-Labeled Galacto and PEGylated RGD Dimers for PET Imaging of αvβ3 Integrin Expression. Mol. Imaging Biol. 2010, 12, 530–538. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S.; Kang, K.W.; Lee, H.-Y.; Han, S.-W.; Kim, T.-Y.; Lee, Y.-S.; Jeong, J.M.; Lee, D.S. Whole-Body Distribution and Radiation Dosimetry of68Ga-NOTA-RGD, a Positron Emission Tomography Agent for Angiogenesis Imaging. Cancer Biotherapy Radiopharm. 2012, 27, 65–71. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, N.; Zhang, J.; Lang, L.; Zhang, W.; Li, S.; Zhao, J.; Niu, G.; Li, F.; Zhu, Z.; et al. 68Ga-NOTA-PRGD2 PET/CT for Integrin Imaging in Patients with Lung Cancer. J. Nucl. Med. 2015, 56, 1823–1827. [Google Scholar] [CrossRef]
- Liapis, H.; Flath, A.; Kitazawa, S. Integrin αvβ3 Expression by Bone-residing Breast Cancer Metastases. Diagn. Mol. Pathol. 1996, 5, 127–135. [Google Scholar] [CrossRef]
- Ghiani, S.; Hawala, I.; Szikra, D.; Trencsényi, G.; Baranyai, Z.; Nagy, G.; Vágner, A.; Stefania, R.; Pandey, S.; Maiocchi, A. Synthesis, radiolabeling, and pre-clinical evaluation of [44Sc]Sc-AAZTA conjugate PSMA inhibitor, a new tracer for high-efficiency imaging of prostate cancer. Eur. J. Nucl. Med. 2021, 48, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; Iori, M.; Capponi, P.C.; Atti, G.; Rubagotti, S.; Martin, R.; Brennauer, A.; Müller, M.; Bergmann, R.; Erba, P.A.; et al. Influence of different chelators on the radiochemical properties of a 68-Gallium labelled bombesin analogue. Nucl. Med. Biol. 2014, 41, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Bartholdi, M.F.; Wu, J.M.; Pu, H.; Troncoso, P.; Eden, P.A.; Feldman, R.I. In situ hybridization for gastrin-releasing peptide receptor (GRP receptor) expression in prostatic carcinoma. Int. J. Cancer 1998, 79, 82–90. [Google Scholar] [CrossRef]
- Chave, H.S.; Gough, A.C.; Palmer, K.; Preston, S.R.; Primrose, J.N. Bombesin family receptor and ligand gene expression in human colorectal cancer and normal mucosa. Br. J. Cancer 2000, 82, 124–130. [Google Scholar] [CrossRef]
- Fleischmann, A.; Läderach, U.; Friess, H.; Buechler, M.W.; Reubi, J.C. Bombesin Receptors in Distinct Tissue Compartments of Human Pancreatic Diseases. Lab. Investig. 2000, 80, 1807–1817. [Google Scholar] [CrossRef]
- Halmos, G.; Wittliff, J.L.; Schally, A.V. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Cancer Res. 1995, 55, 280–287. [Google Scholar]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- Mattei, J.; Achcar, R.D.; Cano, C.H.; Macedo, B.R.; Meurer, L.; Batlle, B.S.; Groshong, S.D.; Kulczynski, J.M.; Roesler, R.; Lago, L.D.; et al. Gastrin-Releasing Peptide Receptor Expression in Lung Cancer. Arch. Pathol. Lab. Med. 2014, 138, 98–104. [Google Scholar] [CrossRef]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Fleischmann, A.; Waser, B.; Reubi, J.C. Overexpression of Gastrin-Releasing Peptide Receptors in Tumor-Associated Blood Vessels of Human Ovarian Neoplasms. Anal. Cell. Pathol. 2007, 29, 421–433. [Google Scholar] [CrossRef]
- Elshafae, S.M.; Hassan, B.B.; Supsavhad, W.; Dirksen, W.P.; Camiener, R.Y.; Ding, H.; Tweedle, M.F.; Rosol, T.J. Gastrin-releasing peptide receptor (GRPr) promotes EMT, growth, and invasion in canine prostate cancer. Prostate 2016, 76, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T.; Moody, T.; Pert, C.; Rivier, J.E.; Gardner, J.D. Interaction of bombesin and litorin with specific membrane receptors on pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 1978, 75, 6139–6143. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Freitas, V.L.; Thomaz, L.D.G.R.; Simoneti, L.E.L.; Malfitano, C.; De Angelis, K.; Ulbrich, J.M.; Schwartsmann, G.; Andrade, C.F. RC-3095, a Selective Gastrin-Releasing Peptide Receptor Antagonist, Does Not Protect the Lungs in an Experimental Model of Lung Ischemia-Reperfusion Injury. Biomed. Res. Int. 2015, 2015, 496378. [Google Scholar] [CrossRef] [PubMed]
- Bajo, A.M.; Schally, A.V.; Groot, K.; Szepeshazi, K. Bombesin antagonists inhibit proangiogenic factors in human experimental breast cancers. Br. J. Cancer 2004, 90, 245–252. [Google Scholar] [CrossRef]
- Heuser, M.; Schlott, T.; Schally, A.; Kahler, E.; Schliephake, R.; Laabs, S.; Hemmerlein, B. Expression of gastrin releasing peptide receptor in renal cell carcinomas: A potential function for the regulation of neoangiogenesis and microvascular perfusion. J. Urol. 2005, 173, 2154–2159. [Google Scholar] [CrossRef]
- Kanashiro, C.A.; Schally, A.; Cai, R.-Z.; Halmos, G. Antagonists of bombesin/gastrin-releasing peptide decrease the expression of angiogenic and anti-apoptotic factors in human glioblastoma. Anti-Cancer Drugs 2005, 16, 159–165. [Google Scholar] [CrossRef]
- Levine, L.; Lucci, J.A., III; Pazdrak, B.; Cheng, J.Z.; Guo, Y.S.; Townsend, C.M., Jr.; Hellmich, M.R. Bombesin stimulates nuclear factor KB activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 2003, 63, 3495–3502. [Google Scholar]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: Internalization of receptor bound125I-(Tyr4) bombesin by tumor cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Kang, J.; Ishola, T.A.; Baregamian, N.; Mourot, J.M.; Rychahou, P.G.; Evers, B.M.; Chung, D.H. Bombesin induces angiogenesis and neuroblastoma growth. Cancer Lett. 2007, 253, 273–281. [Google Scholar] [CrossRef]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular Function of Phosphoinositide 3-Kinases: Implications for Development, Immunity, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef] [PubMed]
- E Lantry, L.; Cappelletti, E.; Maddalena, M.E.; Fox, J.S.; Feng, W.; Chen, J.; Thomas, R.; Eaton, S.M.; Bogdan, N.J.; Arunachalam, T.; et al. 177Lu-AMBA: Synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J. Nucl. Med. 2006, 47, 1144–1152. [Google Scholar]
- Liu, Y.; Hu, X.; Liu, H.; Bu, L.; Ma, X.; Cheng, K.; Li, J.; Tian, M.; Zhang, H.; Cheng, Z. A Comparative Study of Radiolabeled Bombesin Analogs for the PET Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.P.J.; Müller, C.; Reneman, S.; Melis, M.L.; Breeman, W.A.P.; de Blois, E.; Bangma, C.H.; Krenning, E.P.; van Weerden, W.M.; de Jong, M. A standardised study to compare prostate cancer targeting efficacy of five radiolabelled bombesin analogues. Eur. J. Nucl. Med. 2010, 37, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Bologna, M.; Festuccia, C.; Muzi, P.; Biordi, L.; Ciomei, M. Bombesin Stimulates Growth of Human Prostatic Cancer Cells in Vitro. Cancer 1989, 63, 1714–1720. [Google Scholar] [CrossRef]
- Kilgore, W.; Mantyh, P.; Mantyh, C.; McVey, D.; Vigna, S. Bombesin/GRP-preferring and neuromedin B-preferring receptors in the rat urogenital system. Neuropeptides 1993, 24, 43–52. [Google Scholar] [CrossRef]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian Bombesin Receptors: Nomenclature, Distribution, Pharmacology, Signaling, and Functions in Normal and Disease States. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef]
- Johnson, D.E.; Georgieff, M.K. Pulmonary Neuroendocrine Cells: Their Secretory Products and Their Potential Roles in Health and Chronic Lung Disease in Infancy. Am. Rev. Respir. Dis. 1989, 140, 1807–1812. [Google Scholar] [CrossRef]
- Weber, H.C. Regulation and signaling of human bombesin receptors and their biological effects. Curr. Opin. Endocrinol. Diabetes 2009, 16, 66–71. [Google Scholar] [CrossRef]
- Smith, C.; Volkert, W.; Hoffman, T. Gastrin releasing peptide (GRP) receptor targeted radiopharmaceuticals: A concise update. Nucl. Med. Biol. 2003, 30, 861–868. [Google Scholar] [CrossRef]
- Barthel, H.; Wilson, H.; Collingridge, D.R.; Brown, G.; Osman, S.; Luthra, S.K.; Brady, F.; Workman, P.; Price, P.M.; Aboagye, E. In vivo evaluation of [18F]fluoroetanidazole as a new marker for imaging tumour hypoxia with positron emission tomography. Br. J. Cancer 2004, 90, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Wallace, S.; Cherif, A.; Li, C.; Gretzer, M.B.; E Kim, E.; A Podoloff, D. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology 1995, 194, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Ziemer, L.; Evans, S.; Kachur, A.; Shuman, A.; Cardi, C.; Jenkins, W.; Karp, J.; Alavi, A.; Dolbier, W.; Koch, C. Noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18F-EF5. Eur. J. Nucl. Med. 2003, 30, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C.A. The Concentration of Oxygen Dissolved in Tissues at the Time of Irradiation as a Factor in Radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and tumor progression: Pathophysiology and therapeutics. Trends Mol. Med. 2002, 8, S62–S67. [Google Scholar] [CrossRef]
- Sun, X.; Niu, G.; Chan, N.; Shen, B.; Chen, X. Tumor Hypoxia Imaging. Mol. Imaging Biol. 2011, 13, 399–410. [Google Scholar] [CrossRef]
- Koh, W.-J.; Rasey, J.S.; Evans, M.L.; Grierson, J.R.; Lewellen, T.K.; Graham, M.; Krohn, K.A.; Griffin, T.W. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int. J. Radiat. Oncol. 1992, 22, 199–212. [Google Scholar] [CrossRef]
- Reischl, G.; Ehrlichmann, W.; Bieg, C.; Solbach, C.; Kumar, P.; Wiebe, L.I.; Machulla, H.-J. Preparation of the hypoxia imaging PET tracer [18F]FAZA: Reaction parameters and automation. Appl. Radiat. Isot. 2005, 62, 897–901. [Google Scholar] [CrossRef]
- Hoigebazar, L.; Jeong, J.M.; Hong, M.K.; Kim, Y.J.; Lee, J.Y.; Shetty, D.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Lee, M.C. Synthesis of 68Ga-labeled DOTA-nitroimidazole derivatives and their feasibilities as hypoxia imaging PET tracers. Bioorganic Med. Chem. 2011, 19, 2176–2181. [Google Scholar] [CrossRef]
- Cherif, A.; Wallace, S.; Yang, D.; Newman, R.; Harrod, V.; Nornoo, A.; Inoue, T.; Kim, C.; Kuang, L.-R.; Kim, E.; et al. Development of new markers for hypoxic cells:131I]Iodomisonidazole and [131I]Iodoerythronitroimidazole. J. Drug Target. 1996, 4, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-S.; Chou, T.-K.; Chang, C.-H.; Wu, C.-Y.; Chang, C.-W.; Chang, T.-J.; Wang, S.-J.; Lin, W.-J.; Wang, H.-E. Biodistribution, pharmacokinetics and PET Imaging of [18F]FMISO, [18F]FDG and [18F]FAc in a sarcoma- and inflammation-bearing mouse model. Nucl. Med. Biol. 2009, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, H.; Lin, X.; Chu, T.; Luo, R.; Wang, Y.; Yang, Z. Synthesis and radiolabeling of 64Cu-labeled 2-nitroimidazole derivative 64Cu-BMS2P2 for hypoxia imaging. Bioorganic Med. Chem. Lett. 2016, 26, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Nitroimidazole radiopharmaceuticals in bioimaging: Part I: Synthesis and imaging applications. Curr. Radiopharm. 2011, 4, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Hoigebazar, L.; Jeong, J.M.; Choi, S.Y.; Choi, J.Y.; Shetty, D.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Lee, M.C.; Chung, Y.K. Synthesis and Characterization of Nitroimidazole Derivatives for 68Ga-Labeling and Testing in Tumor Xenografted Mice. J. Med. Chem. 2010, 53, 6378–6385. [Google Scholar] [CrossRef] [PubMed]
- Seelam, S.R.; Lee, J.Y.; Lee, Y.-S.; Hong, M.K.; Kim, Y.J.; Banka, V.K.; Lee, D.S.; Chung, J.-K.; Jeong, J.M. Development of 68Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Bioorganic Med. Chem. 2015, 23, 7743–7750. [Google Scholar] [CrossRef]
- Jussila, L.; Alitalo, K. Vascular Growth Factors and Lymphangiogenesis. Physiol. Rev. 2002, 82, 673–700. [Google Scholar] [CrossRef]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef]
- Djordjevic, S.; Driscoll, P.C. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov. Today 2013, 18, 447–455. [Google Scholar] [CrossRef]
- E Bachelder, R.; Crago, A.; Chung, J.; A Wendt, M.; Shaw, L.M.; Robinson, G.; Mercurio, A.M. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001, 61, 5736–5740. [Google Scholar]
- Cao, Y.; Wang, L.; Nandy, D.; Zhang, Y.; Basu, A.; Radisky, D.; Mukhopadhyay, D. Neuropilin-1 Upholds Dedifferentiation and Propagation Phenotypes of Renal Cell Carcinoma Cells by Activating Akt and Sonic Hedgehog Axes. Cancer Res 2008, 68, 8667–8672. [Google Scholar] [CrossRef] [PubMed]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J., Jr.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF–VEGFR2–Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-M.; Chen, Y.-L.; Wu, Y.-Y.; Yuan, A.; Chao, Y.-C.; Chung, Y.-C.; Wu, M.-H.; Yang, S.-C.; Pan, S.-H.; Shih, J.-Y.; et al. Targeting Neuropilin 1 as an Antitumor Strategy in Lung Cancer. Clin. Cancer Res. 2007, 13, 4759–4768. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Bai, Y.; Zhu, Q.; Hu, B.; Xu, Y. Targeting VEGF–neuropilin interactions: A promising antitumor strategy. Drug Discov. Today 2019, 24, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wickström, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef]
- Ito, S.; Miyahara, R.; Takahashi, R.; Nagai, S.; Takenaka, K.; Wada, H.; Tanaka, F. Stromal aminopeptidase N expression: Correlation with angiogenesis in non-small-cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2009, 57, 591–598. [Google Scholar] [CrossRef]
- Sledge, G.W.; Rugo, H.S.; Burstein, H.J. The role of angiogenesis inhibition in the treatment of breast cancer. Clin. Adv. Hematol. Oncol. 2006, 4, 1–10. [Google Scholar]
- Wang, X.; Niu, Z.; Jia, Y.; Cui, M.; Han, L.; Zhang, Y.; Liu, Z.; Bi, D.; Liu, S. Ubenimex inhibits cell proliferation, migration and invasion by inhibiting the expression of APN and inducing autophagic cell death in prostate cancer cells. Oncol. Rep. 2016, 35, 2121–2130. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar]
- Corti, A. Tumor Vasculature Targeting Through NGR Peptide-Based Drug Delivery Systems. Curr. Pharm. Biotechnol. 2011, 12, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, I.; Van de Vijver, P.; Dirksen, A.; Hackeng, T.M. Synthesis and application of cNGR-containing imaging agents for detection of angiogenesis. Bioorganic Med. Chem. 2013, 21, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, Z.; Li, X.; Wei, H.; Chen, Y. Research Progress of Radiolabeled Asn-Gly-Arg (NGR) Peptides for Imaging and Therapy. Mol. Imaging 2020, 19, 1536012120934957. [Google Scholar] [CrossRef] [PubMed]
- Gyuricza, B.; Szabó, J.P.; Arató, V.; Dénes, N.; Szűcs, Á.; Berta, K.; Kis, A.; Szücs, D.; Forgács, V.; Szikra, D.; et al. Synthesis of 68Ga-Labeled cNGR-Based Glycopeptides and In Vivo Evaluation by PET Imaging. Pharmaceutics 2021, 13, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Dénes, N.; Szabó, J.P.; Arató, V.; Jószai, I.; Enyedi, K.N.; Lakatos, S.; Garai, I.; Mező, G.; Kertész, I.; et al. In vivo assessment of aminopeptidase N (APN/CD13) specificity of different 68Ga-labelled NGR derivatives using PET/MRI imaging. Int. J. Pharm. 2020, 589, 119881. [Google Scholar] [CrossRef] [PubMed]
- Máté, G.; Kertész, I.; Enyedi, K.N.; Mező, G.; Angyal, J.; Vasas, N.; Kis, A.; Szabó, É.; Emri, M.; Bíró, T.; et al. In vivo imaging of Aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer 68Ga-NOTA-c(NGR). Eur. J. Pharm. Sci. 2015, 69, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Zong, S.; Wang, J.; Conti, P.S.; Chen, K. MicroPET Imaging of CD13 Expression Using a 64Cu-Labeled Dimeric NGR Peptide Based on Sarcophagine Cage. Mol. Pharm. 2014, 11, 3938–3946. [Google Scholar] [CrossRef]
- Ma, W.; Shao, Y.; Yang, W.; Li, G.; Zhang, Y.; Zhang, M.; Zuo, C.; Chen, K.; Wang, J. Evaluation of 188Re-labeled NGR–VEGI protein for radioimaging and radiotherapy in mice bearing human fibrosarcoma HT-1080 xenografts. Tumor Biol. 2016, 37, 9121–9129. [Google Scholar] [CrossRef]
- Vats, K.; Satpati, A.K.; Sharma, R.; Sarma, H.D.; Satpati, D.; Dash, A. 177 Lu-labeled cyclic Asn-Gly-Arg peptide tagged carbon nanospheres as tumor targeting radio-nanoprobes. J. Pharm. Biomed. Anal. 2018, 152, 173–178. [Google Scholar] [CrossRef]
| Investigated Object | Investigated Phenomenon | Target Molecule | (Radio) Labelled Vector | Imaging Technique | Reference |
|---|---|---|---|---|---|
| PCa PC-3 and HaCaT cell lines | In vitro receptor-binding affinity utilizing in vitro blocking studies with BBN | GRPR | [44Sc]Sc-NODAGA-AMBA and [68Ga]Ga-NODAGA-AMBA, | In vitro gamma counter measurements %ID/million cells units | [75] |
| PCa PC-3 tumor-bearing CB17 SCID mice and healthy control | In vitro and in vivo biodistribution pattern, tumor-targeting capability based on blocking experiments | GRPR | [44Sc]Sc-NODAGA-AMBA and [68Ga]Ga-NODAGA-AMBA | In vivo miniPET imaging, ex vivo radioactivity determination by gamma counter (%ID/g), in vivo and ex vivo blocking studies with BBN | [75] |
| PC-3 cell line | In vitro receptor-binding affinity with blocking studies applying [125I-Tyr4]-BN | GRPR | natSc-DOTA-BN[2-14]NH2 and natGa-DOTA-BN[2-14]NH2 | Competitive displacement cell-binding assay | [33] |
| Healthy male Sprague-Dawley rats, male Copenhagen rats bearing androgen-independent Dunning R-3327-AT-1 prostate cancer tumour | In vivo and ex vivo organ distribution, GRPR-targeting ability applying blocking studies with BBN | GRPR | 44Sc-DOTA-BN[2-14]NH2 and 68Ga-DOTA-BN[2-14]NH2 | In vivo dynamic microPET imaging (in tumourous rats), ex vivo (in healthy rats) radioactivity calculations with a dose calibrator (% ID/g) | [33] |
| U87MG and AR42J tumour-bearing female athymic nude mice (CD-1 nude) | Evaluation of in vitro and in vivo behaviour, in vivo and ex vivo biodistribution | αvβ3 integrin | [44Sc]Sc-DOTA-RGD, [44Sc]Sc-NODAGA-RGD, [68Ga]Ga-DOTA-RGD, [68Ga]Ga-NODAGA-RGD | In vivo PET/CT acquisition, ex vivo gamma counting (%IA/g) | [76] |
| 4T1 tumor-bearing BALB/c mice and healthy control | applicability of chelator AAZTA, in vivo biodistribution | αvβ3 integrin | 44Sc3+, and 44Sc(AAZTA)− and 44Sc(CNAAZTA-c(RGDfK) | in vivo PET/MRI examinations | [77] |
| U87MG cells | In vitro receptor-binding affinity and specificity with blocking studies using 125I-Echistatin | αvβ3 integrin | cRGD, (cRGD)2, and DOTA-(cRGD)2 | In vitro competitive cell-binding assay, gamma counter-based detection of the tracer concentration | [17] |
| U87MG glioblastoma-bearing female athymic nude mice | Tumour-targeting competence, specificity applying in vivo and ex vivo receptor blocking with c(RGD)2, in vivo and ex vivo biodistribution | αvβ3 integrin | [44Sc]Sc-DOTA-c(RGD)2 | In vivo microPET/microCT imaging, ex vivo radioactivity determination (%ID/g) | [17] |
| CB17 SCID adult male mice bearing KB tumours and healthy control mice | Synthesis procedure, comparison of 44Sc- and 68Ga-labelled derivatives, in vivo and ex vivo biodistribution | Hypoxia | [44Sc]Sc-DO3AM-NI and [68Ga]Ga- DO3AM-NI | In vivo PET/MRI studies, ex vivo radiopharmaceutical uptake measurement %ID/g | [78] |
| Pro-angiogenic VEGF-A165/NRP-1 complex formation | Investigation of physicochemical properties and affinity for NRP-1 | NRP-1 | 44Sc-radiocompounds (44Sc-1, 44Sc-1bis, 44Sc-2, 44Sc-3):Sc-DOTA-Ahx-A7R (Sc-1), Lys(DOTA-Sc)-A7R (Sc-1bis), Lys(hArg)-Dab(Ahx-DOTA-Sc)-Pro-Arg (Sc-2) and DR7A-DLys(DOTA-Sc) (Sc-3) | Competitive ELISA test | [79] |
| 18F | 68Ga | 64Cu | 43Sc | 44Sc | 89Zr | |
|---|---|---|---|---|---|---|
| Half-life (h) | 1.83 | 1.13 | 12.7 | 3.89 | 3.97 | 78.41 |
| Decay method (%) | EC (3) ß+ (97) | EC (11.1) ß+ (88.9) | EC (43.9) ß+ (17.6) ß− (38.5) | EC (12) ß+ (88) | EC (5.7) ß+ (94.3) | EC (77.3) ß+ (22.7) |
| β+ endpoint energy, keV (%) | 633.5 (96.7%) | 1899 (87.7) 822 (1.2) | 653 (17.6) | 1200 (70.9) 826 (17.2) | 1474 (94.3) | 902 (22.8) |
| Principal γ energies, keV (Abs.%) | 511 (194) | 511 (177.8) 1077 (3.2) 1261 (0.1) 1883 (0.1) | 511 (35.2) 1346 (0.5) | 372.8 (23) | 511 (188.5) 1157 (99.9) 1499 (0.9) | 511 (45.5) 909 (99.0) 1713 (0.7) 1745 (0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trencsényi, G.; Képes, Z. Scandium-44: Diagnostic Feasibility in Tumor-Related Angiogenesis. Int. J. Mol. Sci. 2023, 24, 7400. https://doi.org/10.3390/ijms24087400
Trencsényi G, Képes Z. Scandium-44: Diagnostic Feasibility in Tumor-Related Angiogenesis. International Journal of Molecular Sciences. 2023; 24(8):7400. https://doi.org/10.3390/ijms24087400
Chicago/Turabian StyleTrencsényi, György, and Zita Képes. 2023. "Scandium-44: Diagnostic Feasibility in Tumor-Related Angiogenesis" International Journal of Molecular Sciences 24, no. 8: 7400. https://doi.org/10.3390/ijms24087400
APA StyleTrencsényi, G., & Képes, Z. (2023). Scandium-44: Diagnostic Feasibility in Tumor-Related Angiogenesis. International Journal of Molecular Sciences, 24(8), 7400. https://doi.org/10.3390/ijms24087400







