Sweat Testing for the Detection of Methylone after Controlled Administrations in Humans
Abstract
1. Introduction
- -
- to develop and validate an LC-MS/MS method for the determination of methylone and its metabolites, MDC and HMMC, in sweat;
- -
- to investigate the excretion of methylone, MDC and HMMC in sweat after the ingestion of increasing controlled doses (50, 100, 150 and 200 mg) to 12 healthy volunteers involved in a clinical trial.
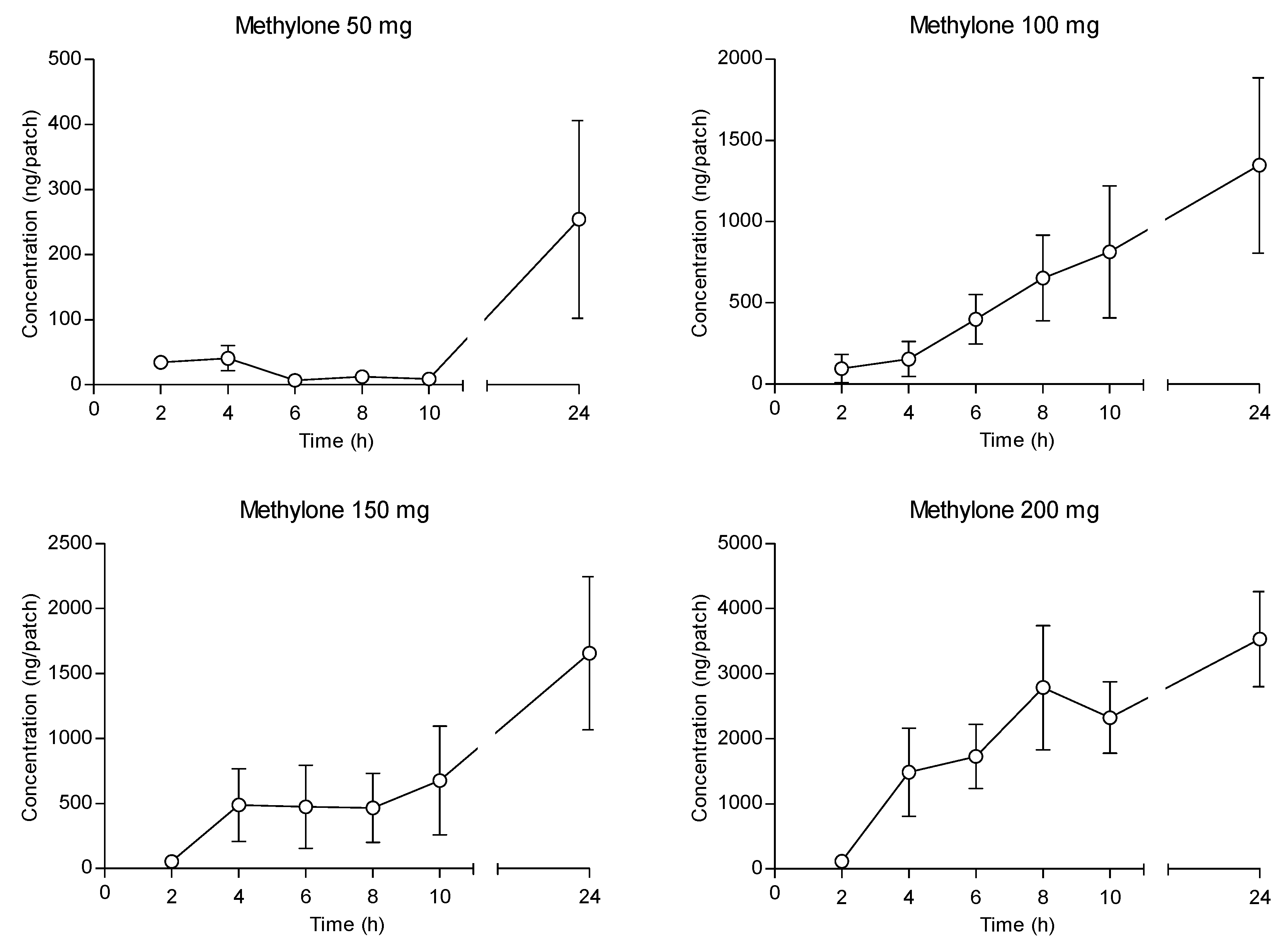
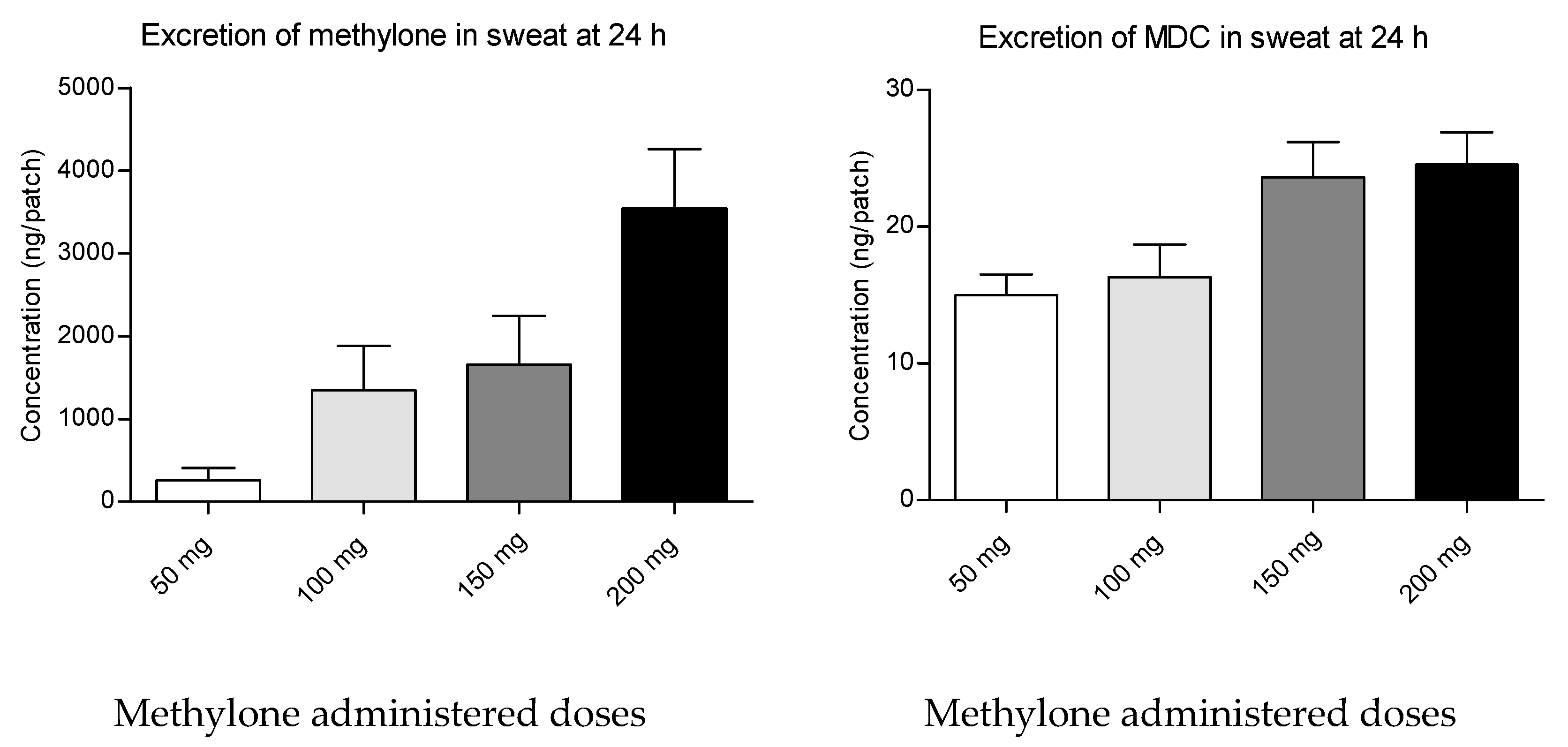
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Chemicals
4.3. Sweat Sample Collection
4.4. Sample Preparation and Analyte Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaami, S.; Giorgetti, R.; Pichini, S.; Pantano, F.; Marinelli, E.; Busardò, F.P. Synthetic Cathinones Related Fatalities: An Update. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Lo Faro, A.F.; Berardinelli, D.; Sprega, G.; Malaca, S.; Pichini, S.; Huestis, M.A.; Papaseit, E.; Pérez-Mañá, C.; Busardò, F.P.; et al. Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans. Int. J. Mol. Sci. 2022, 23, 14636. [Google Scholar] [CrossRef] [PubMed]
- Minaeva, V.; Karaush-Karmazin, N.; Panchenko, O.; Minaev, B.; Ågren, H. Hirshfeld and AIM Analysis of the Methylone Hydrochloride Crystal Structure and Its Impact on the IR Spectrum Combined with DFT Study. Crystals 2023, 13, 383. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P.; Ruoho, A.E. Inhibition of Plasma Membrane Monoamine Transporters by Beta-Ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef]
- Nagai, F.; Nonaka, R.; Satoh Hisashi Kamimura, K. The Effects of Non-Medically Used Psychoactive Drugs on Monoamine Neurotransmission in Rat Brain. Eur. J. Pharmacol. 2007, 559, 132–137. [Google Scholar] [CrossRef]
- Piao, Y.-S.; Hall, F.S.; Moriya, Y.; Ito, M.; Ohara, A.; Kikura-Hanajiri, R.; Goda, Y.; Lesch, K.-P.; Murphy, D.L.; Uhl, G.R.; et al. Methylone-Induced Hyperthermia and Lethal Toxicity: Role of the Dopamine and Serotonin Transporters. Behav. Pharmacol. 2015, 26, 345–352. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; de la Rosa, G.; Martín, S.; Barriocanal, A.M.; Carabias, L.; Kelmendi, B.; Taoussi, O.; et al. Pharmacological Effects of Methylone and MDMA in Humans. Front. Pharmacol. 2023, 14, 328. [Google Scholar] [CrossRef]
- Pearson, J.M.; Hargraves, T.L.; Hair, L.S.; Massucci, C.J.; Frazee, C.C.; Garg, U.; Pietak, B.R. Three Fatal Intoxications Due to Methylone. J. Anal. Toxicol. 2012, 36, 444–451. [Google Scholar] [CrossRef]
- Barrios, L.; Grison-Hernando, H.; Boels, D.; Bouquie, R.; Monteil-Ganiere, C.; Clement, R. Death Following Ingestion of Methylone. Int. J. Legal. Med. 2016, 130, 381–385. [Google Scholar] [CrossRef]
- Kidwell, D.A.; Holland, J.C.; Athanaselis, S. Testing for Drugs of Abuse in Saliva and Sweat. J. Chromatogr. B Biomed. Sci. Appl. 1998, 713, 111–135. [Google Scholar] [CrossRef]
- Cone, E.J. New Developments in Biological Measures of Drug Prevalence. NIDA Res. Monogr. 1997, 167, 108–129. [Google Scholar]
- Pichini, S.; Navarro, M.; Pacifici, R.; Zuccaro, P.; Ortuño, J.; Farré, M.; Roset, P.N.; Segura, J.; de la Torre, R. Usefulness of Sweat Testing for the Detection of MDMA after a Single-Dose Administration*. J. Anal. Toxicol. 2003, 27, 294–303. [Google Scholar] [CrossRef]
- Hong, W.-Y.; Ko, Y.-C.; Lin, M.-C.; Wang, P.-Y.; Chen, Y.-P.; Chiueh, L.-C.; Shih, D.Y.-C.; Chou, H.-K.; Cheng, H.-F. Determination of Synthetic Cathinones in Urine Using Gas Chromatography-Mass Spectrometry Techniques. J. Anal. Toxicol. 2016, 40, 12–16. [Google Scholar] [CrossRef] [PubMed]
- De Castro, A.; Lendoiro, E.; Fernández-Vega, H.; Steinmeyer, S.; López-Rivadulla, M.; Cruz, A. Liquid Chromatography Tandem Mass Spectrometry Determination of Selected Synthetic Cathinones and Two Piperazines in Oral Fluid. Cross Reactivity Study with an on-Site Immunoassay Device. J. Chromatogr. A 2014, 1374, 93–101. [Google Scholar] [CrossRef] [PubMed]
- La Maida, N.; Mannocchi, G.; Pichini, S.; Basile, G.; Di Giorgi, A.; Busardò, F.P.; Marchei, E. Targeted Screening and Quantification of Synthetic Cathinones and Metabolites in Hair by UHPLC-HRMS. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5033–5042. [Google Scholar] [CrossRef] [PubMed]
- Vree, T.B.; Muskens, A.T.; van Rossum, J.M. Excretion of Amphetamines in Human Sweat. Arch Int. Pharmacodyn. Ther. 1972, 199, 311–317. [Google Scholar]
- Uemura, N.; Nath, R.P.; Harkey, M.R.; Henderson, G.L.; Mendelson, J.; Jones, R.T. Cocaine Levels in Sweat Collection Patches Vary by Location of Patch Placement and Decline Over Time*. J. Anal. Toxicol. 2004, 28, 253–259. [Google Scholar] [CrossRef]
- Schwilke, E.W.; Barnes, A.J.; Kacinko, S.L.; Cone, E.J.; Moolchan, E.T.; Huestis, M.A. Opioid Disposition in Human Sweat after Controlled Oral Codeine Administration. Clin. Chem. 2006, 52, 1539–1545. [Google Scholar] [CrossRef]
- Huestis, M.A.; Oyler, J.M.; Cone, E.J.; Wstadik, A.T.; Schoendorfer, D.; Joseph, R.E. Sweat Testing for Cocaine, Codeine and Metabolites by Gas Chromatography-Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 733, 247–264. [Google Scholar] [CrossRef]
- Sprega, G.; Di Giorgi, A.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Tini, A.; Pichini, S.; Busardò, F.P.; Lo Faro, A.F.; Farré, M. Usefulness of Oral Fluid for Measurement of Methylone and Its Metabolites: Correlation with Plasma Drug Concentrations and the Effect of Oral Fluid PH. Metabolites 2023, 13, 468. [Google Scholar] [CrossRef]
- Kintz, P. Excretion of MBDB and BDB in Urine, Saliva, and Sweat Following Single Oral Administration. J. Anal. Toxicol. 1997, 21, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Tracqui, A.; Mangin, P. Sweat Testing for Benzodiazepines. J. Forensic Sci. 1996, 41, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, D.A.; Smith, F.P. Susceptibility of PharmChek Drugs of Abuse Patch to Environmental Contamination. Forensic. Sci. Int. 2001, 116, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.J.; De Martinis, B.S.; Gorelick, D.A.; Goodwin, R.S.; Kolbrich, E.A.; Huestis, M.A. Disposition of MDMA and Metabolites in Human Sweat Following Controlled MDMA Administration. Clin. Chem. 2009, 55, 454–462. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Methylone (Bk-MDMA). Critical Review Report; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; de la Torre, R.; et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology 2021, 10, 788. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.-A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Olesti, E.; Farré, M.; Carbó, M.; Papaseit, E.; Perez-Mañá, C.; Torrens, M.; Yubero-Lahoz, S.; Pujadas, M.; Pozo, Ó.J.; de la Torre, R. Dose-Response Pharmacological Study of Mephedrone and Its Metabolites: Pharmacokinetics, Serotoninergic Effects, and Impact of CYP2D6 Genetic Variation. Clin. Pharmacol. Ther. 2019, 106, 596–604. [Google Scholar] [CrossRef]

| Methylone 50 mg (n = 3) | Methylone 100 mg (n = 5) | Methylone 150 mg (n = 4) | Methylone 200 mg (n = 7) | Placebo (n = 14) | |
|---|---|---|---|---|---|
| Age (years) | 22.3 ± 0.6 (22–23) | 22.7 ± 0.8 (22–24) | 23.4 ± 0.9 (22–24) | 24.0 ± 0.0 (24–24) | 23.3 ± 0.9 (22–24) |
| Weight (kg) | 69.7 ± 14.2 (60.4–86.0) | 71.0 ± 12.3 (60.4–87.0) | 71.9 ± 10.0 (61.9–87.0) | 70.0 ± 3.9 (66.7–76.6) | 70.2 ± 8.7 (60.4–87.0) |
| Height (cm) | 178.4 ± 3.4 (175.0–181.8) | 177.0 ± 2.8 (181.8–174.2) | 176.9 ± 4.8 (172.8–185.0) | 183.6 ± 9.8 (172.8–193.5) | 180.4 ± 7.2 (172.8–193.5) |
| BMI (kg/m2) | 21.9 ± 4.5 (18.3–27.0) | 22.7 ± 3.9 (18.3–27.9) | 23.1 ± 3.7 (19.4–27.9) | 21.0 ± 3.3 (18.0–25.7) | 21.7 ± 3.5 (18.0–27.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giorgi, A.; Sprega, G.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Di Trana, A.; Varì, M.R.; Busardò, F.P.; Pichini, S.; Zaami, S.; et al. Sweat Testing for the Detection of Methylone after Controlled Administrations in Humans. Int. J. Mol. Sci. 2023, 24, 7395. https://doi.org/10.3390/ijms24087395
Di Giorgi A, Sprega G, Poyatos L, Papaseit E, Pérez-Mañá C, Di Trana A, Varì MR, Busardò FP, Pichini S, Zaami S, et al. Sweat Testing for the Detection of Methylone after Controlled Administrations in Humans. International Journal of Molecular Sciences. 2023; 24(8):7395. https://doi.org/10.3390/ijms24087395
Chicago/Turabian StyleDi Giorgi, Alessandro, Giorgia Sprega, Lourdes Poyatos, Esther Papaseit, Clara Pérez-Mañá, Annagiulia Di Trana, Maria Rosaria Varì, Francesco Paolo Busardò, Simona Pichini, Simona Zaami, and et al. 2023. "Sweat Testing for the Detection of Methylone after Controlled Administrations in Humans" International Journal of Molecular Sciences 24, no. 8: 7395. https://doi.org/10.3390/ijms24087395
APA StyleDi Giorgi, A., Sprega, G., Poyatos, L., Papaseit, E., Pérez-Mañá, C., Di Trana, A., Varì, M. R., Busardò, F. P., Pichini, S., Zaami, S., Lo Faro, A. F., & Farré, M. (2023). Sweat Testing for the Detection of Methylone after Controlled Administrations in Humans. International Journal of Molecular Sciences, 24(8), 7395. https://doi.org/10.3390/ijms24087395








