A Rat Model of Clinically Relevant Extracorporeal Circulation Develops Early Organ Dysfunctions
Abstract
1. Introduction
2. Results
2.1. Hemodynamic, Lactate, and Hemoglobinemia Measures in Rats
2.2. Blood Gases, Electrolytes Measure in Whole Blood of Rats
2.3. Patient Demography
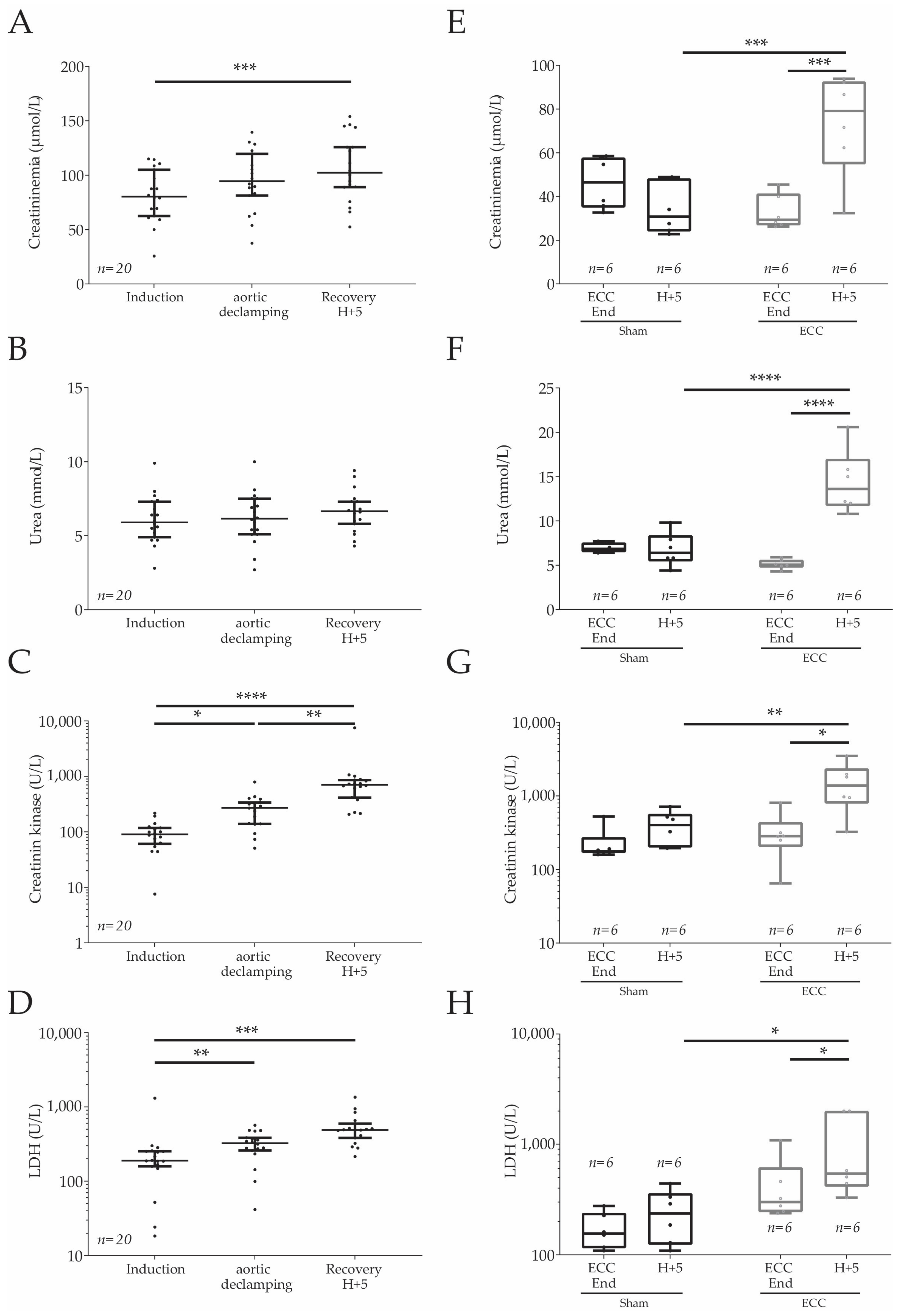
2.4. Plasmatic Markers in Humans and Rats
2.5. DEGSeq Analyses
3. Discussion
Limitations
4. Materials and Methods
4.1. Clinical Study
4.1.1. Ethics Statement and Inclusions
4.1.2. Clinical Data, Blood Sampling, and Processing
4.2. Rat Model Study
4.2.1. Ethical Statement and Animal Care
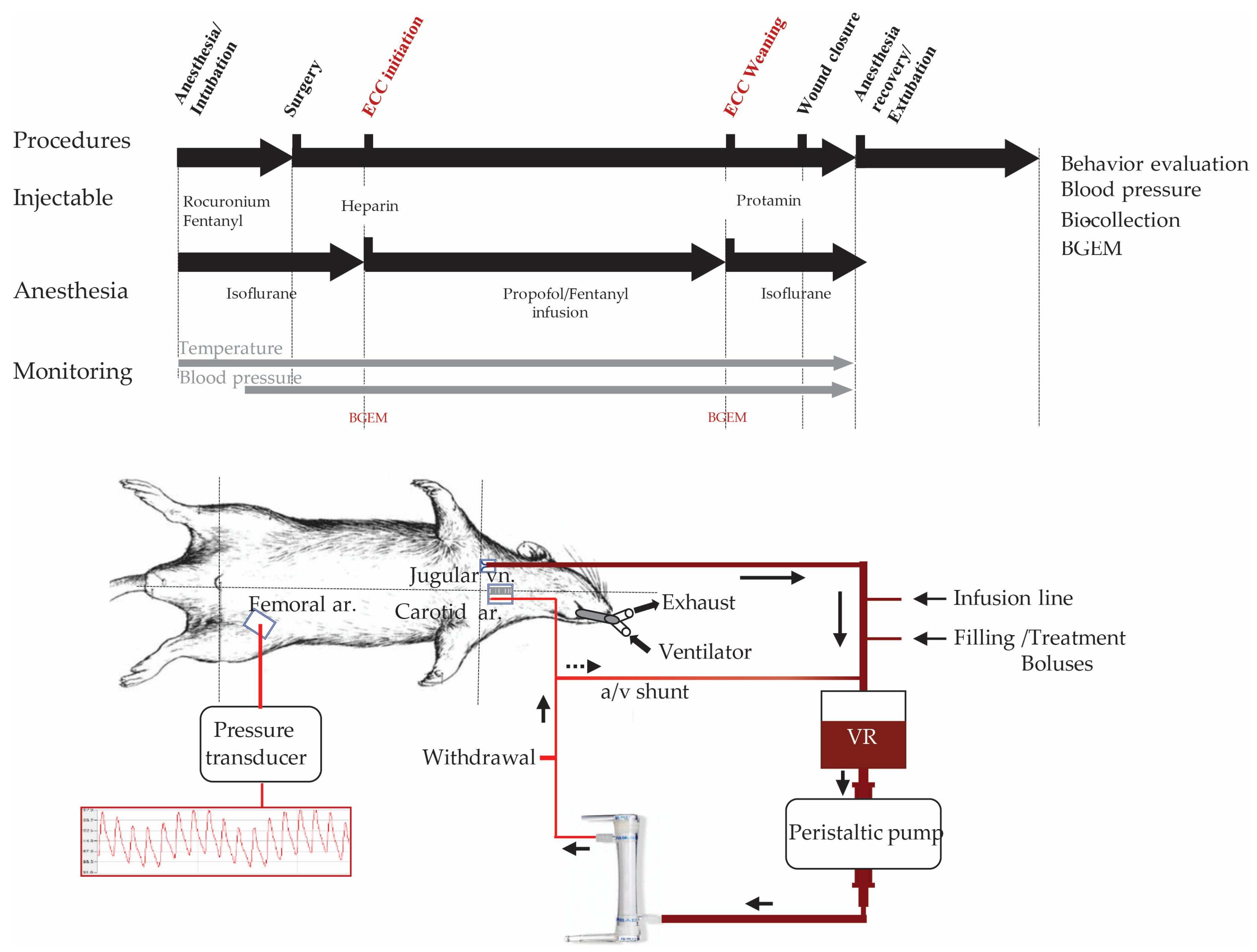
4.2.2. ECC in Rats
4.2.3. Experimental Design and Groups
4.2.4. Arterial Pressure, Temperature Monitoring, and Measure of Blood Samples
4.3. RNA Extraction, Quality Evaluation, and DGESeq Processing and Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, M.M.; Annich, G.M. The Artificial Endothelium. Organogenesis 2011, 7, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Al-Fares, A.; Pettenuzzo, T.; Del Sorbo, L. Extracorporeal Life Support and Systemic Inflammation. Intensive Care Med. Exp. 2019, 7, 46. [Google Scholar] [CrossRef]
- Casey, L.C. Role of Cytokines in the Pathogenesis of Cardiopulmonary-Induced Multisystem Organ Failure. Ann. Thorac. Surg. 1993, 56, S92–S96. [Google Scholar] [CrossRef] [PubMed]
- Esper, S.A.; Subramaniam, K.; Tanaka, K.A. Pathophysiology of Cardiopulmonary Bypass: Current Strategies for the Prevention and Treatment of Anemia, Coagulopathy, and Organ Dysfunction. Semin. Cardiothorac. Vasc. Anesth. 2014, 18, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Riehle, C.; Bauersachs, J. Small Animal Models of Heart Failure. Cardiovasc. Res. 2019, 115, 1838–1849. [Google Scholar] [CrossRef]
- Ponzoni, M.; Coles, J.G.; Maynes, J.T. Rodent Models of Dilated Cardiomyopathy and Heart Failure for Translational Investigations and Therapeutic Discovery. Int. J. Mol. Sci. 2023, 24, 3162. [Google Scholar] [CrossRef]
- Houser, S.R.; Margulies, K.B.; Murphy, A.M.; Spinale, F.G.; Francis, G.S.; Prabhu, S.D.; Rockman, H.A.; Kass, D.A.; Molkentin, J.D.; Sussman, M.A.; et al. Animal Models of Heart Failure: A Scientific Statement From the American Heart Association. Circ. Res. 2012, 111, 131–150. [Google Scholar] [CrossRef]
- Chang, R.-W.; Luo, C.-M.; Yu, H.-Y.; Chen, Y.-S.; Wang, C.-H. Investigation of the Pathophysiology of Cardiopulmonary Bypass Using Rodent Extracorporeal Life Support Model. BMC Cardiovasc. Disord. 2017, 17, 123. [Google Scholar] [CrossRef]
- Madrahimov, N.; Boyle, E.C.; Gueler, F.; Goecke, T.; Knöfel, A.-K.; Irkha, V.; Maegel, L.; Höffler, K.; Natanov, R.; Ismail, I.; et al. Novel Mouse Model of Cardiopulmonary Bypass. Eur. J. Cardio-Thorac. Surg. 2018, 53, 186–193. [Google Scholar] [CrossRef]
- Waterbury, T.; Clark, T.J.; Niles, S.; Farivar, R.S. Rat Model of Cardiopulmonary Bypass for Deep Hypothermic Circulatory Arrest. J. Thorac. Cardiovasc. Surg. 2011, 141, 1549–1551. [Google Scholar] [CrossRef]
- You, X.-M.; Nasrallah, F.; Darling, E.; Robins, M.; Nieman, G.; Searles, B. Rat Cardiopulmonary Bypass Model: Application of a Miniature Extracorporeal Circuit Composed of Asanguinous Prime. J. Extra Corpor. Technol. 2005, 37, 60–65. [Google Scholar] [PubMed]
- Günzinger, R.; Wildhirt, S.M.; Schad, H.; Heimisch, W.; Mendler, N.; Grammer, J.; Lange, R.; Bauernschmitt, R. A Rat Model of Cardiopulmonary Bypass with Cardioplegic Arrest and Hemodynamic Assessment by Conductance Catheter Technique. Basic Res. Cardiol. 2007, 102, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Bouadma, L.; Bouhemad, B.; Brissaud, O.; Dauger, S.; Gibot, S.; Hraiech, S.; Jung, B.; Kipnis, E.; Launey, Y.; et al. Healthcare Associated Pneumonia in the Intensive Care Unit. Anesthésie Réanimation 2018, 4, 421–441. [Google Scholar] [CrossRef]
- Honore, P.M.; Barreto Gutierrez, L.; Kugener, L.; Redant, S.; Attou, R.; Gallerani, A.; De Bels, D. Risk of Harlequin Syndrome during Bi-Femoral Peripheral VA-ECMO: Should We Pay More Attention to the Watershed or Try to Change the Venous Cannulation Site? Crit. Care 2020, 24, 450. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Mogi, M.; Furuya, M.; Kojima, K. Rat Extracorporeal Circulation Model for Evaluation of Systemic Immunotoxicity. Toxicol. Lett. 2000, 115, 63–70. [Google Scholar] [CrossRef]
- Fujii, Y.; Shirai, M.; Tsuchimochi, H.; Pearson, J.T.; Takewa, Y.; Tatsumi, E.; Taenaka, Y. Hyperoxic Condition Promotes an Inflammatory Response During Cardiopulmonary Bypass in a Rat Model: Inflammatory Response During Hyperoxic CPB. Artif. Organs 2013, 37, 1034–1040. [Google Scholar] [CrossRef]
- Fujii, Y. Evaluation of Inflammation Caused by Cardiopulmonary Bypass in a Small Animal Model. Biology 2020, 9, 81. [Google Scholar] [CrossRef]
- Peterss, S.; Guenther, S.; Kellermann, K.; Jungwirth, B.; Lichtinghagen, R.; Haverich, A.; Hagl, C.; Khaladj, N. An Experimental Model of Myocardial Infarction and Controlled Reperfusion Using a Miniaturized Cardiopulmonary Bypass in Rats. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 561–566. [Google Scholar] [CrossRef]
- Fujii, Y.; Shirai, M.; Takewa, Y.; Tatsumi, E. Cardiopulmonary Bypass with Low-Versus High-Priming Volume: Comparison of Inflammatory Responses in a Rat Model. ASAIO J. 2016, 62, 286–290. [Google Scholar] [CrossRef]
- Bronson, S.L.; Riley, J.B.; Blessing, J.P.; Ereth, M.H.; Dearani, J.A. Prescriptive Patient Extracorporeal Circuit and Oxygenator Sizing Reduces Hemodilution and Allogeneic Blood Product Transfusion during Adult Cardiac Surgery. J. Extra Corpor. Technol. 2013, 45, 167–172. [Google Scholar]
- Hare, G.M.T.; Han, K.; Leshchyshyn, Y.; Mistry, N.; Kei, T.; Dai, S.Y.; Tsui, A.K.Y.; Pirani, R.A.; Honavar, J.; Patel, R.P.; et al. Potential Biomarkers of Tissue Hypoxia during Acute Hemodilutional Anemia in Cardiac Surgery: A Prospective Study to Assess Tissue Hypoxia as a Mechanism of Organ Injury. Can. J. Anaesth. 2018, 65, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, J.; Hood, R.R.; Edelstein, S.B. Overcoming Challenges in the Management of Critical Events During Cardiopulmonary Bypass. Semin. Cardiothorac. Vasc. Anesth. 2014, 18, 190–207. [Google Scholar] [CrossRef] [PubMed]
- McEwen, C.C.; Amir, T.; Qiu, Y.; Young, J.; Kennedy, K.; Grocott, H.P.; Kashani, H.; Mazer, D.; Brudney, S.; Kavosh, M.; et al. Morbidity and Mortality in Patients Managed with High Compared with Low Blood Pressure Targets during On-Pump Cardiac Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. J. Anaesth. 2022, 69, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Rodseth, R.N.; Cywinski, J.; Thabane, L.; Sessler, D.I. Relationship between Intraoperative Mean Arterial Pressure and Clinical Outcomes after Noncardiac Surgery: Toward an Empirical Definition of Hypotension. Anesthesiology 2013, 119, 507–515. [Google Scholar] [CrossRef]
- Hori, D.; Nomura, Y.; Ono, M.; Joshi, B.; Mandal, K.; Cameron, D.; Kocherginsky, M.; Hogue, C.W. Optimal Blood Pressure During Cardiopulmonary Bypass Defined By Cerebral Autoregulation Monitoring. J. Thorac. Cardiovasc. Surg. 2017, 154, 1590–1598.e2. [Google Scholar] [CrossRef]
- Sevransky, J. Clinical Assessment of Hemodynamically Unstable Patients. Curr. Opin. Crit. Care 2009, 15, 234–238. [Google Scholar] [CrossRef]
- Matteucci, M.; Ferrarese, S.; Cantore, C.; Cappabianca, G.; Massimi, G.; Mantovani, V.; Rossi, M.B.; Beghi, C. Hyperlactatemia during Cardiopulmonary Bypass: Risk Factors and Impact on Surgical Results with a Focus on the Long-Term Outcome. Perfusion 2020, 35, 756–762. [Google Scholar] [CrossRef]
- Meakins, J.; Long, C.N. Oxygen consumption, oxygen debt and lactic acid in circulatory failure. J. Clin. Investig. 1927, 4, 273–293. [Google Scholar] [CrossRef]
- Parikh, C.R.; Coca, S.G.; Thiessen-Philbrook, H.; Shlipak, M.G.; Koyner, J.L.; Wang, Z.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J. Am. Soc. Nephrol. 2011, 22, 1748–1757. [Google Scholar] [CrossRef]
- Krawczeski, C.D. Cardiopulmonary Bypass and AKI: AKI Is Bad, So Let’s Get Beyond the Diagnosis. Front. Pediatr. 2019, 7, 492. [Google Scholar] [CrossRef]
- Milne, B.; Gilbey, T.; De Somer, F.; Kunst, G. Adverse Renal Effects Associated with Cardiopulmonary Bypass. Perfusion 2023, 026765912311570. [Google Scholar] [CrossRef] [PubMed]
- Schurle, A.; Koyner, J.L. CSA-AKI: Incidence, Epidemiology, Clinical Outcomes, and Economic Impact. J. Clin. Med. 2021, 10, 5746. [Google Scholar] [CrossRef] [PubMed]
- Udzik, J.; Waszczyk, A.; Safranow, K.; Biskupski, A.; Majer, K.; Kwiatkowski, S.; Kwiatkowska, E. Assessment and Prognosis in CSA-AKI Using Novel Kidney Injury Biomarkers: A Prospective Observational Study. Biology 2021, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Lakhal, K.; Rozec, B.; Souab, F.; Senage, T.; Leroy, M.; Legrand, A.; Boissier, E.; Bigot-Corbel, E. Plasma Haemolysis Index and Interleukine-6 for the Early Prediction of Cardiac Surgery-Associated Acute Kidney Injury. A Proof-of-Concept Study. Perfusion 2022, 38, 2676591221083791. [Google Scholar] [CrossRef]
- Claure-Del Granado, R.; Mehta, R.L. Fluid Overload in the ICU: Evaluation and Management. BMC Nephrol. 2016, 17, 109. [Google Scholar] [CrossRef]
- Salunke, B.G. Fluid Overload and Acute Kidney Injury. Indian J. Crit. Care Med. 2020, 24, 94–97. [Google Scholar] [CrossRef]
- Magruder, J.T.; Crawford, T.C.; Harness, H.L.; Grimm, J.C.; Suarez-Pierre, A.; Wierschke, C.; Biewer, J.; Hogue, C.; Whitman, G.R.; Shah, A.S.; et al. A Pilot Goal-Directed Perfusion Initiative Is Associated with Less Acute Kidney Injury after Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 118–125.e1. [Google Scholar] [CrossRef]
- Bouma, H.R.; Samarska, I.V.; Schenk, M.; Dahlem, K.K.K.; van den Bos, H.; Brebenel, I.; Duin, M.; Houwertjes, M.C.; Loef, B.G.; Mungroop, H.E.; et al. Microarray Analysis of Gene Expression Profiles in the Rat Kidney Demonstrates a Local Inflammatory Response Induced by Cardiopulmonary Bypass. Eur. J. Anaesthesiol. EJA 2013, 30, 492–500. [Google Scholar] [CrossRef]
- Kang, P.T.; Chen, C.-L.; Lin, P.; Zhang, L.; Zweier, J.L.; Chen, Y.-R. Mitochondrial Complex I in the Post-Ischemic Heart: Reperfusion-Mediated Oxidative Injury and Protein Cysteine Sulfonation. J. Mol. Cell Cardiol. 2018, 121, 190–204. [Google Scholar] [CrossRef]
- Charpentier, E.; Cornec, M.; Dumont, S.; Meistermann, D.; Bordron, P.; David, L.; Redon, R.; Bonnaud, S.; Bihouée, A. 3’ RNA Sequencing for Robust and Low-Cost Gene Expression Profiling; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Lu, Y.; Liu, P.-Y.; Xiao, P.; Deng, H.-W. Hotelling’s T2 Multivariate Profiling for Detecting Differential Expression in Microarrays. Bioinformatics 2005, 21, 3105–3113. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]


| Start ECC (Mean ± SEM) | End ECC (Mean ± SEM) | H+5 (Mean ± SEM) | ||||
|---|---|---|---|---|---|---|
| pH | Sham | 7.41 ± 0.02 | 7.37 ± 0.03 | 7.40 ± 0.02 | ||
| ECC | 7.42 ± 0.01 | 7.42 ± 0.04 | 7.41 ± 0.03 | |||
| pCO2 (mmHg) | Sham | 45.47 ± 2.94 | 46.13 ± 1.88 | 43.28 ± 3.43 | ||
| ECC | 42.67 ± 2.52 | 39.93 ± 4.14 | 41.15 ± 3.50 | |||
| pO2 (mmHg) | Sham | 287.17 ± 35.86 | 386.68 ± 43.61 | 329.4 ± 70.54 | ||
| ECC | 333.12 ± 48.52 | 436.42 ± 66.41 | 464.62 ± 43.87 | |||
| BE (mmol/L) | Sham | 4.18 ± 0.59 | 2.37 ± 1.87 | 2.15 ± 2.11 | ||
| ECC | 3.15 ± 0.67 | 0.80 ± 1.13 | 1.17 ± 1.14 | |||
| HCO3− (mmol/L) | Sham | 28.72 ± 0.72 | 27.98 ± 1.17 | 27.59 ± 1.77 | ||
| ECC | 27.47 ± 0.79 | 25.4 ± 1.24 | 25.73 ± 1.08 | |||
| Ca2+ (mmol/L) | Sham | 1.32 ± 0.02 | 1.35 ± 0.03 | 1.24 ± 0.03 | ||
| ECC | 1.29 ± 0.02 | 1.40 ± 0.02 | * | 1.10 ± 0.04 | $$$*** | |
| Cl- (mmol/L) | Sham | 101.00 ± 0.73 | 102.33 ± 1.52 | 100.67 ± 1.56 | ||
| ECC | 101.33 ± 0.84 | 102.83 ± 1.17 | 104.17 ± 0.7 | |||
| K+ (mmol/L) | Sham | 4.90 ± 0.11 | 5.07 ± 0.14 | 4.17 ± 0.15 | ||
| ECC | 4.62 ± 0.15 | 4.42 ± 0.11 | 5.04 ± 0.22 | * | ||
| Na+ (mmol/L) | Sham | 139.33 ± 0.56 | 141.33 ± 4.08 | 139.33 ± 0.95 | ||
| ECC | 138.73 ± 0.87 | 139.17 ± 0.79 | 140.17 ± 0.79 | |||
| AGAP | Sham | 10.17 ± 0.79 | 10 ± 0.75 | 12.2 ± 1.45 | ||
| ECC | 10.50 ± 0.92 | 11.17 ± 1.17 | 10.77 ± 0.92 | |||
| AGAPK | Sham | 14.83 ± 0.7 | 15.25 ± 0.84 | 16.2 ± 1.45 | ||
| ECC | 15.17 ± 0.95 | 15.5 ± 1.15 | 15.5 ± 0.99 |
| Overall (n = 41) | |
| Sex (Male) | 36 (87.8%) |
| Age (year) | |
| Mean (SD) | 66.1 (12.9) |
| Range | 32.0–82.0 |
| Body mass index | |
| Mean (SD) | 26.0 (3.6) |
| Range | 20.0–38.0 |
| Surgery Type | |
| Mitral valve repair | 5 (12.2%) |
| Mitral valve repair + tricuspid annuloplasty | 7 (17.1%) |
| Mitral valve replacement + tricuspid annuloplasty | 3 (7.3%) |
| Mitral valve replacement + CABG | 1 (2.4%) |
| Aortic valve replacement | 2 (4.9%) |
| Aortic valve replacement + tricuspid annuloplasty | 3 (7.3%) |
| Aortic valve replacement + CABG | 4 (9.8%) |
| CABG x3 | 5 (12.2) |
| CABG > 3 | 3 (7.3%) |
| Ascending aorta surgery (Bentall, Tirone David, etc.) | 7 (17.1%) |
| Bentall + CABG | 1 (2.4%) |
| Redux | 2 (4.9%) |
| Dyslipidemia | 25 (61.0%) |
| Hypertension | 28 (68.3%) |
| Tobacco | |
| Smoker | 4 (9.8%) |
| Diabetes | 7 (17.1%) |
| Obesity | 5 (12.2%) |
| Familial history | 3 (7.3%) |
| Exogenesis | 3 (7.3%) |
| NYHA | |
| 0 | 4 (9.8%) |
| I | 8 (19.5%) |
| II | 24 (58.5%) |
| III | 5 (12.2%) |
| Arteriopathy | 8 (19.5%) |
| Myocardial infarction | 11 (26.8%) |
| Cerebral dysfunction | 1 (2.4%) |
| Chronic renal failure | 9 (22.0%) |
| Dialysis | 1 (2.4%) |
| BPCO | 2 (4.9%) |
| Asthma | 1 (2.4%) |
| FeVG | |
| Mean (SD) | 60.8 (7.8) |
| Range | 40.0–70.0 |
| EUROSCORE II | |
| Mean (SD) | 2.5 (2.2) |
| Range | 0.8–11.6 |
| CPB time (min) | |
| Mean (SD) | 126.9 (37.5) |
| Range | 63.0–229.0 |
| Cross-clamping time (min) | |
| Mean (SD) | 98.0 (30.2) |
| Range | 45.0–182.0 |
| Catecholamine | 39 (95.1%) |
| Norepinephrine | 37 (90.2%) |
| Dobutamine | 20 (48.8%) |
| Transfusion peri-op | 6 (14.6%) |
| Cell saver | 41 (100.0%) |
| VIS score 6 h post-op | |
| Mean (SD) | 7.6 (18.1) |
| Range | 0.0–112.0 |
| KDIGO 24 h post-op | |
| 0 | 31 (75.6%) |
| 1 | 7 (17.1%) |
| 2 | 2 (4.9%) |
| 3 | 1 (2.4%) |
| Left ventricular dysfunction post-op | 17 (41.5%) |
| Hydroxycortisone | 2 (4.9%) |
| Ventilation duration (h) | |
| Mean (SD) | 13.0 (48.1) |
| Range | 2.0–312.0 |
| Pulmonary infection | 1 (2.4%) |
| Bleeding volume 6 h post-op (mL) | |
| Mean (SD) | 191.8 (116.5) |
| Range | 70.0–500.0 |
| Transfusion in critical care | 4 (9.8%) |
| Intensive care stay (day) | |
| Mean (SD) | 3.8 (5.9) |
| Range | 1.0–34.0 |
| Hospital stay (day) | |
| Mean (SD) | 13.6 (6.1) |
| Range | 6.0–34.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persello, A.; Souab, F.; Dupas, T.; Aillerie, V.; Bigot, E.; Denis, M.; Erraud, A.; Pelé, T.; Blangy-Letheule, A.; Miniou, P.; et al. A Rat Model of Clinically Relevant Extracorporeal Circulation Develops Early Organ Dysfunctions. Int. J. Mol. Sci. 2023, 24, 7338. https://doi.org/10.3390/ijms24087338
Persello A, Souab F, Dupas T, Aillerie V, Bigot E, Denis M, Erraud A, Pelé T, Blangy-Letheule A, Miniou P, et al. A Rat Model of Clinically Relevant Extracorporeal Circulation Develops Early Organ Dysfunctions. International Journal of Molecular Sciences. 2023; 24(8):7338. https://doi.org/10.3390/ijms24087338
Chicago/Turabian StylePersello, Antoine, Fouzia Souab, Thomas Dupas, Virginie Aillerie, Edith Bigot, Manon Denis, Angélique Erraud, Thomas Pelé, Angélique Blangy-Letheule, Pierre Miniou, and et al. 2023. "A Rat Model of Clinically Relevant Extracorporeal Circulation Develops Early Organ Dysfunctions" International Journal of Molecular Sciences 24, no. 8: 7338. https://doi.org/10.3390/ijms24087338
APA StylePersello, A., Souab, F., Dupas, T., Aillerie, V., Bigot, E., Denis, M., Erraud, A., Pelé, T., Blangy-Letheule, A., Miniou, P., Guedat, P., De Waard, M., Abgueguen, E., Rozec, B., & Lauzier, B. (2023). A Rat Model of Clinically Relevant Extracorporeal Circulation Develops Early Organ Dysfunctions. International Journal of Molecular Sciences, 24(8), 7338. https://doi.org/10.3390/ijms24087338








