Protective Effects of Selenium Nanoparticles against Bisphenol A-Induced Toxicity in Porcine Intestinal Epithelial Cells
Abstract
1. Introduction
2. Results
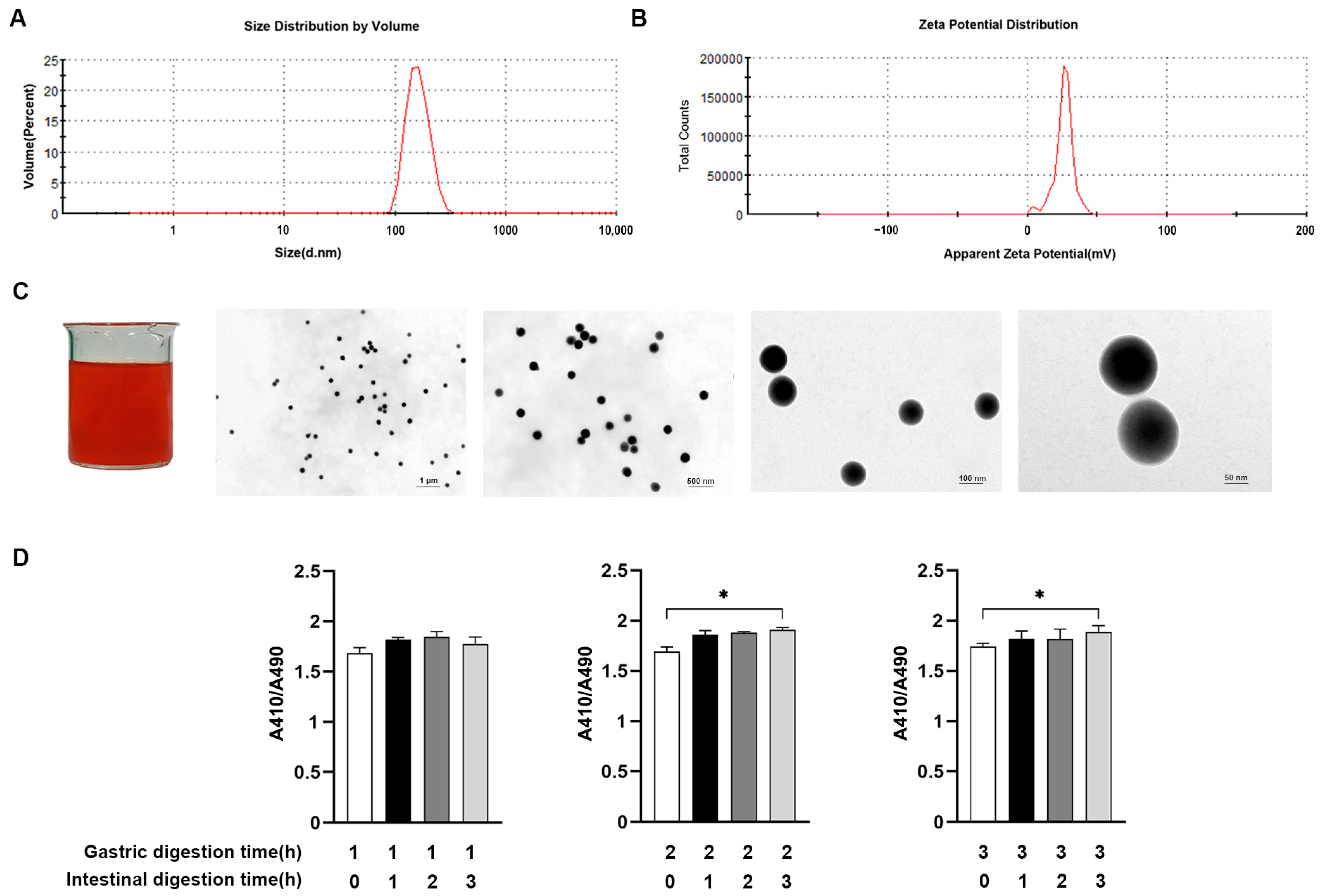
2.1. Preparation and Characterization of SeNPs
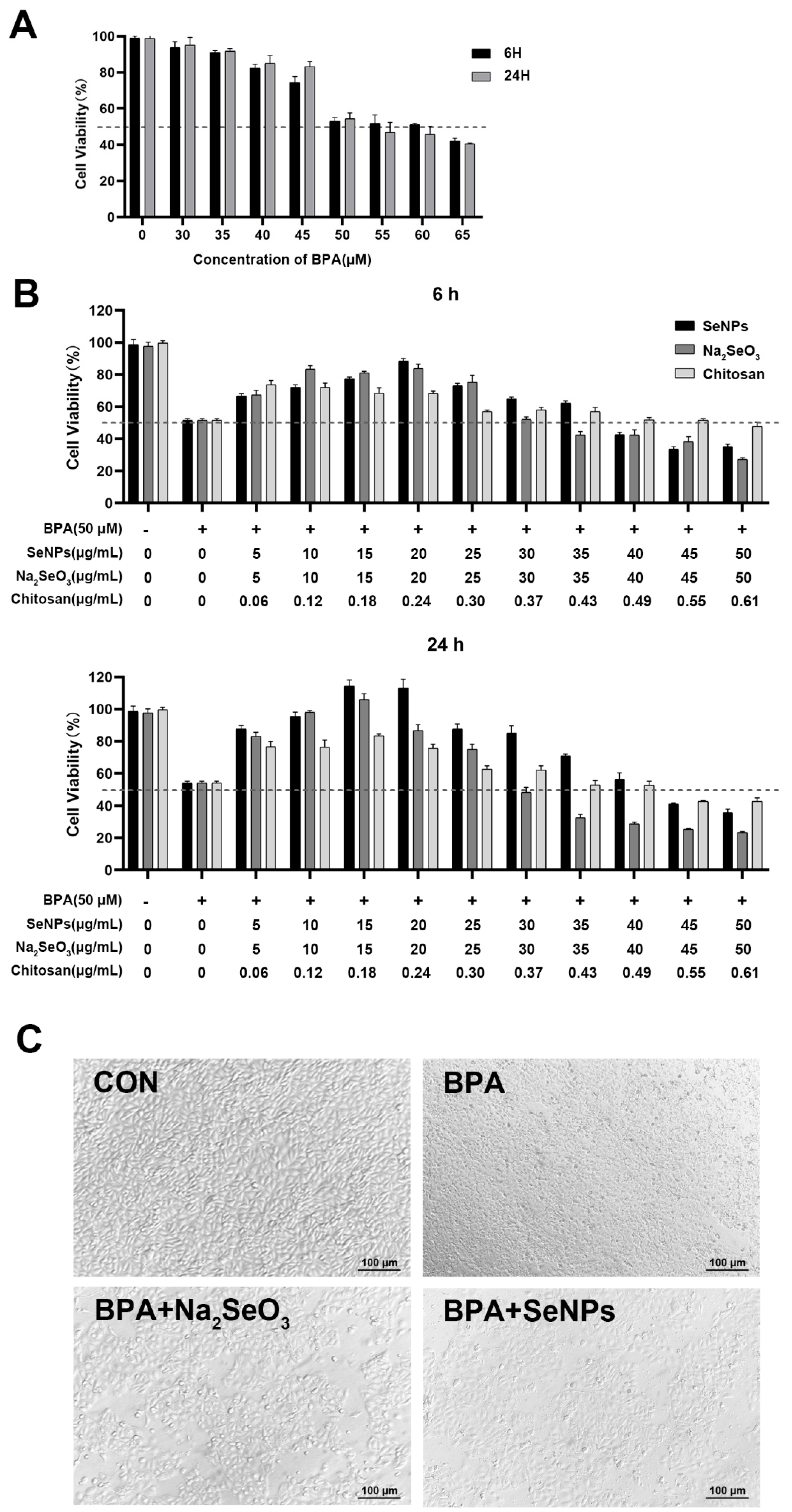
2.2. Establishment of the Model of BPA-Exposed and Na2SeO3 or SeNPs-Treated IPECs
2.3. SeNPs Maintained the TJ Expression in BPA-Exposed IPECs
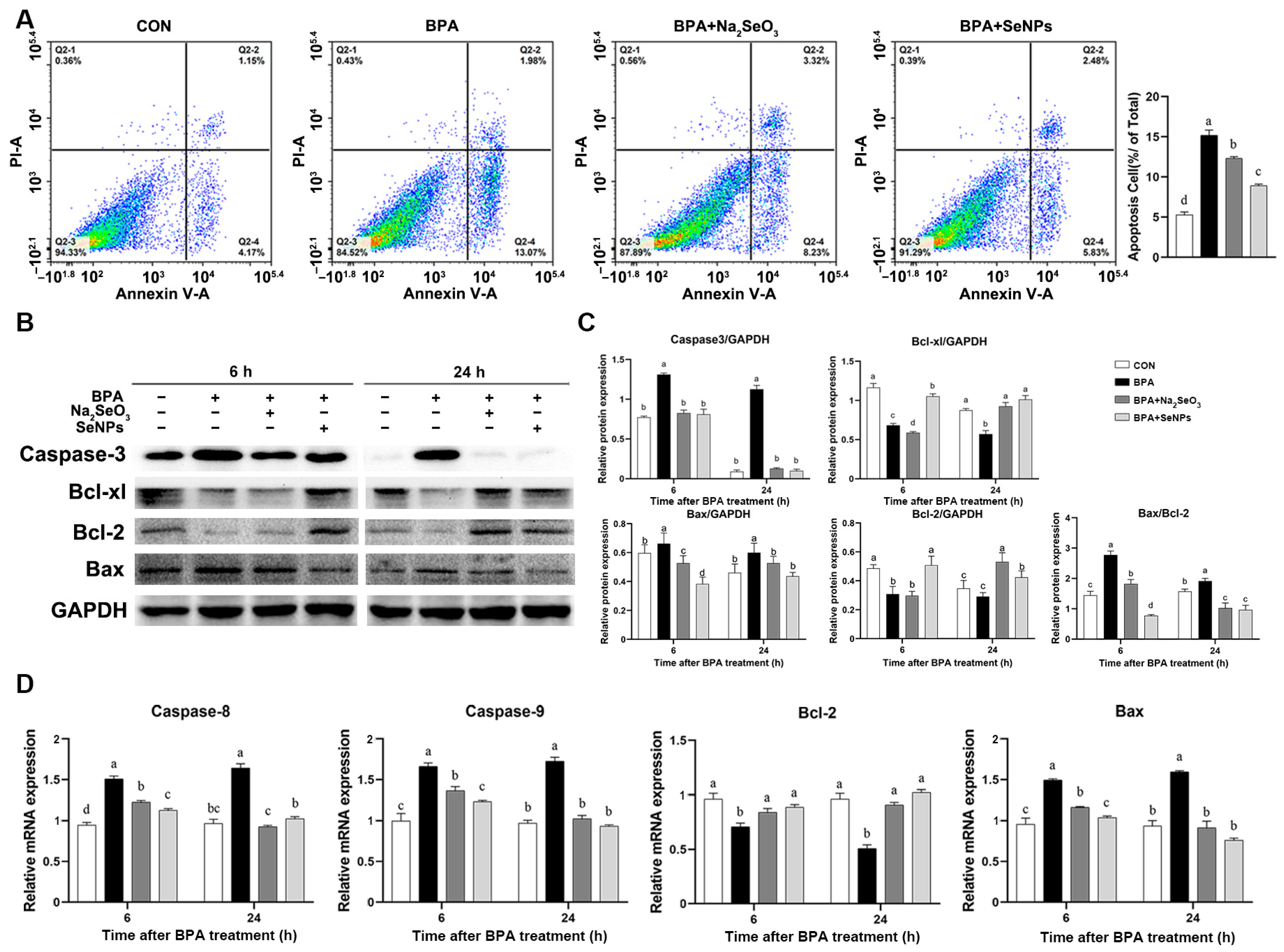
2.4. SeNPs Attenuated the BPA-Induced IPEC Apoptosis
2.5. The Effects of Na2SeO3 and SeNPs on Inflammatory Pathways in BPA-exposed IPECs
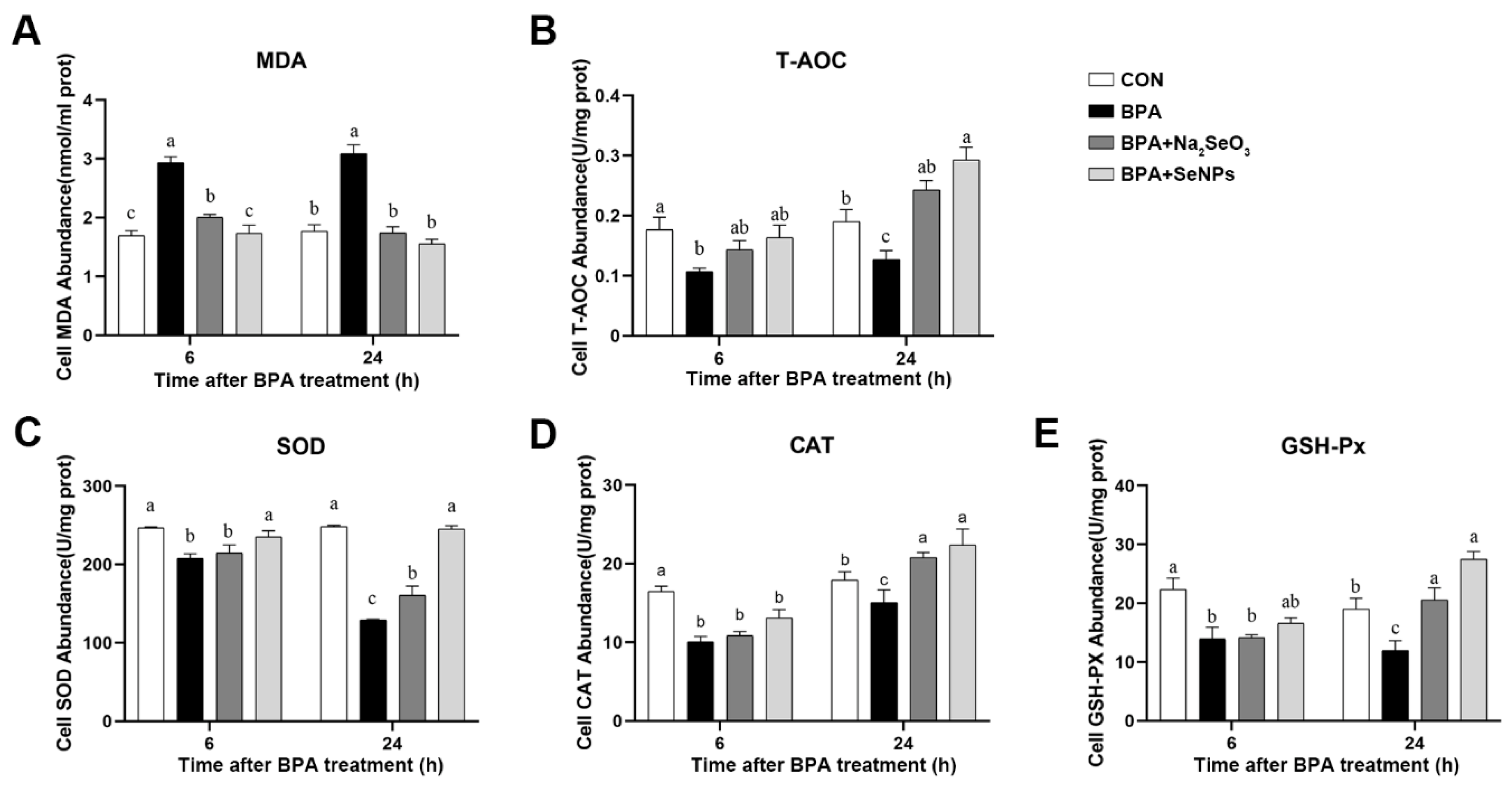
2.6. SeNPs Maintained the Antioxidant Capacity of IPEC-J2 Cells during BPA Exposure
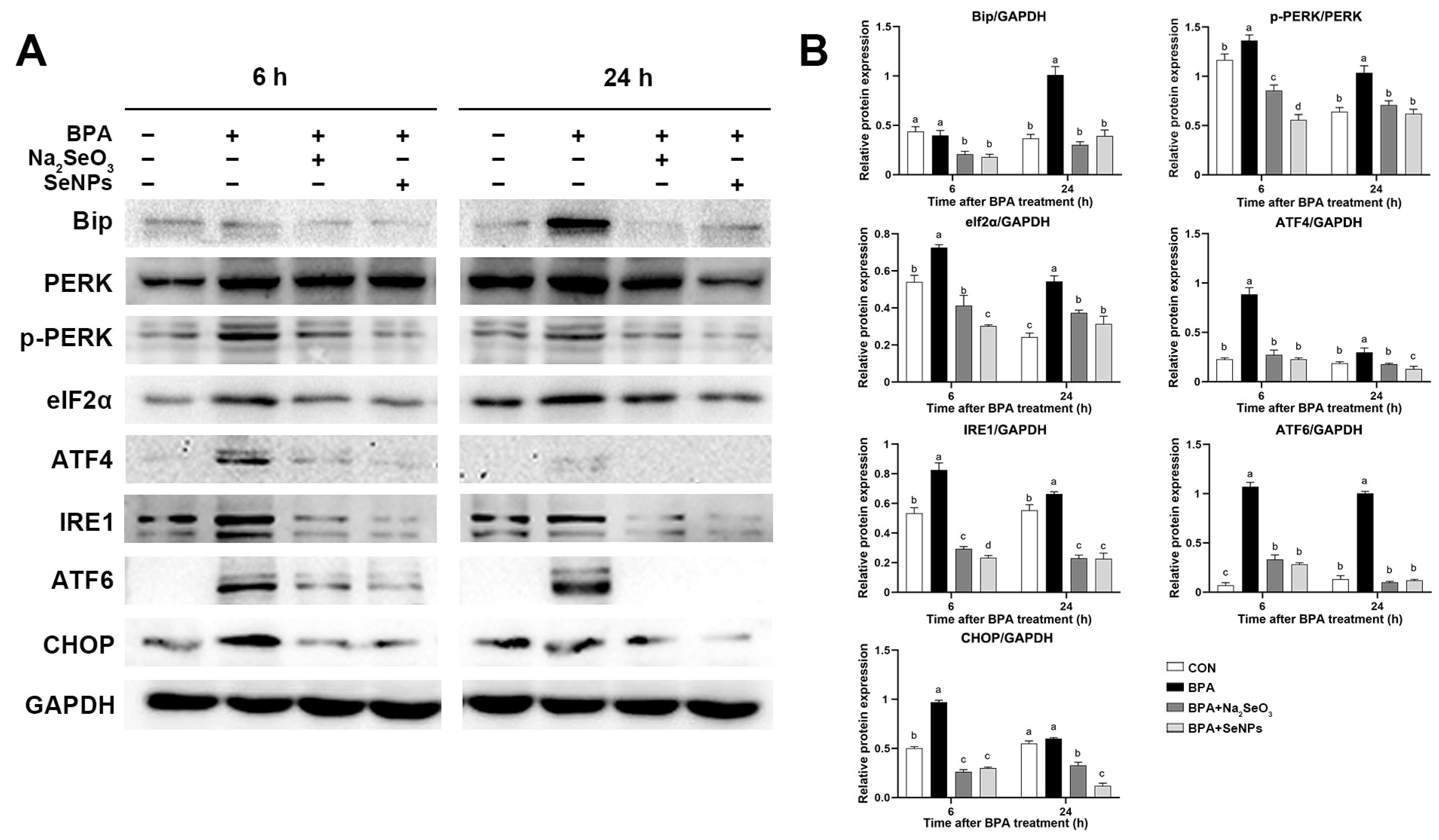
2.7. SeNPs Ameliorated the BPA-Induced ERS in IPEC-J2 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of SeNPs
4.2. Dynamic Light Scattering (DLS)
4.3. Transmission Electron Microscopy (TEM)
4.4. Gastric and Intestinal Fluid Digestion Test
4.5. Cell Culture and Treatment
4.6. Cell Counting Kit-8 (CCK8) Assay
4.7. Morphological Observation
4.8. Western Blotting
4.9. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.10. Antioxidant Determination
4.11. Detection of Apoptosis by Flow Cytometry
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pabst, R.; Russell, M.W.; Brandtzaeg, P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 2008, 29, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Correa, C.A.; Mulett-Vásquez, E.; Miranda, D.A.; Gonzalez-Correa, C.H.; Gómez-Buitrago, P.A. The colon revisited or the key to wellness, health and disease. Med. Hypotheses 2017, 108, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Odle, J. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 2012, 3, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Guo, C.; Yu, S.; Zhu, L.; Wang, Y.; Hu, H.; Deng, J. Progress in mycotoxins affecting intestinal mucosal barrier function. Int. J. Mol. Sci. 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int. J. Mol. Sci. 2020, 21, 16. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef]
- Gonkowski, S. Bisphenol A (BPA)-Induced changes in the number of serotonin-positive cells in the mucosal layer of porcine small intestine-the preliminary studies. Int. J. Mol. Sci. 2020, 21, 3. [Google Scholar] [CrossRef]
- Makowska, K.; Szymańska, K.; Całka, J.; Gonkowski, S. The influence of bisphenol A (BPA) on the occurrence of selected active substances in neuregulin 1 (NRG1)-positive enteric neurons in the porcine large intestine. Int. J. Mol. Sci. 2021, 22, 19. [Google Scholar] [CrossRef]
- Qu, W.; Zhao, Z.; Chen, S.; Zhang, L.; Wu, D.; Chen, Z. Bisphenol A suppresses proliferation and induces apoptosis in colonic epithelial cells through mitochondrial and MAPK/AKT pathways. Life Sci. 2018, 208, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qiu, L.; Zhu, J.; Sun, Q.; Qu, W.; Yu, Y.; Zhao, Z.; Yu, Y.; Shao, G. Environmental contaminant BPA causes intestinal damage by disrupting cellular repair and injury homeostasis in vivo and in vitro. Biomed. Pharmacother. 2021, 137, 111270. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Z.; Sun, K.; Zhang, Y.; Chen, J.; Yang, Y.; Tso, P.; Wu, G.; Wu, Z. Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel disease pathogenesis: An update review. Front. Immunol. 2017, 8, 1271. [Google Scholar] [CrossRef]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Holczer, M.; Márton, M.; Kurucz, A.; Bánhegyi, G.; Kapuy, O. A Comprehensive systems biological study of autophagy-apoptosis crosstalk during endoplasmic reticulum stress. Biomed. Res. Int. 2015, 2015, 319589. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Asahi, J.; Kamo, H.; Baba, R.; Doi, Y.; Yamashita, A.; Murakami, D.; Hanada, A.; Hirano, T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010, 87, 431–438. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, Y.; Zhong, Y.; Gao, X.; Tan, T. Effect of vitamin E on reproductive functions and anti-oxidant activity of adolescent male mice exposed to bisphenol A. Wei Sheng Yan Jiu 2013, 42, 18–22. [Google Scholar] [PubMed]
- Abedelhaffez, A.S.; El-Aziz, E.A.A.; Aziz, M.A.A.; Ahmed, A.M. Lung injury induced by Bisphenol A: A food contaminant, is ameliorated by selenium supplementation. Pathophysiology 2017, 24, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.G.; Yang, R.J.; Yue, W.B.; Xun, W.J.; Zhang, C.X.; Ren, Y.S.; Shi, L.; Lei, F.L. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim. Reprod. Sci. 2010, 118, 248–254. [Google Scholar] [CrossRef] [PubMed]
- El-Deep, M.H.; Ijiri, D.; Ebeid, T.A.; Ohtsuka, A. Effects of dietary nano-selenium supplementation on growth performance, antioxidative status, and immunity in broiler chickens under thermoneutral and high ambient temperature conditions. J. Poult. Sci. 2016, 53, 274–283. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H.; Mozanzadeh, M.T. Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol. Biochem. 2018, 44, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, K.; Zhang, C.; Talukder, M.; Lv, M.W.; Li, J.Y.; Li, J.L. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation ofcadmium-induced inflammation via NF-kB/IκB pathway in heart. Sci. Total Environ. 2021, 773, 145442. [Google Scholar] [CrossRef]
- Ge, J.; Guo, K.; Huang, Y.; Morse, P.D.; Zhang, C.; Lv, M.W.; Li, J.L. Comparison of antagonistic effects of nanoparticle-selenium, selenium-enriched yeast and sodium selenite against cadmium-induced cardiotoxicity via AHR/CAR/PXR/Nrf2 pathways activation. Comp. Study 2022, 105, 108992. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Ahmed, W.; Moselhy, W.A.; Abdel-Halim, B.R.; Ibrahim, M.A. Protective effects of selenium and nano-selenium on bisphenol-induced reproductive toxicity in male rats. Hum. Exp. Toxicol. 2019, 38, 398–408. [Google Scholar] [CrossRef]
- Acaroz, U.; Ince, S.; Arslan-Acaroz, D.; Gurler, Z.; Demirel, H.H.; Kucukkurt, I.; Eryavuz, A.; Kara, R.; Varol, N.; Zhu, K. Bisphenol-A induced oxidative stress, inflammatory gene expression, and metabolic and histopathological changes in male Wistar albino rats: Protective role of boron. Toxicol. Res. 2019, 8, 262–269. [Google Scholar] [CrossRef]
- Soundararajan, A.; Prabu, P.; Mohan, V.; Gibert, Y.; Balasubramanyam, M. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol. Cell. Biochem. 2019, 458, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, B.; Khalaf, A.; Moselhy, W.; Ahmed, W.M. Protective effect of nano-selenium and ionized selenium against the testicular damage, endocrine disruptor and testicular ultrastructure of bisphenol A in albino male rats. Asian J. Anim. Vet. Adv. 2016, 11, 653–664. [Google Scholar] [CrossRef]
- Fahmy, H.A.; Abd El Azim, A.S.; Gharib, O.A. Protective Effects of omega-3 fatty acids and/or nanoselenium on cisplatin and ionizing radiation induced liver toxicity in rats. Indian J. Pharm. Educ. Res. 2016, 50, 649–656. [Google Scholar] [CrossRef]
- Feng, L.; Chen, S.; Zhang, L.; Qu, W.; Chen, Z. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019, 254, 112960. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.; Feng, X. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ. Pollut. 2020, 265, 114880. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- Chen, S.; Xue, Y.; Shen, Y.; Ju, H.; Zhang, X.; Liu, J.; Wang, Y. Effects of different selenium sources on duodenum and jejunum tight junction network and growth performance of broilers in a model of fluorine-induced chronic oxidative stress. Poult. Sci. 2022, 101, 101664. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Saeed, K.; Fatemeh, H. Nano-Bio selenium synthesized by bacillus subtilis modulates broiler performance, intestinal morphology and microbiota, and expression of tight junction’s proteins. Bio. Trace Elem. Res. 2022, 200, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Ayna, A.; Gür, C.; Küçükler, S.; Darendelioğlu, E. Neuroprotective effects of 18β-glycyrrhetinic acid against bisphenol A-induced neurotoxicity in rats: Involvement of neuronal apoptosis, endoplasmic reticulum stress and JAK1/STAT1 signaling pathway. Metab. Brain Dis. 2022, 37, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Jia, R.; He, Q.; Cao, L.; Du, J.; Feng, W.; Jeney, G.; Xu, P.; Yin, G. Alteration of lipid metabolism, autophagy, apoptosis and immune response in the liver of common carp (Cyprinus carpio) after long-term exposure to bisphenol A. Ecotoxicol. Environ. Saf. 2021, 211, 111923. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Su, C.C.; Lee, K.I.; Chen, Y.W. Effects of bisphenol a metabolite 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on lung function and type 2 pulmonary alveolar epithelial cell growth. Sci. Rep. 2016, 6, 39254. [Google Scholar] [CrossRef] [PubMed]
- Harnett, K.G.; Chin, A.; Schuh, S.M. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol. Environ. Saf. 2021, 216, 112210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, B.; Yang, S.; Wang, J.; Chen, J. Bisphenol A (BPA) induces apoptosis of mouse Leydig cells via oxidative stress. Environ. Toxicol. 2023, 38, 312–321. [Google Scholar] [CrossRef]
- Zhang, R.; Yi, R.; Bi, Y.; Xing, L.; Bao, J.; Li, J. The effect of selenium on the Cd-Induced apoptosis via NO-mediated mitochondrial apoptosis pathway in chicken liver. Bio. Trace Elem. Res. 2017, 178, 310–319. [Google Scholar] [CrossRef]
- Nettore, I.C.; De Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium supplementation modulates apoptotic processes in thyroid follicular cells. BioFactors 2017, 43, 415–423. [Google Scholar] [CrossRef]
- Zaldivar, V.; Magri, M.L.; Zárate, S.; Jaita, G.; Eijo, G.; Radl, D.; Ferraris, J.; Pisera, D.; Seilicovich, A. Estradiol increases the Bax/Bcl-2 ratio and induces apoptosis in the anterior pituitary gland. Neuroendocrinology 2009, 90, 292–300. [Google Scholar] [CrossRef]
- Kim, K.Y.; Oh, T.W.; Do, H.J.; Yang, J.H.; Yang, I.J.; Jeon, Y.H.; Go, Y.H.; Ahn, S.C.; Ma, J.Y.; Park, K.I. Acer palmatum thumb. Ethanol extract alleviates interleukin-6-induced barrier dysfunction and dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation. J. Immunol. Res. 2018, 2018, 5718396. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhu, H.; Yao, X.M.; Qian, J.P.; Yang, J.; Pan, X.D.; Chen, X.D. Metformin regulates tight junction of intestinal epithelial cells via MLCK-MLC signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5239–5246. [Google Scholar]
- Araiza, V.; Mendoza, M.S.; Castro, K.E.N.; Cruz, S.M.; Rueda, K.C.; de Leon, C.T.G.; Morales Montor, J. Bisphenol A, an endocrine-disruptor compund, that modulates the immune response to infections. Front. Biosci. 2021, 26, 346–362. [Google Scholar] [CrossRef]
- Ma, Q.; Deng, P.; Lin, M.; Yang, L.; Li, L.; Guo, L.; Zhang, L.; He, M.; Lu, Y.; Pi, H.; et al. Long-term bisphenol A exposure exacerbates diet-induced prediabetes via TLR4-dependent hypothalamic inflammation. J. Hazard. Mater. 2021, 402, 123926. [Google Scholar] [CrossRef]
- Misme-Aucouturier, B.; De Carvalho, M.; Delage, E.; Dijoux, E.; Klein, M.; Brosseau, C.; Bodinier, M.; Guzylack-Piriou, L.; Bouchaud, G. Oral exposure to bisphenol A exacerbates allergic inflammation in a mouse model of food allergy. Toxicology 2022, 472, 153188. [Google Scholar] [CrossRef]
- Raza, A.; Johnson, H.; Singh, A.; Sharma, A.K. Impact of selenium nanoparticles in the regulation of inflammation. Arch. Biochem. Biophys. 2022, 732, 109466. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Liu, T.; Tan, S.; Wan, N.; Zhang, Y.; Li, S. Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency. Biol. Trace Elem. Res. 2018, 182, 364–372. [Google Scholar] [CrossRef]
- Tiwari, D.; Vanage, G. Bisphenol A Induces Oxidative stress in bone marrow cells, lymphocytes, and reproductive organs of holtzman Rats. Int. J. Toxicol. 2017, 36, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dai, S.; Hua, J.; Hu, H.; Wang, S.; Wen, A. Influence of dietary copper methionine concentrations on growth performance, digestibility of nutrients, serum lipid profiles, and immune defenses in broilers. Biol. Trace Elem. Res. 2019, 191, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Huo, B.; He, J.; Shen, X. Effects of selenium-deprived habitat on the immune index and antioxidant capacity of przewalski’s Gazelle. Biol. Trace Elem. Res. 2020, 198, 149–156. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Yan, H.; Qin, B.; Dong, Y.; Li, Z.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; et al. Maternal supplementation of organic selenium during gestation improves sows and offspring antioxidant capacity and inflammatory status and promotes embryo survival. Food Funct. 2020, 11, 7748–7761. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Liu, Y.L.; Xie, C.Y.; Zhang, B.; Huang, Y.Q.; Zhang, Y.W.; Yao, Y.; Huang, R.; Wu, X. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol. Trace Elem. Res. 2019, 189, 548–555. [Google Scholar] [CrossRef]
- Ozkemahli, G.; Erkekoglu, P.; Ercan, A.; Zeybek, N.D.; Yersal, N.; Kocer-Gumusel, B. Effects of single or combined exposure to bisphenol A and mono(2-ethylhexyl) phthalate on oxidant/antioxidant status, endoplasmic reticulum stress, and apoptosis in HepG2 cell line. Environ. Sci. Pollut. Res. Int. 2023, 30, 12189–12206. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Amaral, C.; Correia-da-Silva, G.; Almada, M.; Borges, M.; Cunha, S.C.; Fernandes, J.O.; Teixeira, N. Bisphenols A, F, S and AF trigger apoptosis and/or endoplasmic reticulum stress in human endometrial stromal cells. Toxicology 2022, 478, 153282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Huang, D.G.; Zhu, M.; Gao, C.Q.; Yan, H.C.; Li, X.G.; Wang, X.Q. Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress. J. Cell. Physiol. 2020, 235, 5613–5627. [Google Scholar] [CrossRef]
- Monceaux, K.; Gressette, M.; Karoui, A.; Pires Da Silva, J.; Piquereau, J.; Ventura-Clapier, R.; Garnier, A.; Mericskay, M.; Lemaire, C. Ferulic acid, pterostilbene, and tyrosol protect the heart from ER-stress-induced injury by activating SIRT1-dependent deacetylation of eIF2α. Int. J. Mol. Sci. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, B.; Guan, H.; Jiao, X.; Yang, J.; Cai, J.; Liu, Q.; Zhang, Z. Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. Biofactors 2021, 47, 788–800. [Google Scholar] [CrossRef]
- Yao, L.; Du, Q.; Yao, H.; Chen, X.; Zhang, Z.; Xu, S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 2015, 28, 255–265. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Ji, Y.; Li, J.; Dai, Z.; Wu, Z. Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling. Anim. Nutr. 2022, 8, 1–9. [Google Scholar] [CrossRef]


| Gene Product a | Primer Direction b | Sequence (5′ to 3′) | Product Size (bp) | Accession Number |
|---|---|---|---|---|
| GADPH | F | CCAGAACATCATCCCTGCTT | 229 | XM_021091114 |
| R | GTCCTCAGTGTAGCCCAGGA | |||
| Caspase-8 | F | TGGAGGACGTTTTCACAGGGC | 133 | XM_021074712 |
| R | AGTTGTAACCGGAGGCAAATCC | |||
| Caspase-9 | F R | AACTTCTGCCATGAGTCGGG GAGGTGGCTGGCCTTGG | 135 | XM_013998997 |
| Bcl-2 | F | AGCATGCGGCCTCTATTTGAT | 107 | XM_021099593 |
| R | CACTTATGGCCCAGATAGGCA | |||
| Bax | F | AGCAGATCATGAAGACAGGGG | 137 | XM_003127290 |
| R | ACACTCGCTCAACTTCTTGGT | |||
| IL-6 | F | GGCTGTGCAGATTAGTACC | 124 | JQ_839263 |
| R | CTGTGACTGCAGCTTATCC | |||
| IL-10 | F | CTTGTTGCTGACCGGGTCTC | 110 | HQ_236499 |
| R | TCTCTGCCTTCGGCATTACG | |||
| IL-17 | F | GACGGCCCTCAGATTACTCC | 125 | KF_646141 |
| R | AGCATTGATACAGCCCGAGT | |||
| IL-1β | F | GCCAACGTGCAGTCTATGGAGTG | 91 | XM_021085847 |
| R | GGTGGAGAGCCTTCAGCATGTG | |||
| TNF-α | F | GCCCTTCCACCAACGTTTTC | 97 | JF_831365 |
| R | CAAGGGCTCTTGATGGCAGA | |||
| Ifn-γ | F | CAGGCCATTCAAAGGAGCAT | 150 | MH_538101 |
| R | GAGTTCACTGATGGCTTTGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Huang, J.; Hu, T.; Zhang, Y.; Zhang, L.; Zhang, J.; Cui, D.; Li, L.; Wang, J.; Wu, Q. Protective Effects of Selenium Nanoparticles against Bisphenol A-Induced Toxicity in Porcine Intestinal Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 7242. https://doi.org/10.3390/ijms24087242
Pan Z, Huang J, Hu T, Zhang Y, Zhang L, Zhang J, Cui D, Li L, Wang J, Wu Q. Protective Effects of Selenium Nanoparticles against Bisphenol A-Induced Toxicity in Porcine Intestinal Epithelial Cells. International Journal of Molecular Sciences. 2023; 24(8):7242. https://doi.org/10.3390/ijms24087242
Chicago/Turabian StylePan, Zaozao, Jiaqiang Huang, Ting Hu, Yonghong Zhang, Lingyu Zhang, Jiaxi Zhang, Defeng Cui, Lu Li, Jing Wang, and Qiong Wu. 2023. "Protective Effects of Selenium Nanoparticles against Bisphenol A-Induced Toxicity in Porcine Intestinal Epithelial Cells" International Journal of Molecular Sciences 24, no. 8: 7242. https://doi.org/10.3390/ijms24087242
APA StylePan, Z., Huang, J., Hu, T., Zhang, Y., Zhang, L., Zhang, J., Cui, D., Li, L., Wang, J., & Wu, Q. (2023). Protective Effects of Selenium Nanoparticles against Bisphenol A-Induced Toxicity in Porcine Intestinal Epithelial Cells. International Journal of Molecular Sciences, 24(8), 7242. https://doi.org/10.3390/ijms24087242







