Copper- and Silver-Containing Heterometallic Iodobismuthates: Features of Thermochromic Behavior
Abstract
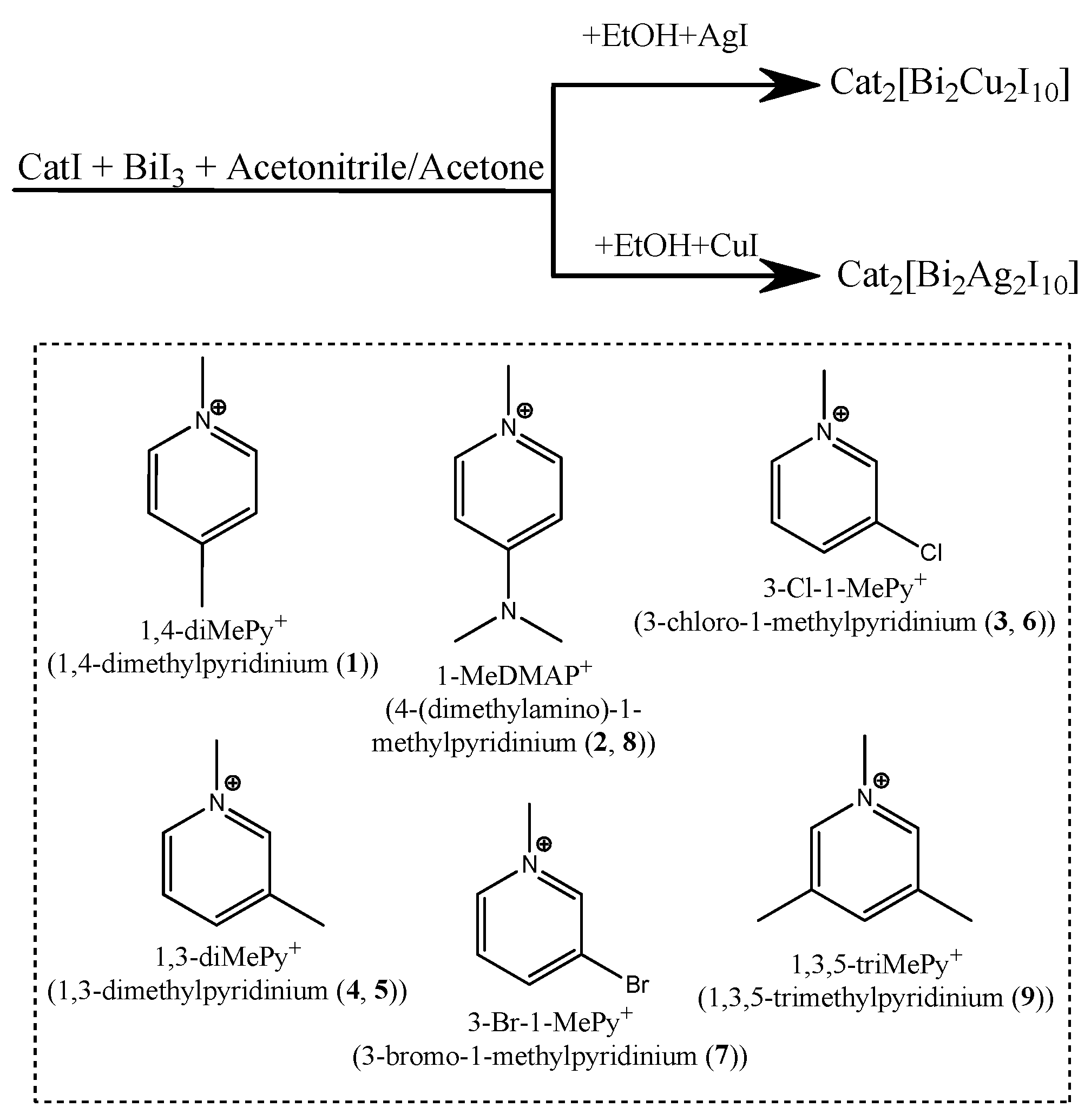
1. Introduction
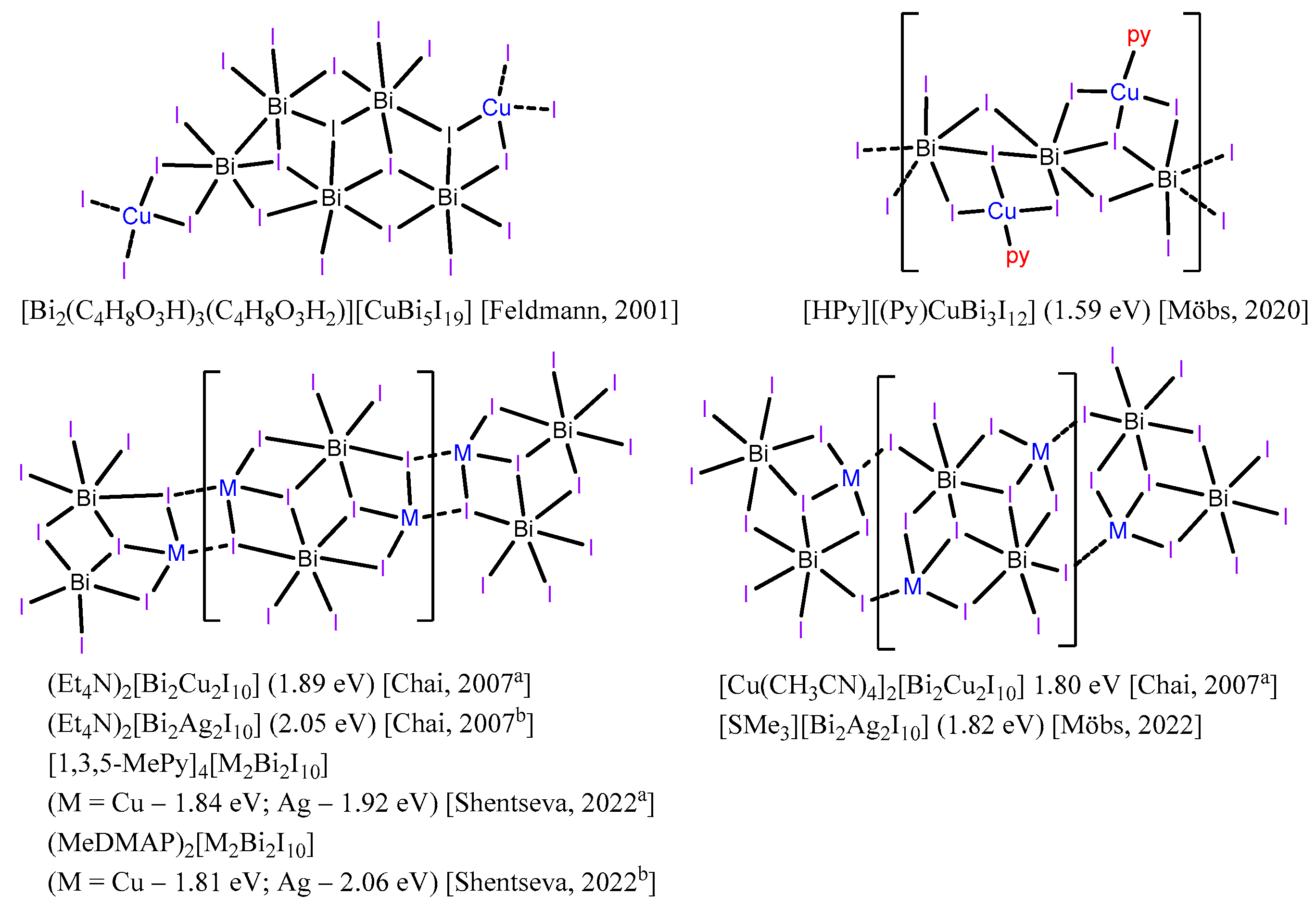
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Compounds 1, 3 and 4
3.2. Preparation of Compounds 5–7
3.3. Thermogravimetric Analysis
3.4. Diffuse Reflectance Spectroscopy
3.5. Single-Crystal X-ray Diffraction Data Collection
3.6. Powder X-ray Diffraction Data Collection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.M.; Wu, X.T.; Chen, L. Structural Overview and Structure-Property Relationships of Iodoplumbate and Iodobismuthate. Coord. Chem. Rev. 2009, 253, 2787–2804. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polynuclear Halide Complexes of Bi(III): From Structural Diversity to the New Properties. Coord. Chem. Rev. 2016, 312, 1–21. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, Z.K.; Liao, L.S. Progress of Lead-Free Halide Double Perovskites. Adv. Energy Mater. 2019, 9, 1803150. [Google Scholar] [CrossRef]
- Szklarz, P.; Jakubas, R.; Gągor, A.; Bator, G.; Cichos, J.; Karbowiak, M. [NH2CHNH2]3Sb2I9: A Lead-Free and Low-Toxicity Organic–Inorganic Hybrid Ferroelectric Based on Antimony(III) as a Potential Semiconducting Absorber. Inorg. Chem. Front. 2020, 7, 1780–1789. [Google Scholar] [CrossRef]
- Owczarek, M.; Szklarz, P.; Jakubas, R. Towards Ferroelectricity-Inducing Chains of Halogenoantimonates(III) and Halogenobismuthates(III). RSC Adv. 2021, 11, 17574–17586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Peng, H.; Liao, W.Q. A Lead-Free Bismuth Iodide Organic-Inorganic Ferroelectric Semiconductor. Chem. Commun. 2021, 57, 647–650. [Google Scholar] [CrossRef]
- Szklarz, P.; Jakubas, R.; Medycki, W.; Gągor, A.; Cichos, J.; Karbowiak, M.; Bator, G. (C3N2H5)3Sb2I9 and (C3N2H5)3Bi2I9: Ferroelastic Lead-Free Hybrid Perovskite-like Materials as Potential Semiconducting Absorbers. Dalt. Trans. 2022, 51, 1850–1860. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Yang, X.; Jia, D.; Zhao, S. Syntheses and Photocatalytic Properties of Polymeric Iodoargentate and Pb-Iodoargentate Hybrids Incorporating Lanthanide Complex. Inorg. Chim. Acta 2022, 537, 120962. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Wang, W.; Chen, X.; Zhang, B.; Li, L. [NH4][Ni(Phen)3]BiI6: Synthesis, Structure, Photocatalytic Property and Theoretical Study of a Discrete Iodobismuthate. Inorg. Chem. Commun. 2021, 130, 108714. [Google Scholar] [CrossRef]
- Jiang, X.F.; Wei, Q.; Ge, B.D.; Fu, A.P.; Li, J.H.; Wang, G.M. Optical and Photocatalytic Properties of Conjugated-Organic-Templates Derived Semiconducting Iodocuprates Hybrids. Opt. Mater. 2020, 109, 110376. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Zhao, J.Q.; Han, Y.F.; Yang, J.T.; Meng, R.R.; Gao, C.S.; Ding, H.; Wang, C.Y.; Chen, W.D. Low-Dimensional Hybrid Cuprous Halides Directed by Transition Metal Complex: Syntheses, Crystal Structures, and Photocatalytic Properties. Cryst. Growth Des. 2015, 15, 5416–5426. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Feng, L.J.; Han, Y.F.; Meng, R.R.; Yang, J.T.; Ding, H.; Gao, C.S.; Wang, C.Y. Syntheses, Crystal Structures and Photocatalytic Properties of Four Hybrid Iodoargentates with Zero- and Two-Dimensional Structures. CrystEngComm 2016, 18, 427–436. [Google Scholar] [CrossRef]
- Skorokhod, A.; Mercier, N.; Allain, M.; Manceau, M.; Katan, C.; Kepenekian, M. From Zero- To One-Dimensional, Opportunities and Caveats of Hybrid Iodobismuthates for Optoelectronic Applications. Inorg. Chem. 2021, 60, 17123–17131. [Google Scholar] [CrossRef] [PubMed]
- Anyfantis, G.C.; Ioannou, A.; Barkaoui, H.; Abid, Y.; Psycharis, V.; Raptopoulou, C.P.; Mousdis, G.A. Hybrid Halobismuthates as Prospective Light-Harvesting Materials: Synthesis, Crystal, Optical Properties and Electronic Structure. Polyhedron 2020, 175, 114180. [Google Scholar] [CrossRef]
- Kotov, V.Y.; Buikin, P.A.; Ilyukhin, A.B.; Korlyukov, A.A.; Dorovatovskii, P.V. Synthesis and First-Principles Study of Structural, Electronic and Optical Properties of Tetragonal Hybrid Halobismuthathes [Py2(XK)]2[Bi2Br10−xIx]. New J. Chem. 2021, 45, 18349–18357. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Yelavik, N.A.; Mironov, A.V.; Kuznetsov, A.N.; Bykov, M.A.; Grigorieva, A.V.; Utochnikova, V.V.; Lepnev, L.S.; Shevelkov, A.V. From Isolated Anions to Polymer Structures through Linking with I2: Synthesis, Structure, and Properties of Two Complex Bismuth(III) Iodine Iodides. Inorg. Chem. 2018, 57, 4077–4087. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Xiu, J.; Song, H.; Gatti, T.; He, Z. A Critical Review on Bismuth and Antimony Halide Based Perovskites and Their Derivatives for Photovoltaic Applications: Recent Advances and Challenges. J. Mater. Chem. A 2020, 8, 16166–16188. [Google Scholar] [CrossRef]
- Dehnhardt, N.; Borkowski, H.; Schepp, J.; Tonner, R.; Heine, J. Ternary Iodido Bismuthates and the Special Role of Copper. Inorg. Chem. 2018, 57, 633–640. [Google Scholar] [CrossRef]
- Feldmann, C. CuBi7I19(C4H8O3H)3(C4H8O3H2), a Novel Complex Bismuth Iodide Containing One-Dimensional [CuBi5I19]3− Chains. Inorg. Chem. 2001, 40, 818–819. [Google Scholar] [CrossRef]
- Dehnhardt, N.; Paneth, H.; Hecht, N.; Heine, J. Multinary Halogenido Bismuthates beyond the Double Perovskite Motif. Inorg. Chem. 2020, 59, 3394–3405. [Google Scholar] [CrossRef] [PubMed]
- Möbs, J.; Heine, J. 11/15/17 Complexes as Molecular Models for Metal Halide Double Perovskite Materials. Inorg. Chem. 2019, 58, 6175–6183. [Google Scholar] [CrossRef] [PubMed]
- Dehnhardt, N.; Klement, P.; Chatterjee, S.; Heine, J. Divergent Optical Properties in an Isomorphous Family of Multinary Iodido Pentelates. Inorg. Chem. 2019, 58, 10983–10990. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.W.; Wheaton, A.M.; Nicholas, A.D.; Barnes, F.H.; Patterson, H.H.; Pike, R.D. Iodobismuthate(III) and Iodobismuthate(III)/Iodocuprate(I) Complexes with Organic Ligands. Eur. J. Inorg. Chem. 2017, 2017, 4990–5000. [Google Scholar] [CrossRef]
- Chai, W.X.; Wu, L.M.; Li, J.Q.; Chen, L. A Series of New Copper Lodobismuthates: Structural Relationships, Optical Band Gaps Affected by Dimensionality, and Distinct Thermal Stabilities. Inorg. Chem. 2007, 46, 8698–8704. [Google Scholar] [CrossRef]
- Chai, W.-X.; Wu, L.-M.; Li, J.-Q.; Chen, L. Silver Iodobismuthates: Syntheses, Structures, Properties, and Theoretical Studies of [Bi2 Ag2I102−]n and [Bi4Ag2I162−]n. Inorg. Chem. 2007, 46, 1042–1044. [Google Scholar] [CrossRef]
- Möbs, J.; Pan, S.; Tonner-Zech, R.; Heine, J. [SMe3]2[Bi2Ag2I10], a Silver Iodido Bismuthate with an Unusually Small Band Gap. Dalt. Trans. 2022, 51, 13771–13778. [Google Scholar] [CrossRef]
- Möbs, J.; Gerhard, M.; Heine, J. (HPy)2(Py)CuBi3I12, a Low Bandgap Metal Halide Photoconductor. Dalt. Trans. 2020, 49, 14397–14400. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, H.; Li, D.; Wu, W.; Li, L.; Luo, J. A Lead-Free I-Based Hybrid Double Perovskite (I-C4H8NH3)4AgBiI8 for X-Ray Detection. J. Mater. Chem. C 2021, 9, 13157–13161. [Google Scholar] [CrossRef]
- Bi, L.-Y.; Hu, Y.-Q.; Li, M.-Q.; Hu, T.-L.; Zhang, H.-L.; Yin, X.-T.; Que, W.-X.; Lassoued, M.S.; Zheng, Y.-Z. Two-Dimensional Lead-Free Iodide-Based Hybrid Double Perovskites: Crystal Growth, Thin-Film Preparation and Photocurrent Responses. J. Mater. Chem. A 2019, 7, 19662–19667. [Google Scholar] [CrossRef]
- Cai, Y.; Chippindale, A.M.; Curry, R.J.; Vaqueiro, P. Multiple Roles of 1,4-Diazabicyclo[2.2.2]Octane in the Solvothermal Synthesis of Iodobismuthates. Inorg. Chem. 2021, 60, 5333–5342. [Google Scholar] [CrossRef] [PubMed]
- Möbs, J.; Luy, J.N.; Shlyaykher, A.; Tonner, R.; Heine, J. The Influence of Copper on the Optical Band Gap of Heterometallic Iodido Antimonates and Bismuthates. Dalt. Trans. 2021, 50, 15855–15869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Pang, M.; Wang, Y.S.; Liu, M.Z.; Zhao, H.M. Four Discrete Silver Iodobismuthates/Bromobismuthates with Metal Complexes: Syntheses, Structures, Photocurrent Responses, and Theoretical Studies. Inorg. Chem. 2022, 61, 406–413. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Pang, M.; Chen, X.; Liu, M.-Z. Two [Co(Bipy)3]3+ -Templated Silver Halobismuthate Hybrids: Syntheses, Structures, Photocurrent Responses, and Theoretical Studies. Inorg. Chem. 2022, 61, 9808–9815. [Google Scholar] [CrossRef] [PubMed]
- Hrizi, C.; Trigui, A.; Abid, Y.; Chniba-Boudjada, N.; Bordet, P.; Chaabouni, S. α- to β-[C6H4(NH3)2]2Bi2I10 Reversible Solid-State Transition, Thermochromic and Optical Studies in the p-Phenylenediamine-Based Iodobismuthate(III) Material. J. Solid State Chem. 2011, 184, 3336–3344. [Google Scholar] [CrossRef]
- Goforth, A.M.; Tershansy, M.A.; Smith, M.D.; Peterson, L.R.; Kelley, J.G.; DeBenedetti, W.J.I.; Zur Loye, H.C. Structural Diversity and Thermochromic Properties of Iodobismuthate Materials Containing D-Metal Coordination Cations: Observation of a High Symmetry [Bi3I11]2− Anion and of Isolated I− Anions. J. Am. Chem. Soc. 2011, 133, 603–612. [Google Scholar] [CrossRef]
- Wang, D.H.; Zhao, L.M.; Lin, X.Y.; Wang, Y.K.; Zhang, W.T.; Song, K.Y.; Li, H.H.; Chen, Z.R. Iodoargentate/Iodobismuthate-Based Materials Hybridized with Lanthanide-Containing Metalloviologens: Thermochromic Behaviors and Photocurrent Responses. Inorg. Chem. Front. 2018, 5, 1162–1173. [Google Scholar] [CrossRef]
- Gagor, A.; Wecławik, M.; Bondzior, B.; Jakubas, R. Periodic and Incommensurately Modulated Phases in a (2-Methylimidazolium)Tetraiodobismuthate(III) Thermochromic Organic-Inorganic Hybrid. CrystEngComm 2015, 17, 3286–3296. [Google Scholar] [CrossRef]
- Yu, T.L.; Guo, Y.M.; Wu, G.X.; Yang, X.F.; Xue, M.; Fu, Y.L.; Wang, M.S. Recent Progress of D10 Iodoargentate(I)/Iodocuprate(I) Hybrids: Structural Diversity, Directed Synthesis, and Photochromic/Thermochromic Properties. Coord. Chem. Rev. 2019, 397, 91–111. [Google Scholar] [CrossRef]
- Shayapov, V.R.; Usoltsev, A.N.; Adonin, S.A.; Sokolov, M.N.; Samsonenko, D.G.; Fedin, V.P. Thermochromism of Bromotellurates(IV): Experimental Insights. New J. Chem. 2019, 43, 3927–3930. [Google Scholar] [CrossRef]
- Shentseva, I.A.; Usoltsev, A.N.; Abramov, P.A.; Shayapov, V.R.; Plyusnin, P.E.; Korolkov, I.V.; Sokolov, M.N.; Adonin, S.A. Homo- and Heterometallic Iodobismuthates(III) with 1,3,5-Trimethylpyridinium Cation: Preparation and Features of Optical Behavior. Polyhedron 2022, 216, 115720. [Google Scholar] [CrossRef]
- Shentseva, I.A.; Usoltsev, A.N.; Abramov, P.A.; Shayapov, V.R.; Korobeynikov, N.A.; Sokolov, M.N.; Adonin, S.A. Copper- and Silver-Containing Heterometallic Iodobismuthates(III) with 4-(Dimethylamino)-1-Methylpyridinium Cation: Structures, Thermal Stability and Optical Properties. Mendeleev Commun. 2022, 32, 754–756. [Google Scholar] [CrossRef]
- Xue, Z.Z.; Meng, X.D.; Li, X.Y.; Han, S.D.; Pan, J.; Wang, G.M. Luminescent Thermochromism and White-Light Emission of a 3D [Ag4Br6] Cluster-Based Coordination Framework with Both Adamantane-like Node and Linker. Inorg. Chem. 2021, 60, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Li, X.X.; Yu, T.L.; Wang, F.; Hao, P.F.; Fu, Y.L. Ultrasensitive Photochromic Iodocuprate(I) Hybrid. Inorg. Chem. 2016, 55, 8271–8273. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Vázquez-García, D.; García-Fernández, A.; Marcos-Cives, I.; Platas-Iglesias, C.; Castro-García, S.; Sánchez-Andújar, M. Diimidazolium Halobismuthates [Dim]2[Bi2X10] (X = Cl−, Br−, or I−): A New Class of Thermochromic and Photoluminescent Materials. Inorg. Chem. 2018, 57, 7655–7664. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Qu, X.J.; Yu, J.H.; Xu, J.Q. A Chained Iodocuprate(I) and Its Photoluminescence Behavior. J. Clust. Sci. 2021, 32, 193–197. [Google Scholar] [CrossRef]
- Chen, X.; Jia, M.; Xu, W.; Pan, G.; Zhu, J.; Tian, Y.; Wu, D.; Li, X.; Shi, Z. Recent Progress and Challenges of Bismuth-Based Halide Perovskites for Emerging Optoelectronic Applications. Adv. Opt. Mater. 2022, 11, 2202153. [Google Scholar] [CrossRef]
- Li, H.; Yu, T.; An, L.; Wang, Y.; Shen, J.; Fu, Y.; Fu, Y. Heterometal Silver/Copper(I) Modulated Thermochromism of Two Isostructural Iodoplumbates. J. Solid State Chem. 2015, 221, 140–144. [Google Scholar] [CrossRef]
- Yu, T.; Fu, Y.; An, L.; Zhang, L.; Shen, J.; Fu, Y. Two Thermochromic Layered Iodoargentate Hybrids Directed by 4- and 3-Cyanopyridinium Cations. Cryst. Growth Des. 2014, 14, 3875–3879. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]




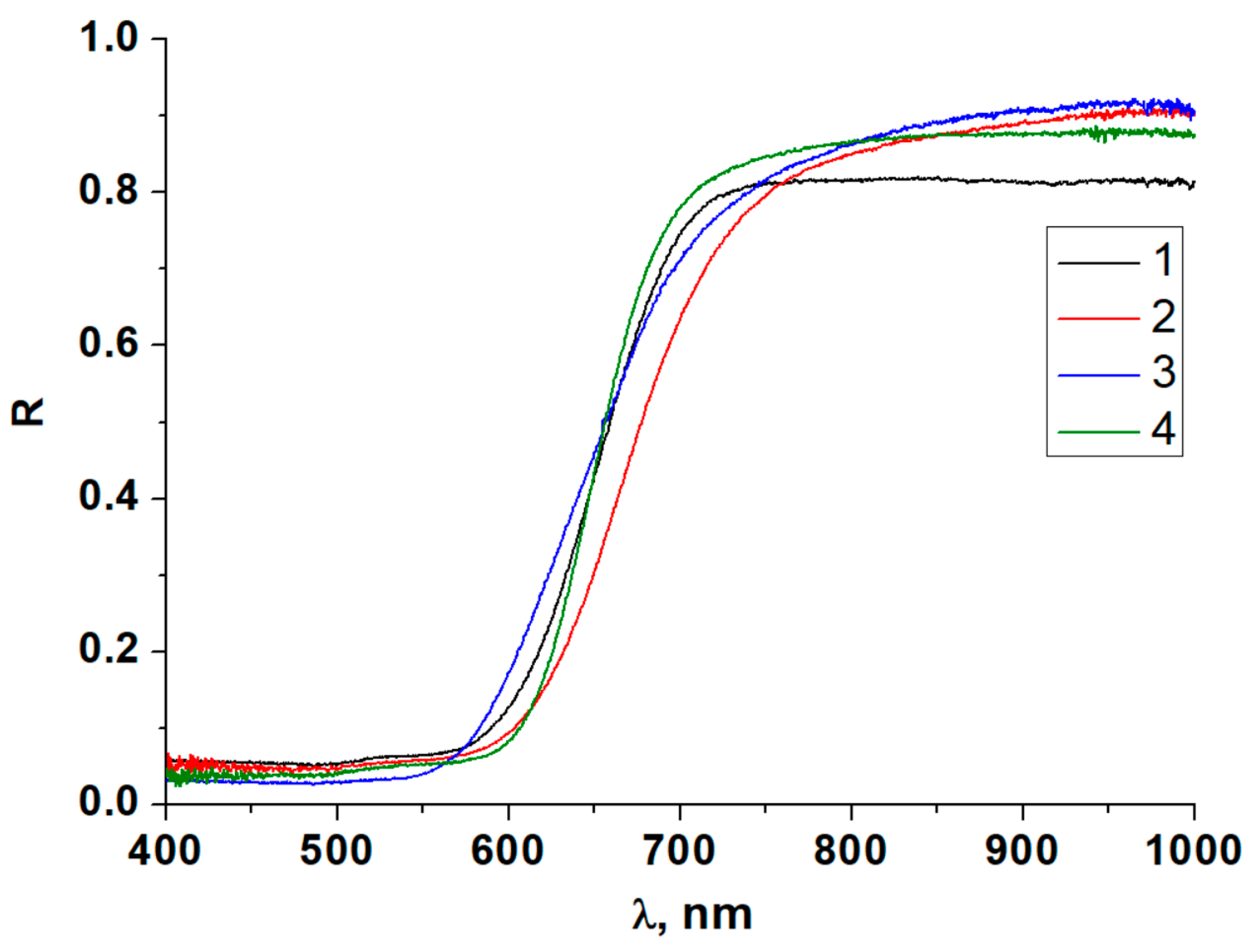
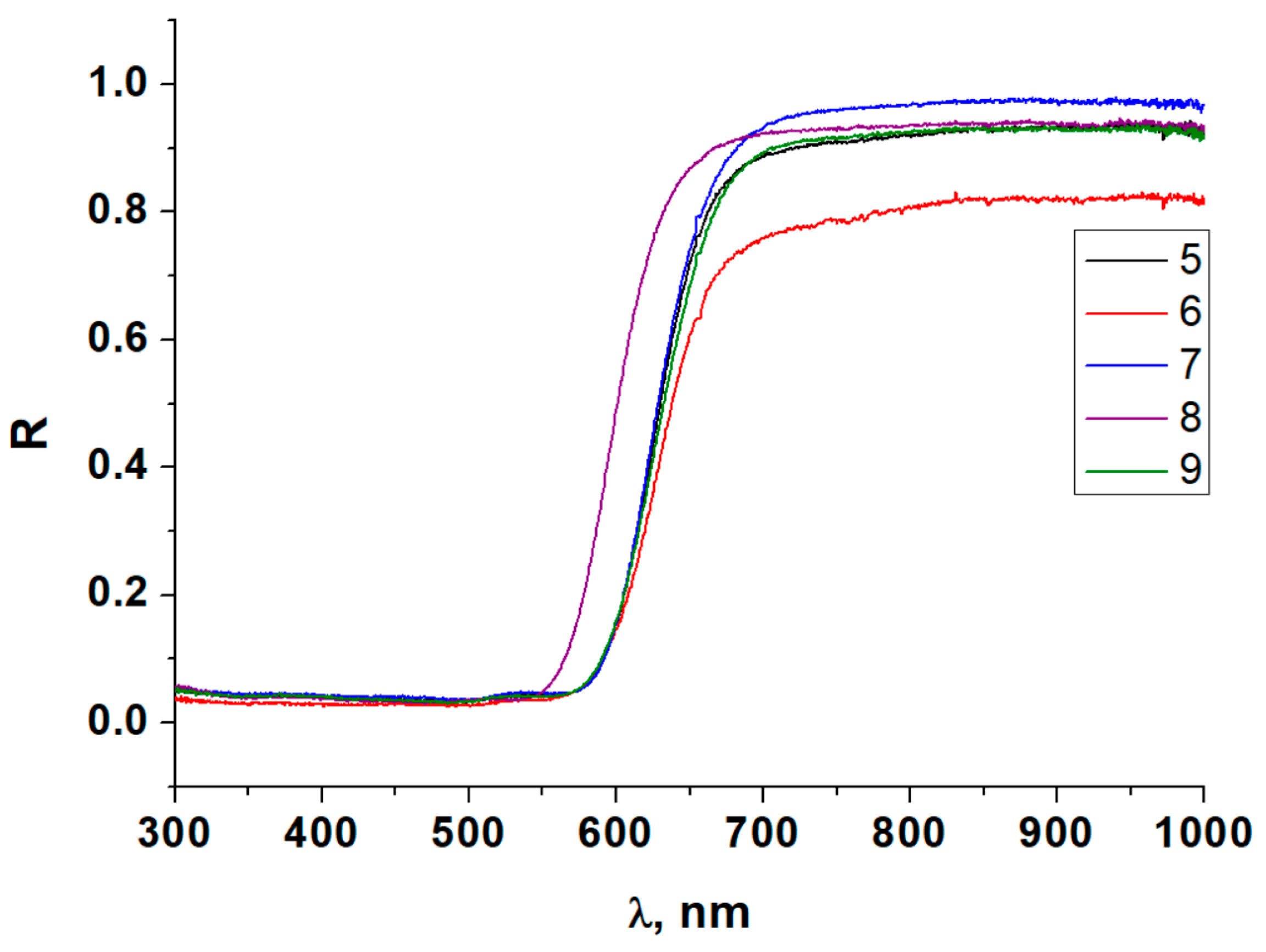

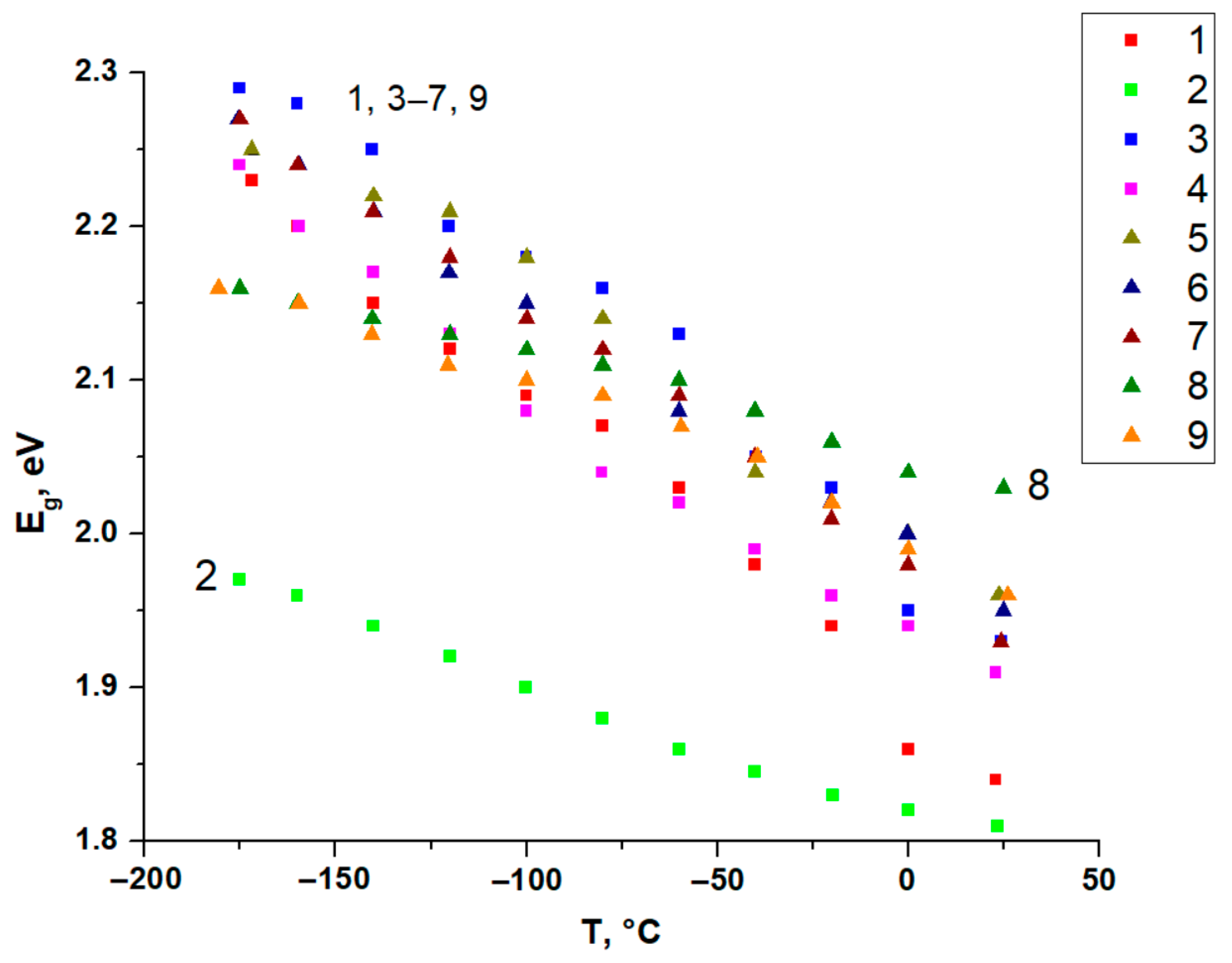
| Compound | TKEg, meV/°C | Compound | TKEg, eV/°C |
|---|---|---|---|
| 1 | −1.97 | 6 | −1.56 |
| 2 | −0.86 | 7 | −1.66 |
| 3 | −1.88 | 8 | −0.66 |
| 4 | −1.67 | 9 | −0.93 |
| 5 | −1.59 |
| Compound | C, H, N, Calculated/Found, % | Compound | C, H, N, Calculated/Found, % |
|---|---|---|---|
| 1 | 8.3, 1.0, 1.4/8.7, 1.2, 1.2 | 5 | 7.9, 1.0, 1.3/8.1, 1.2, 1.6 |
| 3 | 7.0, 0.7, 1.4/7.4, 1.0, 1.5 | 6 | 6.7, 0.7, 1.3/7.0, 1.3, 1.4 |
| 4 | 8.3, 1.0, 1.4/8.1, 1.2, 1.4 | 7 | 6.4, 0.6, 1.2/6.9, 1.0, 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shentseva, I.A.; Usoltsev, A.N.; Korobeynikov, N.A.; Sukhikh, T.S.; Shayapov, V.R.; Sokolov, M.N.; Adonin, S.A. Copper- and Silver-Containing Heterometallic Iodobismuthates: Features of Thermochromic Behavior. Int. J. Mol. Sci. 2023, 24, 7234. https://doi.org/10.3390/ijms24087234
Shentseva IA, Usoltsev AN, Korobeynikov NA, Sukhikh TS, Shayapov VR, Sokolov MN, Adonin SA. Copper- and Silver-Containing Heterometallic Iodobismuthates: Features of Thermochromic Behavior. International Journal of Molecular Sciences. 2023; 24(8):7234. https://doi.org/10.3390/ijms24087234
Chicago/Turabian StyleShentseva, Irina A., Andrey N. Usoltsev, Nikita A. Korobeynikov, Taisiya S. Sukhikh, Vladimir R. Shayapov, Maxim N. Sokolov, and Sergey A. Adonin. 2023. "Copper- and Silver-Containing Heterometallic Iodobismuthates: Features of Thermochromic Behavior" International Journal of Molecular Sciences 24, no. 8: 7234. https://doi.org/10.3390/ijms24087234
APA StyleShentseva, I. A., Usoltsev, A. N., Korobeynikov, N. A., Sukhikh, T. S., Shayapov, V. R., Sokolov, M. N., & Adonin, S. A. (2023). Copper- and Silver-Containing Heterometallic Iodobismuthates: Features of Thermochromic Behavior. International Journal of Molecular Sciences, 24(8), 7234. https://doi.org/10.3390/ijms24087234







