Immunophenotyping of Monocyte Migration Markers and Therapeutic Effects of Selenium on IL-6 and IL-1β Cytokine Axes of Blood Mononuclear Cells in Preoperative and Postoperative Coronary Artery Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Blood Collection, Plasma Isolation, and PBMC Culture
2.3. Selenium Concentration and Treatment
2.4. Quantification of IL1B Gene Expression by Real-Time PCR
2.5. Saphenous Vein Collection and Primary Vascular Endothelial Cell (pVECs) Isolation
2.6. Blood Monocytes and pVEC Phenotyping by Flow Cytometry
2.7. Array of Cytokines, Chemokine and Growth Factor Measurement by Luminex
2.8. Detection of Phosphorylation of STAT-3 and P65 and Cleaved Caspase-1 (p20 Subunit) by Western Blot
2.9. In Vitro Dynamic Analysis Using the Chandler Loop System
2.10. Statistical Analysis
3. Results
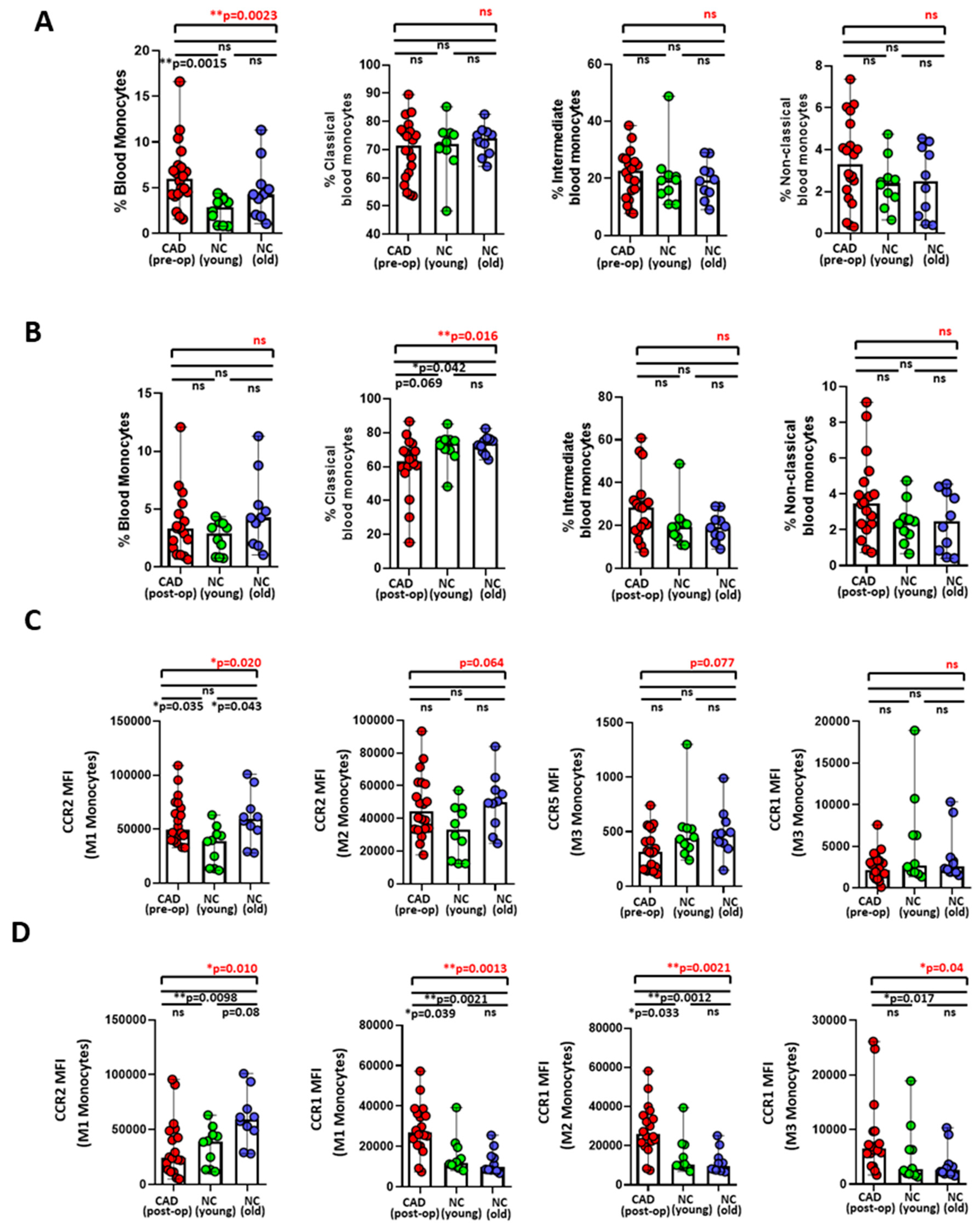
3.1. Differential Expression of Monocyte Migration Markers between Preoperative and Postoperative CAD Patients
3.2. Increased Plasma Levels of Inflammatory Cytokines among Postoperative CAD Patients
3.3. Monocyte Migration Marker, CCR1, and Plasma Cytokines Were Associated with CAD Related Clinical Parameters
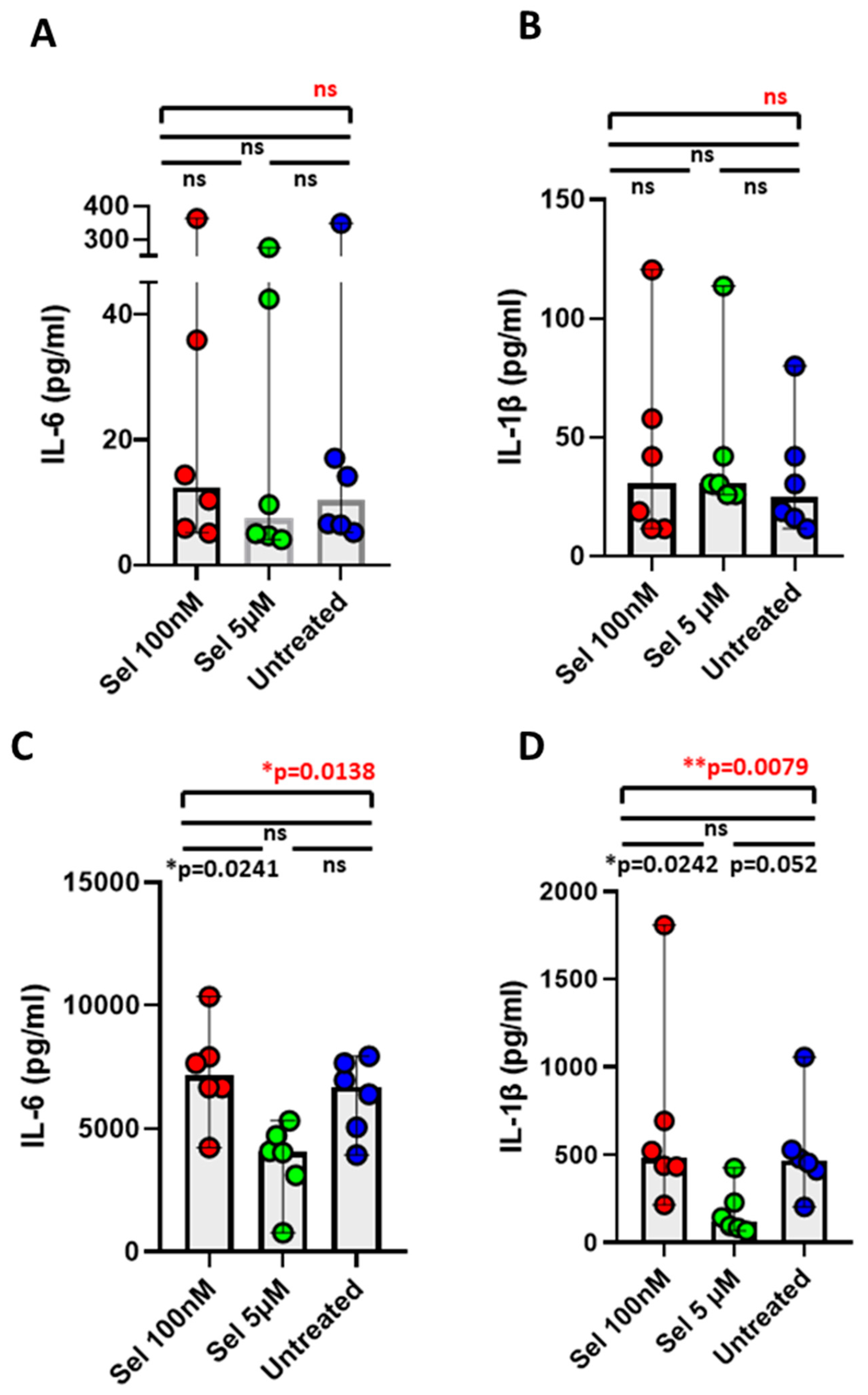
3.4. Effect of Selenium in Minimizing IL-6 and IL-1β Cytokines from Postoperative CAD Mononuclear Cells
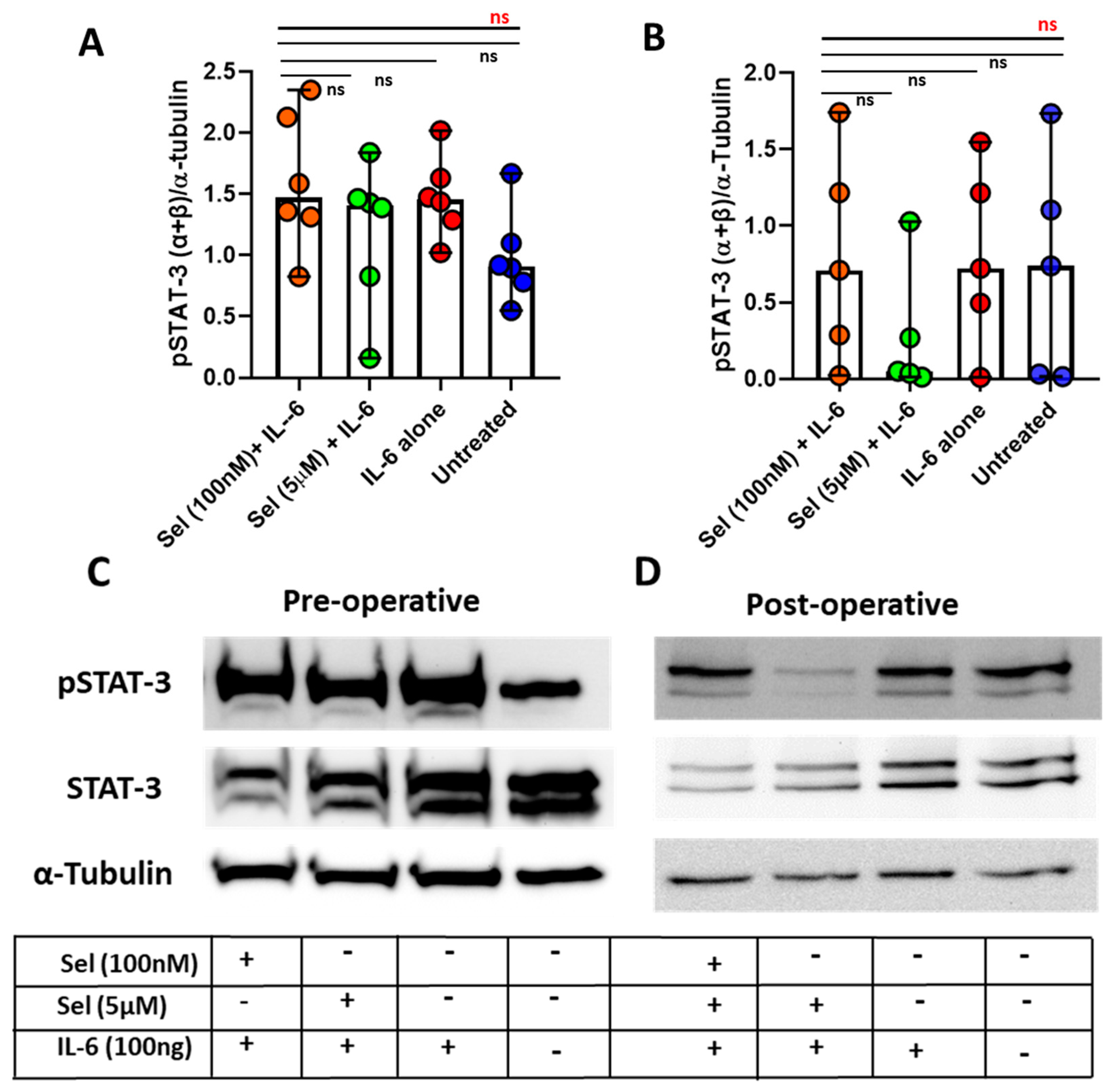
3.5. Markedly Lowered Phosphorylation of STAT-3 under the Influence of Selenium in Postoperative CAD Mononuclear Cells
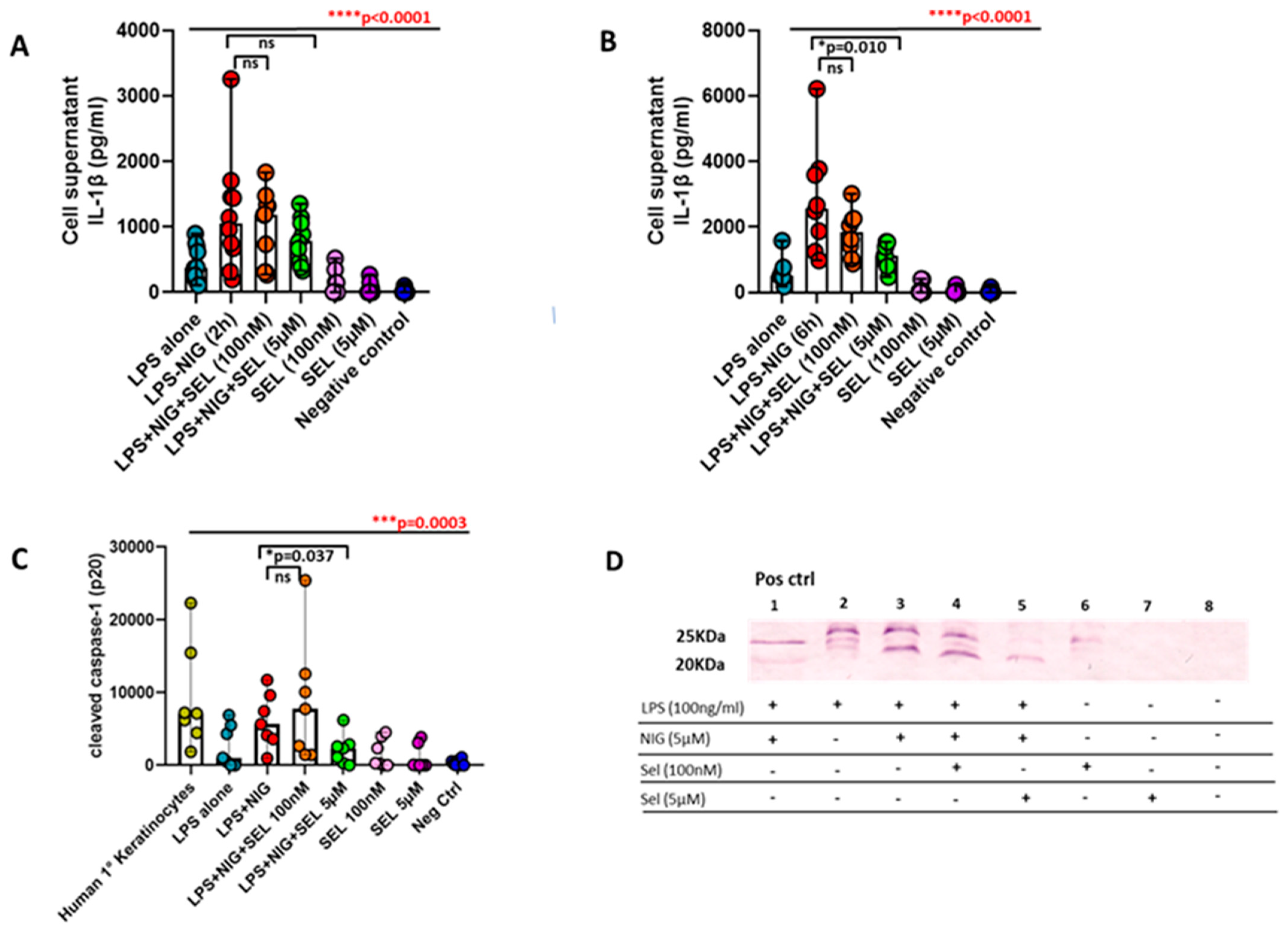
3.6. Curtailed Levels of IL-1β and Reduced Densities of Cleaved Caspase-1 under the Influence of Selenium in Preoperative CAD Mononuclear Cells
3.7. Negligible Effect of Selenium on Human Vascular Endothelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors For Coronary Artery Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Kühn, H.; Hennig, B.; Toborek, M. IL-4 induces apoptosis of endothelial cells through the caspase-3-dependent pathway. FEBS Lett. 2000, 485, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Prasad, K.M.; Butcher, M.; Dobrian, A.; Kolls, J.K.; Ley, K.; Galkina, E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 2010, 121, 1746–1755. [Google Scholar] [CrossRef]
- Mallat, Z.; Taleb, S.; Ait-Oufella, H.; Tedgui, A. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 2009, 50, S364–S369. [Google Scholar] [CrossRef]
- Moss, J.W.; Ramji, D.P. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Future Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, C.; Guo, J.; Yang, Y.; Huang, M.; Li, L.; Wang, Y.; Qin, Y.; Zhang, M. Serum Cytokines Predict the Severity of Coronary Artery Disease Without Acute Myocardial Infarction. Front. Cardiovasc. Med. 2022, 9, 896810. [Google Scholar] [CrossRef]
- Akdoğan, M.F.; Azak, A.; Denizli, N.; Huddam, B.; Koçak, G.; Gücün, M.; Tatlısu, M.A.; Demirci, R.; Yılmaz, B.; Dikeç, M.; et al. MCP-1 and soluble TWEAK levels are independently associated with coronary artery disease severity in patients with chronic kidney disease. Ren. Fail. 2015, 37, 1297–1302. [Google Scholar] [CrossRef]
- Wainstein, M.V.; Mossmann, M.; Araujo, G.N.; Gonçalves, S.C.; Gravina, G.L.; Sangalli, M.; Veadrigo, F.; Matte, R.; Reich, R.; Costa, F.G.; et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol. Metab. Syndr. 2017, 9, 67. [Google Scholar] [CrossRef]
- Herder, C.; de Las Heras Gala, T.; Carstensen-Kirberg, M.; Huth, C.; Zierer, A.; Wahl, S.; Sudduth-Klinger, J.; Kuulasmaa, K.; Peretz, D.; Ligthart, S.; et al. Circulating Levels of Interleukin 1-Receptor Antagonist and Risk of Cardiovascular Disease: Meta-Analysis of Six Population-Based Cohorts. Arter. Thromb. Vasc. Biol. 2017, 37, 1222–1227. [Google Scholar] [CrossRef]
- Schofer, N.; Ludwig, S.; Rübsamen, N.; Schnabel, R.; Lackner, K.J.; Ruprecht, H.J.; Bickel, C.; Landmesser, U.; Blankenberg, S.; Zeller, T. Prognostic impact of Interleukin-1 receptor antagonist in patients with documented coronary artery disease. Int. J. Cardiol. 2018, 257, 24–29. [Google Scholar] [CrossRef]
- Zhu, M.; Lei, L.; Zhu, Z.; Li, Q.; Guo, D.; Xu, J.; Chen, J.; Sha, H.; Zhang, X.; Yang, X.; et al. Excess TNF-α in the blood activates monocytes with the potential to directly form cholesteryl ester-laden cells. Acta Biochim. Biophys. Sin. 2015, 47, 899–907. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.R.; Fliser, D.; Heine, G.H. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 2011, 118, e50–e61. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, N.; Hibi, K.; Endo, M.; Sugano, T.; Ebina, T.; Kosuge, M.; Tsukahara, K.; Okuda, J.; Umemura, S.; Kimura, K. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ. J. 2010, 74, 1384–1391. [Google Scholar] [CrossRef]
- Shantsila, E.; Wrigley, B.; Tapp, L.; Apostolakis, S.; Montoro-Garcia, S.; Drayson, M.T.; Lip, G.Y. Immunophenotypic characterization of human monocyte subsets: Possible implications for cardiovascular disease pathophysiology. J. Thromb. Haemost. 2011, 9, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, A.; Heine, G.H.; Blankenberg, S.; Espinola-Klein, C.; Dopheide, J.F.; Bickel, C.; Lackner, K.J.; Iz, M.; Meyer, J.; Darius, H.; et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb. Haemost. 2004, 92, 419–424. [Google Scholar] [CrossRef]
- Eligini, S.; Cosentino, N.; Fiorelli, S.; Fabbiocchi, F.; Niccoli, G.; Refaat, H.; Camera, M.; Calligaris, G.; De Martini, S.; Bonomi, A.; et al. Biological profile of monocyte-derived macrophages in coronary heart disease patients: Implications for plaque morphology. Sci. Rep. 2019, 9, 8680. [Google Scholar] [CrossRef]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef]
- Combadière, C.; Potteaux, S.; Rodero, M.; Simon, T.; Pezard, A.; Esposito, B.; Merval, R.; Proudfoot, A.; Tedgui, A.; Mallat, Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 2008, 117, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, M.; Winslow, B.T.; Mills, K.; Dauber, I.M. Medical management of stable coronary artery disease. Am. Fam. Physician 2011, 83, 819–826. [Google Scholar]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar] [CrossRef]
- Shekar, P.S. Cardiology patient page. On-pump and off-pump coronary artery bypass grafting. Circulation 2006, 113, e51–e52. [Google Scholar] [CrossRef]
- Squiers, J.J.; Schaffer, J.M.; Banwait, J.K.; Ryan, W.H.; Mack, M.J.; DiMaio, J.M. Long-Term Survival After On-Pump and Off-Pump Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2022, 113, 1943–1952. [Google Scholar] [CrossRef]
- Hobson, C.E.; Yavas, S.; Segal, M.S.; Schold, J.D.; Tribble, C.G.; Layon, A.J.; Bihorac, A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009, 119, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Camacho, F.T.; Wechsler, A.S.; Lahey, S.; Culliford, A.T.; Jordan, D.; Gold, J.P.; Higgins, R.S.; Smith, C.R.; Hannan, E.L. Risk score for predicting long-term mortality after coronary artery bypass graft surgery. Circulation 2012, 125, 2423–2430. [Google Scholar] [CrossRef]
- Hinoue, T.; Yatabe, T.; Nishida, O. Prediction of postoperative atrial fibrillation with the systemic immune-inflammation index in patients undergoing cardiac surgery using cardiopulmonary bypass: A retrospective, single-center study. J. Artif. Organs 2022. [Google Scholar] [CrossRef] [PubMed]
- Kefalogianni, R.; Kamani, F.; Gaspar, M.; Aw, T.C.; Donovan, J.; Laffan, M.; Pickering, M.C.; Arachchillage, D.J. Complement activation during cardiopulmonary bypass and association with clinical outcomes. EJHaem 2022, 3, 86–96. [Google Scholar] [CrossRef]
- Zehr, K.J.; Poston, R.S.; Lee, P.C.; Uthoff, K.; Kumar, P.; Cho, P.W.; Gillinov, A.M.; Redmond, J.M.; Winkelstein, J.A.; Herskowitz, A.; et al. Platelet activating factor inhibition reduces lung injury after cardiopulmonary bypass. Ann. Thorac. Surg. 1995, 59, 328–335. [Google Scholar] [CrossRef]
- Kawamura, T.; Kadosaki, M.; Nara, N.; Kaise, A.; Suzuki, H.; Endo, S.; Wei, J.; Inada, K. Effects of sevoflurane on cytokine balance in patients undergoing coronary artery bypass graft surgery. J. Cardiothorac Vasc. Anesth. 2006, 20, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Wildhirt, S.M.; Schulze, C.; Schulz, C.; Egi, K.; Brenner, P.; Mair, H.; Schütz, A.; Reichart, B. Reduction of systemic and cardiac adhesion molecule expression after off-pump versus conventional coronary artery bypass grafting. Shock 2001, 16 (Suppl. S1), 55–59. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Lamy, A.; Chan, M.T.V.; Allard, R.V.; Lomivorotov, V.V.; Landoni, G.; Zheng, H.; Paparella, D.; McGillion, M.H.; Belley-Côté, E.P.; et al. High-Sensitivity Troponin I after Cardiac Surgery and 30-Day Mortality. N. Engl. J. Med. 2022, 386, 827–836. [Google Scholar] [CrossRef]
- Biglioli, P.; Cannata, A.; Alamanni, F.; Naliato, M.; Porqueddu, M.; Zanobini, M.; Tremoli, E.; Parolari, A. Biological effects of off-pump vs. on-pump coronary artery surgery: Focus on inflammation, hemostasis and oxidative stress. Eur. J. Cardiothorac Surg. 2003, 24, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Taylor, K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int. J. Surg. 2005, 3, 129–140. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Faridhosseini, R.; Mahini, M.; Faridhosseini, F.; Ranjbar, A. Serum Levels of TNF-, IL-6, and Selenium in Patients with Acute and Chronic Coronary Artery Disease. Iran. J. Immunol. 2006, 3, 142–145. [Google Scholar] [PubMed]
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef]
- Tang, X.; Mao, L.; Chen, J.; Zhang, T.; Weng, S.; Guo, X.; Kuang, J.; Yu, B.; Peng, D. High-sensitivity CRP may be a marker of HDL dysfunction and remodeling in patients with acute coronary syndrome. Sci. Rep. 2021, 11, 11444. [Google Scholar] [CrossRef] [PubMed]
- Atakan, A.; Macunluoglu, B.; Kaya, Y.; Ari, E.; Demir, H.; Asicioglu, E.; Kaspar, C. Decreased serum selenium levels are correlated with diminished coronary flow reserve among hemodialysis patients. Biol. Trace. Elem. Res. 2013, 155, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, A.; Coburn, M.; Menon, A.; Rossaint, R.; Heyland, D.; Schälte, G.; Werker, T.; Wonisch, W.; Kiehntopf, M.; Goetzenich, A.; et al. The importance of intraoperative selenium blood levels on organ dysfunction in patients undergoing off-pump cardiac surgery: A randomised controlled trial. PLoS ONE 2014, 9, e104222. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; McDonald, B.; Rex, S.; Manzanares, W.; Whitlock, R.; Fremes, S.; Fowler, R.; Lamarche, Y.; Meybohm, P.; Haberthür, C.; et al. SodiUm SeleniTe Adminstration IN Cardiac Surgery (SUSTAIN CSX-trial): Study design of an international multicenter randomized double-blinded controlled trial of high dose sodium-selenite administration in high-risk cardiac surgical patients. Trials 2014, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.T.M.; Nguyen, V.K.; Oberacher, H.; Fuchs, D.; Hernandez-Vargas, E.A.; Borucki, K.; Waldburg, N.; Wippermann, J.; Schreiber, J.; Bruder, D.; et al. Serum Concentration of the Phytohormone Abscisic Acid Is Associated With Immune-Regulatory Mediators and Is a Potential Biomarker of Disease Severity in Chronic Obstructive Pulmonary Disease. Front. Med. 2021, 8, 676058. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Riedel, J.; Walles, H.; Scherner, M.; Awad, G.; Varghese, S.; Schürlein, S.; Garke, B.; Veluswamy, P.; Wippermann, J.; et al. Comparative Evaluation on Impacts of Fibronectin, Heparin-Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro. Nanomater 2021, 11, 1952. [Google Scholar] [CrossRef]
- Neumann, Y.; Bruns, S.A.; Rohde, M.; Prajsnar, T.K.; Foster, S.J.; Schmitz, I. Intracellular Staphylococcus aureus eludes selective autophagy by activating a host cell kinase. Autophagy 2016, 12, 2069–2084. [Google Scholar] [CrossRef]
- Fink, H.; Hong, J.; Drotz, K.; Risberg, B.; Sanchez, J.; Sellborn, A. An in vitro study of blood compatibility of vascular grafts made of bacterial cellulose in comparison with conventionally-used graft materials. J. Biomed. Mater. Res. A 2011, 97, 52–58. [Google Scholar] [CrossRef]
- Wacker, M.; Betke, U.; Borucki, K.; Hülsmann, J.; Awad, G.; Varghese, S.; Scherner, M.; Hansen, M.; Wippermann, J.; Veluswamy, P. An In Vitro Hemodynamic Loop Model to Investigate the Hemocytocompatibility and Host Cell Activation of Vascular Medical Devices. J. Vis. Exp. 2020, 162, e61570. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Klein, L.W.; Nathan, S. Coronary artery disease in young adults**Editorials published in the Journal of the American College of Cardiologyreflect the views of the authors and do not necessarily represent the views of JACCor the American College of Cardiology. J. Am. Coll. Cardiol. 2003, 41, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.B.; Borden, W.B. Coronary heart disease in young adults. Curr. Atheroscler. Rep. 2012, 14, 140–149. [Google Scholar] [CrossRef]
- Tu, L.N.; Hsieh, L.; Kajimoto, M.; Charette, K.; Kibiryeva, N.; Forero, A.; Hampson, S.; Marshall, J.A.; O’Brien, J.; Scatena, M.; et al. Shear stress associated with cardiopulmonary bypass induces expression of inflammatory cytokines and necroptosis in monocytes. JCI Insight. 2021, 6, e141341. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.L.; Peters, W.; Si, Y.; Slaymaker, S.; Aslanian, A.M.; Weisberg, S.P.; Mack, M.; Charo, I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 2007, 117, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Bernhagen, J.; Heitman, L.H.; Weber, C.; Dichgans, M. Targeting the CCL2-CCR2 axis for atheroprotection. Eur. Heart J. 2022, 43, 1799–1808. [Google Scholar] [CrossRef]
- Breland, U.M.; Michelsen, A.E.; Skjelland, M.; Folkersen, L.; Krohg-Sørensen, K.; Russell, D.; Ueland, T.; Yndestad, A.; Paulsson-Berne, G.; Damås, J.K.; et al. Raised MCP-4 levels in symptomatic carotid atherosclerosis: An inflammatory link between platelet and monocyte activation. Cardiovasc Res. 2010, 86, 265–273. [Google Scholar] [CrossRef]
- Karlmark, K.R.; Zimmermann, H.W.; Roderburg, C.; Gassler, N.; Wasmuth, H.E.; Luedde, T.; Trautwein, C.; Tacke, F. The fractalkine receptor CX₃CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010, 52, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, M.H.; Yi, H.S.; Kim, S.Y.; Kim, H.H.; Kim, J.H.; Yeon, J.E.; Byun, K.S.; Byun, J.S.; Jeong, W.I. CX(3)CR1 differentiates F4/80(low) monocytes into pro-inflammatory F4/80(high) macrophages in the liver. Sci. Rep. 2018, 8, 15076. [Google Scholar] [CrossRef]
- Kaufmann, A.; Salentin, R.; Gemsa, D.; Sprenger, H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J. Leukoc. Biol. 2001, 69, 248–252. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, M.; Xu, X.; Wen, X.; Yang, G.; Li, L.; Sheng, P.; Meng, F. CCL3/CCR1 mediates CD14(+)CD16(−) circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil. 2020, 28, 613–625. [Google Scholar] [CrossRef]
- Mahad, D.J.; Trebst, C.; Kivisäkk, P.; Staugaitis, S.M.; Tucky, B.; Wei, T.; Lucchinetti, C.F.; Lassmann, H.; Ransohoff, R.M. Expression of chemokine receptors CCR1 and CCR5 reflects differential activation of mononuclear phagocytes in pattern II and pattern III multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2004, 63, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Reese, C.; Perry, B.; Heywood, J.; Bonner, M.; Zemskova, M.; Silver, R.M.; Hoffman, S.; Tourkina, E. Enhanced chemokine-receptor expression, function, and signaling in healthy African American and scleroderma-patient monocytes are regulated by caveolin-1. Fibrogenesis Tissue Repair 2015, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, K.; Wada, T.; Sakai, N.; Iwata, Y.; Yoshimoto, K.; Shimizu, M.; Kobayashi, K.; Takasawa, K.; Kida, H.; Takeda, S.I.; et al. Distinct expression of CCR1 and CCR5 in glomerular and interstitial lesions of human glomerular diseases. Am. J. Nephrol. 2000, 20, 291–299. [Google Scholar] [CrossRef]
- Syrbe, U.; Moebes, A.; Scholze, J.; Swidsinski, A.; Dörffel, Y. Effects of the angiotensin II type 1 receptor antagonist telmisartan on monocyte adhesion and activation in patients with essential hypertension. Hypertens. Res. 2007, 30, 521–528. [Google Scholar] [CrossRef]
- Bauer, A.; Korten, I.; Juchem, G.; Kiesewetter, I.; Kilger, E.; Heyn, J. EuroScore and IL-6 predict the course in ICU after cardiac surgery. Eur. J. Med. Res. 2021, 26, 29. [Google Scholar] [CrossRef]
- Wu, Z.K.; Laurikka, J.; Vikman, S.; Nieminen, R.; Moilanen, E.; Tarkka, M.R. High postoperative interleukin-8 levels related to atrial fibrillation in patients undergoing coronary artery bypass surgery. World J. Surg. 2008, 32, 2643–2649. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakusawa, R.; Inada, K. Interleukin-10 and interleukin-1 receptor antagonists increase during cardiac surgery. Can. J. Anaesth. 1997, 44, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Kim, P.H.; Lee, W.H.; Hirani, A.A. Interleukin-4, Oxidative Stress, Vascular Inflammation and Atherosclerosis. Biomol. Ther. 2010, 18, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, A.; Tontonoz, P. Nuclear receptors in macrophage biology: At the crossroads of lipid metabolism and inflammation. Annu. Rev. Cell Dev. Biol. 2004, 20, 455–480. [Google Scholar] [CrossRef]
- Li, A.C.; Binder, C.J.; Gutierrez, A.; Brown, K.K.; Plotkin, C.R.; Pattison, J.W.; Valledor, A.F.; Davis, R.A.; Willson, T.M.; Witztum, J.L.; et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J. Clin. Investig. 2004, 114, 1564–1576. [Google Scholar] [CrossRef]
- Ditiatkovski, M.; Toh, B.H.; Bobik, A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arter. Thromb. Vasc. Biol. 2006, 26, 2337–2344. [Google Scholar] [CrossRef]
- Xu, J.Y.; Xiong, Y.Y.; Tang, R.J.; Jiang, W.Y.; Ning, Y.; Gong, Z.T.; Huang, P.S.; Chen, G.H.; Xu, J.; Wu, C.X.; et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc Res. 2022, 118, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Kudlová, M.; Kolácková, M.; Kunes, P.; Andrýs, C.; Jankovicová, K.; Mand’ák, J.; Lonský, V.; Krejsek, J. Cardiac surgery operations and their influence on serum level of antiinflammatory cytokine interleukin-10. Cas. Lek. Cesk. 2007, 146, 48–55; discussion 46–55. [Google Scholar]
- Rodríguez-Reyna, T.S.; Arrieta, O.; Castillo-Martínez, L.; Orea-Tejeda, A.; Guevara, P.; Rebollar, V.; Granados, J. Tumour Necrosis Factor alpha and Troponin T as predictors of poor prognosis in patients with stable heart failure. Clin. Investig. Med. 2005, 28, 23–29. [Google Scholar]
- Lee, M.M.; Chui, R.K.; Tam, I.Y.; Lau, A.H.; Wong, Y.H. CCR1-mediated STAT3 tyrosine phosphorylation and CXCL8 expression in THP-1 macrophage-like cells involve pertussis toxin-insensitive Gα(14/16) signaling and IL-6 release. J. Immunol. 2012, 189, 5266–5276. [Google Scholar] [CrossRef]
- Tseng, C.K.; Ho, C.T.; Hsu, H.S.; Lin, C.H.; Li, C.I.; Li, T.C.; Liu, C.S.; Lin, C.C.; Lin, W.Y. Selenium is inversely associated with interleukin-6 in the elderly. J. Nutr. Health Aging 2013, 17, 280–284. [Google Scholar] [CrossRef]
- Xiao, J.; Li, N.; Xiao, S.; Wu, Y.; Liu, H. Comparison of Selenium Nanoparticles and Sodium Selenite on the Alleviation of Early Atherosclerosis by Inhibiting Endothelial Dysfunction and Inflammation in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11612. [Google Scholar] [CrossRef]
- Zaghloul, R.A.; Abdelghany, A.M.; Samra, Y.A. Rutin and selenium nanoparticles protected against STZ-induced diabetic nephropathy in rats through downregulating Jak-2/Stat3 pathway and upregulating Nrf-2/HO-1 pathway. Eur. J. Pharmacol. 2022, 933, 175289. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, H.; Hao, Z.; Jing, X.; Zhao, Y.; Cheng, X.; Ma, H.; Wang, J.; Wang, J. Mitigation Effects of Selenium Nanoparticles on Depression-Like Behavior Induced by Fluoride in Mice via the JAK2-STAT3 Pathway. ACS Appl. Mater. Interfaces 2022, 14, 3685–3700. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Lei, Y.; Zhao, J.; Wei, W.; Li, Y. Effects of selenium on myocardial apoptosis by modifying the activity of mitochondrial STAT3 and regulating potassium channel expression. Exp. Ther. Med. 2017, 14, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Dinarello, C.A. Blocking interleukin-1β in acute and chronic autoinflammatory diseases. J. Intern. Med. 2011, 269, 16–28. [Google Scholar] [CrossRef]
- Biasucci, L.M.; Liuzzo, G.; Fantuzzi, G.; Caligiuri, G.; Rebuzzi, A.G.; Ginnetti, F.; Dinarello, C.A.; Maseri, A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation 1999, 99, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Van Zee, K.J.; Marano, M.A.; Rock, C.S.; Kenney, J.S.; Poutsiaka, D.D.; Dinarello, C.A.; Lowry, S.F.; Moldawer, L.L. Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood 1992, 79, 2196–2200. [Google Scholar] [CrossRef]
- Arend, W.P.; Smith, M.F., Jr.; Janson, R.W.; Joslin, F.G. IL-1 receptor antagonist and IL-1 beta production in human monocytes are regulated differently. J. Immunol. 1991, 147, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, A.; Yu, S.; Martin-Sanchez, F.; Diaz-Del-Olmo, I.; Nichols, E.M.; Davis, D.M.; Brough, D.; Lopez-Castejon, G. Priming Is Dispensable for NLRP3 Inflammasome Activation in Human Monocytes In Vitro. Front. Immunol. 2020, 11, 565924. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Samimi, M.; Afshar Ebrahimi, F.; Aghadavod, E.; Mohammadbeigi, R.; Rahimi, M.; Asemi, Z. Effects of Selenium Supplementation on Gene Expression Levels of Inflammatory Cytokines and Vascular Endothelial Growth Factor in Patients with Gestational Diabetes. Biol. Trace Elem Res. 2018, 181, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Veluswamy, P.; Wacker, M.; Scherner, M.; Wippermann, J. Delicate Role of PD-L1/PD-1 Axis in Blood Vessel Inflammatory Diseases: Current Insight and Future Significance. Int. J. Mol. Sci. 2020, 21, 8159. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yang, M.; Wang, Y.-H.; Lande, R.; Gregorio, J.; Perng, O.A.; Qin, X.-F.; Liu, Y.-J.; Gilliet, M. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007, 204, 105–115. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | CAD (Pre-Op) | CAD (Post-Op) | CAD (Vein Donor) | Control (Young) | Control (Old) |
|---|---|---|---|---|---|
| Number of Subjects (n) | 47 | 26 | 4 | 10 | 10 |
| Mean Age (years) (Min–Max) | 65 (40–82) | 71 (59–84) | 74 (71–78) | 27 (21–39) | 56 (50–64) |
| Gender (men/women) | 35/12 | 17/8 | 4/0 | 5/5 | 3/7 |
| Mean body mass index (Min–Max) | 29.4 (21.1–42.4) | 28.5 (21.9–35) | 27.9 (24.4–30.6) | ||
| CAD patient (1/2/3 vessel disease) | (1/5/41) | (0/2/23) | (0/0/4) | ||
| Mean Euroscore II (%) (Min–Max) | 2.57 (0.50–25.85) | 2.38 (0.78–5.13) | 2.08 (0.94–4.20) | ||
| Acute Infarctions No: STEMI < 1 week STEMI > 1 week NSTEMI < 1 week NSTEMI > 1 week | 38 3 1 0 2 | 15 1 1 0 4 | 4 0 0 0 0 | ||
| Leftventricular Ejection Fraction (LVEF) % (Mean) | 52 | 55 | 50 |
| Patient Clinical Paramaters | CAD (Pre-Op) | CAD (Post-Op) | CAD (Vein Donor) |
|---|---|---|---|
| A. Blood parameters | |||
| Leukocytes (Gpt/L) | 9.47 | 9.37 | 6.91 |
| Erythrocytes (Tpt/L) | 4.53 | 4.63 | 4.86 |
| Haemoglobin (mmol/L) | 8.45 | 8.82 | 9.20 |
| Hematocrit (L/L) | 0.40 | 0.41 | 0.44 |
| Mean corpuscular volume (MCV) (fL) | 88.97 | 90.09 | 91.18 |
| Mean corpuscular hemoglobin (MCH) (fmol) | 1.87 | 1.91 | 1.90 |
| Mean corpuscular hemoglobin concentration (MCHC) (mmol/L) | 20.99 | 21.2 | 20.80 |
| Thrombocyte count (Gpt/L) | 240.57 | 241.54 | 205.25 |
| Proportion, large platelets (%) | 30.90 | 31.12 | 27.20 |
| Mean thrombocyte volume (fL) | 10.75 | 10.70 | 10.23 |
| B. Clinical chemistry parameters (plasma) | |||
| Troponin T (ng/mL) | 0.16 | 0.11 | 0.02 |
| Sodium (mmol/L) | 139.06 | 140.95 | 140.50 |
| Potassium (mmol/L) | 4.09 | 3.93 | 4.39 |
| Creatinine (µmol/L) | 110.27 | 96.62 | 85.25 |
| Glucose (mmol/L) | 7.34 | 7.65 | 8.36 |
| Urea (mmol/L) | 6.33 | 5.58 | 6.88 |
| Alanine aminotrasferase (ALAT) (µmol/s.L) | 0.45 | 0.54 | 0.42 |
| Asparate amnotransferase (ASAT) (µmol/s.L) | 0.43 | 0.53 | 0.40 |
| Creatinine kinase (µmol/s.L) | 1.84 | 2.98 | 2.33 |
| Lipase ((µmol/s.L)) | 0.68 | 0.69 | 0.64 |
| Cholinestearse ((µmol/s.L)) | 138.63 | 132.8 | 140.75 |
| Lactate dehydrogenase (LDH) (µmol/s.L) | 3.56 | 3.34 | 3.61 |
| C-reactive protein (CRP) (mg/L) | 8.65 | 5.54 | 4.90 |
| C. Clotting parameters | |||
| Thromboplastin time (%) | 95.30 | 92.29 | 95.25 |
| International normalized ratio | 1.03 | 1.09 | 1.04 |
| Activated partial thromboplastin clot time (s) | 29.83 | 34.55 | 27.98 |
| Type of Medications | Preoperative Cohort (N) | Postoperative Cohort (N) |
|---|---|---|
| A. Regular Medications | ||
| Statins | 32 | 15 |
| Beta blockers | 35 | 11 |
| Oral antidiabetics | 17 | 9 |
| Calcium antagonists | 16 | 3 |
| AT2 receptor antagonists | 20 | 6 |
| Angiotensin converting enzyme (ACE) inhibitors | 18 | 9 |
| Insulin | 5 | 3 |
| Acetylsalicylic acid (ASA) | 43 | 15 |
| Oral antiplatelet therapy (other than ASA) | 6 | 6 |
| Oral anticoagulation | 7 | 2 |
| Thyroid drugs | 11 | 2 |
| Diuretics | 14 | 4 |
| Antibiotics | 0 | 0 |
| B. In-hospital Medications (before surgery) | ||
| Proton Pump Inhibitors (PPI) | All | All |
| Heparin | All | All |
| Antibiotics | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacker, M.; Ball, A.; Beer, H.-D.; Schmitz, I.; Borucki, K.; Azizzadeh, F.; Scherner, M.; Awad, G.; Wippermann, J.; Veluswamy, P. Immunophenotyping of Monocyte Migration Markers and Therapeutic Effects of Selenium on IL-6 and IL-1β Cytokine Axes of Blood Mononuclear Cells in Preoperative and Postoperative Coronary Artery Disease Patients. Int. J. Mol. Sci. 2023, 24, 7198. https://doi.org/10.3390/ijms24087198
Wacker M, Ball A, Beer H-D, Schmitz I, Borucki K, Azizzadeh F, Scherner M, Awad G, Wippermann J, Veluswamy P. Immunophenotyping of Monocyte Migration Markers and Therapeutic Effects of Selenium on IL-6 and IL-1β Cytokine Axes of Blood Mononuclear Cells in Preoperative and Postoperative Coronary Artery Disease Patients. International Journal of Molecular Sciences. 2023; 24(8):7198. https://doi.org/10.3390/ijms24087198
Chicago/Turabian StyleWacker, Max, Anna Ball, Hans-Dietmar Beer, Ingo Schmitz, Katrin Borucki, Faranak Azizzadeh, Maximilian Scherner, George Awad, Jens Wippermann, and Priya Veluswamy. 2023. "Immunophenotyping of Monocyte Migration Markers and Therapeutic Effects of Selenium on IL-6 and IL-1β Cytokine Axes of Blood Mononuclear Cells in Preoperative and Postoperative Coronary Artery Disease Patients" International Journal of Molecular Sciences 24, no. 8: 7198. https://doi.org/10.3390/ijms24087198
APA StyleWacker, M., Ball, A., Beer, H.-D., Schmitz, I., Borucki, K., Azizzadeh, F., Scherner, M., Awad, G., Wippermann, J., & Veluswamy, P. (2023). Immunophenotyping of Monocyte Migration Markers and Therapeutic Effects of Selenium on IL-6 and IL-1β Cytokine Axes of Blood Mononuclear Cells in Preoperative and Postoperative Coronary Artery Disease Patients. International Journal of Molecular Sciences, 24(8), 7198. https://doi.org/10.3390/ijms24087198







