Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes
Abstract
1. Introduction
2. Results
2.1. Immunological Characterisation of the Cells
2.2. Astrocyte Cellular Proteome Analysis after 2 h Exposure to Exogenous sAPPα
2.2.1. The Spectral Library
2.2.2. Changes in Astrocyte Cellular Proteome
2.2.3. Differentially Regulated Proteins of the Proteome Identified by an Individual Peptide
2.3. Astrocyte Secretome Analysis after 6 h Exposure to Exogenous sAPPα
2.3.1. The Spectral Library
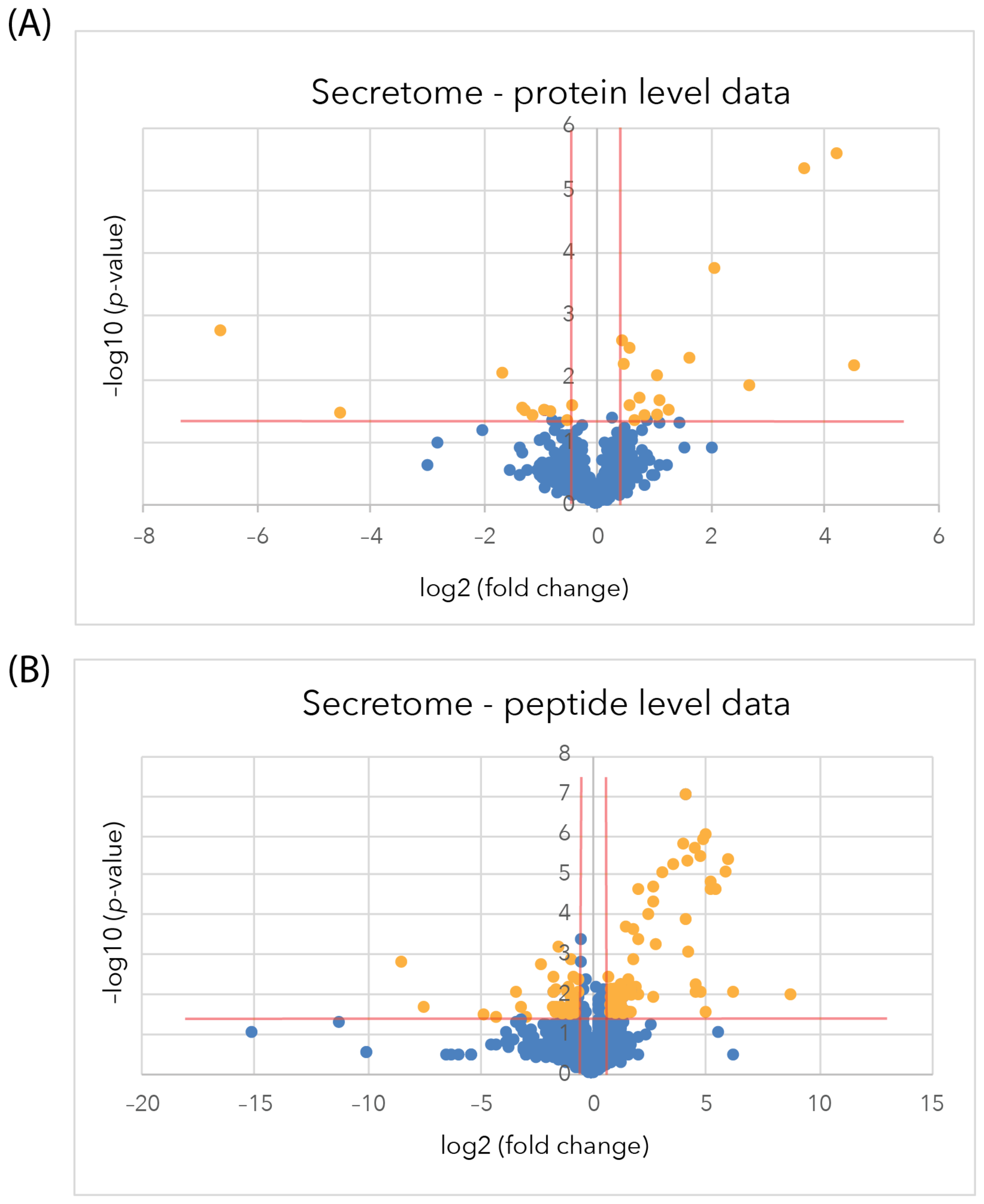
2.3.2. Changes in the Astrocyte Secretome at the Protein Level
2.3.3. Differentially Regulated Secretome Proteins Identified from Individual Peptides
2.4. Comparison of the Proteome and Secretome Differentially Regulated Proteins
3. Discussion
3.1. sAPPα Modulates the Proteome and Secretome of the Astrocyte
3.2. Astrocyte Differentially Regulated Proteins
3.2.1. APP- and AD-Related Proteins
3.2.2. Actin and Vesicle Dynamics
3.2.3. Myelin Sheath
3.2.4. ECM Organization
3.2.5. sAPPα as a Signaling Molecule
3.3. Functions of the Proteins Differentially Regulated in Cellular Proteome and Secretome
3.4. Limitations of the Study
4. Materials and Methods
4.1. Mouse Primary Astrocyte Cell Culture
4.2. Immunocytochemical Characterisation of Astrocytes
4.3. Preparation of the sAPPα Protein Used in the Study
4.4. Study Protocol
4.5. Protein Identification and Quantification by SWATH-MS
4.6. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, L.; Zhang, T.; Fan, K.; Cai, W.; Liu, H. Astrocyte-Neuron Signaling in Synaptogenesis. Front. Cell Dev. Biol. 2021, 9, 680301. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Doron, A.; Rubin, A.; Benmelech-Chovav, A.; Benaim, N.; Carmi, T.; Refaeli, R.; Novick, N.; Kreisel, T.; Ziv, Y.; Goshen, I. Hippocampal astrocytes encode reward location. Nature 2022, 609, 772–778. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nyengaard, J.R.; De Groot, D.M. Total regional and global number of synapses in the human brain neocortex. Synapse 2001, 41, 258–273. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Abraham, W.C. Abraham, Astrocytes and synaptic plasticity in health and disease. Exp. Brain Res. 2017, 235, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Rodnight, R.B.; Gottfried, C. Morphological plasticity of rodent astroglia. J. Neurochem. 2013, 124, 263–275. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.C.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130595. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhäuser, C.; Lee, J.-M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef]
- Kulijewicz-Nawrot, M.; Verkhratsky, A.; Chvátal, A.; Syková, E.; Rodríguez, J.J. Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer’s disease. J. Anat. 2012, 221, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 2010, 58, 831–838. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Scheff, S.W.; Styren, S.D. Structural correlates of cognition in dementia: Quantification and assessment of synapse change. Neurodegeneration 1996, 5, 417–421. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.-I.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Cazal, M.; Adjali, O.; Galéotti, N.; Poncet, J.; Jouin, P.; Homburger, V.; Bockaert, J.; Marin, P. Proteomic Analysis of Astrocytic Secretion in the Mouse: Comparison with the cerebrospinal fluid proteome. J. Biol. Chem. 2003, 278, 24438–24448. [Google Scholar] [CrossRef]
- Greco, T.; Seeholzer, S.H.; Mak, A.; Spruce, L.; Ischiropoulos, H. Quantitative mass spectrometry-based proteomics reveals the dynamic range of primary mouse astrocyte protein secretion. J. Proteome Res. 2010, 9, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Keene, S.D.; Greco, T.; Parastatidis, I.; Lee, S.H.; Hughes, E.; Balice-Gordon, R.J.; Speicher, D.W.; Ischiropoulos, H. Mass spectrometric and computational analysis of cytokine-induced alterations in the astrocyte secretome. Proteomics 2009, 9, 768–782. [Google Scholar] [CrossRef]
- Moore, N.H.; Costa, L.G.; Shaffer, S.A.; Goodlett, D.R.; Guizzetti, M. Shotgun proteomics implicates extracellular matrix proteins and protease systems in neuronal development induced by astrocyte cholinergic stimulation. J. Neurochem. 2009, 108, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes from Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Baez, R.; Hernández-Ortega, K.; Martínez-Martínez, E. Insights into the Proteomic Profiling of Extracellular Vesicles for the Identification of Early Biomarkers of Neurodegeneration. Front. Neurol. 2020, 11, 580030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Q.; Zhang, Y.W.; Xu, H. Proteolytic processing of Alzheimer’s beta-amyloid precursor protein. J. Neurochem. 2012, 120 (Suppl. S1), 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sosa, L.J.; Cáceres, A.; Dupraz, S.; Oksdath, M.; Quiroga, S.; Lorenzo, A. The physiological role of the amyloid precursor protein as an adhesion molecule in the developing nervous system. J. Neurochem. 2017, 143, 11–29. [Google Scholar] [CrossRef]
- Hick, M.; Herrmann, U.; Weyer, S.W.; Mallm, J.P.; Tschäpe, J.A.; Borgers, M.; Mercken, M.; Roth, F.C.; Draguhn, A.; Slomianka, L.; et al. Acute function of secreted amyloid precursor protein fragment APPsalpha in synaptic plasticity. Acta Neuropathol. 2015, 129, 21–37. [Google Scholar] [CrossRef]
- Montagna, E.; Dorostkar, M.M.; Herms, J. The Role of APP in Structural Spine Plasticity. Front. Mol. Neurosci. 2017, 10, 136. [Google Scholar] [CrossRef]
- Steubler, V.; Erdinger, S.; Back, M.K.; Ludewig, S.; Fässler, D.; Richter, M.; Han, K.; Slomianka, L.; Amrein, I.; von Engelhardt, J.; et al. Loss of all three APP family members during development impairs synaptic function and plasticity, disrupts learning, and causes an autism-like phenotype. EMBO J. 2021, 40, e107471. [Google Scholar] [CrossRef]
- Meziane, H.; Dodart, J.C.; Mathis, C.; Little, S.; Clemens, J.; Paul, S.M.; Ungerer, A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc. Natl. Acad. Sci. USA 1998, 95, 12683–12688. [Google Scholar] [CrossRef]
- Taylor, C.J.; Ireland, D.R.; Ballagh, I.; Bourne, K.; Marechal, N.M.; Turner, P.R.; Bilkey, D.K.; Tate, W.P.; Abraham, W.C. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 2008, 31, 250–260. [Google Scholar] [CrossRef]
- Xiong, M.; Jones, O.D.; Peppercorn, K.; Ohline, S.M.; Tate, W.P.; Abraham, W.C. Secreted amyloid precursor protein-alpha can restore novel object location memory and hippocampal LTP in aged rats. Neurobiol. Learn Mem. 2017, 138, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.T.; Mockett, B.G.; Ohline, S.M.; Parfitt, K.D.; Wicky, H.E.; Peppercorn, K.; Schoderboeck, L.; Yahaya, M.F.B.; Tate, W.P.; Hughes, S.M.; et al. Lentivirus-mediated expression of human secreted amyloid precursor protein-alpha prevents development of memory and plasticity deficits in a mouse model of Alzheimer’s disease. Mol. Brain 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Fol, R.; Braudeau, J.; Ludewig, S.; Abel, T.; Weyer, S.W.; Roederer, J.P.; Brod, F.; Audrain, M.; Bemelmans, A.P.; Buchholz, C.J.; et al. Viral gene transfer of APPsalpha rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol. 2016, 131, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Mockett, B.G.; Guévremont, D.; Elder, M.K.; Parfitt, K.D.; Peppercorn, K.; Morrissey, J.; Singh, A.; Hintz, T.J.; Kochen, L.; Dieck, S.T.; et al. Glutamate Receptor Trafficking and Protein Synthesis Mediate the Facilitation of LTP by Secreted Amyloid Precursor Protein-Alpha. J. Neurosci. 2019, 39, 3188–3203. [Google Scholar] [CrossRef]
- Livingstone, R.W.; Elder, M.K.; Singh, A.; Westlake, C.M.; Tate, W.P.; Abraham, W.C.; Williams, J.M. Secreted Amyloid Precursor Protein-Alpha Enhances LTP Through the Synthesis and Trafficking of Ca(2+)-Permeable AMPA Receptors. Front. Mol. Neurosci. 2021, 14, 660208. [Google Scholar] [CrossRef]
- Baratchi, S.; Evans, J.; Tate, W.P.; Abraham, W.C.; Connor, B. Secreted amyloid precursor proteins promote proliferation and glial differentiation of adult hippocampal neural progenitor cells. Hippocampus 2012, 22, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Caillé, I.; Allinquant, B.; Dupont, E.; Bouillot, C.; Langer, A.; Müller, U.; Prochiantz, A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 2004, 131, 2173–2181. [Google Scholar] [CrossRef]
- Demars, M.P.; Bartholomew, A.; Strakova, Z.; Lazarov, O. Soluble amyloid precursor protein: A novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res. Ther. 2011, 2, 36. [Google Scholar] [CrossRef]
- Richter, M.C.; Ludewig, S.; Winschel, A.; Abel, T.; Bold, C.; Salzburger, L.R.; Klein, S.; Han, K.; Weyer, S.W.; Fritz, A.K.; et al. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 2018, 37, e98335. [Google Scholar] [CrossRef] [PubMed]
- Small, D.H.; Nurcombe, V.; Reed, G.; Clarris, H.; Moir, R.; Beyreuther, K.; Masters, C.L. A heparin-binding domain in the amyloid protein precursor of Alzheimer’s disease is involved in the regulation of neurite outgrowth. J. Neurosci. 1994, 14, 2117–2127. [Google Scholar] [CrossRef]
- Young-Pearse, T.L.; Chen, A.C.; Chang, R.; Marquez, C.; Selkoe, D.J. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural. Dev. 2008, 3, 15. [Google Scholar] [CrossRef]
- Dorard, E.; Chasseigneaux, S.; Gorisse-Hussonnois, L.; Broussard, C.; Pillot, T.; Allinquant, B. Soluble Amyloid Precursor Protein Alpha Interacts with alpha3-Na, K-ATPAse to Induce Axonal Outgrowth but Not Neuroprotection: Evidence for Distinct Mechanisms Underlying these Properties. Mol. Neurobiol. 2018, 55, 5594–5610. [Google Scholar] [CrossRef] [PubMed]
- Chasseigneaux, S.; Dinc, L.; Rose, C.; Chabret, C.; Coulpier, F.; Topilko, P.; Mauger, G.; Allinquant, B. Secreted amyloid precursor protein beta and secreted amyloid precursor protein alpha induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS ONE 2011, 6, e16301. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.R.; Bourne, K.; Garama, D.; Carne, A.; Abraham, W.C.; Tate, W.P. Production, purification and functional validation of human secreted amyloid precursor proteins for use as neuropharmacological reagents. J. Neurosci. Methods 2007, 164, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Sopher, B.L.; Rydel, R.E.; Begley, J.G.; Pham, D.G.; Martin, G.M.; Fox, M.; Mattson, M.P. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J. Neurochem. 1996, 67, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Rice, H.C.; De Malmazet, D.; Schreurs, A.; Frere, S.; Van Molle, I.; Volkov, A.N.; Creemers, E.; Vertkin, I.; Nys, J.; Ranaivoson, F.M.; et al. Secreted amyloid-β precursor protein functions as a GABA(B)R1a ligand to modulate synaptic transmission. Science 2019, 363, eaao4827. [Google Scholar] [CrossRef]
- Ishibashi, M.; Egawa, K.; Fukuda, A. Diverse Actions of Astrocytes in GABAergic Signaling. Int. J. Mol. Sci. 2019, 20, 2964. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; McIntire, J.; Ryan, S.; Dunah, A.; Loring, R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J. Neuroinflamm. 2017, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Gustafsen, C.; Glerup, S.; Pallesen, L.T.; Olsen, D.; Andersen, O.M.; Nykjær, A.; Madsen, P.; Petersen, C.M. Sortilin and SorLA display distinct roles in processing and trafficking of amyloid precursor protein. J. Neurosci. 2013, 33, 64–71. [Google Scholar] [CrossRef]
- Larsen, J.V.; Petersen, C.M. SorLA in Interleukin-6 Signaling and Turnover. Mol. Cell. Biol. 2017, 37, e00641-16. [Google Scholar] [CrossRef]
- Hartl, D.; Klatt, S.; Roch, M.; Konthur, Z.; Klose, J.; Willnow, T.E.; Rohe, M. Soluble alpha-APP (sAPPalpha) regulates CDK5 expression and activity in neurons. PLoS ONE 2013, 8, e65920. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.E.; Helms, H.C.; Harazin, A.; Johnsen, K.B.; Goldeman, C.; Burkhart, A.; Thomsen, M.S.; Kempen, P.J.; Klepe, A.; Lipka, D.V.; et al. Sortilin regulates blood-brain barrier integrity. FEBS J. 2021, 289, 1062–1079. [Google Scholar] [CrossRef] [PubMed]
- Milosch, N.; Tanriöver, G.; Kundu, A.; Rami, A.; Francois, J.C.; Baumkötter, F.; Weyer, S.W.; Samanta, A.; Jäschke, A.; Brod, F.; et al. Holo-APP and G-protein-mediated signaling are required for sAPPα-induced activation of the Akt survival pathway. Cell Death Dis. 2014, 5, e1391. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.D.; Anders, N.J.; DeCarli, C.; Chan, S.L.; Mattson, M.P.; Johnson, J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: Support for the amyloid hypothesis. J. Neurosci. 2004, 24, 7707–7717. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.M.; Morris, G.P.; Mockett, B.G.; Bourne, K.; Abraham, W.C.; Tate, W.P.; Williams, J.M. Time-dependent changes in gene expression induced by secreted amyloid precursor protein-alpha in the rat hippocampus. BMC Genom. 2013, 14, 376. [Google Scholar] [CrossRef]
- Ryan, M.; Tan, V.T.; Thompson, N.; Guévremont, D.; Mockett, B.G.; Tate, W.P.; Abraham, W.C.; Hughes, S.M.; Williams, J. Lentivirus-Mediated Expression of Human Secreted Amyloid Precursor Protein-Alpha Promotes Long-Term Induction of Neuroprotective Genes and Pathways in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2021, 79, 1075–1090. [Google Scholar] [CrossRef]
- Peppercorn, K.; Kleffmann, T.; Jones, O.; Hughes, S.; Tate, W. Secreted Amyloid Precursor Protein Alpha, a Neuroprotective Protein in the Brain Has Widespread Effects on the Transcriptome and Proteome of Human Inducible Pluripotent Stem Cell-Derived Glutamatergic Neurons Related to Memory Mechanisms. Front. Neurosci. 2022, 16, 858524. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.W.; Harmon, A.D. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997, 388, 878–881. [Google Scholar] [CrossRef]
- Koopmans, F.; van Nierop, P.; Andres-Alonso, M.; Byrnes, A.; Cijsouw, T.; Coba, M.P.; Cornelisse, L.N.; Farrell, R.J.; Goldschmidt, H.L.; Howrigan, D.P.; et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 2019, 103, 217–234.e4. [Google Scholar] [CrossRef]
- Brohée, S.; van Helden, J. Evaluation of clustering algorithms for protein-protein interaction networks. BMC Bioinform. 2006, 7, 488. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, W.-J.; Liao, J.-M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef]
- Dowell, J.A.; Johnson, J.A.; Li, L. Identification of Astrocyte Secreted Proteins with a Combination of Shotgun Proteomics and Bioinformatics. J. Proteome. Res. 2009, 8, 4135–4143. [Google Scholar] [CrossRef]
- Sapkota, D.; Sakers, K.; Liu, Y.; Lake, A.M.; Khazanchi, R.; Khankan, R.R.; Zhang, Y.; Dougherty, J.D.; Dougherty, J. Activity dependent translation in astrocytes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sapkota, D.; Kater, M.S.; Sakers, K.; Nygaard, K.R.; Liu, Y.; Koester, S.K.; Fass, S.B.; Lake, A.M.; Khazanchi, R.; Khankan, R.R.; et al. Activity-dependent translation dynamically alters the proteome of the perisynaptic astrocyte process. Cell Rep. 2022, 41, 111474. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Hung, A.; Selkoe, D. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J. Neurosci. 1991, 11, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Megias, L.; Guerri, C.; Fornas, E.; Azorin, I.; Bendala, E.; Sancho-Tello, M.; Durán, J.M.; Tomás, M.; Gomez-Lechon, M.J.; Renau-Piqueras, J. Endocytosis and transcytosis in growing astrocytes in primary culture. Possible implications in neural development. Int. J. Dev. Biol. 2000, 44, 209–221. [Google Scholar]
- Lushnikova, I.; Skibo, G.; Muller, D.; Nikonenko, I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus 2009, 19, 753–762. [Google Scholar] [CrossRef]
- Livingstone, R.W.; Elder, M.K.; Barrett, M.C.; Westlake, C.M.; Peppercorn, K.; Tate, W.P.; Abraham, W.C.; Williams, J.M. Secreted Amyloid Precursor Protein-Alpha Promotes Arc Protein Synthesis in Hippocampal Neurons. Front. Mol. Neurosci. 2019, 12, 198. [Google Scholar] [CrossRef]
- Moss, M.L.; Powell, G.; Miller, M.A.; Edwards, L.; Qi, B.; Sang, Q.-X.A.; De Strooper, B.; Tesseur, I.; Lichtenthaler, S.F.; Taverna, M.; et al. ADAM9 inhibition increases membrane activity of ADAM10 and controls α-secretase processing of amyloid precursor protein. J. Biol. Chem. 2011, 286, 40443–40451. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Olabarria, M.; Noristani, H.N.; Yeh, C.Y.; Rodriguez, J.J. Astrocytes in Alzheimer’s disease. Neurotherapeutics 2010, 7, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Chanaday, N.L.; Cousin, M.A.; Milosevic, I.; Watanabe, S.; Morgan, J.R. The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms. J. Neurosci. 2019, 39, 8209. [Google Scholar] [CrossRef]
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J. Neurosci. 2011, 31, 7275–7290. [Google Scholar] [CrossRef]
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front. Cell. Neurosci. 2018, 12, 526. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): Potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef]
- Traiffort, E.; Kassoussi, A.; Zahaf, A.; Laouarem, Y. Astrocytes and Microglia as Major Players of Myelin Production in Normal and Pathological Conditions. Front. Cell. Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef]
- Ishibashi, T.; Dakin, K.A.; Stevens, B.; Lee, P.R.; Kozlov, S.V.; Stewart, C.L.; Fields, R.D. Astrocytes promote myelination in response to electrical impulses. Neuron 2006, 49, 823–832. [Google Scholar] [CrossRef]
- Wujek, J.R.; Akeson, R.A. Extracellular matrix derived from astrocytes stimulates neuritic outgrowth from PC12 cells in vitro. Dev. Brain Res. 1987, 34, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kurshan, P.T.; Phan, A.Q.; Wang, G.J.; Crane, M.M.; Lu, H.; Shen, K. Regulation of synaptic extracellular matrix composition is critical for proper synapse morphology. J. Neurosci. 2014, 34, 12678–12689. [Google Scholar] [CrossRef] [PubMed]
- Soleman, S.; Filippov, M.; Dityatev, A.; Fawcett, J. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 2013, 253, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Stern, S.A.; Garcia-Osta, A.; Saunier-Rebori, B.; Pollonini, G.; Bambah-Mukku, D.; Blitzer, R.D.; Alberini, C.M. A critical role for IGF-II in memory consolidation and enhancement. Nature 2011, 469, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Beletskiy, A.; Chesnokova, E.; Bal, N. Insulin-Like Growth Factor 2 As a Possible Neuroprotective Agent and Memory Enhancer-Its Comparative Expression, Processing and Signaling in Mammalian CNS. Int. J. Mol. Sci. 2021, 22, 1849. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.L.M.; Sancho, L.; Deng, J.; Bosworth, A.; Miglietta, A.; Diedrich, J.K.; Shokhirev, M.N.; Allen, N.J. Aberrant astrocyte protein secretion contributes to altered neuronal development in multiple models of neurodevelopmental disorders. Nat. Neurosci. 2022, 25, 1163–1178. [Google Scholar] [CrossRef]
- Morris, G. Secreted Amyloid Precursor Protein-α Mediates Neuroprotection and Gene Expression, in Biochemistry. Ph.D. Dissertation, University of Otago, Otago, New Zealand, 2011. [Google Scholar]
- Mizui, T.; Kojima, N.; Yamazaki, H.; Katayama, M.; Hanamura, K.; Shirao, T. Drebrin E is involved in the regulation of axonal growth through actin-myosin interactions. J. Neurochem. 2009, 109, 611–622. [Google Scholar] [CrossRef]
- Hayashi, K.; Ishikawa, R.; Ye, L.-H.; He, X.-L.; Takata, K.; Kohama, K.; Shirao, T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J. Neurosci. 1996, 16, 7161–7170. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.; Esclapez, M.; Pellegrino, C.; Shirao, T.; Ferhat, L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J. Cell Sci. 2009, 122 Pt 4, 524–534. [Google Scholar] [CrossRef]
- Takahashi, H.; Mizui, T.; Shirao, T. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J. Neurochem. 2006, 97 (Suppl. S1), 110–115. [Google Scholar] [CrossRef]
- Harigaya, Y.; Shoji, M.; Shirao, T.; Hirai, S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J. Neurosci. Res. 1996, 43, 87–92. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.F.; Zhang, L.; Huang, L.; Yu, P.; Zhu, H.; Deng, W.; Qin, C. Effective expression of Drebrin in hippocampus improves cognitive function and alleviates lesions of Alzheimer’s disease in APP (swe)/PS1 (ΔE9) mice. CNS Neurosci. Ther. 2017, 23, 590–604. [Google Scholar] [CrossRef]
- Stevens, R.J.; Littleton, J.T. Synaptic growth: Dancing with adducin. Curr. Biol. 2011, 21, R402–R405. [Google Scholar] [CrossRef] [PubMed]
- Kosturko, L.D.; Maggipinto, M.J.; Korza, G.; Lee, J.W.; Carson, J.H.; Barbarese, E. Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs. Mol. Biol. Cell 2006, 17, 3521–3533. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Seo, J.Y.; Ryu, H.G.; Kim, D.Y.; Lee, K.H.; Kim, K.T. BDNF-induced local translation of GluA1 is regulated by HNRNP A2/B1. Sci. Adv. 2020, 6, eabd2163. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, F.; Wang, W.; Ni, Y.; Gao, M.; Guo, M.; Sun, G. hnRNPA2B1-Mediated Extracellular Vesicles Sorting of miR-122-5p Potentially Promotes Lung Cancer Progression. Int. J. Mol. Sci. 2021, 22, 12866. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.E.; Kielian, T. Astrocytes in juvenile neuronal ceroid lipofuscinosis (CLN3) display metabolic and calcium signaling abnormalities. J. Neurochem. 2019, 148, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, J.; Piers, T.; Pocock, J. Selective Depletion of Microglia from Cerebellar Granule Cell Cultures Using L-leucine Methyl Ester. J. Vis. Exp. 2015, e52983. [Google Scholar]
- Demmer, J.; Dragunow, M.; Lawlor, P.A.; Mason, S.E.; Leah, J.D.; Abraham, W.C.; Tate, W.P. Differential expression of immediate early genes after hippocampal long-term potentiation in awake rats. Brain Res. Mol. Brain Res. 1993, 17, 279–286. [Google Scholar] [CrossRef]
- Wiśniewski, J.R. Filter-Aided Sample Preparation: The Versatile and Efficient Method for Proteomic Analysis. Methods Enzymol. 2017, 585, 15–27. [Google Scholar]
- Sweetman, E.; Kleffmann, T.; Edgar, C.; de Lange, M.; Vallings, R.; Tate, W. A SWATH-MS analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome peripheral blood mononuclear cell proteomes reveals mitochondrial dysfunction. J. Transl. Med. 2020, 18, 365. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808-15. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.A.; Mockett, B.G.; Singh, A.; Kweon, D.; Ohline, S.M.; Tate, W.P.; Hughes, S.M.; Abraham, W.C. A C-terminal peptide from secreted amyloid precursor protein-alpha enhances long-term potentiation in rats and a transgenic mouse model of Alzheimer’s disease. Neuropharmacology 2019, 157, 107670. [Google Scholar] [CrossRef] [PubMed]



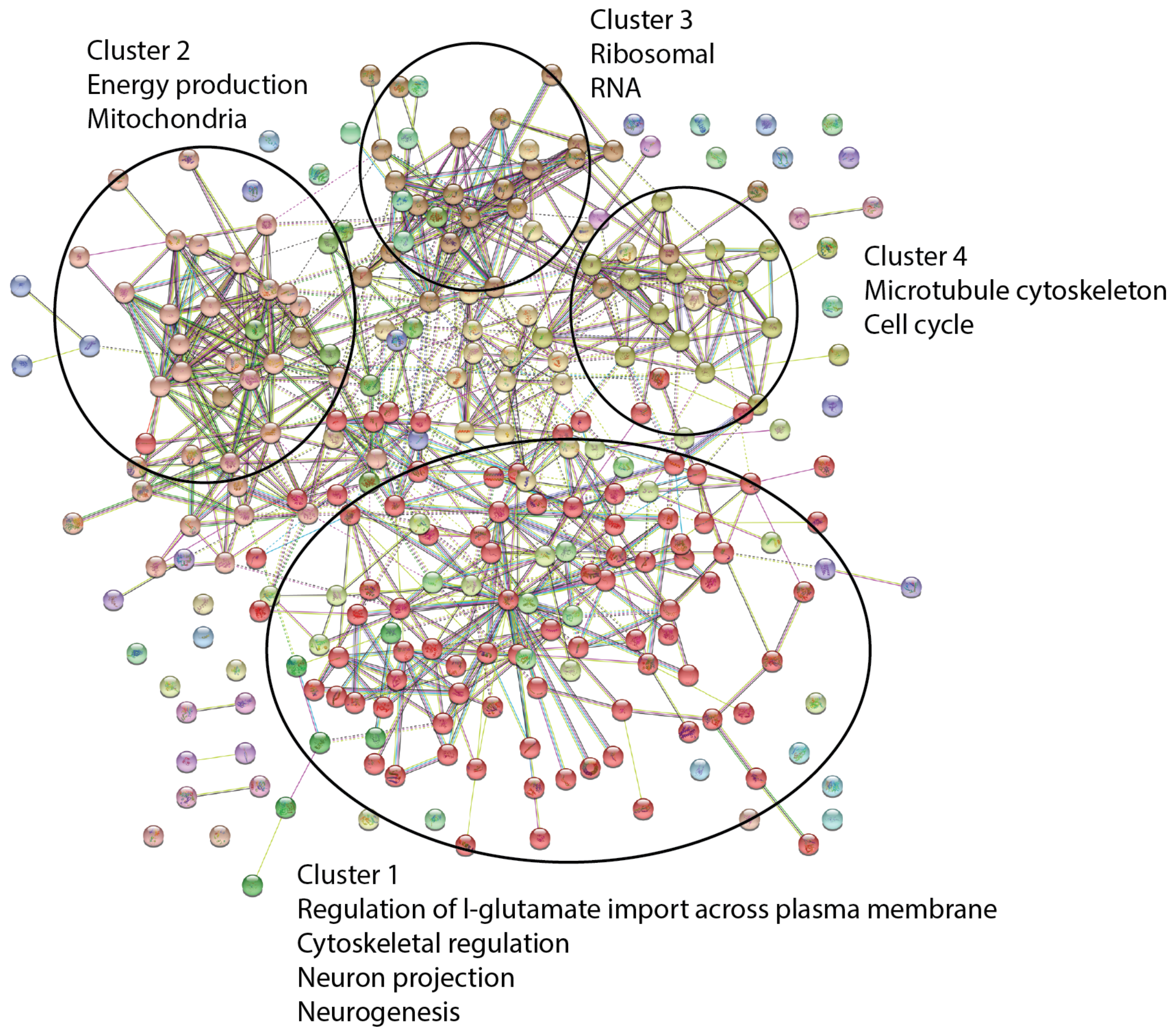
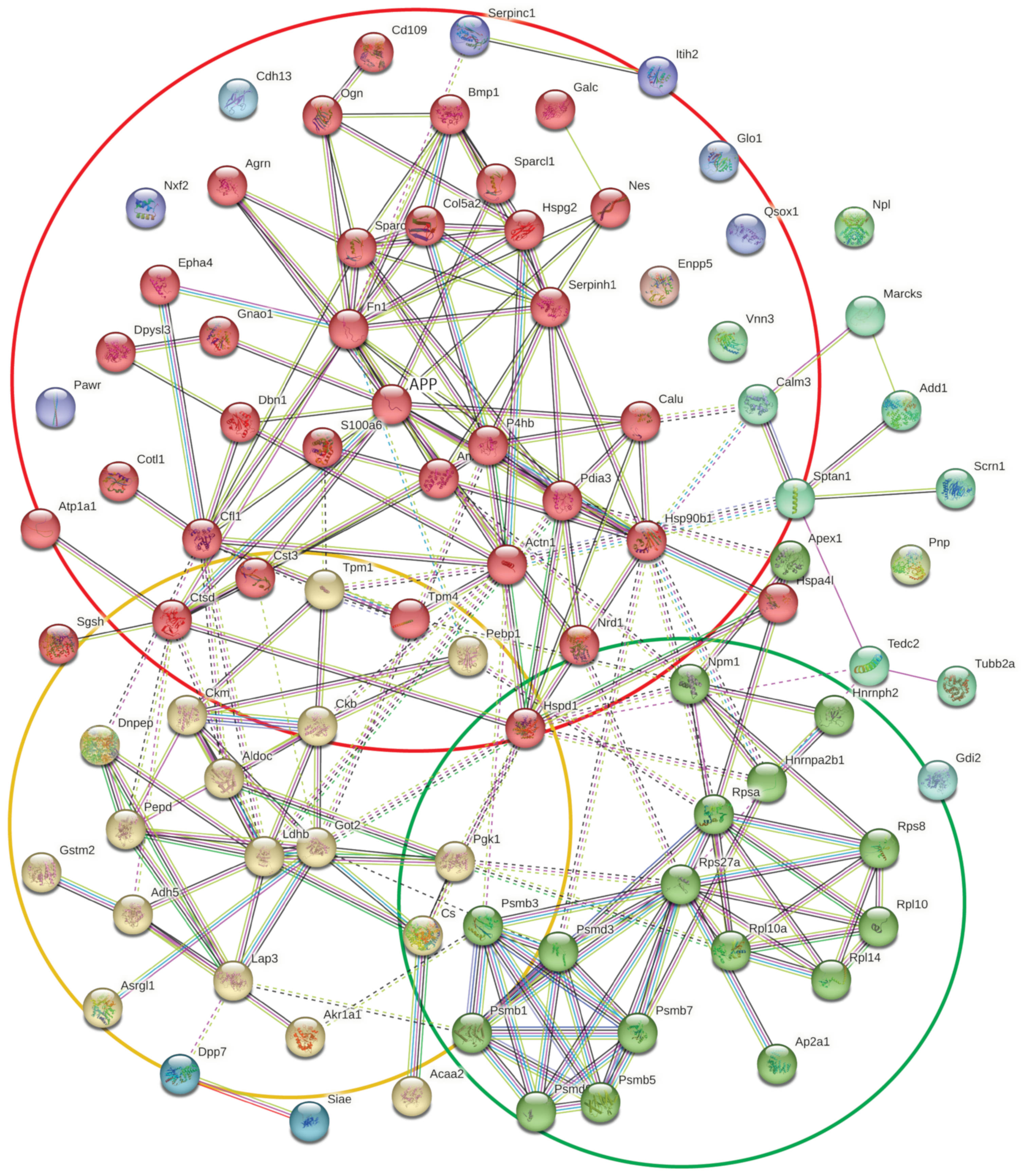
| Category | GO Term ID | GO Term Description | OGC | BGC | Strength | FDR |
|---|---|---|---|---|---|---|
| GO Component | GO:0045202 | Synapse | 48 | 1492 | 0.42 | 1.11 × 10−7 |
| GO:0030054 | Cell junction | 58 | 2050 | 0.37 | 1.50 × 10−7 | |
| GO:0030424 | Axon | 26 | 791 | 0.43 | 0.00031 | |
| GO:0005856 | Cytoskeleton | 57 | 2060 | 0.36 | 3.72 × 10−7 | |
| GO:0015630 | Microtubule cytoskeleton | 32 | 1190 | 0.35 | 0.0011 | |
| GO:0098794 | Postsynapse | 28 | 761 | 0.48 | 1.78 × 10−5 | |
| GO:0030135 | Coated vesicle | 12 | 210 | 0.67 | 0.00074 | |
| GO:0031982 | Vesicle | 46 | 2007 | 0.28 | 0.00097 | |
| GO:0098978 | Glutamatergic synapse | 19 | 501 | 0.5 | 0.00078 | |
| GO:0099572 | Postsynaptic specialization | 17 | 449 | 0.5 | 0.0018 | |
| GO:0043209 | Myelin sheath | 11 | 212 | 0.63 | 0.0026 | |
| GO:0043005 | Neuron projection | 37 | 1583 | 0.29 | 0.0033 | |
| GO:0120025 | Plasma-membrane-bounded cell projection | 53 | 2387 | 0.26 | 0.00058 | |
| GO:0043197 | Dendritic spine | 10 | 212 | 0.59 | 0.0083 | |
| GO:0098984 | Neuron-to-neuron synapse | 15 | 446 | 0.44 | 0.0097 | |
| GO:0014069 | Postsynaptic density | 14 | 414 | 0.45 | 0.0128 | |
| GO:0099571 | Postsynaptic cytoskeleton | 3 | 17 | 1.16 | 0.0254 | |
| GO:0030425 | Dendrite | 19 | 753 | 0.32 | 0.0348 | |
| GO Process | GO:0007010 | Cytoskeleton organization | 33 | 1064 | 0.41 | 0.00026 |
| GO:0007015 | Actin filament organization | 14 | 243 | 0.68 | 0.0006 | |
| GO:0030036 | Actin cytoskeleton organization | 19 | 496 | 0.5 | 0.0025 | |
| GO:0030029 | Actin filament-based process | 20 | 551 | 0.48 | 0.0031 | |
| GO:0097435 | Supramolecular fibre organization | 22 | 469 | 0.59 | 3.82 × 10−5 | |
| GO:0008064 | Regulation of actin polymerization or depolymerization | 11 | 181 | 0.7 | 0.0033 | |
| GO:0030705 | Cytoskeleton-dependent intracellular transport | 11 | 186 | 0.69 | 0.004 | |
| GO:0110053 | Regulation of actin filament organization | 12 | 266 | 0.57 | 0.0154 | |
| GO:0051493 | Regulation of cytoskeleton organization | 18 | 541 | 0.44 | 0.0161 | |
| GO:0010970 | Transport along microtubule | 9 | 155 | 0.68 | 0.0175 | |
| GO:1902683 | Regulation of receptor localization to synapse | 5 | 25 | 1.22 | 0.0039 | |
| KEGG | mmu05014 | Amyotrophic lateral sclerosis | 20 | 364 | 0.66 | 7.34 × 10−6 |
| mmu05012 | Parkinson disease | 16 | 239 | 0.74 | 9.20 × 10−6 | |
| mmu05016 | Huntington disease | 17 | 296 | 0.68 | 2.15 × 10−5 | |
| mmu05010 | Alzheimer disease | 18 | 359 | 0.62 | 5.02 × 10−5 | |
| Reactome | MMU-195721 | Signaling by WNT | 13 | 230 | 0.67 | 0.0013 |
| Monarch MPO | MP:0010768 | Mortality/aging | 124 | 5549 | 0.27 | 2.04 × 10−10 |
| Node1 | Node2 | Coexpression | Experimentally Determined Interaction | Database Annotated | Automated Textmining | Combined Score |
|---|---|---|---|---|---|---|
| APP | CAV1 | 0.098 | 0.091 | 0 | 0.371 | 0.439 |
| APP | SNX27 | 0 | 0.13 | 0 | 0.4 | 0.455 |
| APP | FUS | 0 | 0 | 0 | 0.474 | 0.474 |
| APP | GSK3 | 0.061 | 0 | 0 | 0.468 | 0.479 |
| APP | DBN1 | 0.116 | 0 | 0 | 0.528 | 0.565 |
| APP | STUB1 | 0 | 0.439 | 0 | 0.317 | 0.6 |
| APP | ADAM9 | 0.076 | 0.053 | 0 | 0.61 | 0.629 |
| APP | ERC1 | 0.062 | 0 | 0 | 0.679 | 0.686 |
| APP | SOD1 | 0 | 0 | 0 | 0.72 | 0.72 |
| APP | SNX17 | 0 | 0.061 | 0 | 0.72 | 0.726 |
| APP | BIN1 | 0.098 | 0 | 0.54 | 0.681 | 0.856 |
| APP | GSK3B | 0.056 | 0.427 | 0 | 0.79 | 0.876 |
| APP | KLC1 | 0.118 | 0.402 | 0 | 0.905 | 0.946 |
| APP | NFIB | 0.062 | 0 | 0 | 0.962 | 0.963 |
| APP | PSEN2 | 0.048 | 0.623 | 0.502 | 0.933 | 0.986 |
 indicates the protein was classified to be in that category.
indicates the protein was classified to be in that category.
 indicates the protein was classified to be in that category.
indicates the protein was classified to be in that category.| (A) Up-Regulated | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Protein Name | FC | p-Value | APP Related | Synapse | Neuron Projection | Myelin Sheath | Cytoskeleton | Alzheimer’s Disease |
| DBN1 | Drebrin 1 | 9.51 | 2.30 × 10−2 |  |  |  | |||
| CYC1 | Cytochrome c-1 | 8.31 | 3.57 × 10−4 |  | |||||
| ERC1 | ELKS/Rab6-interacting/CAST family member 1 | 3.82 | 3.41 × 10−2 |  |  |  | |||
| RPS7 | Ribosomal protein S7 | 3.47 | 3.29 × 10−2 |  |  | ||||
| RPL26 | 60S ribosomal protein L26 | 2.86 | 2.93 × 10−2 |  |  | ||||
| ADNP | Activity-dependent neuroprotective protein | 2.67 | 3.89 × 10−2 |  | |||||
| TBCA | Tubulin cofactor A | 2.65 | 2.66 × 10−2 |  | |||||
| MYL6 | Myosin light polypeptide 6 | 2.64 | 1.21 × 10−2 |  | |||||
| XRN1 | 5′-3′ exoribonuclease 1 | 2.62 | 9.61 × 10−3 |  |  | ||||
| PRC1 | Protein regulator of cytokinesis 1 | 2.54 | 5.44 × 10−3 |  | |||||
| WASL | WASP-like actin-nucleation-promoting factor | 2.35 | 3.04 × 10−2 |  |  | ||||
| PRKAA1 | Protein kinase, AMP-activated, alpha 1 catalytic subunit | 2.34 | 2.13 × 10−2 |  | |||||
| SOD1 | Superoxide dismutase 1, soluble | 2.33 | 6.13 × 10−4 |  |  |  | |||
| NDUFS8 | NADH:ubiquinone oxidoreductase core subunit S8 | 2.27 | 2.02 × 10−2 |  | |||||
| BRK1 | BRICK1, SCAR/WAVE actin-nucleating complex subunit | 2.25 | 4.68 × 10−4 |  | |||||
| TMOD3 | Tropomodulin 3 | 2.22 | 3.60 × 10−3 |  | |||||
| TACC3 | Transforming, acidic coiled-coil containing protein 3 | 2.16 | 2.57 × 10−2 |  | |||||
| RIF1 | Telomere-associated protein RIF1 | 2.09 | 6.85 × 10−3 |  | |||||
| Erbin | Erbb2 interacting protein/LAP2 | 2.04 | 4.71 × 10−2 |  | |||||
| HYPK | Huntingtin interacting protein K | 2.03 | 1.45 × 10−2 |  | |||||
| PSMD4 | Proteasome 26S subunit, non-ATPase, 4 | 2.02 | 5.66 × 10−3 |  | |||||
| SEPTIN2 | Septin-2 | 1.99 | 1.53 × 10−2 |  |  |  |  | ||
| MPP1 | Membrane protein, palmitoylated | 1.98 | 2.34 × 10−3 |  |  | ||||
| FNTA | Farnesyltransferase, CAAX box, alpha | 1.98 | 3.18 × 10−3 |  | |||||
| PLEKHA7 | Pleckstrin homology domain containing family A member 7 | 1.95 | 8.09 × 10−3 |  | |||||
| SRI | Sorcin | 1.94 | 4.84 × 10−2 |  |  | ||||
| NEXN | Nexilin | 1.92 | 3.68 × 10−2 |  |  | ||||
| KLC1 | Kinesin light chain 1 | 1.89 | 2.35 × 10−2 |  |  |  |  | ||
| EPB4.1 | Erythrocyte membrane protein band 4.1 | 1.89 | 4.72 × 10−2 |  |  | ||||
| RPS19 | 40S ribosomal protein S19 | 1.87 | 3.56 × 10−3 |  | |||||
| WDR1 | WD repeat domain 1 | 1.85 | 2.38 × 10−2 |  |  | ||||
| SNX17 | Sorting nexin 17 | 1.78 | 1.62 × 10−3 |  | |||||
| COX6B1 | Cytochrome c oxidase, subunit 6B1 | 1.77 | 3.64 × 10−2 |  | |||||
| MYO1E | Myosin IE | 1.77 | 3.65 × 10−2 |  | |||||
| SEPTIN11 | Septin 11 | 1.74 | 3.61 × 10−2 |  |  |  | |||
| HNRNPC | Heterogeneous nuclear ribonucleoprotein C | 1.74 | 2.84 × 10−2 |  | |||||
| MTPN | Myotrophin | 1.74 | 1.76 × 10−2 |  | |||||
| VSP4B | Vacuolar protein sorting 4B | 1.74 | 3.73 × 10−2 |  | |||||
| DCTN2 | Dynactin 2 | 1.73 | 4.21 × 10−3 |  |  | ||||
| RPL17 | Ribosomal protein L17 | 1.73 | 2.74 × 10−4 |  | |||||
| YWHAH | Tryptophan 5-monooxygenase activation protein | 1.71 | 1.25 × 10−2 |  | |||||
| AP3B1 | Adaptor-related protein complex 3, beta 1 subunit | 1.71 | 2.06 × 10−2 |  |  | ||||
| STK39 | STE20/SPS1-related proline-alanine-rich protein kinase | 1.70 | 4.09 × 10−2 |  | |||||
| ARL6IP5 | ADP-ribosylation factor-like 6 interacting protein 5 | 1.68 | 4.25 × 10−2 |  | |||||
| ITGB5 | Integrin beta 5 | 1.68 | 4.91 × 10−3 |  | |||||
| PPP2CA | Protein phosphatase 2 (formerly 2A), catalytic subunit | 1.67 | 1.06 × 10−2 |  |  |  | |||
| ELAVL1 | ELAV (embryonic lethal, abnormal vision)-like 1 | 1.65 | 4.34 × 10−2 |  | |||||
| NEFM | Neurofilament medium polypeptide | 1.65 | 2.08 × 10−2 |  |  |  |  | ||
| TBCEL | Tubulin-specific chaperone cofactor E-like protein | 1.65 | 2.47 × 10−2 |  | |||||
| TWF1 | Twinfilin actin-binding protein 1 | 1.63 | 2.01 × 10−2 |  | |||||
| BIN1 | Bridging integrator 1 | 1.61 | 4.03 × 10−2 |  |  |  |  | ||
| TUFM | Tu translation elongation factor, mitochondrial | 1.60 | 2.22 × 10−2 |  |  | ||||
| MCM3 | Minichromosome maintenance complex component 3 | 1.59 | 1.39 × 10−2 |  | |||||
| SH3GLB1 | SH3-domain GRB2-like B1 (endophilin) | 1.59 | 2.92 × 10−2 |  |  | ||||
| TPM4 | Tropomyosin 4 | 1.59 | 2.61 × 10−2 |  | |||||
| CDC42 | Cell division cycle 42 | 1.59 | 5.83 × 10−4 |  |  |  |  | ||
| CLTA | Cathrin light chain A | 1.59 | 1.32 × 10−2 |  | |||||
| NPM3 | Nucleoplasmin 3 | 1.59 | 2.29 × 10−4 |  | |||||
| RPLP2 | Ribosomal protein, large P2 | 1.59 | 4.35 × 10−2 |  | |||||
| RPL29 | 60S ribosomal protein L29 | 1.58 | 1.81 × 10−2 |  |  | ||||
| PSMD2 | proteasome 26S subunit, non-ATPase, 2 | 1.58 | 9.68 × 10−3 |  | |||||
| DDAH2 | N(G),N(G)-dimethylarginine dimethylaminohydrolase 2 | 1.58 | 3.30 × 10−2 |  | |||||
| NPTN | Neuroplastin | 1.56 | 9.91 × 10−3 |  | |||||
| KPNA2 | Karyopherin (importin) alpha 2 | 1.54 | 4.27 × 10−2 |  | |||||
| MDH2 | Malate dehydrogenase 2, NAD (mitochondrial) | 1.52 | 8.93 × 10−3 |  | |||||
| MAP2K1 | Mitogen-activated protein kinase kinase 1 | 1.52 | 4.53 × 10−2 |  |  |  |  | ||
| (B) Down-regulated | |||||||||
| Symbol | Protein Name | FC | p-Value | APP Related | Synapse | Neuron Projection | Myelin Sheath | Cytoskeleton | Alzheimer’s Disease |
| RPS6KA1 | Ribosomal protein S6 kinase polypeptide 1 | 0.003 | 1.16 × 10−2 |  | |||||
| GPC4 | Glypican 4 | 0.003 | 2.21 × 10−3 |  | |||||
| SNX27 | Sorting nexin family member 27 | 0.20 | 7.92 × 10−3 |  |  | ||||
| PCNA | Proliferating cell nuclear antigen | 0.22 | 1.77 × 10−7 |  | |||||
| NDUFB9 | NADH:ubiquinone oxidoreductase subunit B9 | 0.27 | 4.10 × 10−2 |  | |||||
| NEK9 | NIMA (never in mitosis gene a)-related expressed kinase 9 | 0.27 | 5.78 × 10−4 |  | |||||
| HNRNPA2B1 | Heterogeneous nuclear ribonucleoproteins A2/B1 | 0.30 | 8.82 × 10−4 |  |  | ||||
| FARP1 | FERM, RhoGEF (Arhgef) and pleckstrin domain protein 1 | 0.33 | 1.65 × 10−2 |  |  |  | |||
| GSK3 | Glycogen synthase kinase-3 | 0.37 | 3.43 × 10−6 |  |  |  |  |  | |
| GSK3 | Lysosomal-associated membrane protein 2 | 0.38 | 2.43 × 10−2 |  | |||||
| NDUFV1 | NADH:ubiquinone oxidoreductase core subunit V1 | 0.38 | 2.03 × 10−2 |  | |||||
| STOML2 | Stomatin (Epb7.2)-like 2 | 0.40 | 8.65 × 10−3 |  | |||||
| GLS | Glutaminase | 0.41 | 1.87 × 10−2 |  | |||||
| STUB1 | STIP1 homology and U-Box containing protein 1 | 0.42 | 1.09 × 10−2 |  | |||||
| DARS | Aspartyl-tRNA synthetase | 0.42 | 3.17 × 10−2 |  | |||||
| NDUFS2 | NADH:ubiquinone oxidoreductase core subunit S2 | 0.42 | 8.08 × 10−4 |  | |||||
| COPG2 | Coatomer protein complex, subunit gamma 2 | 0.43 | 1.53 × 10−2 |  | |||||
| ENAH | Protein-enabled homolog | 0.43 | 1.59 × 10−2 |  |  | ||||
| PSEN2 | Presenilin 2 | 0.44 | 2.04 × 10−2 |  |  |  |  | ||
| ND4 | NADH dehydrogenase 4 | 0.46 | 1.49 × 10−3 |  | |||||
| GLRX5 | Glutaredoxin 5 | 0.47 | 3.15 × 10−2 |  | |||||
| LAMC1 | Laminin subunit gamma-1 precursor | 0.48 | 1.35 × 10−2 |  | |||||
| NFIB | Nuclear factor I/B | 0.49 | 2.03 × 10−3 |  |  | ||||
| ATP5D | ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit | 0.49 | 3.26 × 10−2 |  | |||||
| NDRG2 | Protein NDRG2, cytoplasmic protein Ndr1 | 0.52 | 1.64 × 10−2 |  |  | ||||
| DMD | Dystrophin, muscular dystrophy | 0.56 | 1.33 × 10−2 |  |  |  | |||
| COX5B | Cytochrome c oxidase subunit 5B | 0.57 | 1.45 × 10−2 |  | |||||
| FUS | Fused in sarcoma | 0.58 | 2.30 × 10−2 |  |  |  | |||
| PSMD3 | Proteasome 26S subunit, non-ATPase, 3 | 0.59 | 8.54 × 10−3 |  | |||||
| LIMA1 | LIM domain and actin-binding 1 | 0.61 | 3.95 × 10−2 |  | |||||
| COX7A2 | Cytochrome c oxidase subunit 7A2 | 0.61 | 2.63 × 10−2 |  | |||||
| ADAM9 | a disintegrin and metallopeptidase domain 9 | 0.62 | 3.11 × 10−2 |  | |||||
| ITSN1 | Intersectin 1 (SH3 domain protein 1A) | 0.62 | 2.74 × 10−2 |  |  |  | |||
| RPL18A | Ribosomal protein L18A | 0.63 | 3.74 × 10−2 |  | |||||
| CAV1 | Caveolin 1, caveolae protein | 0.64 | 1.70 × 10−2 |  | |||||
| CKB | Creatine kinase | 0.65 | 1.48 × 10−3 |  |  | ||||
| ARHGAP39 | rho GTPase-activating protein 39 | 0.65 | 3.28 × 10−2 |  |  | ||||
| PHB2 | Prohibitin 2 | 0.66 | 2.09 × 10−2 |  |  | ||||
| ATP6V1B2 | ATPase, H+ transporting, lysosomal V1 subunit B2 | 0.66 | 7.47 × 10−3 |  | |||||
| NEDD1 | Neural precursor cell-expressed, developmentally down-regulated gene 1 | 0.66 | 7.56 × 10−3 |  | |||||
| ADD1 | Adducin 1 (alpha) | 0.66 | 3.22 × 10−2 |  |  |  | |||
| SLC25A4 | Solute carrier family 25 | 0.67 | 3.22 × 10−2 |  |  | ||||
| Category | GO Term ID | GO Term Description | OGC | BGC | Strength | FDR |
|---|---|---|---|---|---|---|
| GO Process | GO:0050807 | Regulation of synapse organization | 7 | 275 | 0.79 | 0.0278 |
| GO:0050808 | Synapse organization | 7 | 303 | 0.75 | 0.0412 | |
| GO:0030036 | Actin cytoskeleton organization | 11 | 496 | 0.73 | 0.0038 | |
| GO:0007010 | Cytoskeleton organization | 14 | 1064 | 0.5 | 0.0241 | |
| GO:0034330 | Cell junction organization | 9 | 481 | 0.66 | 0.0304 | |
| GO Component | GO:0005576 | Extracellular region | 34 | 2229 | 0.57 | 9.14 × 10−9 |
| GO:0005615 | Extracellular space | 24 | 1424 | 0.61 | 7.45 × 10−7 | |
| GO:0043209 | Myelin sheath | 12 | 212 | 1.14 | 5.67 × 10−8 | |
| GO:0045202 | Synapse | 24 | 1492 | 0.59 | 1.57 × 10−6 | |
| GO:0098794 | Postsynapse | 12 | 761 | 0.58 | 0.0034 | |
| GO:0098978 | Glutamatergic synapse | 9 | 501 | 0.64 | 0.0085 | |
| GO:0030424 | Axon | 13 | 791 | 0.6 | 0.0015 | |
| GO:0043005 | Neuron projection | 19 | 1583 | 0.46 | 0.0015 | |
| GO:0030426 | Growth cone | 7 | 224 | 0.88 | 0.0024 | |
| GO:0150034 | Distal axon | 9 | 399 | 0.74 | 0.0024 | |
| GO:0031982 | Vesicle | 21 | 2007 | 0.4 | 0.0028 | |
| COMPARTMENTS | GOCC:1903561 | Extracellular vesicle | 5 | 93 | 1.11 | 0.0052 |
| GO:0043197 | Dendritic spine | 6 | 212 | 0.84 | 0.0089 | |
| GO:0044295 | Axonal growth cone | 3 | 42 | 1.24 | 0.0194 | |
| GOCC:0120025 | Plasma-membrane-bounded cell projection | 17 | 1774 | 0.37 | 0.0364 | |
| GO:0014069 | Postsynaptic density | 7 | 414 | 0.61 | 0.0335 | |
| KEGG | mmu00330 | Arginine and proline metabolism | 4 | 53 | 1.26 | 0.0033 |
| mmu05010 | Alzheimer disease | 8 | 359 | 0.73 | 0.0043 | |
| mmu05014 | Amyotrophic lateral sclerosis | 9 | 364 | 0.78 | 0.0015 | |
| mmu05016 | Huntington disease | 8 | 296 | 0.82 | 0.0017 | |
| mmu05017 | Spinocerebellar ataxia | 6 | 140 | 1.02 | 0.0017 | |
| mmu05012 | Parkinson disease | 9 | 239 | 0.96 | 0.00014 | |
| Reactome | MMU-381426 | Regulation of IGF transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) | 10 | 122 | 1.3 | 6.95 × 10−8 |
| MMU-422475 | Axon guidance | 7 | 278 | 0.79 | 0.0021 | |
| MMU-1474228 | Degradation of the extracellular matrix | 6 | 144 | 1 | 0.00047 | |
| MMU-1474244 | Extracellular matrix organization | 11 | 295 | 0.96 | 1.30 × 10−6 | |
| MMU-5687128 | MAPK6/MAPK4 signaling | 7 | 74 | 1.36 | 1.11 × 10−6 | |
| MMU-3858494 | Beta-catenin-independent WNT signaling | 10 | 127 | 1.28 | 8.04 × 10−8 | |
| MMU-5676590 | NIK → noncanonical NF-kB signaling | 7 | 57 | 1.47 | 5.37 × 10−7 | |
| Monarch MPO | MP:0010768 | Mortality/aging | 44 | 5549 | 0.28 | 0.009 |
 indicates the protein was classified to be in that category.
indicates the protein was classified to be in that category.
 indicates the protein was classified to be in that category.
indicates the protein was classified to be in that category.| (A) Down-Regulated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | Protein Name | FC | p-Value | APP Related | Synapse | Neuron Projection | Myelin Sheath | Alzheimer’s Disease | ECM Organisation | IGF Regulation | Actin Cytoskeleton |
| CDH13 | Cadherin-13 | 0.003 | 1.73 × 10−3 |  | |||||||
| GDI2 | Rab GDP dissociation inhibitor beta | 0.01 | 2.34 × 10−2 |  |  | ||||||
| DPYSL3 | Dihydropyrimidinase-related protein 3 | 0.13 | 4.03 × 10−2 |  |  |  | |||||
| QSOX1 | Sulfhydryl oxidase 1 | 0.22 | 2.11 × 10−3 |  | |||||||
| CS | Citrate synthase | 0.33 | 8.97 × 10−3 |  | |||||||
| SERPINC1 | Antithrombin-III | 0.38 | 6.83 × 10−4 |  | |||||||
| EPHA4 | Ephrin type-A receptor 4 | 0.39 | 2.36 × 10−2 |  |  |  | |||||
| CKB | Creatine kinase B-type | 0.40 | 3.63 × 10−2 |  |  | ||||||
| PSMB3 | Proteasome subunit beta type-3 | 0.48 | 6.94 × 10−4 |  | |||||||
| RPL10A | Large-subunit ribosomal protein l10ae | 0.52 | 1.25 × 10−2 |  | |||||||
| ANXA5 | Annexin A5 | 0.54 | 3.18 × 10−2 |  |  | ||||||
| CKM | Creatine kinase M-type | 0.55 | 1.91 × 10−2 |  | |||||||
| CALU | Calumenin | 0.56 | 1.57 × 10−2 |  |  | ||||||
| ENPP5 | Ectonucleotide pyrophosphatase | 0.57 | 2.92 × 10−2 | ||||||||
| PGK1 | Phosphoglycerate kinase 1 | 0.59 | 2.11 × 10−2 |  | |||||||
| AGRN | Agrin | 0.59 | 4.81 × 10−2 |  |  |  |  | ||||
| RPS27A | Ubiquitin-40S ribosomal protein S27a | 0.61 | 3.85 × 10−2 |  | |||||||
| ASRGL1 | Isoaspartyl peptidase/L-asparaginase | 0.61 | 4.45 × 10−2 |  | |||||||
| PSMB5 | Proteasome subunit beta type-5 | 0.66 | 1.06 × 10−2 |  | |||||||
| HSPA4L | Heat shock 70 kDa protein 4L | 0.67 | 4.72 × 10−3 |  | |||||||
| GOT2 | Glutamatic-oxaloacetic transaminase 2 | 0.67 | 1.38 × 10−2 |  | |||||||
| (B) Up-regulated | |||||||||||
| Symbol | Protein Name | FC | p-Value | APP Related | Synapse | Neuron Projection | Myelin Sheath | Alzheimer’s Disease | ECM Organisation | IGF Regulation | Actin Cytoskeleton |
| SPARC | SPARC | 1.57 | 1.88 × 10−2 |  |  |  | |||||
| PDIA3 | Protein disulfide-isomerase A3 | 1.58 | 2.92 × 10−2 |  |  | ||||||
| AKR1A1 | Aldehyde reductase | 1.62 | 4.35 × 10−2 |  | |||||||
| ITIH2 | Inter-alpha-trypsin inhibitor heavy chain | 1.65 | 1.62 × 10−2 |  | |||||||
| CST3 | Cystatin-C | 1.68 | 1.20 × 10−2 |  |  |  | |||||
| GNAO1 | Guanine nucleotide-binding protein G(o) | 1.68 | 3.98 × 10−2 |  |  |  | |||||
| SPTAN1 | Spectrin alpha chain, non-erythrocytic 1 | 1.72 | 2.70 × 10−2 |  |  |  |  | ||||
| CALM3 | Calmodulin-1; Calmodulin 3 | 1.73 | 1.06 × 10−2 |  |  |  |  | ||||
| PEBP1 | Phosphatidylethanolamine-binding protein | 1.73 | 2.37 × 10−2 |  |  |  | |||||
| DBN1 | Drebrin 1; Drebrin | 1.73 | 1.96 × 10−2 |  |  |  |  | ||||
| PSMB7 | Proteasome subunit, beta | 1.73 | 1.40 × 10−2 |  | |||||||
| TPM1 | Tropomyosin alpha-1 chain | 1.76 | 3.22 × 10−2 |  | |||||||
| S100A6 | Protein S100-A6 | 1.76 | 4.37 × 10−2 |  | |||||||
| PAWR | PRKC apoptosis WT1 regulator protein | 1.77 | 1.38 × 10−2 |  |  | ||||||
| ACTN1 | Actinin alpha 1/4 (Alpha-actinin-1) | 1.80 | 2.70 × 10−2 |  |  | ||||||
| COL5A2 | Collagen alpha-2(V) chain | 1.85 | 4.53 × 10−2 |  | |||||||
| HSP90B1 | Heat shock protein 90, Endoplasmin | 1.85 | 4.18 × 10−2 |  |  |  | |||||
| RPL14 | 60S ribosomal protein L14 | 1.88 | 1.04 × 10−2 |  | |||||||
| PSMD3 | Proteasome 26s subunit, non-atpase, 3 | 1.89 | 4.72 × 10−2 |  | |||||||
| COTL1 | Coactosin-like 1 (dictyostelium) | 1.96 | 4.92 × 10−2 |  |  | ||||||
| SCRN1 | Secernin-1 | 1.98 | 8.80 × 10−3 |  | |||||||
| TPM4 | Tropomyosin alpha-4 chain | 2.00 | 3.81 × 10−2 |  | |||||||
| ADD1 | Adducin 1 (alpha); Alpha-adducin | 2.14 | 9.37 × 10−3 |  |  |  |  | ||||
| LDHB | Lactate dehydrogenase B | 2.17 | 1.28 × 10−2 |  | |||||||
| MARCKS | Myristoylated ala-rich C-kinase substrate | 2.17 | 9.25 × 10−3 |  | |||||||
| PSMD6 | Proteasome 26s subunit, non-atpase, 6 | 2.19 | 2.24 × 10−2 |  | |||||||
| BMP1 | Bone morphogenetic protein 1 | 2.25 | 3.66 × 10−2 |  |  | ||||||
| ATP1A1 | Na/K-transporting ATPase subunit alpha-1 | 2.34 | 2.78 × 10−2 |  |  | ||||||
| NES | Nestin | 2.36 | 1.04 × 10−2 |  | |||||||
| GALC | Galactosylceramidase | 2.49 | 3.20 × 10−2 |  | |||||||
| SPARCL1 | SPARC-like 1 | 2.65 | 1.41 × 10−2 |  |  |  | |||||
| FN1 | Fibronectin | 2.67 | 2.79 × 10−2 |  |  |  | |||||
| HNRNPA2B | Heterogeneous nuclear ribonucleoprotein | 2.67 | 7.84 × 10−3 |  |  | ||||||
| PSMB1 | Proteasome subunit, beta type 1 | 2.78 | 2.27 × 10−3 |  | |||||||
| RPSA | 40S ribosomal protein SA | 3.10 | 3.00 × 10−2 |  | |||||||
| HSPD1 | 60 kDa heat shock protein, mitochondrial | 3.39 | 9.18 × 10−3 |  |  | ||||||
| NRD1 | Nardilysin | 4.17 | 4.66 × 10−4 |  | |||||||
| SERPINH1 | Serpin H1 | 4.31 | 1.17 × 10−2 |  |  | ||||||
| TUBB2A | Tubulin beta-2A chain | 4.37 | 2.47 × 10−5 |  | |||||||
| CTSD | Cathepsin D | 19.32 | 9.23 × 10−4 |  | |||||||
| APP | Amyloid-beta A4 protein | 23.22 | 3.41 × 10−6 |  |  |  |  |  | |||
| RPL10 | Ribosomal protein L10 | 23.90 | 6.53 × 10−3 |  | |||||||
| P4HB | Protein disulfide-isomerase | 24.03 | 1.03 × 10−2 |  |  | ||||||
| ALDOC | Fructose-bisphosphate aldolase, class i | 34.84 | 3.39 × 10−2 |  |  | ||||||
| CFL1 | Cofilin-1 | >100 | 1.10 × 10−2 |  |  |  |  | ||||
| Protein | Proteome (Fold Change) | Secretome (Fold Change) |
|---|---|---|
| * CKB | 0.68 | 0.40 |
| * DBN1 | 9.51 | 1.73 |
| * TPM4 | 1.59 | 2.0 |
| COL5a | 0.37 | 1.85 |
| ADD | 0.66 | 2.14 |
| PSMD3 | 0.59 | 1.89 |
| HNRMP2b1 | 0.30 | 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peppercorn, K.; Kleffmann, T.; Hughes, S.M.; Tate, W.P. Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes. Int. J. Mol. Sci. 2023, 24, 7165. https://doi.org/10.3390/ijms24087165
Peppercorn K, Kleffmann T, Hughes SM, Tate WP. Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes. International Journal of Molecular Sciences. 2023; 24(8):7165. https://doi.org/10.3390/ijms24087165
Chicago/Turabian StylePeppercorn, Katie, Torsten Kleffmann, Stephanie M. Hughes, and Warren P. Tate. 2023. "Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes" International Journal of Molecular Sciences 24, no. 8: 7165. https://doi.org/10.3390/ijms24087165
APA StylePeppercorn, K., Kleffmann, T., Hughes, S. M., & Tate, W. P. (2023). Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes. International Journal of Molecular Sciences, 24(8), 7165. https://doi.org/10.3390/ijms24087165






