Zinc in Cardiovascular Functions and Diseases: Epidemiology and Molecular Mechanisms for Therapeutic Development
Abstract
1. Introduction
2. Epidemiological Studies of Zinc Level and CVDs
3. Zinc-Mediated Intracellular Processes Related on Cardiovascular Functions
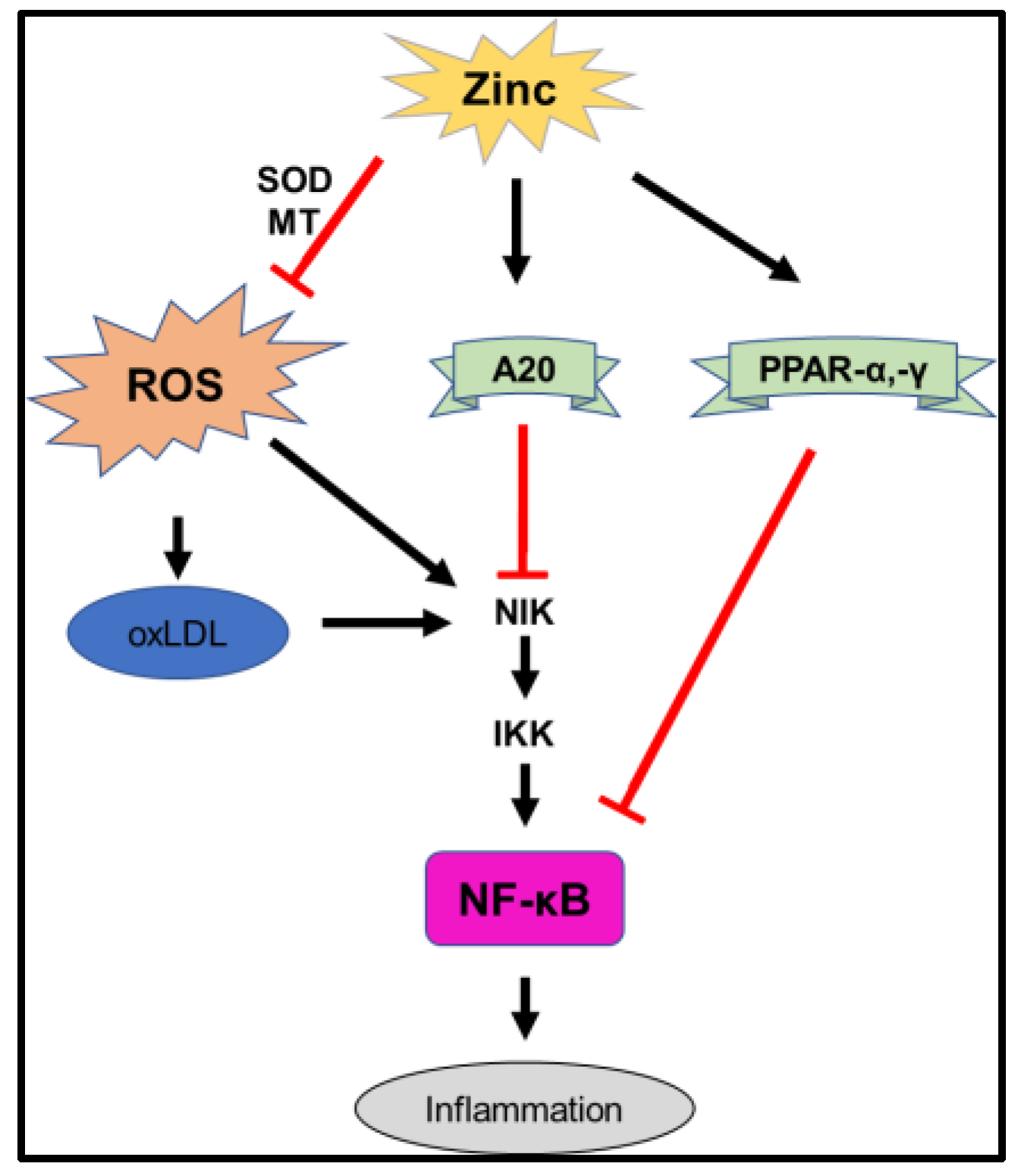
3.1. Oxidative Stress
3.2. Metallothionein (MT)
4. Zinc Transporters Regulating Cardiovascular Functions
4.1. ZnT1
4.2. ZnT-5
4.3. ZnT7 and ZIP7
4.4. ZIP2
4.5. ZIP8
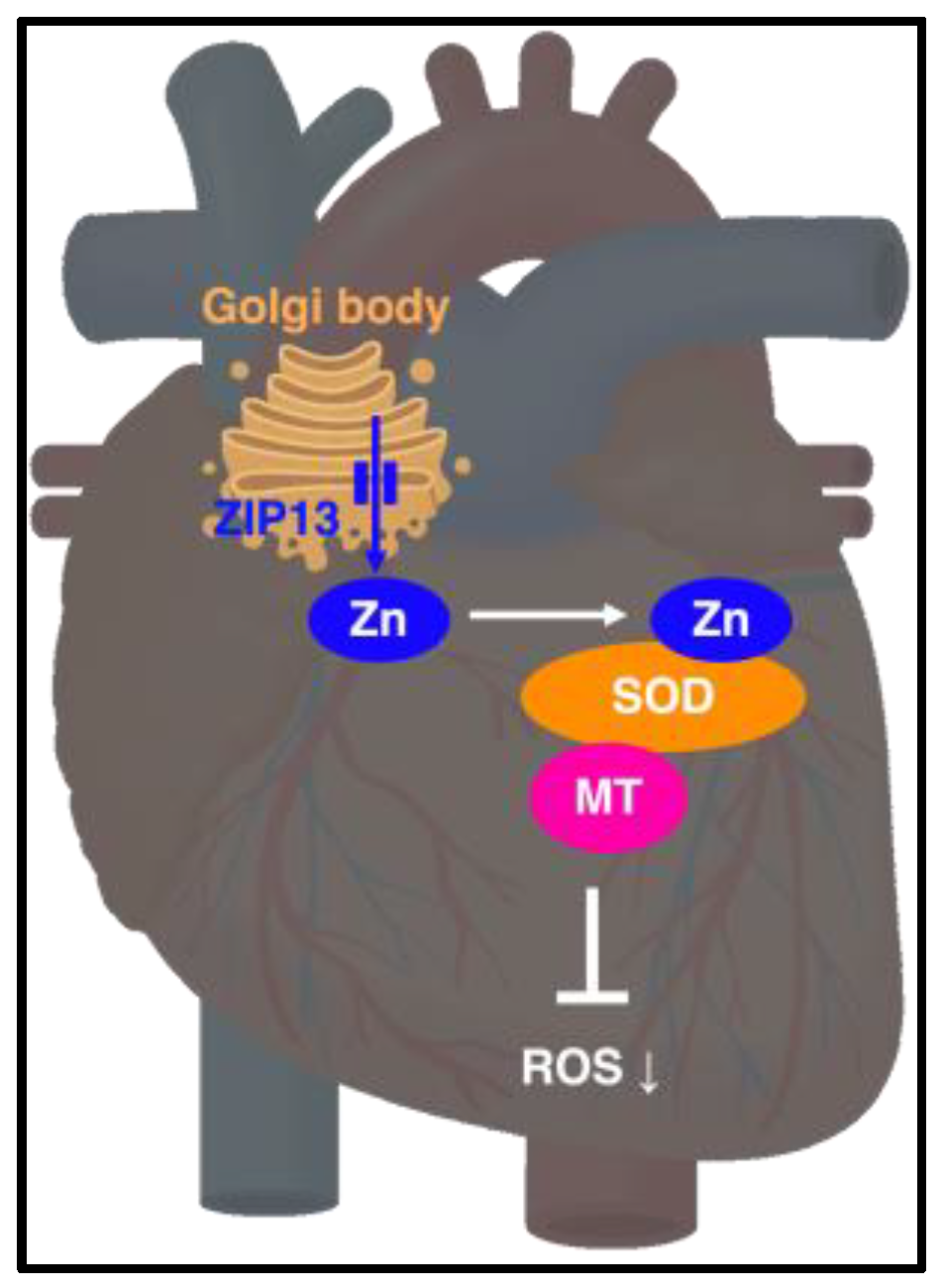
4.6. ZIP13
5. Membrane Proteins Regulating Zinc Homeostasis in Calcium Homeostasis (Other Membrane Proteins Involved in the Regulation of Zinc Homeostasis)
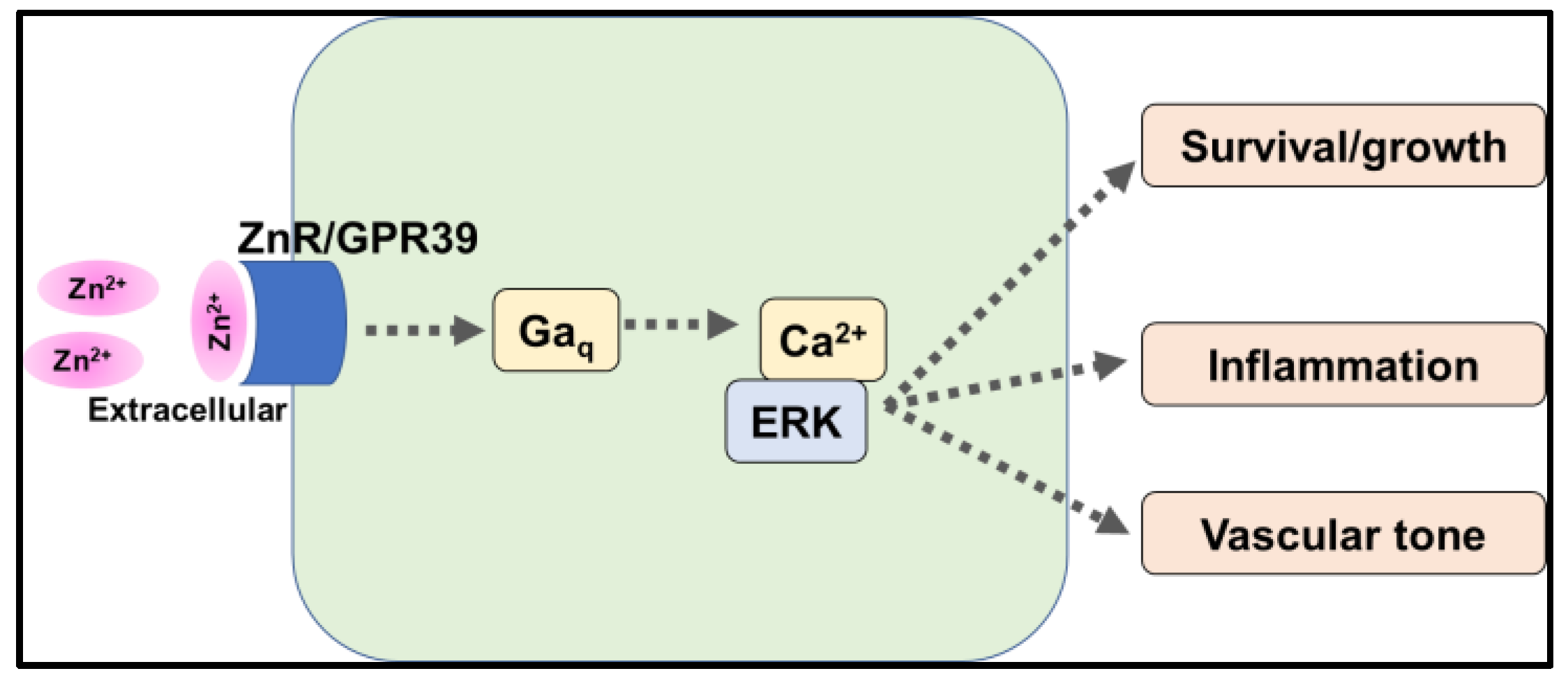
5.1. G-Protein Coupled Receptor 39 (GPR39)
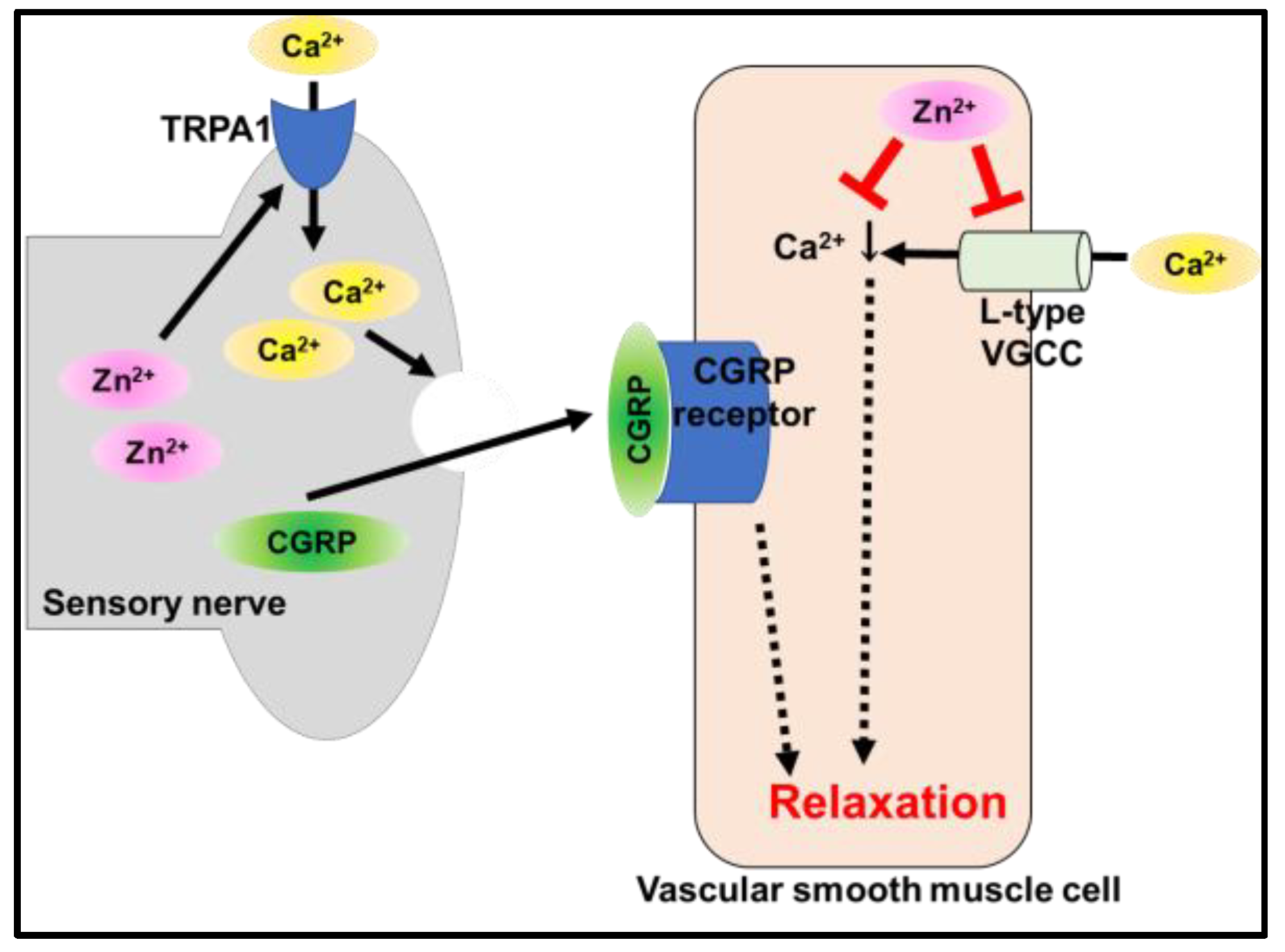
5.2. Transient Receptor Potential Ankyrin 1 (TRPA1)
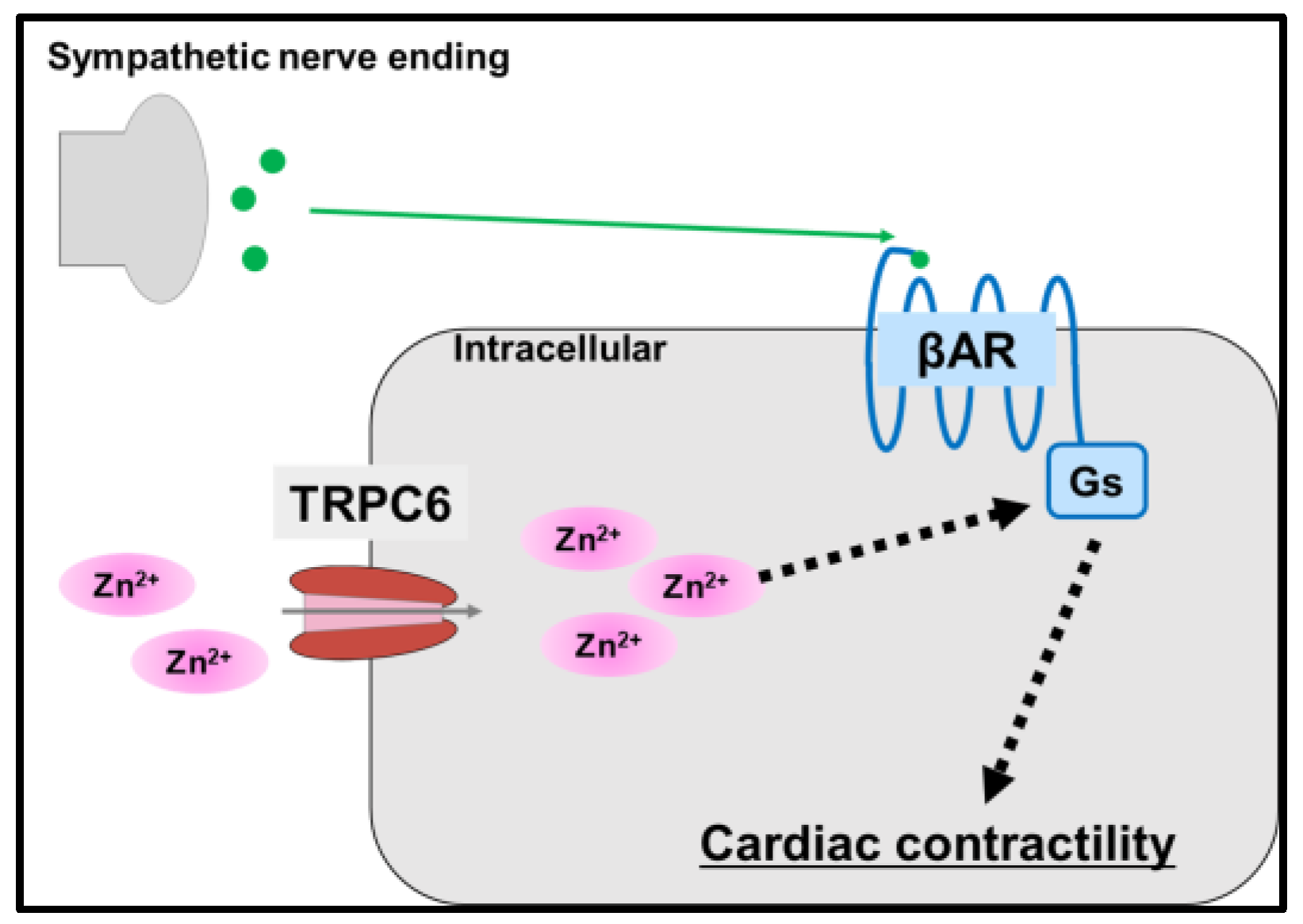
5.3. Transient Receptor Potential Cation Channel 6 (TRPC6)
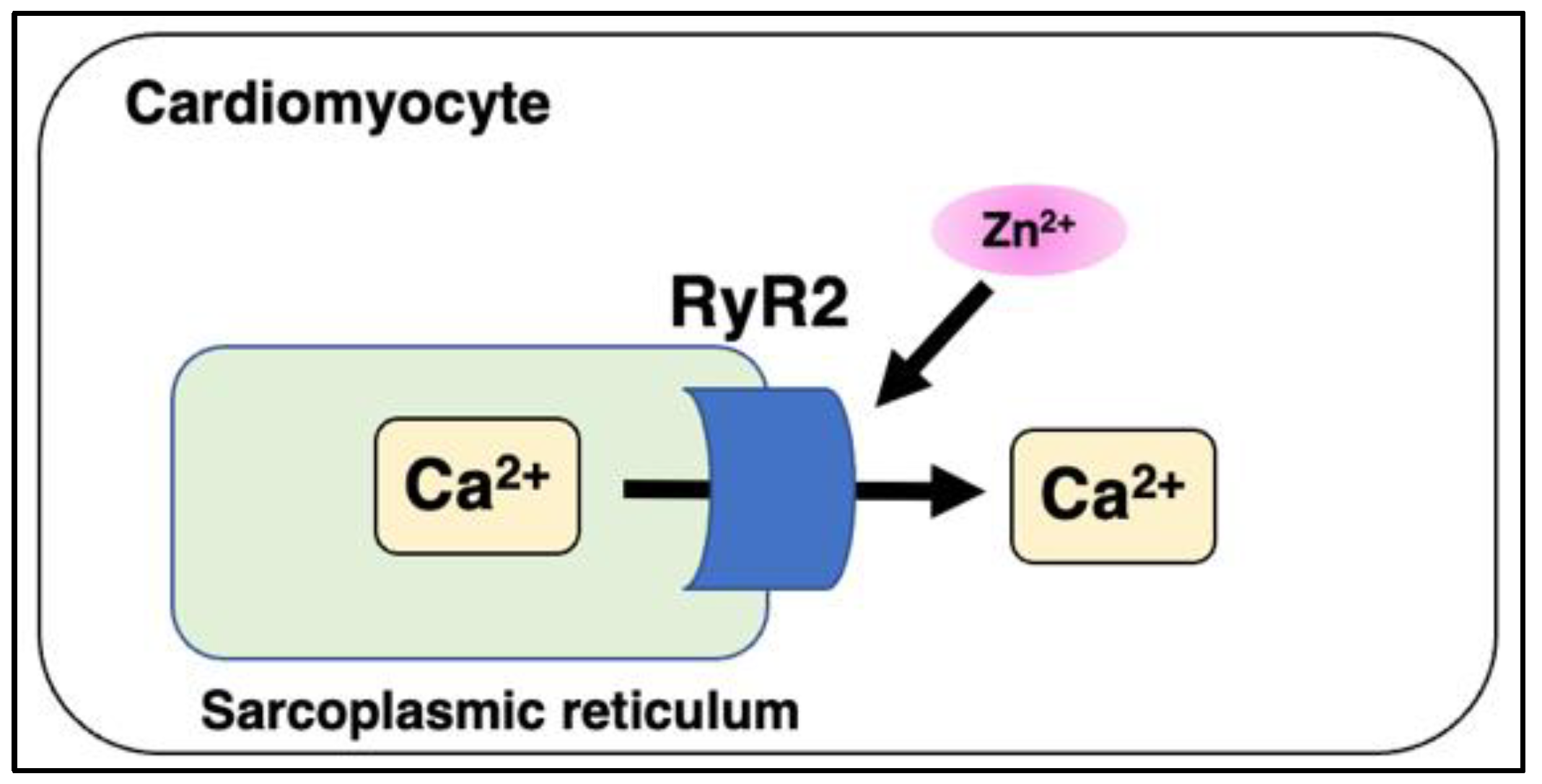
5.4. Ryanodine Receptor 2 (RyR2) and Mitsugumin 23 (MG23)
6. Therapeutic Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Hara, T.; Yoshigai, E.; Ohashi, T.; Fukada, T. Zinc transporters as potential therapeutic targets: An updated review. J. Pharmacol. Sci. 2022, 148, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsujimura, N.; Hashimoto, A.; Itsumura, N.; Nakagawa, M.; Miyazaki, S.; Kizu, K.; Goto, T.; Komatsu, Y.; Matsunaga, A.; et al. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985, 5, 341–363. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Pompano, L.M.; Boy, E. Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 141–160. [Google Scholar] [CrossRef]
- Brown, K.H.; Peerson, J.M.; Rivera, J.; Allen, L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002, 75, 1062–1071. [Google Scholar] [CrossRef]
- Barnett, J.B.; Dao, M.C.; Hamer, D.H.; Kandel, R.; Brandeis, G.; Wu, D.; E Dallal, G.; Jacques, P.F.; Schreiber, R.; Kong, E.; et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016, 103, 942–951. [Google Scholar] [CrossRef]
- Hongo, T.; Suzuki, T.; Ishida, H.; Kabuto, M.; Neriishi, K. Diurnal Variation of Plasma Minerals and Trace Elements in a Group of Japanese Male Adults. J. Nutr. Sci. Vitaminol. 1993, 39, 33–46. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Michael Hambidge, K.; Westcott, J.L.; Kern, D.L.; Marshall, G. Daily Variation in Plasma Zinc Concentrations in Women Fed Meals at Six-Hour Intervals1,2,3. J. Nutr. 1994, 124, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, W.K.; Merchant, A.T.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am. J. Med. 2004, 116, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Folsom, A.R.; Jacobs, D.R. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: The Iowa Women’s Health Study. Am. J. Clin. Nutr. 2005, 81, 787–791. [Google Scholar] [CrossRef]
- Sun, Q.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care 2009, 32, 629–634. [Google Scholar] [CrossRef]
- Mursu, J.; Robien, K.; Harnack, L.J.; Park, K.; Jacobs, D.R. Dietary supplements and mortality rate in older women: The Iowa Women’s Health Study. Arch. Intern. Med. 2011, 171, 1625–1633. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Q.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Chen, H. Multivitamins, individual vitamin and mineral supplements, and risk of diabetes among older U.S. adults. Diabetes Care 2011, 34, 108–114. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Alonso, A.; Lee, D.-H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef]
- Vashum, K.P.; McEvoy, M.; Shi, Z.; Milton, A.H.; Islam, R.; Sibbritt, D.; Patterson, A.; Byles, J.; Loxton, D.; Attia, J. Is dietary zinc protective for type 2 diabetes? Results from the Australian longitudinal study on women’s health. BMC Endocr. Disord. 2013, 13, 40. [Google Scholar] [CrossRef]
- Park, J.S.; Xun, P.; Li, J.; Morris, S.J.; Jacobs, D.R.; Liu, K.; He, K. Longitudinal association between toenail zinc levels and the incidence of diabetes among American young adults: The CARDIA Trace Element Study. Sci. Rep. 2016, 6, 23155. [Google Scholar] [CrossRef]
- Kok, F.J.; Van Duijn, C.M.; Hofman, A.; Van Der Voet, G.B.; De Wolff, F.A.; Paays, C.H.C.; Valkenburg, H.A. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am. J. Epidemiol. 1988, 128, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Gupta, U.C.; Mittal, N.; A Niaz, M.; Ghosh, S.; Rastogi, V. Epidemiologic study of trace elements and magnesium on risk of coronary artery disease in rural and urban Indian populations. J. Am. Coll. Nutr. 1997, 16, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Marniemi, J.; Järvisalo, J.; Toikka, T.; Räihä, I.; Ahotupa, M.; Sourander, L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int. J. Epidemiol. 1998, 27, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Bahjri, S.M.; Ahmed, W.H.; Al-Ama, N.; Ferns GA, A. Trace element status in Saudi patients with established atherosclerosis. J. Trace Elem. Med. Biol. 2006, 20, 105–114. [Google Scholar] [CrossRef]
- Kazemi-Bajestani, S.M.R.; Ghayour-Mobarhan, M.; Ebrahimi, M.; Moohebati, M.; Esmaeili, H.A.; Parizadeh, M.R.; Aghacizadeh, R.; Ferns, G.A. Serum copper and zinc concentrations are lower in Iranian patients with angiographically defined coronary artery disease than in subjects with a normal angiogram. J. Trace Elem. Med. Biol. 2007, 21, 22–28. [Google Scholar] [CrossRef]
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyörälä, K.; Lehto, S.; Ronnemaa, T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Winklhofer-Roob, B.M.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; März, W. Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br. J. Nutr. 2009, 101, 1534–1540. [Google Scholar] [CrossRef]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef]
- Alexanian, I.; Parissis, J.; Farmakis, D.; Athanaselis, S.; Pappas, L.; Gavrielatos, G.; Mihas, C.; Paraskevaidis, I.; Sideris, A.; Kremastinos, D.; et al. Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clin. Res. Cardiol. 2014, 103, 938–949. [Google Scholar] [CrossRef]
- De Paula, R.C.; Aneni, E.C.; Costa, A.P.R.; Figueiredo, V.N.; Moura, F.A.; Freitas, W.M.; Quaglia, L.A.; Santos, S.N.; Soares, A.A.; Nadruz, W.; et al. Low zinc levels is associated with increased inflammatory activity but not with atherosclerosis, arteriosclerosis or endothelial dysfunction among the very elderly. BBA Clin. 2014, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cai, Z.-Q.; Zhou, Y.-M. Deficient zinc levels and myocardial infarction: Association between deficient zinc levels and myocardial infarction: A meta-analysis. Biol. Trace Elem. Res. 2015, 165, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Foster, M.; Samman, S. Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus-A Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 707. [Google Scholar] [CrossRef]
- Yary, T.; Virtanen, J.K.; Ruusunen, A.; Tuomainen, T.-P.; Voutilainen, S. Serum zinc and risk of type 2 diabetes incidence in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. J. Trace Elem. Med. Biol. 2016, 33, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Teng, T.; Bian, B.; Yao, W.; Yu, X.; Wang, Z.; Xu, Z.; Sun, Y. Zinc Levels in Left Ventricular Hypertrophy. Biol. Trace Elem. Res. 2017, 176, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, L.; Zhao, J.; Wang, Z.; Yao, W.; Wu, X.; Huang, J.; Bian, B. The Relationship between Serum Zinc Level and Heart Failure: A Meta-Analysis. BioMed Res. Int. 2018, 2018, 2739014. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Ceylan-Isik, A.F.; Zhao, P.; Zhang, B.; Xiao, X.; Su, G.; Ren, J. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J. Mol. Cell. Cardiol. 2010, 48, 367–378. [Google Scholar] [CrossRef]
- Hu, N.; Han, X.; Lane, E.K.; Gao, F.; Zhang, Y.; Ren, J. Cardiac-specific overexpression of metallothionein rescues against cigarette smoking exposure-induced myocardial contractile and mitochondrial damage. PLoS ONE 2013, 8, e57151. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y.; Zhou, G.; Chen, T.; Song, Y.; Li, X.; Kang, Y.J. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1688–1697. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Men, H.; Tan, Y.; Lin, Q.; Gozal, E.; Zheng, Y.; Cai, L. Cardiac metallothionein overexpression rescues diabetic cardiomyopathy in Akt2-knockout mice. J. Cell. Mol. Med. 2021, 25, 6828–6840. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef] [PubMed]
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 expression and function lead to impaired intracellular zinc homeostasis in cancer. Cell Death Discov. 2019, 5, 144. [Google Scholar] [CrossRef]
- Beharier, O.; Etzion, Y.; Levi, S.; Mor, M.; Mor, M.; Dror, S.; Kahn, J.; Katz, A.; Moran, A. The involvement of ZnT-1, a new modulator of cardiac L-type calcium channels, in [corrected] atrial tachycardia remodeling. [corrected]. Ann. N. Y. Acad. Sci. 2010, 1188, 87–95. [Google Scholar] [CrossRef]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef]
- Ohashi, W.; Kimura, S.; Iwanaga, T.; Furusawa, Y.; Irié, T.; Izumi, H.; Watanabe, T.; Hijikata, A.; Hara, T.; Ohara, O.; et al. Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet. 2016, 12, e1006349. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Seo, J.; Kim, S.Y.; Lee, E.; Park, K.; Choi, D.H.; Takagishi, T.; Hara, T.; Hwang, D.; et al. Requirement of Zinc Transporter SLC39A7/ZIP7 for Dermal Development to Fine-Tune Endoplasmic Reticulum Function by Regulating Protein Disulfide Isomerase. J. Investig. Dermatol. 2017, 137, 1682–1691. [Google Scholar] [CrossRef]
- Tuncay, E.; Bitirim, C.V.; Olgar, Y.; Durak, A.; Rutter, G.A.; Turan, B. Zn2+-transporters ZIP7 and ZnT7 play important role in progression of cardiac dysfunction via affecting sarco(endo)plasmic reticulum-mitochondria coupling in hyperglycemic cardiomyocytes. Mitochondrion 2019, 44, 41–52. [Google Scholar] [CrossRef]
- Nolin, E.; Gans, S.; Llamas, L.; Bandyopadhyay, S.; Brittain, S.M.; Bernasconi-Elias, P.; Carter, K.P.; Loureiro, J.J.; Thomas, J.R.; Schirle, M.; et al. Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat. Chem. Biol. 2019, 15, 179–188. [Google Scholar] [CrossRef]
- Du, L.; Zhang, H.; Zhao, H.; Cheng, X.; Qin, J.; Teng, T.; Yang, Q.; Xu, Z. The critical role of the zinc transporter Zip2 (SLC39A2) in ischemia/reperfusion injury in mouse hearts. J. Mol. Cell. Cardiol. 2019, 132, 136–145. [Google Scholar] [CrossRef]
- Peters, J.L.; Dufner-Beattie, J.; Xu, W.; Geiser, J.; Lahner, B.; Salt, D.E.; Andrews, G.K. Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron, and calcium homeostasis. Genesis 2007, 45, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hasegawa, S.; Ban, S.; Yamada, T.; Date, Y.; Mizutani, H.; Nakata, S.; Tanaka, M.; Hirashima, N. ZIP2 protein, a zinc transporter, is associated with keratinocyte differentiation. J. Biol. Chem. 2014, 289, 21451–21462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, R.; Zhang, S.; Zhang, H.; Yang, Q.; Xu, Z. Upregulation of p67phox in response to ischemia/reperfusion is cardioprotective by increasing ZIP2 expression via STAT3. Free. Radic. Res. 2022, 56, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Küry, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, D.; Cheng, L.; Li, L.; Liu, F.; Hand, N.J.; Epstein, J.A.; Rader, D.J. Zinc transporter Slc39a8 is essential for cardiac ventricular compaction. J. Clin. Investig. 2018, 128, 826–833. [Google Scholar] [CrossRef]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The Zinc Transporter SLC39A13/ZIP13 Is Required for Connective Tissue Development; Its Involvement in BMP/TGF-β Signaling Pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Bin, B.-H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef]
- Dusanic, M.; Dekomien, G.; Lücke, T.; Vorgerd, M.; Weis, J.; Epplen, J.T.; Köhler, C.; Hoffjan, S. Novel Nonsense Mutation in SLC39A13 Initially Presenting as Myopathy: Case Report and Review of the Literature. MSY 2018, 9, 100–109. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, X.; Zhao, H.; Yang, Q.; Xu, Z. Downregulation of the zinc transporter SLC39A13 (ZIP13) is responsible for the activation of CaMKII at reperfusion and leads to myocardial ischemia/reperfusion injury in mouse hearts. J. Mol. Cell. Cardiol. 2021, 152, 69–79. [Google Scholar] [CrossRef]
- Hirose, T.; Shimazaki, T.; Takahashi, N.; Fukada, T.; Watanabe, T.; Tangkawattana, P.; Takehana, K. Morphometric analysis of thoracic aorta in Slc39a13/Zip13-KO mice. Cell Tissue Res. 2019, 376, 137–141. [Google Scholar] [CrossRef]
- Hara, T.; Yamada, I.; Ohashi, T.; Tamura, M.; Hijikata, A.; Watanabe, T.; Gao, M.; Ito, K.; Kawamata, S.; Azuma, S.; et al. Role of Scl39a13/ZIP13 in cardiovascular homeostasis. PLoS ONE 2022, 17, e0276452. [Google Scholar] [CrossRef] [PubMed]
- Traylor, M.; Persyn, E.; Tomppo, L.; Klasson, S.; Abedi, V.; Bakker, M.K.; Torres, N.; Li, L.; Bell, S.; Rutten-Jacobs, L.; et al. Genetic basis of lacunar stroke: A pooled analysis of individual patient data and genome-wide association studies. Lancet Neurol. 2021, 20, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Zheng, Y.; Fu, B.M.; Tang, L.; Qin, Y.-X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am. J. Physiol. Cell Physiol. 2018, 314, C404–C414. [Google Scholar] [CrossRef] [PubMed]
- Venkata, S.P.M.; Li, H.; Xu, L.; Koh, J.Y.; Nguyen, H.; Minjares, M.; Li, C.; Kowluru, A.; Milligan, G.; Wang, J.-M. Inhibition of GPR39 restores defects in endothelial cell-mediated neovascularization under the duress of chronic hyperglycemia: Evidence for regulatory roles of the sonic hedgehog signaling axis. Proc. Natl. Acad. Sci. USA 2023, 120, e2208541120. [Google Scholar] [CrossRef]
- Liao, H.; Gao, W.; Ma, J.; Xue, H.; Wang, Y.; Huang, D.; Yan, F.; Ye, Y. GPR39 promotes cardiac hypertrophy by regulating the AMPK-mTOR pathway and protein synthesis. Cell Biol. Int. 2021, 45, 1211–1219. [Google Scholar] [CrossRef]
- Betrie, A.H.; Brock, J.A.; Harraz, O.F.; Bush, A.I.; He, G.-W.; Nelson, M.T.; Angus, J.A.; Wright, C.E.; Ayton, S. Zinc drives vasorelaxation by acting in sensory nerves, endothelium and smooth muscle. Nat. Commun. 2021, 12, 3296. [Google Scholar] [CrossRef]
- Gibon, J.; Tu, P.; Bohic, S.; Richaud, P.; Arnaud, J.; Zhu, M.; Boulay, G.; Bouron, A. The over-expression of TRPC6 channels in HEK-293 cells favours the intracellular accumulation of zinc. Biochim. Biophys. Acta (BBA)—Biomembr. 2011, 1808, 2807–2818. [Google Scholar] [CrossRef]
- Oda, S.; Nishiyama, K.; Furumoto, Y.; Yamaguchi, Y.; Nishimura, A.; Tang, X.; Kato, Y.; Numaga-Tomita, T.; Kaneko, T.; Mangmool, S.; et al. Myocardial TRPC6-mediated Zn2+ influx induces beneficial positive inotropy through β-adrenoceptors. Nat. Commun. 2022, 13, 6374. [Google Scholar] [CrossRef]
- Reilly-O’Donnell, B.; Robertson, G.B.; Karumbi, A.; McIntyre, C.; Bal, W.; Nishi, M.; Takeshima, H.; Stewart, A.J.; Pitt, S.J. Dysregulated Zn2+ homeostasis impairs cardiac type-2 ryanodine receptor and mitsugumin 23 functions, leading to sarcoplasmic reticulum Ca2+ leakage. J. Biol. Chem. 2017, 292, 13361–13373. [Google Scholar] [CrossRef]
- Pitt, S.J.; Stewart, A.J. Examining a new role for zinc in regulating calcium release in cardiac muscle. Biochem. Soc. Trans. 2015, 43, 359–363. [Google Scholar] [CrossRef]
- Pujol-Giménez, J.; Poirier, M.; Bühlmann, S.; Schuppisser, C.; Bhardwaj, R.; Awale, M.; Visini, R.; Javor, S.; Hediger, M.A.; Reymond, J. Inhibitors of Human Divalent Metal Transporters DMT1 (SLC11A2) and ZIP8 (SLC39A8) from a GDB-17 Fragment Library. ChemMedChem 2021, 16, 3306–3314. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Ohkura, K.; Takahashi, M.; Kizu, K.; Narita, H.; Enomoto, S.; Miyamae, Y.; Masuda, S.; Nagao, M.; Irie, K.; et al. Soybean extracts increase cell surface ZIP4 abundance and cellular zinc levels: A potential novel strategy to enhance zinc absorption by ZIP4 targeting. Biochem. J. 2015, 472, 183–193. [Google Scholar] [CrossRef] [PubMed]

| Zinc Status | Population | Baseline Age (Year) | Sex (%) | Follow-Up (Year) | No. of Cases | Outcome | References | Prospective Risk of CVD or T2D |
|---|---|---|---|---|---|---|---|---|
| Zinc supplementation | Health professionals, excluded participants with cancer, MI and CVD | 40–75 | Male | 12 | 39,633 | Risk of CVD | Al-Delaimy et al., 2004 [13] | No association |
| Zinc intake | Postmenopausal women without having angina, heart disease or heart attack | 55–69 | Female | 18 | 34,492 | CVD mortality | Lee, D.H., et al., 2005 [14] | ↓ risk |
| Zinc intake | Nurses free of diabetes, cancer or CVD at baseline | 33–60 | Female | 24 | 82,297 | Risk of T2D | Q. Sun et al., 2009 [15] | ↓ risk |
| Zinc supplementation | Mostly postmenopausal women | 55–69 | Female | 10 | 38,772 | CVD mortality | J. Mursu et al., 2011 [16] | No association |
| Zinc supplementation | NIH-AARP cohort (Frequent use of any multivitamins including zinc) | 50–71 | Female (64.5%) | 10 | 232,007 | Risk of T2D | Y. Song et al., 2011 [17] | No association |
| Zinc intake | Population-based sample, free of clinical CVD | 61.8 ± 10.3 | - | 10 | 5285 | Risk of CVD | M.C. Otto et al., 2012 [18] | ↓ risk |
| Zinc intake | Women | 45–50 | Female | 6 | 8921 | Risk of T2D | K.P. Vashum et al., 2013 [19] | No association |
| Zinc intake | African American and Caucasian men and women | 27.03 ± 3.61 | Female (52.5%) | 23 | 3960 | Risk of T2D | J.S. Park et al., 2016 [20] | ↓ risk |
| Zinc Status | Population | Baseline Age (Year) | Sex (%) | Follow-Up (Year) | No. of Cases | Outcome | References | Prospective Risk of CVD |
|---|---|---|---|---|---|---|---|---|
| Serum zinc level | A Dutch prospective follow-up study | N/A | N/A | 6–9 | 10,658 | Risk of death from CVD | F.J. Kok et al., 1988 [21] | No association |
| Serum zinc level | Rural and urban Indian populations | 26–65 | Female (53%) | - | 314 | Coronary artery disease (CAD) | R.B. Singh et al., 1997 [22] | ↓ risk |
| Plasma/serum zinc level | Community-living age ≥ 65 years | ≥65 | Female (47%) | 13 | 344 | serum zinc level and vascular mortality | J. Marniemi et al. 1998 [23] | No association |
| Plasma/serum zinc level | Men aged ≥ 30 year | 30–60 | Male | 20 | 4035 | diabetes, and CVD history | N. Leone et al., 2006 [24] | No association |
| Serum zinc/urine zinc | Saudi male subjects | N/A | Male | - | 260 | CVD and serum zinc | E.M. Alissa et al., 2006 [25] | Association |
| Serum zinc | Iranian subjects undergoing coronary angiography | N/A | Female (41%) | - | 114 | CAD: lower serum zinc | S.M. Kazemi-Bajestani et al., 2007 [26] | Association |
| Plasma/serum zinc level | Patients with Type 2 DM | 45–64 | Female (45%) | 7 | 1050 | Higher baseline serum zinc level was associated with reduction in risk of CHD death. | M. Soinio et al., 2007 [27] | ↓ risk |
| Plasma/serum zinc level | German ancestry who was referred to coronary angiography | >60 (median) | Female (30%) | 7.75 | 3274 | Increased CVD mortality and serum zinc. | S. Pilz et al., 2009 [28] | Association |
| Plasma/serum zinc level | Community-living participants Age ≥ 65 years | 65–99 | Female (49%) | 14 | 741 | Risk of vascular disease mortality | C.J. Bates et al., 2011 [29] | Association |
| Plasma/serum zinc level | A group of 21 subjects free of symptoms, signs and objective evidence of HF | 69 ± 11 | Male (71%) | 6 | 125 | HF and patients with left ventricular diastolic function | I. Alexanian et al., 2014 [30] | No association |
| Plasm zinc | Subjects with uneventful cardiovascular history and without anti-inflammatory treatments | 80–102 | - | - | 201 | coronary calcium score, | R.C. De Paula et al., 2014 [31] | No association |
| Serum zinc level | 2886 subjects from 41 case-control studies (Meta-analysis of 13 articles) | - | - | - | 2886 | Myocardial infarction and serum zinc | B. Liu et al., 2015 [32] | Association |
| Plasma/ Serum zinc | Summarize prospective cohort studies from 14 articles | - | - | 12.4 | 91,708 | CVD and zinc status | A. Chu et al., 2016 [33] | Association |
| Plasma/serum zinc level | Finnish men | 42–60 | Male | 20 | 2220 | Incidence of T2D and serum zinc and | T. Yary et al., 2016 [34] | Association |
| Plasma/serum zinc level | Left ventricular hypertrophy (LVH) patients, especially in the eccentric LVH and concentric LVH patients | 61.8 ± 13.2 | Female (44%) | - | 519 | left ventricular hypertrophy (LVH) | L. Huang et al., 2017 [35] | ↓ risk |
| Serum zinc level | 1453 subjects from 27 case-control studies (Meta-analysis of 12 articles) | - | - | - | 1453 | HF and low serum zinc | X. Yu et al., 2018 [36] | Association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, T.; Yoshigai, E.; Ohashi, T.; Fukada, T. Zinc in Cardiovascular Functions and Diseases: Epidemiology and Molecular Mechanisms for Therapeutic Development. Int. J. Mol. Sci. 2023, 24, 7152. https://doi.org/10.3390/ijms24087152
Hara T, Yoshigai E, Ohashi T, Fukada T. Zinc in Cardiovascular Functions and Diseases: Epidemiology and Molecular Mechanisms for Therapeutic Development. International Journal of Molecular Sciences. 2023; 24(8):7152. https://doi.org/10.3390/ijms24087152
Chicago/Turabian StyleHara, Takafumi, Emi Yoshigai, Takuto Ohashi, and Toshiyuki Fukada. 2023. "Zinc in Cardiovascular Functions and Diseases: Epidemiology and Molecular Mechanisms for Therapeutic Development" International Journal of Molecular Sciences 24, no. 8: 7152. https://doi.org/10.3390/ijms24087152
APA StyleHara, T., Yoshigai, E., Ohashi, T., & Fukada, T. (2023). Zinc in Cardiovascular Functions and Diseases: Epidemiology and Molecular Mechanisms for Therapeutic Development. International Journal of Molecular Sciences, 24(8), 7152. https://doi.org/10.3390/ijms24087152







