Osteogenic Differentiation Effect of Human Periodontal Ligament Stem-Cell Initial Cell Density on Autologous Cells and Human Bone Marrow Stromal Cells
Abstract
1. Introduction
2. Results
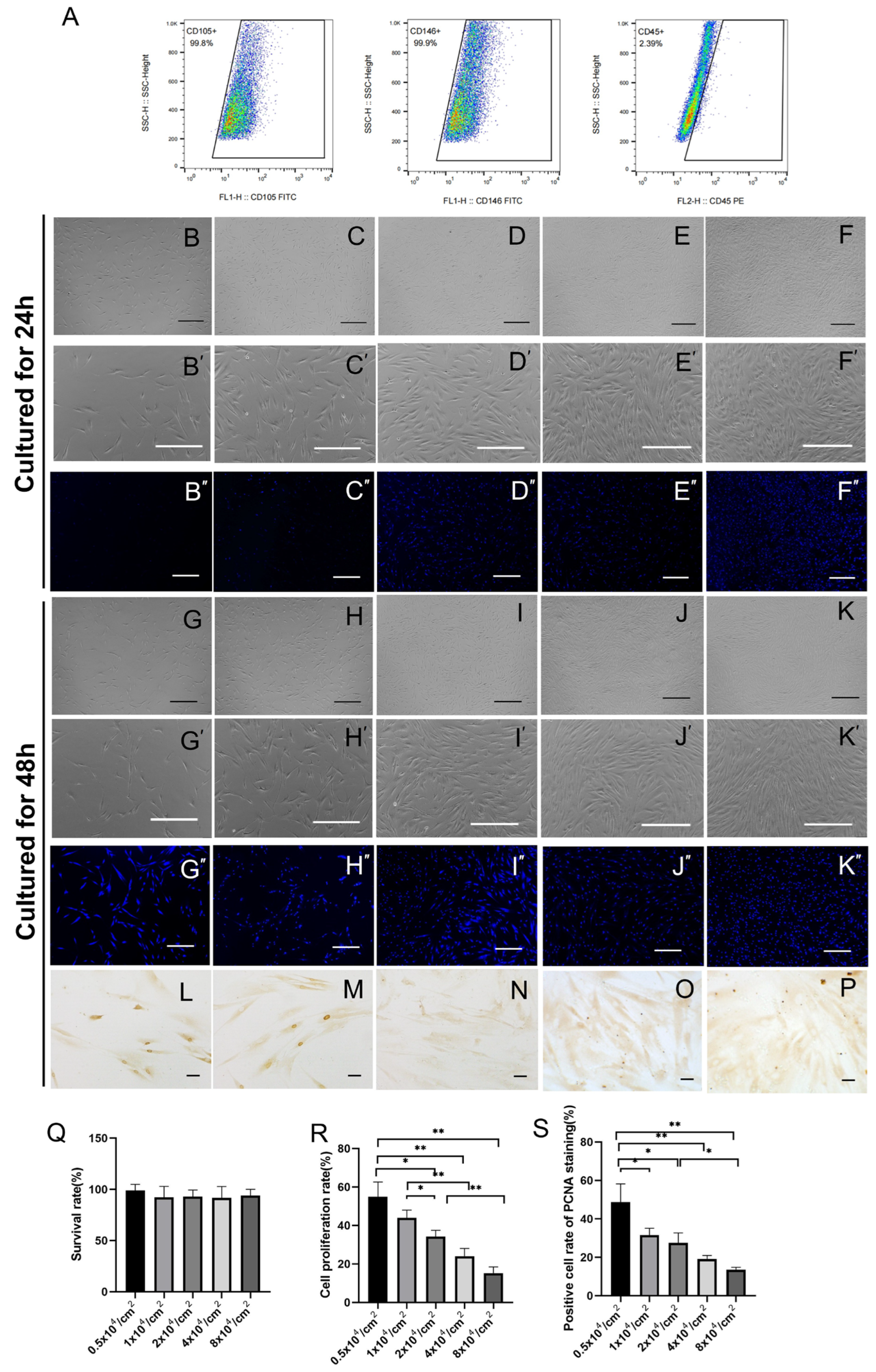
2.1. hPDLSC Proliferation as a Function of Culture Density
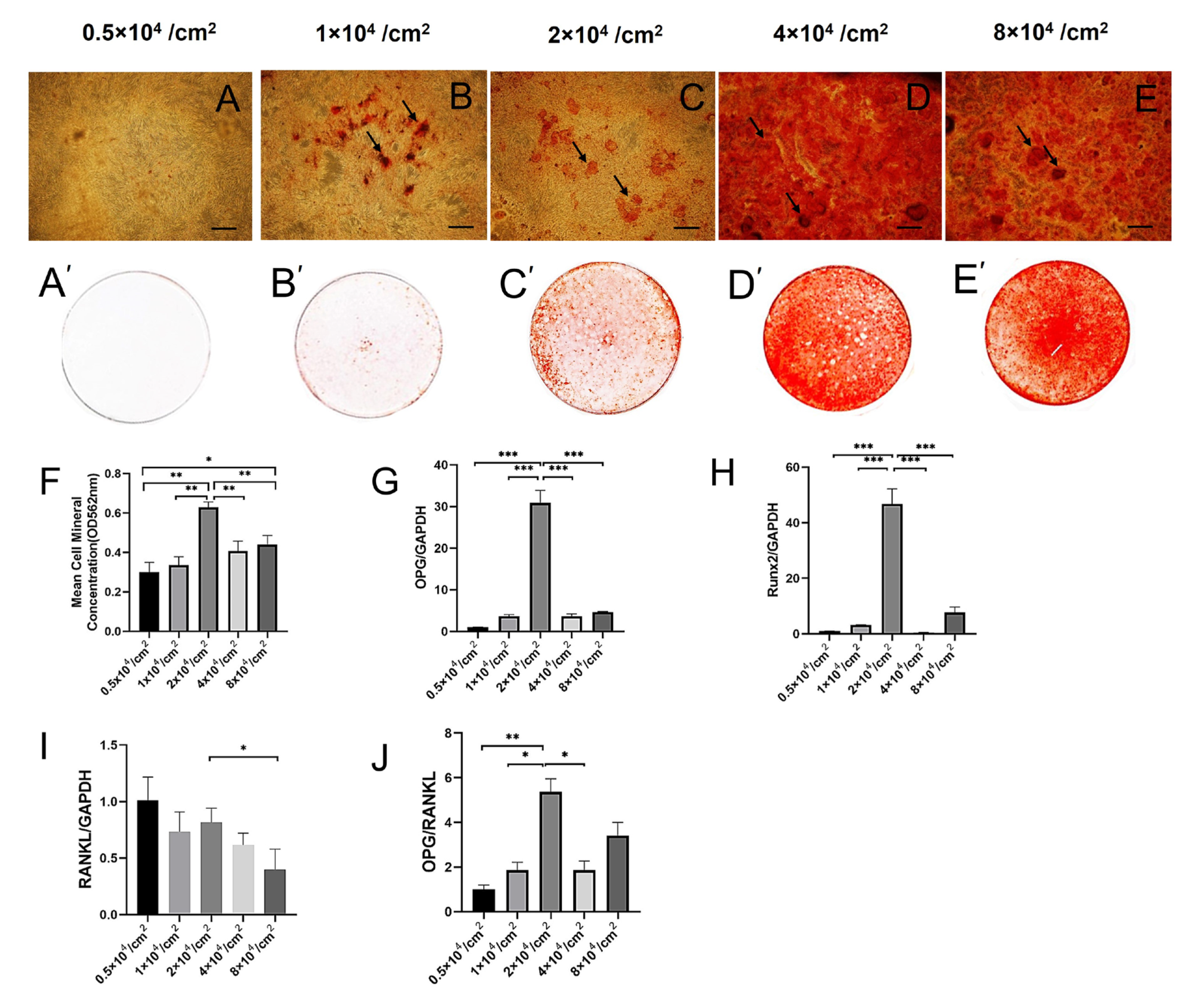
2.2. Effect of Cell Density on the Expression of OPG, RUNX2, and OPG/RANKL during Osteogenesis of hPDLSCs
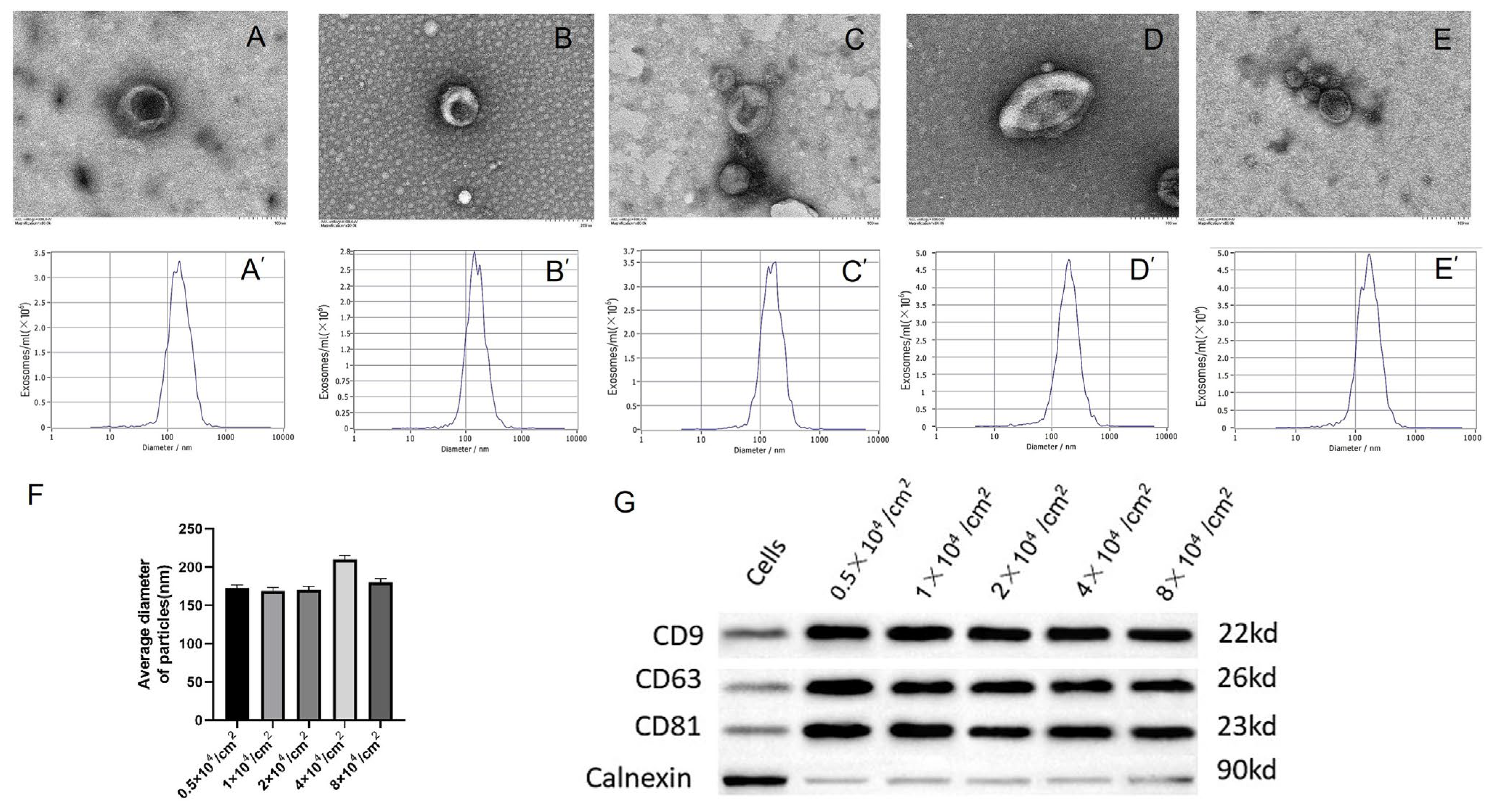
2.3. hPDLSCs with Different Initial Culture Densities Enable High-Yield Production of Exosomes
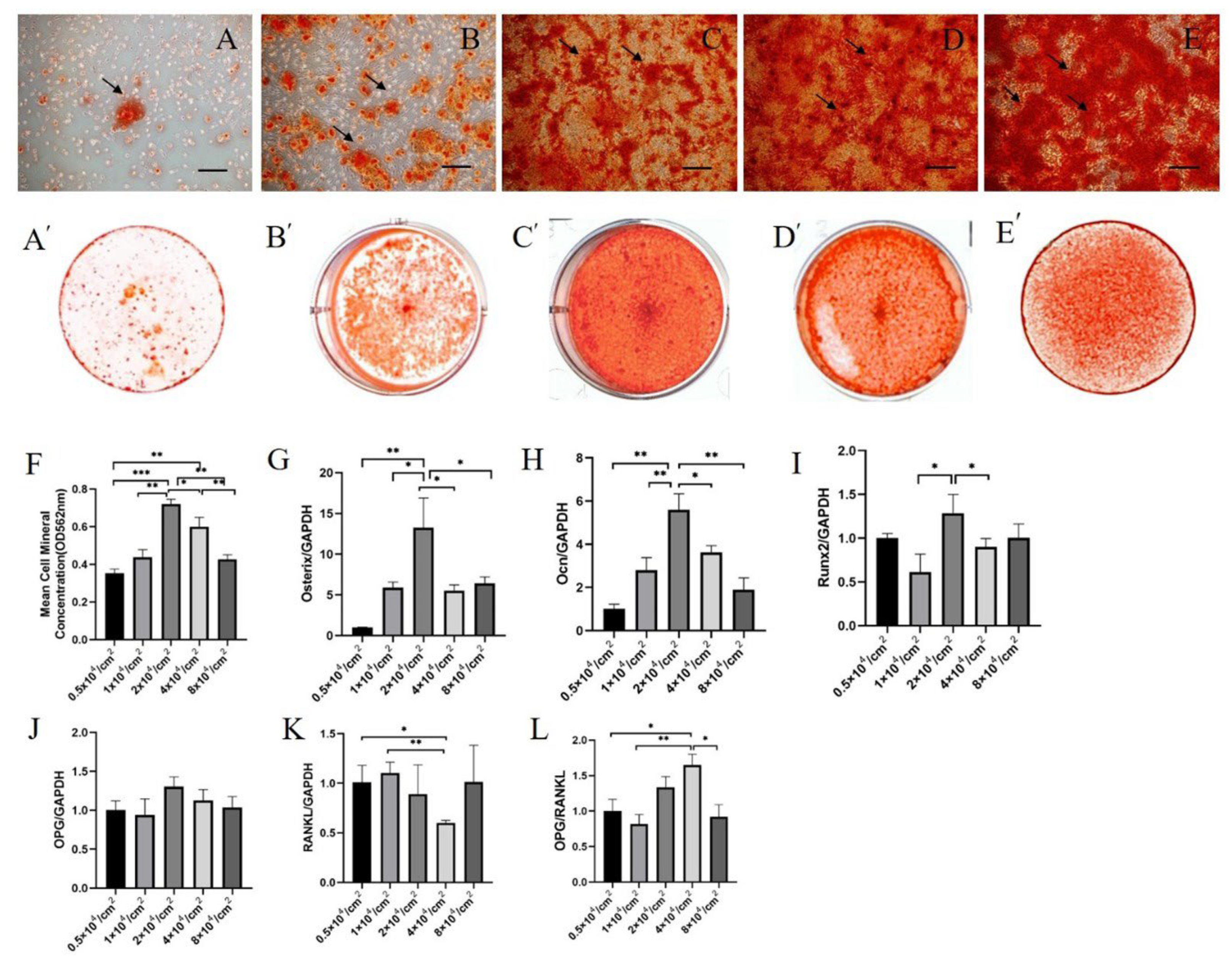
2.4. Osteogenic Effects of hPDLSC Exosomes Derived from Cultures with Different Initial Cell Densities on hBMSCs
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Identification of hPDLSCs and hBMSCs
4.2. Cell Proliferation Test
4.3. Isolation of Exosomes
4.4. Characteristics of Exosomes
4.5. Alizarin Red Staining Test
4.6. Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Li, Y.; Han, G. Advances of Mesenchymal Stem Cells Released Extracellular Vesicles in Periodontal Bone Remodeling. DNA Cell Biol. 2022, 41, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Brooke, G.; Cook, M.; Blair, C.; Han, R.; Heazlewood, C.; Jones, B.; Kambouris, M.; Kollar, K.; McTaggart, S.; Pelekanos, R.; et al. Therapeutic applications of mesenchymal stromal cells. Semin. Cell Dev. Biol. 2007, 18, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, C.G.; de la Serna, E.L.; Dunn, A.R. How cells tell up from down and stick together to construct multicellular tissues—Interplay between apicobasal polarity and cell-cell adhesion. J. Cell Sci. 2021, 134, jcs248757. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A.; Helin, K. Cell cycle, differentiation and disease. Curr. Opin. Cell Biol. 2013, 25, 673–675. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2020, 11, 604274. [Google Scholar] [CrossRef]
- Chen, J.; Mo, Q.; Sheng, R.; Zhu, A.; Ling, C.; Luo, Y.; Zhang, A.; Chen, Z.; Yao, Q.; Cai, Z.; et al. The application of human periodontal ligament stem cells and biomimetic silk scaffold for in situ tendon regeneration. Stem Cell Res. Ther. 2021, 12, 596. [Google Scholar] [CrossRef]
- Song, I.S.; Han, Y.S.; Lee, J.-H.; Um, S.; Kim, H.Y.; Seo, B.M. Periodontal Ligament Stem Cells for Periodontal Regeneration. Curr. Oral Health Rep. 2015, 2, 236–244. [Google Scholar] [CrossRef]
- Cheng, J.; Feng, Y.; Feng, X.; Wu, D.; Lu, X.; Rao, Z.; Li, C.; Lin, N.; Jia, C.; Zhang, Q. Improving the immunomodulatory function of mesenchymal stem cells by defined chemical approach. Front. Immunol. 2022, 13, 1005426. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Amon, A. Relevance and Regulation of Cell Density. Trends Cell Biol. 2020, 30, 213–225. [Google Scholar] [CrossRef]
- He, L.; Yao, Y.; Wang, N.; Nan, G. Effects of electric charge on fracture healing. Sci. Rep. 2022, 12, 15839. [Google Scholar] [CrossRef]
- Loza, A.J.; Koride, S.; Schimizzi, G.V.; Li, B.; Sun, S.X.; Longmore, G.D. Cell density and actomyosin contractility control the organization of migrating collectives within an epithelium. Mol. Biol. Cell 2016, 27, 3459–3470. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Liu, Y.; Wang, J.; Huang, X. Effect of MC3T3 cell density on osteoclastic differentiation of mouse bone marrow cells. Tissue Cell 2022, 75, 101724. [Google Scholar] [CrossRef]
- Zhou, H.; Weir, M.D.; Xu, H.H. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement-fiber scaffold. Tissue Eng. Part A 2011, 17, 2603–2613. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Zhong, J.Y.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Yuan, L.Q. Exosomes as Mediators of Cell-to-Cell Communication in Thyroid Disease. Int. J. Endocrinol. 2020, 2020, 4378345. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Screven, R.; Kenyon, E.; Myers, M.J.; Yancy, H.F.; Skasko, M.; Boxer, L.; Bigley, E.C., III; Borjesson, D.L.; Zhu, M. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet. Immunol. Immunopathol. 2014, 161, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.H.; Ding, Y.N.; Wang, A.; Li, X.; Wang, Y.; Shi, F.G.; Lu, Y.F. Cinnabar protects serum-nutrient starvation induced apoptosis by improving intracellular oxidative stress and inhibiting the expression of CHOP and PERK. Biochem. Biophys. Rep. 2021, 27, 101055. [Google Scholar] [CrossRef] [PubMed]
- Solodushko, V.; Khader, H.A.; Fouty, B.W. Serum can overcome contact inhibition in confluent human pulmonary artery smooth muscle cells. PLoS ONE 2013, 8, e71490. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Fullgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.; Garrod, D.R. The sorting out of embryonic cells in monolayer, the differential adhesion hypothesis and the non-specificity of cell adhesion. J. Cell Sci. 1979, 9, 249–266. [Google Scholar] [CrossRef]
- Wang, G.; Han, J.; Zhuang, L.; Li, S.; Gong, Q.; Chen, Y. Serum starvation induces cell death in NSCLC via miR-224. Onco Targets Ther. 2019, 12, 3953–3962. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, M.W.; Ko, Y.J.; Chun, Y.H.; Kim, H.J.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Cell culture density affects the proliferation activity of human adipose tissue stem cells. Cell Biochem. Funct. 2016, 34, 16–24. [Google Scholar] [CrossRef]
- Bost, L.M.; Hjelmeland, L.M. Cell density regulates differential production of bFGF transcripts. Growth Factors 1993, 9, 195–203. [Google Scholar] [CrossRef]
- Liu, T.; Hu, W.; Zou, X.; Xu, J.; He, S.; Chang, L.; Li, X.; Yin, Y.; Tian, M.; Li, Z.; et al. Human Periodontal Ligament Stem Cell-Derived Exosomes Promote Bone Regeneration by Altering MicroRNA Profiles. Stem Cells Int. 2020, 2020, 8852307. [Google Scholar] [CrossRef]
- Abdi, S.; Binbaz, R.A.; Mohammed, A.K.; Ansari, M.G.A.; Wani, K.; Amer, O.E.; Alnaami, A.M.; Aljohani, N.; Al-Daghri, N.M. Association of RANKL and OPG Gene Polymorphism in Arab Women with and without Osteoporosis. Genes 2021, 12, 200. [Google Scholar] [CrossRef]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.L.; Li, T.T.; Yang, F.; Tzeng, C.M. Baicalin Ameliorates Dexamethasone-Induced Osteoporosis by Regulation of the RANK/RANKL/OPG Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 195–206. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Hou, Y.F.; Shan, C.; Zhuang, S.Y.; Zhuang, Q.Q.; Ghosh, A.; Zhu, K.C.; Kong, X.K.; Wang, S.M.; Gong, Y.L.; Yang, Y.Y.; et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson's disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Bruzzaniti, A. Molecular signaling in bone cells: Regulation of cell differentiation and survival. Adv. Protein Chem. Struct. Biol. 2019, 116, 237–281. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Kohrt, W.; Levasseur, R.; Warren, M.; Whiting, S.; Kraenzlin, M. Biochemical markers for assessment of calcium economy and bone metabolism: Application in clinical trials from pharmaceutical agents to nutritional products. Nutr. Res. Rev. 2014, 27, 252–267. [Google Scholar] [CrossRef]
- Zubaidah, N.; Pratiwi, D.D.; Masa, M.; Setiawatie, E.M.; Kunarti, S. The Osteogenesis Mechanisms of Dental Alveolar Bone Socket Post Induction with Hydroxyapatite Bovine Tooth Graft: An Animal Experimental in Rattus norvegicus Strain Wistar. Eur. J. Dent. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Xu, Y.; Liu, H.; Wang, Z.; Ma, T.; Li, Z.; Sun, L.; Huang, Q.; Zhang, K.; Zhang, C.; et al. Effects of runt-related transcription factor 2 (RUNX2) on the autophagy of rapamycin-treated osteoblasts. Bioengineered 2022, 13, 5262–5276. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Li, J.; Cheng, Y.; Wang, Z.; Feng, T.; Lu, G.; Wang, S.; Song, J.; Xia, P.; et al. Biological Functions of Let-7e-5p in Promoting the Differentiation of MC3T3-E1 Cells. Front. Cell Dev. Biol. 2021, 9, 671170. [Google Scholar] [CrossRef]
- Wang, K.; Kong, X.; Du, M.; Yu, W.; Wang, Z.; Xu, B.; Yang, J.; Xu, J.; Liu, Z.; Cheng, Y.; et al. Novel Soy Peptide CBP: Stimulation of Osteoblast Differentiation via TbetaRI-p38-MAPK-Depending RUNX2 Activation. Nutrients 2022, 14, 1940. [Google Scholar] [CrossRef]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tian, W.; Li, Y.; Tang, W.; Zhang, C. Osteoblast-specific transcription factor Osterix (Osx) and HIF-1alpha cooperatively regulate gene expression of vascular endothelial growth factor (VEGF). Biochem. Biophys. Res. Commun. 2012, 424, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Chen, W.; Luo, Y.; Wu, J.; Zhang, Y.; McVicar, A.; McConnell, M.; Liu, Y.; Zhou, H.D.; Li, Y.P. Runx1 up-regulates chondrocyte to osteoblast lineage commitment and promotes bone formation by enhancing both chondrogenesis and osteogenesis. Biochem. J. 2020, 477, 2421–2438. [Google Scholar] [CrossRef] [PubMed]
- Perez-Campo, F.M.; Santurtun, A.; Garcia-Ibarbia, C.; Pascual, M.A.; Valero, C.; Garces, C.; Sanudo, C.; Zarrabeitia, M.T.; Riancho, J.A. Osterix and RUNX2 are Transcriptional Regulators of Sclerostin in Human Bone. Calcif. Tissue Int. 2016, 99, 302–309. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J.; et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Faulkner, B.; Astleford, K.; Mansky, K.C. Regulation of Osteoclast Differentiation and Skeletal Maintenance by Histone Deacetylases. Molecules 2019, 24, 1355. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, M.W.; Lee, T.H.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed. Rep. 2017, 6, 300–306. [Google Scholar] [CrossRef]
- Xu, H.; Chai, Q.; Xu, X.; Li, Z.; Bao, W.; Man, Z.; Li, W. Exosome-Functionalized Ti6Al4V Scaffolds Promoting Osseointegration by Modulating Endogenous Osteogenesis and Osteoimmunity. ACS Appl. Mater. Interfaces 2022, 14, 46161–46175. [Google Scholar] [CrossRef]
- Byrnes, K.; Blessinger, S.; Bailey, N.T.; Scaife, R.; Liu, G.; Khambu, B. Therapeutic regulation of autophagy in hepatic metabolism. Acta Pharm. Sin. B 2022, 12, 33–49. [Google Scholar] [CrossRef]
- Kuah, D.; Sivell, S.; Longworth, T.; James, K.; Guermazi, A.; Cicuttini, F.; Wang, Y.; Craig, S.; Comin, G.; Robinson, D.; et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 2018, 16, 49. [Google Scholar] [CrossRef]

| Gene (Human) | Forward Primer | Reverse Primer |
|---|---|---|
| OPG | 5′-GAGTCCGATCCAGCCAAGA-3′ | 5′-GTACGGCGGAAACTCACAG-3′ |
| OCN | 5′-TAGTGAAGAGACCCAGGCGCT-3′ | 5′-ATAGGCCTCCTGAAAGCCGA-3′ |
| RUNX2 | 5′-GGAATGCCTCTGCTGTTATGAA-3′ | 5′-GCTTCTGTCTGTGCCTTCTG-3′ |
| Osterix | 5′-CTCCTGCGACTGCCCTAAT-3′ | 5′-CTCATCCGAACGAGTGAACCT-3′ |
| RANKL | 5′-TCGCTGGGAAACAACACTG-3′ | 5′-GGGAAGGGAAAGGTAGATGC-3′ |
| GAPDH | 5′-CTGGGCTACACTGAGCACC-3′ | 5′-AAGTGGTCGTTGAGGGCAATG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qiao, Q.; Sun, Y.; Yu, W.; Wang, J.; Zhu, M.; Yang, K.; Huang, X.; Bai, Y. Osteogenic Differentiation Effect of Human Periodontal Ligament Stem-Cell Initial Cell Density on Autologous Cells and Human Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2023, 24, 7133. https://doi.org/10.3390/ijms24087133
Wang J, Qiao Q, Sun Y, Yu W, Wang J, Zhu M, Yang K, Huang X, Bai Y. Osteogenic Differentiation Effect of Human Periodontal Ligament Stem-Cell Initial Cell Density on Autologous Cells and Human Bone Marrow Stromal Cells. International Journal of Molecular Sciences. 2023; 24(8):7133. https://doi.org/10.3390/ijms24087133
Chicago/Turabian StyleWang, Jing, Qingchen Qiao, Yaxi Sun, Wenting Yu, Jiran Wang, Minjia Zhu, Kai Yang, Xiaofeng Huang, and Yuxing Bai. 2023. "Osteogenic Differentiation Effect of Human Periodontal Ligament Stem-Cell Initial Cell Density on Autologous Cells and Human Bone Marrow Stromal Cells" International Journal of Molecular Sciences 24, no. 8: 7133. https://doi.org/10.3390/ijms24087133
APA StyleWang, J., Qiao, Q., Sun, Y., Yu, W., Wang, J., Zhu, M., Yang, K., Huang, X., & Bai, Y. (2023). Osteogenic Differentiation Effect of Human Periodontal Ligament Stem-Cell Initial Cell Density on Autologous Cells and Human Bone Marrow Stromal Cells. International Journal of Molecular Sciences, 24(8), 7133. https://doi.org/10.3390/ijms24087133






