COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters
Abstract
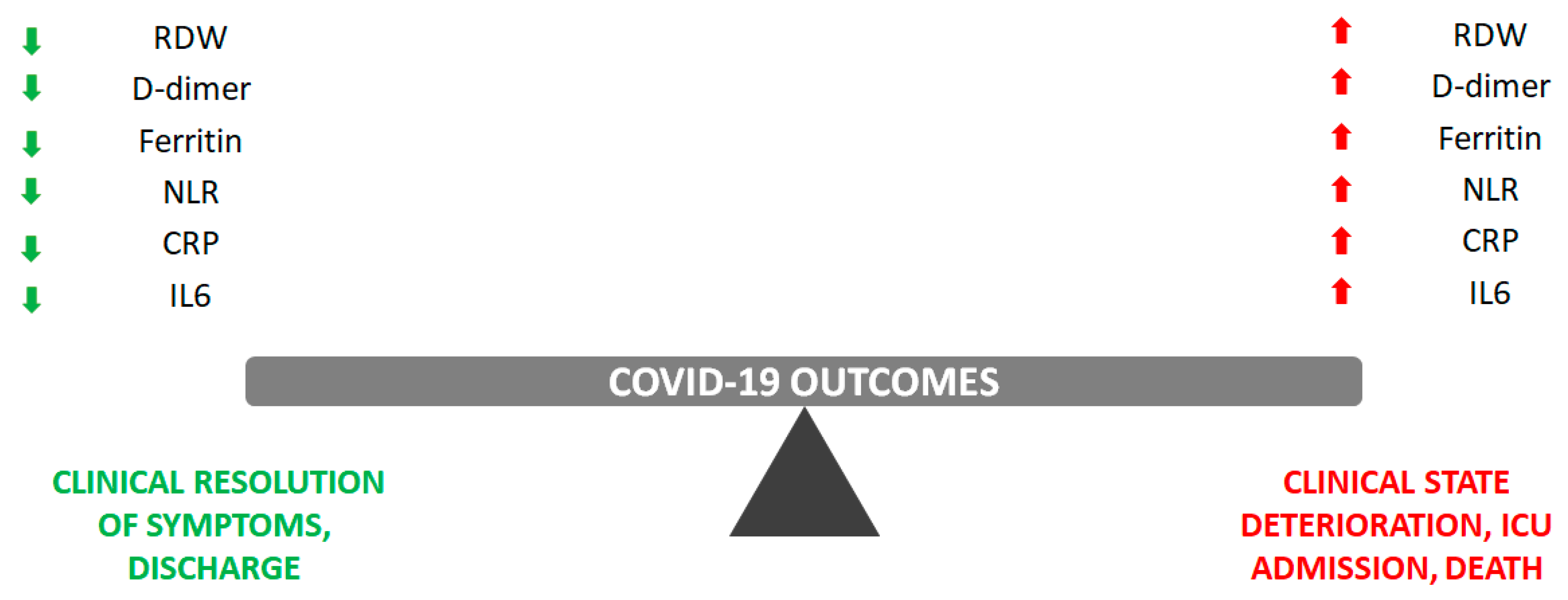
1. Background
2. Currently Validated Biomarkers in Clinical Practice
2.1. Red Cell Distribution Width (RDW)
2.2. D-Dimer
2.3. Ferritin
2.4. Neutrophil-to-Lymphocyte Ratio (NLR)
2.5. C-Reactive Protein (CRP)
2.6. Interleukin 6 (IL6)
3. Proposed Biomarkers Not Yet Implemented in Clinical Practice
3.1. IFN-Inducible Protein 10 (IP10)
3.2. Growth Arrest-Specific Gene 6 (Gas6)
3.3. SARS-CoV-2 Viremia
3.4. Osteopontin (OPN)
3.5. Calcitonin Gene-Related Peptide (CGRP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernandez, A.; Costa, R.A.; Esquer Garrigos, Z.; Marcelin, J.R.; Vijayvargiya, P. COVID-19 pathogenesis and clinical manifestations. Infect. Dis. Clin. N. Am. 2022, 36, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, A.; Shafqat, S.; Al Salameh, S.; Kashir, J.; Alkattan, K.; Yaqinuddin, A. Mechanistic insights into the immune pathophysiology of COVID-19; an in-depth review. Front. Immunol. 2022, 13, 835104. [Google Scholar] [CrossRef] [PubMed]
- Jamison, D.A.; Narayanan, S.A.; Trovão, N.S.; Guarnieri, J.W.; Topper, M.J.; Moraes-Vieira, P.M.; Zaksas, V.; Singh, K.K.; Wurtele, E.S.; Beheshti, A. A comprehensive SARS-CoV-2 and COVID-19 review, Part 1: Intracellular overdrive for SARS-CoV-2 infection. Eur. J. Hum. Genet. 2022, 30, 889–898. [Google Scholar] [CrossRef]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe covid-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Aldè, M.; Barozzi, S.; Di Berardino, F.; Zuccotti, G.; Consonni, D.; Ambrosetti, U.; Socci, M.; Bertoli, S.; Battezzati, A.; Foppiani, A.; et al. Prevalence of symptoms in 1512 COVID-19 patients: Have dizziness and vertigo been underestimated thus far? Intern. Emerg. Med. 2022, 17, 1343–1353. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Jia, L.; Zhang, Y. Potential mechanism of SARS-CoV-2-associated central and peripheral nervous system impairment. Acta. Neurol. Scand. 2022, 146, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Murgolo, N.; Therien, A.G.; Howell, B.; Klein, D.; Koeplinger, K.; Lieberman, L.A.; Adam, G.C.; Flynn, J.; McKenna, P.; Swaminathan, G.; et al. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLoS Pathog. 2021, 17, e1009225. [Google Scholar] [CrossRef]
- de Morais Batista, F.; Puga, M.A.M.; da Silva, P.V.; Oliveira, R.; Dos Santos, P.C.P.; da Silva, B.O.; Tatara, M.B.; Tsuha, D.H.; Dos Santos Pires, M.A.; Gonçalves, C.C.M.; et al. Serum biomarkers associated with SARS-CoV-2 severity. Sci. Rep. 2022, 12, 15999. [Google Scholar] [CrossRef]
- Geraili, Z.; Hajian-Tilaki, K.; Bayani, M.; Hosseini, S.R.; Khafri, S.; Ebrahimpour, S.; Javanian, M.; Babazadeh, A.; Shokri, M. Prognostic accuracy of inflammatory markers in predicting risk of ICU admission for COVID-19: Application of time-dependent receiver operating characteristic curves. J. Int. Med. Res. 2022, 50, 3000605221102217. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, L.; Ye, J.; Gu, Z.; Wang, S.; Xia, J.; Xie, Y.; Li, Q.; Xu, R.; Lin, N. Predictors of mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 663. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Dong, J.; Ren, Y.; Tian, M.; Li, W.; Hu, J.; Li, Y. The value of clinical parameters in predicting the severity of COVID-19. J. Med. Virol. 2020, 92, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine profiles as potential prognostic and therapeutic markers in SARS-CoV-2-induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine storm in COVID-19: Immunopathogenesis and therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Shcherbak, S.G.; Anisenkova, A.Y.; Mosenko, S.V.; Glotov, O.S.; Chernov, A.N.; Apalko, S.V.; Urazov, S.P.; Garbuzov, E.Y.; Khobotnikov, D.N.; Klitsenko, O.A.; et al. Basic predictive risk factors for cytokine storms in COVID-19 patients. Front. Immunol. 2021, 12, 745515. [Google Scholar] [CrossRef]
- Asaduzzaman, M.D.; Romel Bhuia, M.; Nazmul Alam, Z.; Zabed Jillul Bari, M.; Ferdousi, T. Significance of hemogram-derived ratios for predicting in-hospital mortality in COVID-19: A multicenter study. Health Sci. Rep. 2022, 5, e663. [Google Scholar] [CrossRef]
- Corradini, E.; Ventura, P.; Ageno, W.; Cogliati, C.B.; Muiesan, M.L.; Girelli, D.; Pirisi, M.; Gasbarrini, A.; Angeli, P.; Querini, P.R.; et al. SIMI-COVID-19 Collaborators. Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: Results from the SIMI-COVID-19 study of the Italian Society of Internal Medicine (SIMI). Intern. Emerg. Med. 2021, 16, 1005–1015. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Song, C.; Lu, R.; Zhao, Y.; Lin, F.; Han, D.; Chen, L.; Pan, P.; Dai, M. Early prediction of disease progression in patients with severe COVID-19 using C-reactive protein to albumin ratio. Dis. Markers 2021, 2021, 6304189. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis-a narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Lucijanić, M.; Jordan, A.; Jurin, I.; Piskač Živković, N.; Sorić, E.; Hadžibegović, I.; Atić, A.; Stojić, J.; Rudan, D.; Jakšić, O.; et al. Red cell distribution width is a potent prognostic parameter for in-hospital and post-discharge mortality in hospitalized coronavirus disease 2019 patients: A registry-based cohort study on 3941 patients. Croat. Med. J. 2022, 63, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jandaghian, S.; Vaezi, A.; Manteghinejad, A.; Nasirian, M.; Vaseghi, G.; Haghjooy Javanmard, S. Red blood cell distribution width (RDW) as a predictor of in-hospital mortality in COVID-19 patients; a cross sectional study. Arch. Acad. Emerg. Med. 2021, 9, e67. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.M.; Sanchis-Gomar, F. Red blood cell distribution is a significant predictor of severe illness in coronavirus disease 2019. Acta. Haematol. 2021, 144, 360–364. [Google Scholar] [CrossRef]
- Foy, B.H.; Carlson, J.C.T.; Reinertsen, E.; Padros, I.; Valls, R.; Pallares Lopez, R.; Palanques-Tost, E.; Mow, C.; Westover, M.B.; Aguirre, A.D.; et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw. Open 2020, 3, e2022058. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Platelet and red blood cell volume indices in patients with rheumatoid arthritis: A systematic review and meta-analysis. Diagnostics 2022, 12, 2633. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Zecca, E.; Croce, A.; Bonometti, R.; Pedrazzoli, R.; Sola, D.; Rigamonti, C.; Castello, L.M.; Avanzi, G.C.; et al. Association between red cell distribution width and response to methotrexate in rheumatoid arthritis. Reumatismo 2020, 72, 16–20. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Lu, Z. Study on the red blood cell distribution width in connective tissue disease associated with interstitial lung disease. Biomed. Res. Int. 2020, 2020, 8130213. [Google Scholar] [CrossRef]
- Bellan, M.; Giubertoni, A.; Piccinino, C.; Dimagli, A.; Grimoldi, F.; Sguazzotti, M.; Burlone, M.E.; Smirne, C.; Sola, D.; Marino, P.; et al. Red cell distribution width and platelet count as biomarkers of pulmonary arterial hypertension in patients with connective tissue disorders. Dis. Markers 2019, 2019, 4981982. [Google Scholar] [CrossRef]
- Haybar, H.; Pezeshki, S.M.S.; Saki, N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp. Mol Pathol. 2019, 110, 104267. [Google Scholar] [CrossRef]
- Ai, L.; Mu, S.; Hu, Y. Prognostic role of RDW in hematological malignancies: A systematic review and meta-analysis. Cancer Cell Int. 2018, 18, 61. [Google Scholar] [CrossRef]
- Goyal, H.; Lippi, G.; Gjymishka, A.; John, B.; Chhabra, R.; May, E. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J. Gastroenterol. 2017, 23, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.C.; Ahmed, A.; Brunner, U.; Jäger, B.; Aicher, G.; Equiluz-Bruck, S.; Spiel, A.O.; Funk, G.C.; Gschwantler, M.; Fasching, P.; et al. Red cell distribution width upon hospital admission predicts short-term mortality in hospitalized patients with COVID-19: A single-center experience. Front. Med. 2021, 8, 652707. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chung, H.J.; Kim, K.; Jo, Y.H.; Rhee, J.E.; Kim, Y.J.; Kang, K.W. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am. J. Emerg. Med. 2013, 31, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Banon, T.; Wortsman, J.; Ben Moshe, S.; Gazit, S.; Peretz, A.; Ben Tov, A.; Chodick, G.; Perez, G.; Patalon, T. Evaluating red blood cell distribution width from community blood tests as a predictor of hospitalization and mortality in adults with SARS-CoV-2: A cohort study. Ann. Med. 2021, 53, 1410–1418. [Google Scholar] [CrossRef]
- Bellan, M.; Azzolina, D.; Hayden, E.; Gaidano, G.; Pirisi, M.; Acquaviva, A.; Aimaretti, G.; Aluffi Valletti, P.; Angilletta, R.; Arioli, R.; et al. Simple parameters from complete blood count predict in-hospital mortality in COVID-19. Dis. Markers 2021, 2021, 8863053. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Montazerin, S.M.; Jamil, A.; Jamil, U.; Marszalek, J.; Chuang, M.L.; Chi, G. Association between red blood cell distribution width and mortality and severity among patients with COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 2513–2522. [Google Scholar] [CrossRef]
- Berger, J.S.; Kunichoff, D.; Adhikari, S.; Ahuja, T.; Amoroso, N.; Aphinyanaphongs, Y.; Cao, M.; Goldenberg, R.; Hindenburg, A.; Horowitz, J.; et al. Prevalence and Outcomes of D-Dimer Elevation in Hospitalized Patients With COVID-19. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2539–2547. [Google Scholar] [CrossRef]
- Johnson, E.D.; Schell, J.C.; Rodgers, G.M. The D-dimer assay. Am. J. Hematol. 2019, 94, 833–839. [Google Scholar] [CrossRef]
- Favresse, J.; Lippi, G.; Roy, P.M.; Chatelain, B.; Jacqmin, H.; Ten Cate, H.; Mullier, F. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit. Rev. Clin. Lab. Sci. 2018, 55, 548–577. [Google Scholar] [CrossRef]
- Weitz, J.I.; Fredenburgh, J.C.; Eikelboom, J.W. A test in context: D-dimer. J. Am. Coll. Cardiol. 2017, 70, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, K.; Duan, H.; Yue, P.; Zheng, X.; Liu, L.; Liao, H.; Wu, J.; Li, J.; Hua, Y.; et al. Value of D-dimer in predicting various clinical outcomes following community-acquired pneumonia: A network meta-analysis. PLoS ONE 2022, 17, e0263215. [Google Scholar] [CrossRef] [PubMed]
- Soomro, A.Y.; Guerchicoff, A.; Nichols, D.J.; Suleman, J.; Dangas, G.D. The current role and future prospects of D-dimer biomarker. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- He, X.; Yao, F.; Chen, J.; Wang, Y.; Fang, X.; Lin, X.; Long, H.; Wang, Q.; Wu, Q. The poor prognosis and influencing factors of high D-dimer levels for COVID-19 patients. Sci. Rep. 2021, 11, 1830. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Noubouossie, D.F.; Gandotra, S.; Cao, L. Elevated plasma fibrinogen is associated with excessive inflammation and disease severity in COVID-19 patients. Front. Cell Infect. Microbiol. 2021, 11, 734005. [Google Scholar] [CrossRef]
- Thachil, J. The protective rather than prothrombotic fibrinogen in COVID-19 and other inflammatory states. J. Thromb. Haemost. 2020, 18, 1849–1852. [Google Scholar] [CrossRef]
- Chowdary, P. COVID-19 coagulopathy-what should we treat? Exp. Physiol. 2022, 107, 749–758. [Google Scholar] [CrossRef]
- Chocron, R.; Duceau, B.; Gendron, N.; Ezzouhairi, N.; Khider, L.; Trimaille, A.; Goudot, G.; Weizman, O.; Alsac, J.M.; Pommier, T.; et al. Critical COVID-19 France investigators. D-dimer at hospital admission for COVID-19 are associated with in-hospital mortality, independent of venous thromboembolism: Insights from a French multicenter cohort study. Arc. Cardiovasc. Dis. 2021, 114, 381–393. [Google Scholar] [CrossRef]
- Poudel, A.; Poudel, Y.; Adhikari, A.; Aryal, B.B.; Dangol, D.; Bajracharya, T.; Maharjan, A.; Gautam, R. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE 2021, 16, e0256744. [Google Scholar] [CrossRef]
- Lin, K.; Xu, K.; Daoust, R.; Taylor, J.; Rosychuk, R.J.; Hau, J.P.; Davis, P.; Clark, G.; McRae, A.D.; Hohl, C.M. Canadian COVID-19 Emergency Department Rapid Response Network (CCEDRRN) investigators for the Network of Canadian Emergency Researchers, the Canadian Critical Care Trials Group. Prognostic association between d-dimer thresholds and 30-day pulmonary embolism diagnosis among emergency department patients with suspected SARS-CoV-2 infection: A Canadian COVID-19 Emergency Department Rapid Response Network study. CJEM 2023, 25, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.P.; Beeker, N.; Porcher, R.; Jilet, L.; Fournier, L.; Rance, B.; Chassagnon, G.; Fontenay, M.; Sanchez, O. AP-HP /Universities/Inserm COVID-19 research collaboration, AP-HP Covid CDR Initiative. What level of D-dimers can safely exclude pulmonary embolism in COVID-19 patients presenting to the emergency department? Eur. Radiol. 2022, 32, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Qeadan, F.; Tingey, B.; Gu, L.Y.; Packard, A.H.; Erdei, E.; Saeed, A.I. Prognostic values of serum ferritin and D-dimer trajectory in patients with COVID-19. Viruses 2021, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Manwaring, D.; Curreri, P.W. The role of platelet aggregation and release in fragment D-induced pulmonary dysfunction. Ann. Surg. 1980, 192, 103–107. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Costanzo, S.; Antinori, A.; Berselli, N.; Blandi, L.; Bonaccio, M.; Cauda, R.; Guaraldi, G.; Menicanti, L.; Mennuni, M.; et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: The multicenter italian CORIST study. Thromb. Haemost. 2021, 121, 1054–1065. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. HEP-COVID Investigators. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: The HEP-COVID randomized clinical trial. JAMA Intern. Med. 2021, 181, 1612–1620. [Google Scholar] [CrossRef]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with Covid-19. J. Thromb. Thrombolysis 2020, 50, 298–301. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Aslan, E.S.; Aydın, H.; Tekin, Y.K.; Keleş, S.; White, K.N.; Hekim, N. Association between iron metabolism and SARS-COV-2 infection, determined by ferritin, hephaestin and hypoxia-induced factor-1 alpha levels in COVID-19 patients. Mol. Biol. Rep. 2023, 50, 2471–2478. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin-from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- DePalma, R.G.; Hayes, V.W.; O’Leary, T.J. Optimal serum ferritin level range: Iron status measure and inflammatory biomarker. Metallomics 2021, 13, mfab030. [Google Scholar] [CrossRef] [PubMed]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and biology of ferritin. Metallomics 2021, 13, mfab021. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef]
- Yadav, D.; Pvsn, K.K.; Tomo, S.; Sankanagoudar, S.; Charan, J.; Purohit, A.; Nag, V.; Bhatia, P.; Singh, K.; Dutt, N.; et al. Association of iron-related biomarkers with severity and mortality in COVID-19 patients. J. Trace Elem. Med. Biol. 2022, 74, 127075. [Google Scholar] [CrossRef]
- Volfovitch, Y.; Tsur, A.M.; Gurevitch, M.; Novick, D.; Rabinowitz, R.; Mandel, M.; Achiron, A.; Rubinstein, M.; Shoenfeld, Y.; Amital, H. The intercorrelations between blood levels of ferritin, sCD163, and IL-18 in COVID-19 patients and their association to prognosis. Immunol. Res. 2022, 70, 817–828. [Google Scholar] [CrossRef]
- Zhou, S.; Li, H.; Li, S. The associations of iron related biomarkers with risk, clinical severity and mortality in SARS-CoV-2 patients: A meta-analysis. Nutrients 2022, 14, 3406. [Google Scholar] [CrossRef]
- Alroomi, M.; Rajan, R.; Omar, A.A.; Alsaber, A.; Pan, J.; Fatemi, M.; Zhanna, K.D.; Aboelhassan, W.; Almutairi, F.; Alotaibi, N.; et al. Ferritin level: A predictor of severity and mortality in hospitalized COVID-19 patients. Immun. Inflamm. Dis. 2021, 9, 1648–1655. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Asghar, M.S.; Akram, M.; Yasmin, F.; Najeeb, H.; Naeem, U.; Gaddam, M.; Jafri, M.S.; Tahir, M.J.; Yasin, I.; Mahmood, H.; et al. Comparative analysis of neutrophil to lymphocyte ratio and derived neutrophil to lymphocyte ratio with respect to outcomes of in-hospital coronavirus disease 2019 patients: A retrospective study. Front. Med. 2022, 9, 951556. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Khanna, P.; Singh, A.K. The impact of neutrophil-lymphocyte count ratio in COVID-19: A systematic review and meta-analysis. J. Intensive Care Med. 2022, 37, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Prozan, L.; Shusterman, E.; Ablin, J.; Mitelpunkt, A.; Weiss-Meilik, A.; Adler, A.; Choshen, G.; Kehat, O. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Sci. Rep. 2021, 11, 21519. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Rojas, S.; Salazar-Talla, L.; Romero-Cerdán, A.; Soto-Becerra, P.; Díaz-Vélez, C.; Urrunaga-Pastor, D.; Maguiña, J.L. The neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio as predictors of mortality in older adults hospitalized with COVID-19 in Peru. Dis. Markers 2022, 2022, 2497202. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, A.; Padukudru, S.; Arunachal, S.; Basavaraj, C.K.; Krishna, M.T.; Ganguly, K.; Upadhyay, S.; Anand, M.P. The role of neutrophil-to-lymphocyte ratio in risk stratification and prognostication of COVID-19: A systematic review and meta-analysis. Vaccines 2022, 10, 1233. [Google Scholar] [CrossRef]
- Al-Mazedi, M.S.; Rajan, R.; Al-Jarallah, M.; Dashti, R.; Al Saber, A.; Pan, J.; Zhanna, K.D.; Abdelnaby, H.; Aboelhassan, W.; Almutairi, F.; et al. Neutrophil to lymphocyte ratio and in-hospital mortality among patients with SARS-CoV-2: A retrospective study. Ann. Med. Surg. 2022, 82, 104748. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Feng, S.D.; Chen, G.P.; Wu, J.N. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19: A prospective cohort study. BMC Infect. Dis. 2021, 21, 80. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef]
- Mosquera-Sulbaran, J.A.; Pedreañez, A.; Carrero, Y.; Callejas, D. C-reactive protein as an effector molecule in Covid-19 pathogenesis. Rev. Med. Virol. 2021, 31, e2221. [Google Scholar] [CrossRef] [PubMed]
- Dyer, E.M.; Waterfield, T.; Baynes, H. How to use C-reactive protein. Arch. Dis. Child Educ. Pract. Ed. 2019, 104, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Kushner, I.; Samols, D. C-reactive protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. CRP after 2004. Mol. Immunol. 2005, 42, 927–930. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Bouayed, M.Z.; Laaribi, I.; Chatar, C.E.M.; Benaini, I.; Bouazzaoui, M.A.; Oujidi, Y.; Berrichi, S.; El Aidouni, G.; Bkiyar, H.; Abda, N.; et al. C-reactive protein (CRP): A poor prognostic biomarker in COVID-19. Front. Immunol. 2022, 13, 1040024. [Google Scholar] [CrossRef]
- Stringer, D.; Braude, P.; Myint, P.K.; Evans, L.; Collins, J.T.; Verduri, A.; Quinn, T.J.; Vilches-Moraga, A.; Stechman, M.J.; Pearce, L.; et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021, 50, 420–429. [Google Scholar] [CrossRef]
- Wang, L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020, 50, 332–334. [Google Scholar] [CrossRef]
- Zhang, J.N.; Gao, Y.; Wang, X.T.; Li, N.N.; Du, X.; Tang, Y.J.; Lai, Q.Q.; Chen, P.F.; Yue, C.S.; Wu, J.H.; et al. Lymphocyte-C-reactive protein ratio can differentiate disease severity of COVID-19 patients and serve as an assistant screening tool for hospital and ICU admission. Front. Immunol. 2022, 13, 957407. [Google Scholar] [CrossRef]
- Tan, C.; Huang, Y.; Shi, F.; Tan, K.; Ma, Q.; Chen, Y.; Jiang, X.; Li, X. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020, 92, 856–862. [Google Scholar] [CrossRef]
- Rizzi, M.; Costanzo, M.; Tonello, S.; Matino, E.; Casciaro, F.G.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.; Vassia, V.; et al. Prognostic markers in hospitalized COVID-19 patients: The role of IP-10 and C-reactive protein. Dis. Markers 2022, 2022, 3528312. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.Y.; Yin, C.H.; Yao, Y.M. Update. Advances on C-reactive protein in COVID-19 and other viral infections. Front. Immunol. 2021, 12, 720363. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, K.I.; Liu, S.; Yan, Z.; Xu, C.; Qiao, Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Heart J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Narazaki, M.; Kishimoto, T. The two-faced cytokine IL-6 in host defense and diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Rachman, A.; Rinaldi, I. Coagulopathy in dengue infection and the role of interleukin-6. Acta Med. Indones. 2006, 38, 105–108. [Google Scholar]
- Bryushkova, E.A.; Skatova, V.D.; Mutovina, Z.Y.; Zagrebneva, A.I.; Fomina, D.S.; Kruglova, T.S.; Akopyan, A.A.; Strazhesko, I.D.; Lukyanov, S.A.; Tkacheva, O.N.; et al. Tocilizumab, netakimab, and baricitinib in patients with mild-to-moderate COVID-19: An observational study. PLoS ONE 2022, 17, e0273340. [Google Scholar] [CrossRef]
- Zizzo, G.; Tamburello, A.; Castelnovo, L.; Laria, A.; Mumoli, N.; Faggioli, P.M.; Stefani, I.; Mazzone, A. Immunotherapy of COVID-19: Inside and beyond IL-6 signalling. Front. Immunol. 2022, 13, 795315. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Sayah, W.; Berkane, I.; Guermache, I.; Sabri, M.; Lakhal, F.Z.; Yasmine Rahali, S.; Djidjeli, A.; Lamara Mahammed, L.; Merah, F.; Belaid, B.; et al. Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19. Cytokine 2021, 141, 155428. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J.; Sangroongruangsri, S.; Chaikledkaew, U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: Systematic review with meta-analysis. J. Clin. Immunol. 2021, 41, 11–22. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; Rezaei, N. Interleukin-6 and severe COVID-19: A systematic review and meta-analysis. Eur. Cytokine Netw. 2020, 31, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Recovery Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, K.; Dai, R.; Li, R.; Li, M.; Lv, X.; Yu, Q. Specific Interleukin-1 inhibitors, specific interleukin-6 inhibitors, and GM-CSF blockades for COVID-19 (at the edge of sepsis): A systematic review. Front. Pharmacol. 2022, 12, 804250. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yang, Y.; Yang, J. Effectiveness of tocilizumab in the treatment of hospitalized adults COVID-19: A systematic review and meta-analysis. Medicine 2022, 101, e28967. [Google Scholar] [CrossRef]
- Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 chemokine: A critical player in RNA and DNA viral infections. Viruses 2022, 14, 2445. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Giuggioli, D.; Ferrannini, E.; Ferri, C.; Fallahi, P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun. Rev. 2014, 13, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, Z.H.; Song, Y.W. The interaction between CXCL10 and cytokines in chronic inflammatory arthritis. Autoimmun. Rev. 2013, 12, 554–557. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor. Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Chen, J.; Subbarao, K. The Immunobiology of SARS. Annu. Rev. Immunol. 2007, 25, 443–472. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef]
- Tang, N.L.; Chan, P.K.; Wong, C.K.; To, K.F.; Wu, A.K.; Sung, Y.M.; Hui, D.S.; Sung, J.J.; Lam, C.W. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005, 51, 2333–2340. [Google Scholar] [CrossRef]
- Basheer, M.; Saad, E.; Kananeh, M.; Asad, L.; Khayat, O.; Badarne, A.; Abdo, Z.; Arraf, N.; Milhem, F.; Bassal, T.; et al. Cytokine patterns in COVID-19 patients: Which cytokines predict mortality and which protect against? Curr. Issues Mol. Biol. 2022, 44, 4735–4747. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.S.; Vishwakarma, S.; Trimbake, D.; Gurav, Y.K.; Potdar, V.A.; Mokashi, N.D.; Patsute, S.D.; Kaushal, H.; Choudhary, M.L.; Tilekar, B.N.; et al. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: Biomarkers of SARS-CoV-2 infection. Arch. Virol. 2021, 166, 3301–3310. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy. Clin. Immunol. 2020, 146, 119–127.e4. [Google Scholar] [CrossRef]
- Bellan, M.; Cittone, M.G.; Tonello, S.; Rigamonti, C.; Castello, L.M.; Gavelli, F.; Pirisi, M.; Sainaghi, P.P. Gas6/TAM system: A key modulator of the interplay between inflammation and fibrosis. Int. J. Mol. Sci. 2019, 20, 5070. [Google Scholar] [CrossRef] [PubMed]
- Law, L.A.; Graham, D.K.; Di Paola, J.; Branchford, B.R. GAS6/TAM pathway signaling in hemostasis and thrombosis. Front. Med. 2018, 5, 137. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Lombino, F.; Alciato, F.; Carecchio, M.; Galimberti, D.; Fenoglio, C.; Scarpini, E.; Cantello, R.; Pirisi, M.; et al. Growth arrest specific 6 concentration is increased in the cerebrospinal fluid of patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 59–65. [Google Scholar] [CrossRef]
- van der Meer, J.H.; van der Poll, T.; van’t Veer, C. TAM receptors, Gas6, and protein S: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Collimedaglia, L.; Alciato, F.; Molinari, R.; Sola, D.; Ranza, E.; Naldi, P.; Monaco, F.; Leone, M.; Pirisi, M.; et al. Growth arrest specific gene 6 protein concentration in cerebrospinal fluid correlates with relapse severity in multiple sclerosis. Mediators Inflamm. 2013, 2013, 406483. [Google Scholar] [CrossRef] [PubMed]
- Alciato, F.; Sainaghi, P.P.; Sola, D.; Castello, L.; Avanzi, G.C. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J. Leukoc. Biol. 2010, 87, 869–875. [Google Scholar] [CrossRef]
- Hurtado, B.; de Frutos, P.G. GAS6 in systemic inflammatory diseases: With and without infection. Crit. Care 2010, 14, 1003. [Google Scholar] [CrossRef]
- Alciato, F.; Sainaghi, P.P.; Castello, L.; Bergamasco, L.; Carnieletto, S.; Avanzi, G.C. Development and validation of an ELISA method for detection of growth arrest specific 6 (GAS6) protein in human plasma. J. Immunoass. Immunochem. 2008, 29, 167–180. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Collimedaglia, L.; Alciato, F.; Leone, M.A.; Puta, E.; Naldi, P.; Castello, L.; Monaco, F.; Avanzi, G.C. Elevation of Gas6 protein concentration in cerebrospinal fluid of patients with chronic inflammatory demyelinating polyneuropathy (CIDP). J. Neurol. Sci. 2008, 269, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Tonello, S.; D’Onghia, D.; Sainaghi, P.P. Gas6/TAM axis involvement in modulating inflammation and fibrosis in COVID-19 patients. Int. J. Mol. Sci. 2023, 24, 951. [Google Scholar] [CrossRef] [PubMed]
- Poświata, A.; Kozik, K.; Miączyńska, M.; Zdżalik-Bielecka, D. Endocytic trafficking of GAS6-AXL complexes is associated with sustained AKT activation. Cell. Mol. Life Sci. 2022, 79, 316. [Google Scholar] [CrossRef]
- Bellan, M.; Dimagli, A.; Piccinino, C.; Giubertoni, A.; Ianniello, A.; Grimoldi, F.; Sguazzotti, M.; Nerviani, A.; Barini, M.; Carriero, A.; et al. Role of Gas6 and TAM receptors in the identification of cardiopulmonary involvement in systemic sclerosis and scleroderma spectrum disorders. Dis. Markers 2020, 2020, 2696173. [Google Scholar] [CrossRef]
- Bellan, M.; Quaglia, M.; Nerviani, A.; Mauro, D.; Lewis, M.; Goegan, F.; Gibbin, A.; Pagani, S.; Salmi, L.; Molinari, L.; et al. Increased plasma levels of Gas6 and its soluble tyrosine kinase receptors Mer and Axl are associated with immunological activity and severity of lupus nephritis. Clin. Exp. Rheumatol. 2021, 39, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ye, J.; Zhao, G.; Hong, G.; Hu, X.; Cao, K.; Wu, Y.; Lu, Z. Gas6 attenuates lipopolysaccharide-induced TNF-α expression and apoptosis in H9C2 cells through NF-κB and MAPK inhibition via the Axl/PI3K/Akt pathway. Int. J. Mol. Med. 2019, 44, 982–994. [Google Scholar] [CrossRef]
- Bellan, M.; Pirisi, M.; Sainaghi, P.P. The Gas6/TAM System and Multiple Sclerosis. Int. J. Mol. Sci. 2016, 17, 1807. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Fernández-Fernández, L.; Bellido-Martín, L.; García de Frutos, P. Growth arrest-specific gene 6 (GAS6). An outline of its role in haemostasis and inflammation. Thromb. Haemost. 2008, 100, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Baricich, A.; Borg, M.B.; Cuneo, D.; Cadario, E.; Azzolina, D.; Balbo, P.E.; Bellan, M.; Zeppegno, P.; Pirisi, M.; Cisari, C.; et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2021, 57, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Baricich, A.; Patrucco, F.; Zeppegno, P.; Gramaglia, C.; Balbo, P.E.; Carriero, A.; Amico, C.S.; Avanzi, G.C.; Barini, M.; et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci. Rep. 2021, 11, 22666. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Tonello, S.; Rizzi, M.; Matino, E.; Costanzo, M.; Casciaro, G.F.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.; Vassia, V.; et al. Baseline plasma Gas6 protein elevation predicts adverse outcomes in hospitalized COVID-19 patients. Dis. Markers 2022, 2022, 1568352. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, S.; Bos, L.D.; van Roon, M.A.; Tuip-de Boer, A.M.; Schuurman, A.R.; Koel-Simmelinck, M.J.A.; Bogaard, H.J.; Tuinman, P.R.; van Agtmael, M.A.; Hamann, J.; et al. Clinical features and prognostic factors in Covid-19: A prospective cohort study. EBioMedicine 2021, 67, 103378. [Google Scholar] [CrossRef] [PubMed]
- Huckriede, J.; Anderberg, S.B.; Morales, A.; de Vries, F.; Hultström, M.; Bergqvist, A.; Ortiz-Pérez, J.T.; Sels, J.W.; Wichapong, K.; Lipcsey, M.; et al. Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients. Sci. Rep. 2021, 11, 15701. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Rojo Rello, S.; Cristóbal, H.; Fiz-López, A.; Arribas, E.; Marí, M.; Tutusaus, A.; de la Cal-Sabater, P.; Nicolaes, G.A.F.; Ortiz-Pérez, J.T.; et al. Growth arrest-specific factor 6 (GAS6) is increased in COVID-19 patients and predicts clinical outcome. Biomedicines 2021, 9, 335. [Google Scholar] [CrossRef]
- Ekman, C.; Linder, A.; Akesson, P.; Dahlbäck, B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit. Care 2010, 14, R158. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Iba, T.; Olson, L.B.; Corey, K.M.; Ghadimi, K.; Connors, J.M. COVID-19: Thrombosis, thromboinflammation, and anticoagulation considerations. Int. J. Lab. Hematol. 2021, 43 (Suppl. S1), 29–35. [Google Scholar] [CrossRef]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.B.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef]
- Maarifi, G.; Martin, M.F.; Zebboudj, A.; Boulay, A.; Nouaux, P.; Fernandez, J.; Lagisquet, J.; Garcin, D.; Gaudin, R.; Arhel, N.J.; et al. Identifying enhancers of innate immune signaling as broad-spectrum antivirals active against emerging viruses. Cell Chem. Biol. 2022, 29, 1113–1125. [Google Scholar] [CrossRef]
- Bohan, D.; Van Ert, H.; Ruggio, N.; Rogers, K.J.; Badreddine, M.; Aguilar Briseño, J.A.; Elliff, J.M.; Rojas Chavez, R.A.; Gao, B.; Stokowy, T.; et al. Phosphatidylserine receptors enhance SARS-CoV-2 infection. PLoS Pathog. 2021, 17, e1009743. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.; Franco, R.; Prados, M.; Rodgers, J.B.; Hand, D.T.; Walter, L.A. Hepatitis C active viremia over time in an ED-based testing programme: Impact, disparities and surveillance tool. J. Viral. Hepat. 2022, 29, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Tosiano, M.A.; Jacobs, J.L.; Shutt, K.A.; Cyktor, J.C.; Mellors, J.W. A simpler and more sensitive single-copy HIV-1 RNA assay for quantification of persistent HIV-1 viremia in individuals on suppressive antiretroviral therapy. J. Clin. Microbiol. 2019, 57, e01714–e01718. [Google Scholar] [CrossRef]
- Conway, J.M.; Perelson, A.S. Residual Viremia in Treated HIV+ Individuals. PLoS Comput. Biol 2016, 12, e1004677. [Google Scholar] [CrossRef]
- de la Cruz-Hernández, S.I.; Flores-Aguilar, H.; González-Mateos, S.; López-Martinez, I.; Alpuche-Aranda, C.; Ludert, J.E.; del Angel, R.M. Determination of viremia and concentration of circulating nonstructural protein 1 in patients infected with dengue virus in Mexico. Am. J. Trop. Med. Hyg. 2013, 88, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.R.; Garson, J.A.; Tedder, R.S.; Chan, P.K.; Tam, J.S.; Sung, J.J. Detection of SARS coronavirus in plasma by real-time RT-PCR. N. Engl. J. Med. 2003, 349, 2468–2469. [Google Scholar] [CrossRef]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A.; Scorza, C.A.; Fiorini, A.C. Extrapulmonary onset manifestations of COVID-19. Clinics 2021, 76, e2900. [Google Scholar] [CrossRef]
- Yazdanpanah, F.; Garg, A.; Shadman, S.; Asmarz, H.Y. Literature review of COVID-19, pulmonary and extrapulmonary disease. Am. J. Med. Sci. 2021, 361, 567–574. [Google Scholar] [CrossRef]
- Ram-Mohan, N.; Kim, D.; Zudock, E.J.; Hashemi, M.M.; Tjandra, K.C.; Rogers, A.J.; Blish, C.A.; Nadeau, K.C.; Newberry, J.A.; Quinn, J.V.; et al. Stanford COVID-19 Biobank Study Group. SARS-CoV-2 RNAemia predicts clinical deterioration and extrapulmonary complications from COVID-19. Clin. Infect. Dis. 2022, 74, 218–226. [Google Scholar] [CrossRef]
- van Riel, D.; Embregts, C.W.E.; Sips, G.J.; van den Akker, J.P.C.; Endeman, H.; van Nood, E.; Raadsen, M.; Bauer, L.; van Kampen, J.; Molenkamp, R.; et al. Temporal kinetics of RNAemia and associated systemic cytokines in hospitalized COVID-19 patients. Msphere 2021, 6, e0031121. [Google Scholar] [CrossRef]
- Gutmann, C.; Takov, K.; Burnap, S.A.; Singh, B.; Ali, H.; Theofilatos, K.; Reed, E.; Hasman, M.; Nabeebaccus, A.; Fish, M.; et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat. Commun. 2021, 12, 3406. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.A.; Meyer-Schwickerath, C.; Heger, E.; Knops, E.; Lehmann, C.; Rybniker, J.; Schommers, P.; Eichenauer, D.A.; Kurth, F.; Ramharter, M.; et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients. Viruses 2020, 12, 1045. [Google Scholar] [CrossRef]
- Rizzi, M.; Patrucco, F.; Trevisan, M.; Faolotto, G.; Mercandino, A.; Strola, C.; Ravanini, P.; Costanzo, M.; Tonello, S.; Matino, E.; et al. Baseline plasma SARS-CoV-2 RNA detection predicts an adverse COVID-19 evolution in moderate to severe hospitalized patients. Panminerva Med. 2022, 64, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Colagrossi, L.; Antonello, M.; Renica, S.; Merli, M.; Matarazzo, E.; Travi, G.; Vecchi, M.; Colombo, J.; Muscatello, A.; Grasselli, G.; et al. SARS-CoV-2 RNA in plasma samples of COVID-19 affected individuals: A cross-sectional proof-of-concept study. BMC Infect. Dis 2021, 21, 184. [Google Scholar] [CrossRef]
- Miki, S.; Sasaki, H.; Horiuchi, H.; Miyata, N.; Yoshimura, Y.; Miyazaki, K.; Matsumura, T.; Takahashi, Y.; Suzuki, T.; Matano, T.; et al. On-admission SARS-CoV-2 RNAemia as a single potent predictive marker of critical condition development and mortality in COVID-19. PLoS ONE 2021, 16, e0254640. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- Kawasuji, H.; Morinaga, Y.; Tani, H.; Yoshida, Y.; Takegoshi, Y.; Kaneda, M.; Murai, Y.; Kimoto, K.; Ueno, A.; Miyajima, Y.; et al. SARS-CoV-2 RNAemia with a higher nasopharyngeal viral load is strongly associated with disease severity and mortality in patients with COVID-19. J. Med. Virol. 2022, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B.; et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 1937–1942. [Google Scholar] [CrossRef]
- Roy-Vallejo, E.; Cardeñoso, L.; Triguero-Martínez, A.; Chicot Llano, M.; Zurita, N.; Ávalos, E.; Barrios, A.; Hernando, J.; Ortiz, J.; Rodríguez-García, S.C.; et al. SARS-CoV-2 viremia precedes an IL6 response in severe COVID-19 patients: Results of a longitudinal prospective cohort. Front. Med. 2022, 9, 855639. [Google Scholar] [CrossRef]
- Reisner, A.; Blackwell, L.S.; Sayeed, I.; Myers, H.E.; Wali, B.; Heilman, S.; Figueroa, J.; Lu, A.; Hussaini, L.; Anderson, E.J.; et al. Osteopontin as a biomarker for COVID-19 severity and multisystem inflammatory syndrome in children: A pilot study. Exp. Biol. Med. 2022, 247, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Piccinino, C.; Tonello, S.; Minisini, R.; Giubertoni, A.; Sola, D.; Pedrazzoli, R.; Gagliardi, I.; Zecca, E.; Calzaducca, E.; et al. Role of osteopontin as a potential biomarker of pulmonary arterial hypertension in patients with systemic sclerosis and other connective tissue diseases (CTDs). Pharmaceuticals 2021, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Abreu, H.; Raineri, D.; Scotti, L.; Castello, L.; Vaschetto, R.; Chiocchetti, A. High levels of circulating osteopontin in inflammatory lung disease regardless of Sars-CoV-2 infection. EMBO Mol. Med 2021, 13, e14124. [Google Scholar] [CrossRef]
- Hayek, S.S.; Roderburg, C.; Blakely, P.; Launius, C.; Eugen-Olsen, J.; Tacke, F.; Ktena, S.; Keitel, V.; Leudde, M.; Giamarellos-Bourboulis, E.J.; et al. Circulating osteopontin levels and outcomes in patients hospitalized for COVID-19. J. Clin. Med. 2021, 10, 3907. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in cardiovascular diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Varim, C.; Demirci, T.; Cengiz, H.; Hacibekiroglu, I.; Tuncer, F.B.; Cokluk, E.; Toptan, H.; Karabay, O.; Yildirim, I. Relationship between serum osteopontin levels and the severity of COVID-19 infection. Wien. Klin. Wochenschr. 2021, 133, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Shi, L.; Wang, X.; He, J. Regulatory roles of osteopontin in production of monocyte-origin MCP-1. Cell Transpl. 2018, 27, 1185–1194. [Google Scholar] [CrossRef]
- Karabulut Uzunçakmak, S.; Aksakal, A.; Kerget, F.; Aydın, P.; Halıcı, Z. Evaluation of IGFBP5 expression and plasma osteopontin level in COVID-19 patients. Adv. Med. Sci. 2023, 68, 31–37. [Google Scholar] [CrossRef]
- Nakatsuka, Y.; Shiba, M.; Nishikawa, H.; Terashima, M.; Kawakita, F.; Fujimoto, M.; Suzuki, H.; PSEED Group. Acute-phase plasma osteopontin as an independent predictor for poor outcome after aneurysmal subarachnoid hemorrhage. Mol. Neurobiol. 2018, 55, 6841–6849. [Google Scholar] [CrossRef]
- Roderburg, C.; Benz, F.; Cardenas, D.V.; Lutz, M.; Hippe, H.J.; Luedde, T.; Trautwein, C.; Frey, N.; Koch, A.; Tacke, F.; et al. Persistently elevated osteopontin serum levels predict mortality in critically ill patients. Crit. Care 2015, 19, 271. [Google Scholar] [CrossRef]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Furushima, D.; Niki, T.; Matsuba, T.; Maeda, Y.; Takahashi, A.; Hattori, T.; Ashino, Y. High levels of the cleaved form of galectin-9 and osteopontin in the plasma are associated with inflammatory markers that reflect the severity of COVID-19 pneumonia. Int. J. Mol. Sci. 2021, 22, 4978. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; De Biasi, S.; Paolini, A.; Borella, R.; Boraldi, F.; Mattioli, M.; Lo Tartaro, D.; Fidanza, L.; Caro-Maldonado, A.; Meschiari, M.; et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020, 12, e13001. [Google Scholar] [CrossRef]
- Tonello, S.; D’Onghia, D.; Apostolo, D.; Matino, E.; Costanzo, M.; Casciaro, G.F.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.R.; et al. Baseline plasma osteopontin protein elevation predicts adverse outcomes in hospitalized COVID-19 patients. Viruses 2023, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, O.F.; Uctepe, E.; Opoku, G.; Wake, H.; Ikemura, K.; Ohtsuki, T.; Inagaki, J.; Gunduz, M.; Gunduz, E.; Watanabe, S.; et al. Osteopontin silencing attenuates bleomycin-induced murine pulmonary fibrosis by regulating epithelial-mesenchymal transition. Biomed. Pharmacother. 2021, 139, 111633. [Google Scholar] [CrossRef]
- Pardo, A.; Gibson, K.; Cisneros, J.; Richards, T.J.; Yang, Y.; Becerril, C.; Yousem, S.; Herrera, I.; Ruiz, V.; Selman, M.; et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005, 2, e251. [Google Scholar] [CrossRef]
- Takahashi, F.; Takahashi, K.; Okazaki, T.; Maeda, K.; Ienaga, H.; Maeda, M.; Kon, S.; Uede, T.; Fukuchi, Y. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2001, 24, 264–271. [Google Scholar] [CrossRef]
- Khodabakhsh, P.; Asgari Taei, A.; Mohseni, M.; Bahrami Zanjanbar, D.; Khalili, H.; Masoumi, K.; Haji Abbas Shirazi, A.; Dargahi, L. Vasoactive peptides: Role in COVID-19 pathogenesis and potential use as biomarkers and therapeutic targets. Arch. Med. Res. 2021, 52, 777–787. [Google Scholar] [CrossRef]
- Holzmann, B. Modulation of immune responses by the neuropeptide CGRP. Amino Acids 2013, 45, 1–7. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Y.W.; Peng, W.J.; Peng, J.; Chen, Q.Q.; Li, D.; Deng, H.W.; Li, Y.J. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul. Pept. 2008, 150, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, H.; Inaba, K.; Muramatsu, S. Calcitonin gene-related peptide enhances cytokine-induced IL-6 production by fibroblasts. Cell. Immunol. 1995, 165, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E. Could CGRP Antagonists Be Helpful in the Fight Against COVID-19? Headache 2020, 60, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; García-Sanmartín, J.; Villoslada-Blanco, P.; Íñiguez, M.; Pérez-Matute, P.; Pujadas, E.; Fowkes, M.E.; Brody, R.; Oteo, J.A.; Martínez, A. Circulating levels of calcitonin gene-related peptide are lower in COVID-19 patients. J. Endocr. Soc. 2021, 5, bvaa199. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, G.S.; Padilla, B.; Pikios, S.; Roosterman, D.; Steinhoff, M.; Grady, E.F.; Bunnett, N.W. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J. Biol. Chem. 2007, 282, 12260–12271. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Tonello, S.; Morani, F.; Rizzi, E.; Casciaro, G.F.; Matino, E.; Costanzo, M.; Zecca, E.; Croce, A.; Pedrinelli, A.; et al. CGRP plasma levels correlate with the clinical evolution and prognosis of hospitalized acute COVID-19 patients. Viruses 2022, 14, 2123. [Google Scholar] [CrossRef]
- Bolay, H.; Karadas, Ö.; Oztürk, B.; Sonkaya, R.; Tasdelen, B.; Bulut, T.D.S.; Gülbahar, Ö.; Özge, A.; Baykan, B. HMGB1, NLRP3, IL-6 and ACE2 levels are elevated in COVID-19 with headache: A window to the infection-related headache mechanism. J. Headache Pain. 2021, 22, 94. [Google Scholar] [CrossRef]
- Cardillo, G.; Viggiano, G.V.; Russo, V.; Mangiacapra, S.; Cavalli, A.; Castaldo, G.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Antithrombotic and anti-inflammatory effects of fondaparinux and enoxaparin in hospitalized COVID-19 patients: The FONDENOXAVID Study. J. Blood Med. 2021, 12, 69–75. [Google Scholar] [CrossRef]
- Buijsers, B.; Yanginlar, C.; Maciej-Hulme, M.L.; de Mast, Q.; van der Vlag, J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine 2020, 59, 102969. [Google Scholar] [CrossRef]
- Gozzo, L.; Viale, P.; Longo, L.; Vitale, D.C.; Drago, F. The potential role of heparin in patients with COVID-19: Beyond the anticoagulant effect. A Review. Front. Pharmacol. 2020, 11, 1307. [Google Scholar] [CrossRef]
- Albuquerque, A.M.; Eckert, I.; Tramujas, L.; Butler-Laporte, G.; McDonald, E.G.; Brophy, J.M.; Lee, T.C. Effect of tocilizumab, sarilumab, and baricitinib on mortality among patients hospitalized for COVID-19 treated with corticosteroids: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Barkas, F.; Christaki, E.; Liberopoulos, E.; Kosmidou, M.; Milionis, H. Anakinra in COVID-19: A step closer to the cure. Eur. J. Intern. Med. 2022, 96, 113–114. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An Update. Molecules 2022, 27, 8562. [Google Scholar] [CrossRef]
- Cherian, J.J.; Eerike, M.; Bagepally, B.S.; Das, S.; Panda, S. Efficacy and safety of baricitinib and tocilizumab in hospitalized patients with COVID-19: A comparison using systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1004308. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Huet, T.; Cavalli, G.; Gori, A.; Kyprianou, M.; Pickkers, P.; Eugen-Olsen, J.; Clerici, M.; Veas, F.; Chatellier, G.; et al. Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021, 3, e690–e697. [Google Scholar] [CrossRef]
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. A Clinical Update on SARS-CoV-2: Pathology and Development of Potential Inhibitors. Curr. Issues Mol. Biol. 2023, 45, 400–433. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ding, C.; Jiang, L.; Tang, W.; Liu, Y.; Zhao, L.; Yi, Z.; Ren, H.; Li, C.; He, Y.; et al. Discovery of potential anti-SARS-CoV-2 drugs based on large-scale screening in vitro and effect evaluation in vivo. Sci. China Life. Sci. 2022, 65, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, M.; Lee, J.S.; Whig, K.; Segrist, E.; Li, M.; Kamalia, B.; Castellana, L.; Ayyanathan, K.; Cardenas-Diaz, F.L.; Morrisey, E.E.; et al. Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2. Cell Rep. 2021, 35, 108959. [Google Scholar] [CrossRef]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Welson, N.N. Pathophysiology of Post-COVID syndromes: A new perspective. Virol. J. 2022, 19, 158. [Google Scholar] [CrossRef]
- Queiroz, M.A.F.; Neves, P.F.M.D.; Lima, S.S.; Lopes, J.D.C.; Torres, M.K.D.S.; Vallinoto, I.M.V.C.; Bichara, C.D.A.; Dos Santos, E.F.; de Brito, M.T.F.M.; da Silva, A.L.S.; et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front. Cell. Infect. Microbiol. 2022, 12, 922422. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Knauer, M.J.; Nicholson, M.; Daley, M.; Van Nynatten, L.R.; Martin, C.; Patterson, E.K.; Cepinskas, G.; Seney, S.L.; Dobretzberger, V.; et al. Elevated vascular transformation blood biomarkers in long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol. Med. 2022, 28, 122. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, M.; D’Onghia, D.; Tonello, S.; Minisini, R.; Colangelo, D.; Bellan, M.; Castello, L.M.; Gavelli, F.; Avanzi, G.C.; Pirisi, M.; et al. COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters. Int. J. Mol. Sci. 2023, 24, 7099. https://doi.org/10.3390/ijms24087099
Rizzi M, D’Onghia D, Tonello S, Minisini R, Colangelo D, Bellan M, Castello LM, Gavelli F, Avanzi GC, Pirisi M, et al. COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters. International Journal of Molecular Sciences. 2023; 24(8):7099. https://doi.org/10.3390/ijms24087099
Chicago/Turabian StyleRizzi, Manuela, Davide D’Onghia, Stelvio Tonello, Rosalba Minisini, Donato Colangelo, Mattia Bellan, Luigi Mario Castello, Francesco Gavelli, Gian Carlo Avanzi, Mario Pirisi, and et al. 2023. "COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters" International Journal of Molecular Sciences 24, no. 8: 7099. https://doi.org/10.3390/ijms24087099
APA StyleRizzi, M., D’Onghia, D., Tonello, S., Minisini, R., Colangelo, D., Bellan, M., Castello, L. M., Gavelli, F., Avanzi, G. C., Pirisi, M., & Sainaghi, P. P. (2023). COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters. International Journal of Molecular Sciences, 24(8), 7099. https://doi.org/10.3390/ijms24087099







