Appropriate Macronutrients or Mineral Elements Are Beneficial to Improve Depression and Reduce the Risk of Depression
Abstract
1. Introduction
2. Overdose or Deficiency of Macronutrients Elements Increase the Risk of Depression
2.1. Dietary Sugars
2.2. Dietary Fat
2.3. Dietary Protein
3. Overdose or Deficiency of Mineral Element Increase the Risk in Depression
3.1. Zinc(Zn)
3.2. Magnesium (Mg)
3.3. Copper(Cu)
3.4. Iron(Fe)
4. Appropriate Supplementation of Some Mineral Elements or as Medication Can Help Alleviate Depression
4.1. Selenium(Se) Supplementation
4.2. Zinc(Zn) Supplementation
4.3. Magnesium (Mg) Supplementation
4.4. Lithium(Li) as a Psychotropic Medication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depression. Available online: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed on 1 May 2021).
- Mrazek, D.A.; Hornberger, J.C.; Altar, C.A.; Degtiar, I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr. Serv. 2014, 65, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Z.-Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Dwyer, J.B.; Aftab, A.; Radhakrishnan, R.; Widge, A.; Rodriguez, C.I.; Carpenter, L.L.; Nemeroff, C.B.; McDonald, W.M.; Kalin, N.H. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am. J. Psychiatry 2020, 177, 686–705. [Google Scholar] [CrossRef]
- Aly, J.; Engmann, O. The Way to a Human’s Brain Goes Through Their Stomach: Dietary Factors in Major Depressive Disorder. Front. Neurosci. 2020, 14, 582853. [Google Scholar] [CrossRef] [PubMed]
- Shayganfard, M. Are Essential Trace Elements Effective in Modulation of Mental Disorders? Update and Perspectives. Biol. Trace Elem. Res. 2022, 200, 1032–1059. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Guo, X.; Park, Y.; Freedman, N.D.; Sinha, R.; Hollenbeck, A.R.; Blair, A.; Chen, H. Sweetened beverages, coffee, and tea and depression risk among older US adults. PLoS ONE 2014, 9, e94715. [Google Scholar] [CrossRef]
- Vermeulen, E.; Stronks, K.; Snijder, M.B.; Schene, A.H.; Lok, A.; de Vries, J.H.; Visser, M.; Brouwer, I.A.; Nicolaou, M. A combined high-sugar and high-saturated-fat dietary pattern is associated with more depressive symptoms in a multi-ethnic population: The HELIUS (Healthy Life in an Urban Setting) study. Public Health Nutr. 2017, 20, 2374–2382. [Google Scholar] [CrossRef]
- Shimmura, N.; Nanri, A.; Kashino, I.; Kochi, T.; Eguchi, M.; Kabe, I.; Mizoue, T. Prospective association of confectionery intake with depressive symptoms among Japanese workers: The Furukawa Nutrition and Health Study. Br. J. Nutr. 2022, 128, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kashino, I.; Kochi, T.; Imamura, F.; Eguchi, M.; Kuwahara, K.; Nanri, A.; Kurotani, K.; Akter, S.; Hu, H.; Miki, T.; et al. Prospective association of soft drink consumption with depressive symptoms. Nutrition 2021, 81, 110860. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cheng, L.; Jiang, W. Sugar-sweetened beverages consumption and the risk of depression: A meta-analysis of observational studies. J. Affect. Disord. 2019, 245, 348–355. [Google Scholar] [CrossRef]
- Yu, B.; He, H.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Wang, C.; Shi, H.; Xia, Y.; Guo, X.; et al. Soft drink consumption is associated with depressive symptoms among adults in China. J. Affect. Disord. 2015, 172, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.; Xiao, Y.; Jing, D.; Huang, Y.; Chen, L.; Luo, D.; Chen, X.; Shen, M. Daily intake of soft drinks is associated with symptoms of anxiety and depression in Chinese adolescents. Public Health Nutr. 2019, 22, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Zazpe, I.; Santiago, S.; Perez-Cornago, A.; Martinez-Gonzalez, M.A.; Lahortiga-Ramos, F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. Br. J. Nutr. 2018, 119, 211–221. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, E. Association between Soft-Drink Intake and Obesity, Depression, and Subjective Health Status of Male and Female Adults. Int. J. Environ. Res. Public Health 2021, 18, 10415. [Google Scholar] [CrossRef]
- Pinna, F.; Suprani, F.; Deiana, V.; Lai, L.; Manchia, M.; Paribello, P.; Somaini, G.; Diana, E.; Nicotra, E.F.; Farci, F.; et al. Depression in Diabetic Patients: What Is the Link With Eating Disorders? Results of a Study in a Representative Sample of Patients With Type 1 Diabetes. Front. Psychiatry 2022, 13, 848031. [Google Scholar] [CrossRef]
- Borgland, S.L. Can treatment of obesity reduce depression or vice versa? J. Psychiatry Neurosci. 2021, 46, E313–E318. [Google Scholar] [CrossRef]
- Peng, Y.-F.; Xiang, Y.; Wei, Y.-S. The significance of routine biochemical markers in patients with major depressive disorder. Sci. Rep. 2016, 6, 34402. [Google Scholar] [CrossRef]
- Inam, Q.-u.-A.; Jabeen, B.; Haleem, M.A.; Haleem, D.J. Long-term consumption of sugar-rich diet decreases the effectiveness of somatodendritic serotonin-1A receptors. Nutr. Neurosci. 2008, 11, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.; Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—A central role for the serotonin transporter? Pharmacol. Ther. 2015, 147, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Sabedra Sousa, F.S.; Birmann, P.T.; Bampi, S.R.; Fronza, M.G.; Balaguez, R.; Alves, D.; Leite, M.R.; Nogueira, C.W.; Brüning, C.A.; Savegnago, L. Lipopolysaccharide-induced depressive-like, anxiogenic-like and hyperalgesic behavior is attenuated by acute administration of α-(phenylselanyl) acetophenone in mice. Neuropharmacology 2019, 146, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Domingues, M.; de Andrade Lourenço, D.; Birmann, P.T.; Padilha, N.; Vieira, B.; Begnini, K.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Depression- and anxiogenic-like behaviors induced by lipopolysaccharide in mice are reversed by a selenium-containing indolyl compound: Behavioral, neurochemical and computational insights involving the serotonergic system. J. Psychiatr. Res. 2019, 115, 1–12. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Sen, S.; Duman, R.; Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, H.P.; Won, S.D.; Park, E.Y.; Lee, H.Y.; Lee, B.H.; Lee, S.W.; Yoon, D.; Han, C.; Kim, D.J.; et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 78–85. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ochoa, E.; Hernández-Ortega, K.; Ferrera, P.; Morimoto, S.; Arias, C. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J. Cereb. Blood Flow Metab. 2014, 34, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Speed, M.S.; Jefsen, O.H.; Børglum, A.D.; Speed, D.; Østergaard, S.D. Investigating the association between body fat and depression via Mendelian randomization. Transl. Psychiatry 2019, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatry 2016, 21, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Prospective Associations between Depression and Obesity for Adolescent Males and Females—A Systematic Review and Meta-Analysis of Longitudinal Studies. PLoS ONE 2016, 11, e0157240. [Google Scholar] [CrossRef]
- Panth, N.; Dias, C.B.; Wynne, K.; Singh, H.; Garg, M.L. Medium-chain fatty acids lower postprandial lipemia: A randomized crossover trial. Clin. Nutr. 2020, 39, 90–96. [Google Scholar] [CrossRef]
- Oh, J.; Kim, T.-S. Serum lipid levels in depression and suicidality: The Korea National Health and Nutrition Examination Survey (KNHANES) 2014. J. Affect. Disord. 2017, 213, 51–58. [Google Scholar] [CrossRef]
- Enko, D.; Brandmayr, W.; Halwachs-Baumann, G.; Schnedl, W.J.; Meinitzer, A.; Kriegshäuser, G. Prospective plasma lipid profiling in individuals with and without depression. Lipids Health Dis. 2018, 17, 149. [Google Scholar] [CrossRef]
- So, H.-C.; Chau, C.K.-L.; Cheng, Y.-Y.; Sham, P.C. Causal relationships between blood lipids and depression phenotypes: A Mendelian randomisation analysis. Psychol. Med. 2021, 51, 2357–2369. [Google Scholar] [CrossRef]
- Braga, S.P.; Delanogare, E.; Machado, A.E.; Prediger, R.D.; Moreira, E.L.G. Switching from high-fat feeding (HFD) to regular diet improves metabolic and behavioral impairments in middle-aged female mice. Behav. Brain Res. 2021, 398, 112969. [Google Scholar] [CrossRef]
- Yu, H.; Qin, X.; Yu, Z.; Chen, Y.; Tang, L.; Shan, W. Effects of high-fat diet on the formation of depressive-like behavior in mice. Food Funct. 2021, 12, 6416–6431. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Solskov, L.; Volke, V.; Harvey, B.H.; Lund, S.; Wegener, G. A high-fat diet exacerbates depressive-like behavior in the Flinders Sensitive Line (FSL) rat, a genetic model of depression. Psychoneuroendocrinology 2011, 36, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kim, J.; Park, J.; Lee, H.; Yaicharoen, P.; Suidasari, S.; Yokozawa, M.; Yamauchi, K. Olive leaf extract prevents obesity, cognitive decline, and depression and improves exercise capacity in mice. Sci. Rep. 2021, 11, 12495. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lv, W.; Pan, Q.; Kalavagunta, P.K.; Liu, Q.; Qin, G.; Cai, M.; Zhou, L.; Wang, T.; Xia, Z.; et al. Simvastatin therapy in adolescent mice attenuates HFD-induced depression-like behavior by reducing hippocampal neuroinflammation. J. Affect. Disord. 2019, 243, 83–95. [Google Scholar] [CrossRef]
- Hersey, M.; Woodruff, J.L.; Maxwell, N.; Sadek, A.T.; Bykalo, M.K.; Bain, I.; Grillo, C.A.; Piroli, G.G.; Hashemi, P.; Reagan, L.P. High-fat diet induces neuroinflammation and reduces the serotonergic response to escitalopram in the hippocampus of obese rats. Brain Behav. Immun. 2021, 96, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Liu, Q.; Wan, R.; Kalavagunta, P.K.; Liu, L.; Lv, W.; Qiao, T.; Shang, J.; Wu, H. Selective inhibition of intestinal 5-HT improves neurobehavioral abnormalities caused by high-fat diet mice. Metab. Brain Dis. 2019, 34, 747–761. [Google Scholar] [CrossRef]
- Liu, S.; Xiu, J.; Zhu, C.; Meng, K.; Li, C.; Han, R.; Du, T.; Li, L.; Xu, L.; Liu, R.; et al. Fat mass and obesity-associated protein regulates RNA methylation associated with depression-like behavior in mice. Nat. Commun. 2021, 12, 6937. [Google Scholar] [CrossRef]
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal control of obesity and anxiety-depressive disorder via a GABA and serotonin neural circuit. Mol. Psychiatry 2021, 26, 2837–2853. [Google Scholar] [CrossRef]
- Tsai, S.-F.; Hsu, P.-L.; Chen, Y.-W.; Hossain, M.S.; Chen, P.-C.; Tzeng, S.-F.; Chen, P.-S.; Kuo, Y.-M. High-fat diet induces depression-like phenotype via astrocyte-mediated hyperactivation of ventral hippocampal glutamatergic afferents to the nucleus accumbens. Mol. Psychiatry 2022, 27, 4372–4384. [Google Scholar] [CrossRef]
- Rebai, R.; Jasmin, L.; Boudah, A. Agomelatine effects on fat-enriched diet induced neuroinflammation and depression-like behavior in rats. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 135, 111246. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, J.; Liu, Q.Z.; Wang, L.L.; Shang, J. Simvastatin and Bezafibrate ameliorate Emotional disorder Induced by High fat diet in C57BL/6 mice. Sci. Rep. 2017, 7, 2335. [Google Scholar] [CrossRef] [PubMed]
- Arcego, D.M.; Toniazzo, A.P.; Krolow, R.; Lampert, C.; Berlitz, C.; Dos Santos Garcia, E.; do Couto Nicola, F.; Hoppe, J.B.; Gaelzer, M.M.; Klein, C.P.; et al. Impact of High-Fat Diet and Early Stress on Depressive-Like Behavior and Hippocampal Plasticity in Adult Male Rats. Mol. Neurobiol. 2018, 55, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Vagena, E.; Ryu, J.K.; Baeza-Raja, B.; Walsh, N.M.; Syme, C.; Day, J.P.; Houslay, M.D.; Baillie, G.S. A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl. Psychiatry 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Y.; Zhou, Y.; Du, H.; Zhang, C.; Zhao, Z.; Chen, Y.; Zhou, Z.; Mei, J.; Wu, W.; et al. High fat diet-induced obesity leads to depressive and anxiety-like behaviors in mice via AMPK/mTOR-mediated autophagy. Exp. Neurol. 2022, 348, 113949. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.; Xia, J.; Xue, X.; Wang, H.; Qi, Z.; Ji, L. Leptin receptor knockout-induced depression-like behaviors and attenuated antidepressant effects of exercise are associated with STAT3/SOCS3 signaling. Brain Behav. Immun. 2017, 61, 297–305. [Google Scholar] [CrossRef]
- Guo, M.; Huang, T.-Y.; Garza, J.C.; Chua, S.C.; Lu, X.-Y. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int. J. Neuropsychopharmacol. 2013, 16, 857–867. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, D.X.; Jiang, H.; Pan, F.; Ho, C.S.; Ho, R.C. The Effects of High-fat-diet Combined with Chronic Unpredictable Mild Stress on Depression-like Behavior and Leptin/LepRb in Male Rats. Sci. Rep. 2016, 6, 35239. [Google Scholar] [CrossRef]
- Gallego-Landin, I.; García-Baos, A.; Castro-Zavala, A.; Valverde, O. Reviewing the Role of the Endocannabinoid System in the Pathophysiology of Depression. Front. Pharm. 2021, 12, 762738. [Google Scholar] [CrossRef]
- Valverde, O.; Torrens, M. CB1 receptor-deficient mice as a model for depression. Neuroscience 2012, 204, 193–206. [Google Scholar] [CrossRef]
- Gawliński, D.; Gawlińska, K.; Smaga, I. Maternal High-Fat Diet Modulates Gene Expression in Male Rat Offspring. Nutrients 2021, 13, 2885. [Google Scholar] [CrossRef]
- Oh, J.; Yun, K.; Chae, J.-H.; Kim, T.-S. Association Between Macronutrients Intake and Depression in the United States and South Korea. Front. Psychiatry 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.R.; Arroyo, C.; Tedders, S.H.; Li, Y.; Dai, Q.; Zhang, J. Dietary protein and protein-rich food in relation to severely depressed mood: A 10 year follow-up of a national cohort. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Li, S.; Zhang, D. Association between dietary protein intake and the risk of depressive symptoms in adults. Br. J. Nutr. 2020, 123, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Eguchi, M.; Kuwahara, K.; Kochi, T.; Kurotani, K.; Ito, R.; Pham, N.M.; Tsuruoka, H.; Akter, S.; Jacka, F.; et al. Macronutrient intake and depressive symptoms among Japanese male workers: The Furukawa Nutrition and Health Study. Psychiatry Res. 2014, 220, 263–268. [Google Scholar] [CrossRef]
- Nucci, D.; Fatigoni, C.; Amerio, A.; Odone, A.; Gianfredi, V. Red and Processed Meat Consumption and Risk of Depression: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6686. [Google Scholar] [CrossRef]
- Ciarambino, T.; Ferrara, N.; Castellino, P.; Paolisso, G.; Coppola, L.; Giordano, M. Effects of a 6-days-a-week low protein diet regimen on depressive symptoms in young-old type 2 diabetic patients. Nutrition 2011, 27, 46–49. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Zhang, D. Associations of different types of dairy intakes with depressive symptoms in adults. J. Affect. Disord. 2020, 274, 326–333. [Google Scholar] [CrossRef]
- Badawy, A.A. B. Tryptophan: The key to boosting brain serotonin synthesis in depressive illness. J. Psychopharmacol. 2013, 27, 878–893. [Google Scholar] [CrossRef]
- Reuter, M.; Zamoscik, V.; Plieger, T.; Bravo, R.; Ugartemendia, L.; Rodriguez, A.B.; Kirsch, P. Tryptophan-rich diet is negatively associated with depression and positively linked to social cognition. Nutr. Res. 2021, 85, 14–20. [Google Scholar] [CrossRef]
- Franklin, M.; Bermudez, I.; Murck, H.; Singewald, N.; Gaburro, S. Sub-chronic dietary tryptophan depletion—An animal model of depression with improved face and good construct validity. J. Psychiatr. Res. 2012, 46, 239–247. [Google Scholar] [CrossRef]
- Papakostas, G.I. Dopaminergic-based pharmacotherapies for depression. Eur. Neuropsychopharmacol. 2006, 16, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Vekovischeva, O.Y.; Peuhkuri, K.; Bäckström, P.; Sihvola, N.; Pilvi, T.; Korpela, R. The effects of native whey and α-lactalbumin on the social and individual behaviour of C57BL/6J mice. Br. J. Nutr. 2013, 110, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Matsunaga, K.; Sugiyama, A. Antidepressant-like effect of milk-derived lactoferrin in the repeated forced-swim stress mouse model. J. Vet. Med. Sci. 2017, 79, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Kubera, M.; Nowak, G. The role of zinc in neurodegenerative inflammatory pathways in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Xin, X.; Song, X.; Zhang, D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J. Affect. Disord. 2018, 228, 68–74. [Google Scholar] [CrossRef]
- Vashum, K.P.; McEvoy, M.; Milton, A.H.; McElduff, P.; Hure, A.; Byles, J.; Attia, J. Dietary zinc is associated with a lower incidence of depression: Findings from two Australian cohorts. J. Affect. Disord. 2014, 166, 249–257. [Google Scholar] [CrossRef]
- Anbari-Nogyni, Z.; Bidaki, R.; Madadizadeh, F.; Sangsefidi, Z.S.; Fallahzadeh, H.; Karimi-Nazari, E.; Nadjarzadeh, A. Relationship of zinc status with depression and anxiety among elderly population. Clin. Nutr. ESPEN 2020, 37, 233–239. [Google Scholar] [CrossRef]
- Nakamura, M.; Miura, A.; Nagahata, T.; Shibata, Y.; Okada, E.; Ojima, T. Low Zinc, Copper, and Manganese Intake is Associated with Depression and Anxiety Symptoms in the Japanese Working Population: Findings from the Eating Habit and Well-Being Study. Nutrients 2019, 11, 847. [Google Scholar] [CrossRef]
- Miki, T.; Kochi, T.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Kurotani, K.; Ito, R.; Akter, S.; Kashino, I.; Pham, N.M.; et al. Dietary intake of minerals in relation to depressive symptoms in Japanese employees: The Furukawa Nutrition and Health Study. Nutrition 2015, 31, 686–690. [Google Scholar] [CrossRef]
- Maserejian, N.N.; Hall, S.A.; McKinlay, J.B. Low dietary or supplemental zinc is associated with depression symptoms among women, but not men, in a population-based epidemiological survey. J. Affect. Disord. 2012, 136, 781–788. [Google Scholar] [CrossRef]
- Thi Thu Nguyen, T.; Miyagi, S.; Tsujiguchi, H.; Kambayashi, Y.; Hara, A.; Nakamura, H.; Suzuki, K.; Yamada, Y.; Shimizu, Y.; Nakamura, H. Association between Lower Intake of Minerals and Depressive Symptoms among Elderly Japanese Women but Not Men: Findings from Shika Study. Nutrients 2019, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Al-Fartusie, F.S.; Al-Bairmani, H.K.; Al-Garawi, Z.S.; Yousif, A.H. Evaluation of Some Trace Elements and Vitamins in Major Depressive Disorder Patients: A Case-Control Study. Biol. Trace Elem. Res. 2019, 189, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.M.A.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.K.M.; Islam, M.S.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Whittle, N.; Lubec, G.; Singewald, N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids 2009, 36, 147–158. [Google Scholar] [CrossRef]
- Tassabehji, N.M.; Corniola, R.S.; Alshingiti, A.; Levenson, C.W. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008, 95, 365–369. [Google Scholar] [CrossRef]
- Młyniec, K.; Nowak, G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. PR 2012, 64, 249–255. [Google Scholar] [CrossRef]
- Thingholm, T.E.; Rönnstrand, L.; Rosenberg, P.A. Why and how to investigate the role of protein phosphorylation in ZIP and ZnT zinc transporter activity and regulation. Cell Mol. Life Sci. 2020, 77, 3085–3102. [Google Scholar] [CrossRef]
- Rafalo-Ulinska, A.; Piotrowska, J.; Kryczyk, A.; Opoka, W.; Sowa-Kucma, M.; Misztak, P.; Rajkowska, G.; Stockmeier, C.A.; Datka, W.; Nowak, G.; et al. Zinc transporters protein level in postmortem brain of depressed subjects and suicide victims. J. Psychiatr. Res. 2016, 83, 220–229. [Google Scholar] [CrossRef]
- McAllister, B.B.; Dyck, R.H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 2017, 80, 329–350. [Google Scholar] [CrossRef]
- Dou, X.; Tian, X.; Zheng, Y.; Huang, J.; Shen, Z.; Li, H.; Wang, X.; Mo, F.; Wang, W.; Wang, S.; et al. Psychological stress induced hippocampus zinc dyshomeostasis and depression-like behavior in rats. Behav. Brain Res. 2014, 273, 133–138. [Google Scholar] [CrossRef]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588. [Google Scholar] [CrossRef]
- Laitakari, A.; Liu, L.; Frimurer, T.M.; Holst, B. The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target. Int. J. Mol. Sci. 2021, 22, 3872. [Google Scholar] [CrossRef] [PubMed]
- Siodłak, D.; Nowak, G.; Mlyniec, K. Interaction between zinc, the GPR39 zinc receptor and the serotonergic system in depression. Brain Res. Bull. 2021, 170, 146–154. [Google Scholar] [CrossRef]
- Młyniec, K.; Gaweł, M.; Nowak, G. Study of antidepressant drugs in GPR39 (zinc receptor−/−) knockout mice, showing no effect of conventional antidepressants, but effectiveness of NMDA antagonists. Behav. Brain Res. 2015, 287, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Młyniec, K.; Budziszewska, B.; Holst, B.; Ostachowicz, B.; Nowak, G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int. J. Neuropsychopharmacol. 2014, 18, pyu002. [Google Scholar] [CrossRef] [PubMed]
- Mlyniec, K. Interaction between Zinc, GPR39, BDNF and Neuropeptides in Depression. Curr. Neuropharmacol. 2021, 19, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Doboszewska, U.; Szewczyk, B.; Sowa-Kućma, M.; Noworyta-Sokołowska, K.; Misztak, P.; Gołębiowska, J.; Młyniec, K.; Ostachowicz, B.; Krośniak, M.; Wojtanowska-Krośniak, A.; et al. Alterations of Bio-elements, Oxidative, and Inflammatory Status in the Zinc Deficiency Model in Rats. Neurotox. Res. 2016, 29, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Cabral, D.; Galvão, M.C.; Monteiro, R.; Bondan, E.F.; Bernardi, M.M. Zinc, but not paracetamol, prevents depressive-like behavior and sickness behavior, and inhibits interferon-gamma and astrogliosis in rats. Brain Behav. Immun. 2020, 87, 489–497. [Google Scholar] [CrossRef]
- Doboszewska, U.; Szewczyk, B.; Sowa-Kućma, M.; Młyniec, K.; Rafało, A.; Ostachowicz, B.; Lankosz, M.; Nowak, G. Antidepressant activity of fluoxetine in the zinc deficiency model in rats involves the NMDA receptor complex. Behav. Brain Res. 2015, 287, 323–330. [Google Scholar] [CrossRef]
- Doboszewska, U.; Sowa-Kućma, M.; Młyniec, K.; Pochwat, B.; Hołuj, M.; Ostachowicz, B.; Pilc, A.; Nowak, G.; Szewczyk, B. Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 254–263. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Kennedy, A.G.; Rose, G.L.; Crocker, A.; Littenberg, B. The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, R.; Li, Z.; Zhang, D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 2019, 246, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Singewald, N.; Sinner, C.; Hetzenauer, A.; Sartori, S.B.; Murck, H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice—Influence of desipramine and Hypericum perforatum extract. Neuropharmacology 2004, 47, 1189–1197. [Google Scholar] [CrossRef]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Del Chierico, F.; Trapani, V.; Petito, V.; Reddel, S.; Pietropaolo, G.; Graziani, C.; Masi, L.; Gasbarrini, A.; Putignani, L.; Scaldaferri, F.; et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota. Nutrients 2021, 13, 4188. [Google Scholar] [CrossRef]
- Ghafari, M.; Whittle, N.; Miklósi, A.G.; Kotlowski, C.; Kotlowsky, C.; Schmuckermair, C.; Berger, J.; Bennett, K.L.; Singewald, N.; Lubec, G. Dietary magnesium restriction reduces amygdala-hypothalamic GluN1 receptor complex levels in mice. Brain Struct. Funct. 2015, 220, 2209–2221. [Google Scholar] [CrossRef]
- Whittle, N.; Li, L.; Chen, W.-Q.; Yang, J.-W.; Sartori, S.B.; Lubec, G.; Singewald, N. Changes in brain protein expression are linked to magnesium restriction-induced depression-like behavior. Amino Acids 2011, 40, 1231–1248. [Google Scholar] [CrossRef]
- Opanković, A.; Milovanović, S.; Radosavljević, B.; Čavić, M.; Besu Žižak, I.; Bukumirić, Z.; Latas, M.; Medić, B.; Vučković, S.; Srebro, D.; et al. Correlation of Ionized Magnesium with the Parameters of Oxidative Stress as Potential Biomarkers in Patients with Anxiety and Depression: A Pilot Study. Dose Response 2022, 20, 15593258221116741. [Google Scholar] [CrossRef]
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; You, Y.; Chen, J.; Zhang, L. Copper in depressive disorder: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2018, 267, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Szkup, M.; Jurczak, A.; Brodowska, A.; Brodowska, A.; Noceń, I.; Chlubek, D.; Laszczyńska, M.; Karakiewicz, B.; Grochans, E. Analysis of Relations Between the Level of Mg, Zn, Ca, Cu, and Fe and Depressiveness in Postmenopausal Women. Biol. Trace Elem. Res. 2017, 176, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Styczeń, K.; Sowa-Kućma, M.; Siwek, M.; Dudek, D.; Reczyński, W.; Misztak, P.; Szewczyk, B.; Topór-Mądry, R.; Opoka, W.; Nowak, G. Study of the Serum Copper Levels in Patients with Major Depressive Disorder. Biol. Trace Elem. Res. 2016, 174, 287–293. [Google Scholar] [CrossRef]
- Xu, J.; He, K.; Zhang, K.; Yang, C.; Nie, L.; Dan, D.; Liu, J.; Zhang, C.-E.; Yang, X. Low-Dose Copper Exposure Exacerbates Depression-Like Behavior in ApoE4 Transgenic Mice. Oxidative Med. Cell. Longev. 2021, 2021, 6634181. [Google Scholar] [CrossRef]
- Lamtai, M.; Zghari, O.; Azirar, S.; Ouakki, S.; Mesfioui, A.; El Hessni, A.; Berkiks, I.; Marmouzi, I.; Ouichou, A. Melatonin modulates copper-induced anxiety-like, depression-like and memory impairments by acting on hippocampal oxidative stress in rat. Drug Chem. Toxicol. 2021, 45, 1707–1715. [Google Scholar] [CrossRef]
- Liu, X.; Lin, C.; Wang, S.; Yu, X.; Jia, Y.; Chen, J. Effects of high levels of copper on the depression-related memory disorders. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 78, 611–618. [Google Scholar] [CrossRef]
- Barks, A.; Hall, A.M.; Tran, P.V.; Georgieff, M.K. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatr. Res. 2019, 85, 176–182. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Song, X.; Zhang, D. Dietary zinc and iron intake and risk of depression: A meta-analysis. Psychiatry Res. 2017, 251, 41–47. [Google Scholar] [CrossRef]
- Portugal-Nunes, C.; Castanho, T.C.; Amorim, L.; Moreira, P.S.; Mariz, J.; Marques, F.; Sousa, N.; Santos, N.C.; Palha, J.A. Iron Status is Associated with Mood, Cognition, and Functional Ability in Older Adults: A Cross-Sectional Study. Nutrients 2020, 12, 3594. [Google Scholar] [CrossRef]
- Hameed, S.; Naser, I.A.; Al Ghussein, M.A.; Ellulu, M.S. Is iron deficiency a risk factor for postpartum depression? A case-control study in the Gaza Strip, Palestine. Public Health Nutr. 2021, 25, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zheng, Z.; Ma, C. The effectiveness of iron supplementation for postpartum depression: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23603. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Saito, K.; Asano, S.; Kunugi, H. Association between iron-deficiency anemia and depression: A web-based Japanese investigation. Psychiatry Clin. Neurosci. 2018, 72, 513–521. [Google Scholar] [CrossRef]
- Bergis, D.; Tessmer, L.; Badenhoop, K. Iron deficiency in long standing type 1 diabetes mellitus and its association with depression and impaired quality of life. Diabetes Res. Clin. Pract. 2019, 151, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Y.; Li, Q.; Xu, J.; Yan, S.; Cai, J.; Jiaerken, Y.; Lou, M. Brain Iron Deposits in Thalamus Is an Independent Factor for Depressive Symptoms Based on Quantitative Susceptibility Mapping in an Older Adults Community Population. Front. Psychiatry 2019, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharm. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Texel, S.J.; Camandola, S.; Ladenheim, B.; Rothman, S.M.; Mughal, M.R.; Unger, E.L.; Cadet, J.L.; Mattson, M.P. Ceruloplasmin deficiency results in an anxiety phenotype involving deficits in hippocampal iron, serotonin, and BDNF. J. Neurochem. 2012, 120, 125–134. [Google Scholar] [CrossRef]
- Tran, P.V.; Carlson, E.S.; Fretham, S.J.B.; Georgieff, M.K. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J. Nutr. 2008, 138, 2495–2501. [Google Scholar] [CrossRef]
- Tran, P.V.; Fretham, S.J.B.; Carlson, E.S.; Georgieff, M.K. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr. Res. 2009, 65, 493–498. [Google Scholar] [CrossRef]
- Mehrpouya, S.; Nahavandi, A.; Khojasteh, F.; Soleimani, M.; Ahmadi, M.; Barati, M. Iron administration prevents BDNF decrease and depressive-like behavior following chronic stress. Brain Res. 2015, 1596, 79–87. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Li, Y.; Li, Y.; Gong, H.; Li, J.; Zhang, Y.; Zhang, C.; Yan, F.; Sun, B.; et al. Alterations in brain iron deposition with progression of late-life depression measured by magnetic resonance imaging (MRI)-based quantitative susceptibility mapping. Quant. Imaging Med. Surg. 2022, 12, 3873–3888. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B. H. Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases. J. Neural Transm. 2018, 125, 1719–1733. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Vázquez, G.H. Pharmacological treatment of adult bipolar disorder. Mol. Psychiatry 2019, 24, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Barroilhet, S.A.; Ghaemi, S.N. When and how to use lithium. Acta Psychiatr. Scand. 2020, 142, 161–172. [Google Scholar] [CrossRef]
- Memon, A.; Rogers, I.; Fitzsimmons, S.M.D.D.; Carter, B.; Strawbridge, R.; Hidalgo-Mazzei, D.; Young, A.H. Association between naturally occurring lithium in drinking water and suicide rates: Systematic review and meta-analysis of ecological studies. Br. J. Psychiatry J. Ment. Sci. 2020, 217, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Birkenhäger, T.K.; van den Broek, W.W.; Mulder, P.G.; Bruijn, J.A.; Moleman, P. Comparison of two-phase treatment with imipramine or fluvoxamine, both followed by lithium addition, in inpatients with major depressive disorder. Am. J. Psychiatry 2004, 161, 2060–2065. [Google Scholar] [CrossRef]
- Taylor, R.W.; Marwood, L.; Oprea, E.; DeAngel, V.; Mather, S.; Valentini, B.; Zahn, R.; Young, A.H.; Cleare, A.J. Pharmacological Augmentation in Unipolar Depression: A Guide to the Guidelines. Int. J. Neuropsychopharmacol. 2020, 23, 587–625. [Google Scholar] [CrossRef] [PubMed]
- Undurraga, J.; Sim, K.; Tondo, L.; Gorodischer, A.; Azua, E.; Tay, K.H.; Tan, D.; Baldessarini, R.J. Lithium treatment for unipolar major depressive disorder: Systematic review. J. Psychopharmacol. 2019, 33, 167–176. [Google Scholar] [CrossRef]
- Tiihonen, J.; Tanskanen, A.; Hoti, F.; Vattulainen, P.; Taipale, H.; Mehtälä, J.; Lähteenvuo, M. Pharmacological treatments and risk of readmission to hospital for unipolar depression in Finland: A nationwide cohort study. Lancet Psychiatry 2017, 4, 547–553. [Google Scholar] [CrossRef]
- Maruki, T.; Utsumi, T.; Takeshima, M.; Fujiwara, Y.; Matsui, M.; Aoki, Y.; Toda, H.; Watanabe, N.; Watanabe, K.; Takaesu, Y. Efficacy and safety of adjunctive therapy to lamotrigine, lithium, or valproate monotherapy in bipolar depression: A systematic review and meta-analysis of randomized controlled trials. Int. J. Bipolar Disord. 2022, 10, 24. [Google Scholar] [CrossRef]
- Rakofsky, J.J.; Lucido, M.J.; Dunlop, B.W. Lithium in the treatment of acute bipolar depression: A systematic review and meta-analysis. J. Affect. Disord. 2022, 308, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.H.; Bahji, A.; Undurraga, J.; Tondo, L.; Baldessarini, R.J. Efficacy and Tolerability of Combination Treatments for Major Depression: Antidepressants plus Second-Generation Antipsychotics vs. Esketamine vs. Lithium. J. Psychopharmacol. 2021, 35, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Denoth-Lippuner, A.; Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 2021, 22, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; Jäger, M.; Smajstrlova, I.; Born, C.; Bottlender, R.; Palladino, T.; Reiser, M.; Möller, H.-J.; Meisenzahl, E.M. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: A 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 2008, 33, 423–430. [Google Scholar] [PubMed]
- Egeland, M.; Guinaudie, C.; Du Preez, A.; Musaelyan, K.; Zunszain, P.A.; Fernandes, C.; Pariante, C.M.; Thuret, S. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry 2017, 7, e1101. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kawauchi, S.; Kameda, M.; Hosomoto, K.; Tomita, Y.; Umakoshi, M.; Kuwahara, K.; Kin, I.; Kidani, N.; et al. Lithium counteracts depressive behavior and augments the treatment effect of selective serotonin reuptake inhibitor in treatment-resistant depressed rats. Brain Res. 2019, 1717, 52–59. [Google Scholar] [CrossRef]
- Ricken, R.; Adli, M.; Lange, C.; Krusche, E.; Stamm, T.J.; Gaus, S.; Koehler, S.; Nase, S.; Bschor, T.; Richter, C.; et al. Brain-derived neurotrophic factor serum concentrations in acute depressive patients increase during lithium augmentation of antidepressants. J. Clin. Psychopharmacol. 2013, 33, 806–809. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Q.-Q.; Wang, D.; Song, S.-P.; Yang, X.-N.; Hu, S.-W.; Wang, Z.-Y.; Xu, Z.; Liu, H.; Yang, J.-X.; et al. Mesocortical BDNF signaling mediates antidepressive-like effects of lithium. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 1557–1566. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Y.; Du, L. Blood-Brain Barrier Dysfunction in the Pathogenesis of Major Depressive Disorder. Cell. Mol. Neurobiol. 2021, 42, 2571–2591. [Google Scholar] [CrossRef]
- Taler, M.; Aronovich, R.; Henry Hornfeld, S.; Dar, S.; Sasson, E.; Weizman, A.; Hochman, E. Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord. 2021, 23, 55–65. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Ghimire, S.; Baral, B.K.; Feng, D.; Sy, F.S.; Rodriguez, R. Is selenium intake associated with the presence of depressive symptoms among US adults? Findings from National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrition 2019, 62, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, Y.; Unverzagt, F.W.; Liang, C.; Hall, K.S.; Cao, J.; Ma, F.; Murrell, J.R.; Cheng, Y.; Li, P.; et al. Selenium level and depressive symptoms in a rural elderly Chinese cohort. BMC Psychiatry 2012, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Almeida, T.L.; Petarli, G.B.; Cattafesta, M.; Zandonade, E.; Bezerra, O.M.d.P.A.; Tristão, K.G.; Salaroli, L.B. Association of Selenium Intake and Development of Depression in Brazilian Farmers. Front. Nutr. 2021, 8, 671377. [Google Scholar] [CrossRef]
- Conner, T.S.; Richardson, A.C.; Miller, J.C. Optimal serum selenium concentrations are associated with lower depressive symptoms and negative mood among young adults. J. Nutr. 2015, 145, 59–65. [Google Scholar] [CrossRef]
- Leung, B.M.Y.; Kaplan, B.J.; Field, C.J.; Tough, S.; Eliasziw, M.; Gomez, M.F.; McCargar, L.J.; Gagnon, L. Prenatal micronutrient supplementation and postpartum depressive symptoms in a pregnancy cohort. BMC Pregnancy Childbirth 2013, 13, 2. [Google Scholar] [CrossRef]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Majdi, M.R.; Tavallaie, S.; Azimi-Nezhad, M.; et al. Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2011, 24, 104–108. [Google Scholar] [CrossRef]
- Colangelo, L.A.; He, K.; Whooley, M.A.; Daviglus, M.L.; Morris, S.; Liu, K. Selenium exposure and depressive symptoms: The Coronary Artery Risk Development in Young Adults Trace Element Study. Neurotoxicology 2014, 41, 167–174. [Google Scholar] [CrossRef]
- Kędzierska, E.; Dudka, J.; Poleszak, E.; Kotlińska, J.H. Antidepressant and anxiolytic-like activity of sodium selenite after acute treatment in mice. Pharmacol. Rep. PR 2017, 69, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, E.; Dąbkowska, L.; Obierzyński, P.; Polakowska, M.; Poleszak, E.; Wlaź, P.; Szewczyk, K.; Kotlińska, J. Synergistic Action of Sodium Selenite with some Antidepressants and Diazepam in Mice. Pharmaceutics 2018, 10, 270. [Google Scholar] [CrossRef]
- Samad, N.; Rao, T.; Rehman, M.H.U.; Bhatti, S.A.; Imran, I. Inhibitory Effects of Selenium on Arsenic-Induced Anxiety-/Depression-Like Behavior and Memory Impairment. Biol. Trace Elem. Res. 2022, 200, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Yan, S.; Guo, X.; Zhao, Y.; Shi, B. The protective effect of selenium on the lipopolysaccharide-induced oxidative stress and depressed gene expression related to milk protein synthesis in bovine mammary epithelial cells. Biol. Trace Elem. Res. 2020, 197, 141–148. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Hao, Z.; Jing, X.; Zhao, Y.; Cheng, X.; Ma, H.; Wang, J.; Wang, J. Mitigation Effects of Selenium Nanoparticles on Depression-Like Behavior Induced by Fluoride in Mice via the JAK2-STAT3 Pathway. ACS Appl. Mater. Interfaces 2022, 14, 3685–3700. [Google Scholar] [CrossRef]
- Yosaee, S.; Clark, C.C.T.; Keshtkaran, Z.; Ashourpour, M.; Keshani, P.; Soltani, S. Zinc in depression: From development to treatment: A comparative/dose response meta-analysis of observational studies and randomized controlled trials. Gen. Hosp. Psychiatry 2022, 74, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, E.; Shams, J.; Sabetkasaei, M.; M-Shirazi, M.; Rashidkhani, B.; Mostafavi, A.; Bornak, E.; Nasrollahzadeh, J. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr. Neurosci. 2014, 17, 65–71. [Google Scholar] [CrossRef]
- Donig, A.; Hautzinger, M. Zinc as an adjunct to antidepressant medication: A meta-analysis with subgroup analysis for different levels of treatment response to antidepressants. Nutr. Neurosci. 2022, 25, 1785–1795. [Google Scholar] [CrossRef]
- Misztak, P.; Sowa-Kućma, M.; Pańczyszyn-Trzewik, P.; Szewczyk, B.; Nowak, G. Antidepressant-like Effects of Combined Fluoxetine and Zinc Treatment in Mice Exposed to Chronic Restraint Stress Are Related to Modulation of Histone Deacetylase. Molecules 2021, 27, 22. [Google Scholar] [CrossRef]
- Rafało-Ulińska, A.; Poleszak, E.; Szopa, A.; Serefko, A.; Rogowska, M.; Sowa, I.; Wójciak, M.; Muszyńska, B.; Krakowska, A.; Gdula-Argasińska, J.; et al. Imipramine Influences Body Distribution of Supplemental Zinc Which May Enhance Antidepressant Action. Nutrients 2020, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef]
- Skalski, M.; Mach, A.; Januszko, P.; Ryszewska-Pokraśniewicz, B.; Biernacka, A.; Nowak, G.; Pilc, A.; Poleszak, E.; Radziwoń-Zaleska, M. Pharmaco-Electroencephalography-Based Assessment of Antidepressant Drug Efficacy-The Use of Magnesium Ions in the Treatment of Depression. J. Clin. Med. 2021, 10, 3135. [Google Scholar] [CrossRef]
- Poleszak, E. Modulation of antidepressant-like activity of magnesium by serotonergic system. J. Neural Transm. 2007, 114, 1129–1134. [Google Scholar] [CrossRef]
- Chen, J.-L.; Zhou, X.; Liu, B.-L.; Wei, X.-H.; Ding, H.-L.; Lin, Z.-J.; Zhan, H.-L.; Yang, F.; Li, W.-B.; Xie, J.-C.; et al. Normalization of magnesium deficiency attenuated mechanical allodynia, depressive-like behaviors, and memory deficits associated with cyclophosphamide-induced cystitis by inhibiting TNF-α/NF-κB signaling in female rats. J. Neuroinflamm. 2020, 17, 99. [Google Scholar] [CrossRef]
- Pochwat, B.; Szewczyk, B.; Sowa-Kucma, M.; Siwek, A.; Doboszewska, U.; Piekoszewski, W.; Gruca, P.; Papp, M.; Nowak, G. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: Alterations in the NMDA receptor subunits. Int. J. Neuropsychopharmacol. 2014, 17, 393–405. [Google Scholar] [CrossRef]
- Ronaldson, A.; Arias de la Torre, J.; Gaughran, F.; Bakolis, I.; Hatch, S.L.; Hotopf, M.; Dregan, A. Prospective associations between vitamin D and depression in middle-aged adults: Findings from the UK Biobank cohort. Psychol. Med. 2022, 52, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Y. Associations of Dietary Vitamin C and E Intake With Depression. A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 857823. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Li, S.; Zhang, D. Associations of dietary vitamin B1, vitamin B2, vitamin B6, and vitamin B12 with the risk of depression: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The potential use of folate and its derivatives in treating psychiatric disorders: A systematic review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- Smaga, I.; Frankowska, M.; Filip, M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 2021, 178, 2569–2594. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Esposito, C.; El-Seedi, H.R.; Khalifa, S.A.M.; Baldi, A.; Greco, A.; Santonastaso, S.; Cioffi, V.; Sperandeo, R.; et al. Efficacy of a food supplement based on S-adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild-to-moderate depression: A monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2022, 156, 113930. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, Y.; Zhang, S.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Sun, S.; Wang, X.; Zhou, M.; et al. Associations between different types and sources of dietary fibre intake and depressive symptoms in a general population of adults: A cross-sectional study. Br. J. Nutr. 2021, 125, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
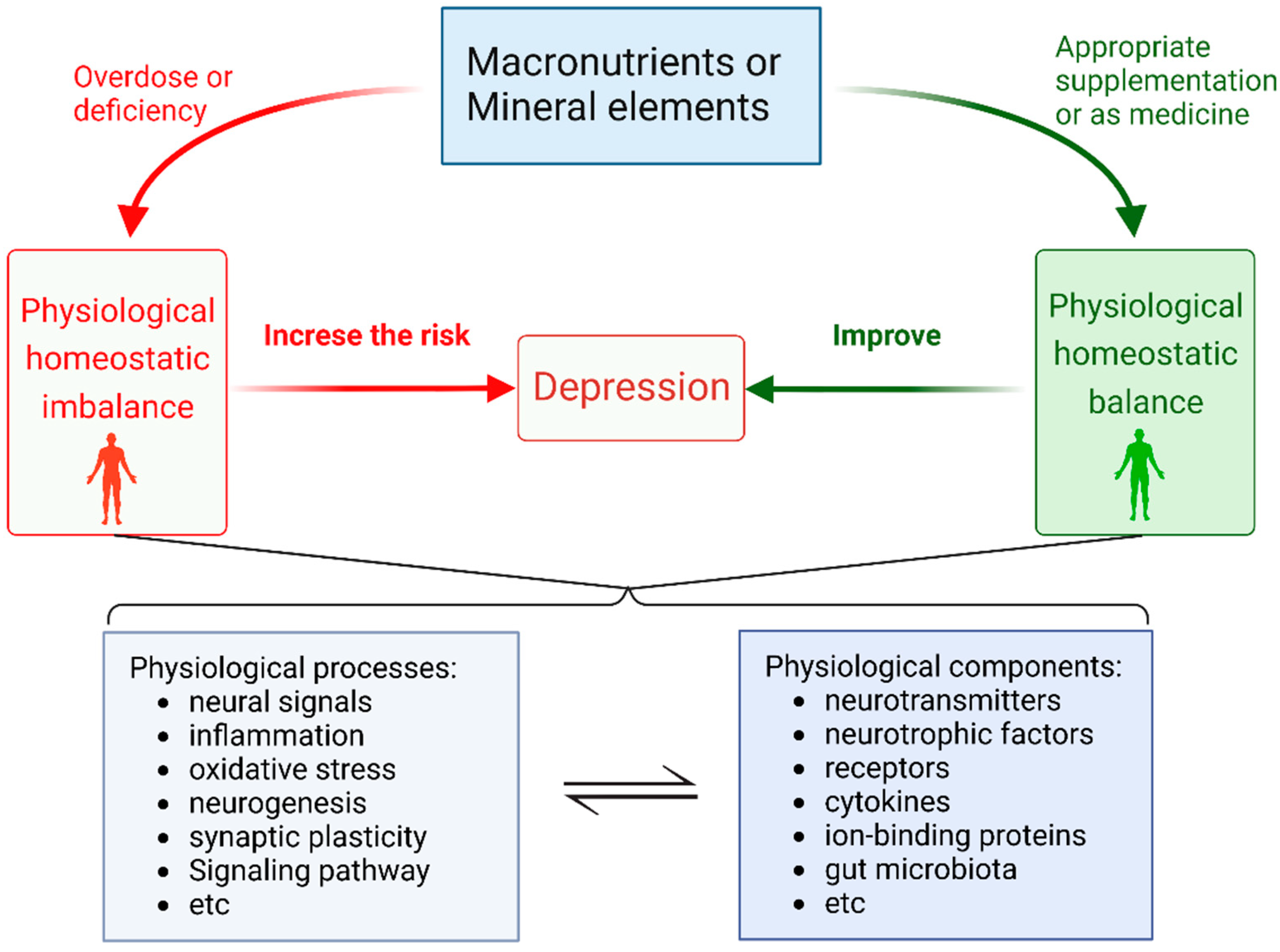
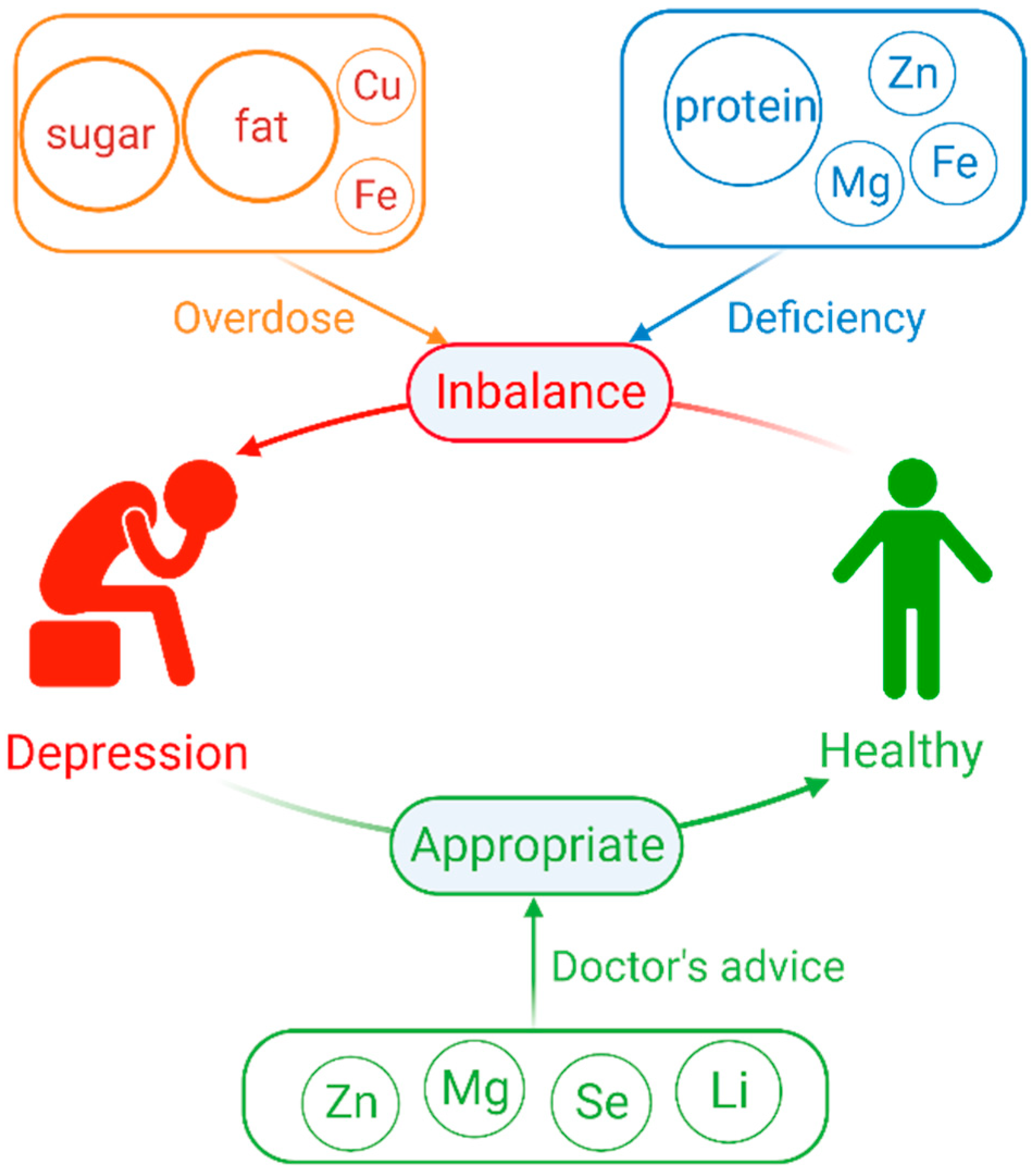
| Category | How to Increase the Risk of Depression | Serum or Blood Levels in Healthy Subjects | Serum or Blood Levels in Major Depressed Patients | Physiological Processes and Physiological Components | |
|---|---|---|---|---|---|
| Macronutrients | Dietary sugars | Overdose | FBG: 4.52 ± 0.43 mmol/L | FBG:4.73 ± 0.45 mmol/L ** [21] | 1. Neural signals: 5-HT↓ [22] |
| 2. Inflammation: pro-inflammatory factors such as IL-6, TNF-α, etc. ↑ [24]; gut microbiota [27] | |||||
| 3. Synaptic plasticity: synapsin I and BDNF↓ [32]; Dendrite spines and dendritic branches↓ [33] | |||||
| Dietary fat | Overdose | TG: 1.08 [0.76–1.54] g/L | TG: 0.84 [0.63–1.32] g/L * [39] | 1. Neural signals: 5-HT↓ [45]; 5-HT reabsorption↓ [46]; Intestinal 5-HT↑ [47]; GlutA2 and GAD65↓ [48]; desensitization of GABAergic AgRP neuron [49]; GLT-1↓ [50] | |
| 2. Inflammation: pro-inflammatory factors such as IL-6, IL-1, TNF-α, etc. ↑ [45,46,51,52] | |||||
| 3. Oxidative stress: TABRS, CAT, GPX↑ [51] | |||||
| HDL-C: 1.24 ± 0.30 mmol/L | HDL-C: 1.31 ± 0.32 mmol/L ** [21] | 4. Synaptic plasticity: synapsin I and BDNF↓ [32]; βIII-tubulin, PSD-95, SNAP-25, and Neurotrophin-3↓ [48] | |||
| 5. Signaling pathway: Akt/GSK3β↓ [53]; cAMP/PKA↓ [54]; AMPK↓ [55]. | |||||
| 6. Other related receptor proteins: LepRb↓ [58], CNR1↓ [61] | |||||
| Dietary protein | Deficiency | TP: 68.72 ± 5.23 g/L | TP: 66.72 ± 5.10 g/L ** [21] | May be related to synthesis of 5-HT and dopamine [71,72] | |
| Mineral elements | Zinc | Deficiency | 0.96 ± 0.11 mg/L | 0.72 ± 0.08 mg/L ** [83] | 1. ZnT3↓ [89,91]; ZnT3 knockout induced decreased hippocampal neurogenesis [92] |
| 2. GPR39 knockout [95]; GPR39 knockout induced decreased CREB and BDNF expression [96] | |||||
| 3. Oxidation/inflammation parameters: IL-1 and TBARS↑ [98] | |||||
| 4. Neural signals: NMDAR(GluN2A, GluN2B) ↑ [100,111] | |||||
| Magnesium | Deficiency | 1.64 ± 0. 15 mg/L | 1.10 ± 0.11 mg/L ** [83] | 1. Gut microbiota [107] | |
| 2. Neural signals: GluN1↓ [109] | |||||
| 3. Oxidative stress: DDAH1, MnSOD, and GDH1↑ [110]; GPX↑ [111] | |||||
| Copper | Overdose | 1.12 ± 0.13 mg/L | 1.55 ± 0.12 mg/L ** [83] | 1. Inflammation↑ [116] | |
| 2. Oxidative stress: SOD and CAT↑ [117] | |||||
| 3. Synaptic plasticity: GluN2B, PSD95↓ [118] | |||||
| Iron | Deficiency/overdose | 1.30 ± 0.03 mg/L | 1.02 ± 0.02 mg/L * [84] | May be related to BDNF↓ [129,130] and oxidative stress↑ [133] | |
| Category | Daily Dose and Course of Treatment in Depressed Patients in Clinical Trials | Physiological Processes and Physiological Components | ||
|---|---|---|---|---|
| Mineral elements | Proper supplementation | Selenium | — | 1. Anti-oxidative stress [144,145] |
| 2. Anti-inflammatory: pro-inflammatory factors IL-1↓ [146] | ||||
| Zinc | 25~220 mg for 8 to 12 weeks [147,148,149] | 1. Anti-inflammatory: IFN-γ↓ [99] | ||
| 2. BDNF↑ [151] | ||||
| Magnesium | 248~500 mg for 6 to 8 weeks [152,153] | 1. 5-HT↑ [155] | ||
| 2. Anti-inflammation: TNF-α and IL6↓ [156] | ||||
| 3. Glutamate signaling↑ [157] | ||||
| Psychotropic medication | 1. Hippocampal neurogenesis↑ [171] | |||
| Lithium | 600~1200 mg for 1 to 6 weeks [167] | 2. BDNF↑ [172,173] | ||
| 3. Protects the blood–brain barrier [175] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, Z.; Li, H.; Quan, Z.; Qing, H. Appropriate Macronutrients or Mineral Elements Are Beneficial to Improve Depression and Reduce the Risk of Depression. Int. J. Mol. Sci. 2023, 24, 7098. https://doi.org/10.3390/ijms24087098
Quan Z, Li H, Quan Z, Qing H. Appropriate Macronutrients or Mineral Elements Are Beneficial to Improve Depression and Reduce the Risk of Depression. International Journal of Molecular Sciences. 2023; 24(8):7098. https://doi.org/10.3390/ijms24087098
Chicago/Turabian StyleQuan, Zhengyang, Hui Li, Zhenzhen Quan, and Hong Qing. 2023. "Appropriate Macronutrients or Mineral Elements Are Beneficial to Improve Depression and Reduce the Risk of Depression" International Journal of Molecular Sciences 24, no. 8: 7098. https://doi.org/10.3390/ijms24087098
APA StyleQuan, Z., Li, H., Quan, Z., & Qing, H. (2023). Appropriate Macronutrients or Mineral Elements Are Beneficial to Improve Depression and Reduce the Risk of Depression. International Journal of Molecular Sciences, 24(8), 7098. https://doi.org/10.3390/ijms24087098






