3D-Cultured Adipose-Derived Stem Cell Spheres Using Calcium-Alginate Scaffolds for Osteoarthritis Treatment in a Mono-Iodoacetate-Induced Rat Model
Abstract
1. Introduction
2. Results
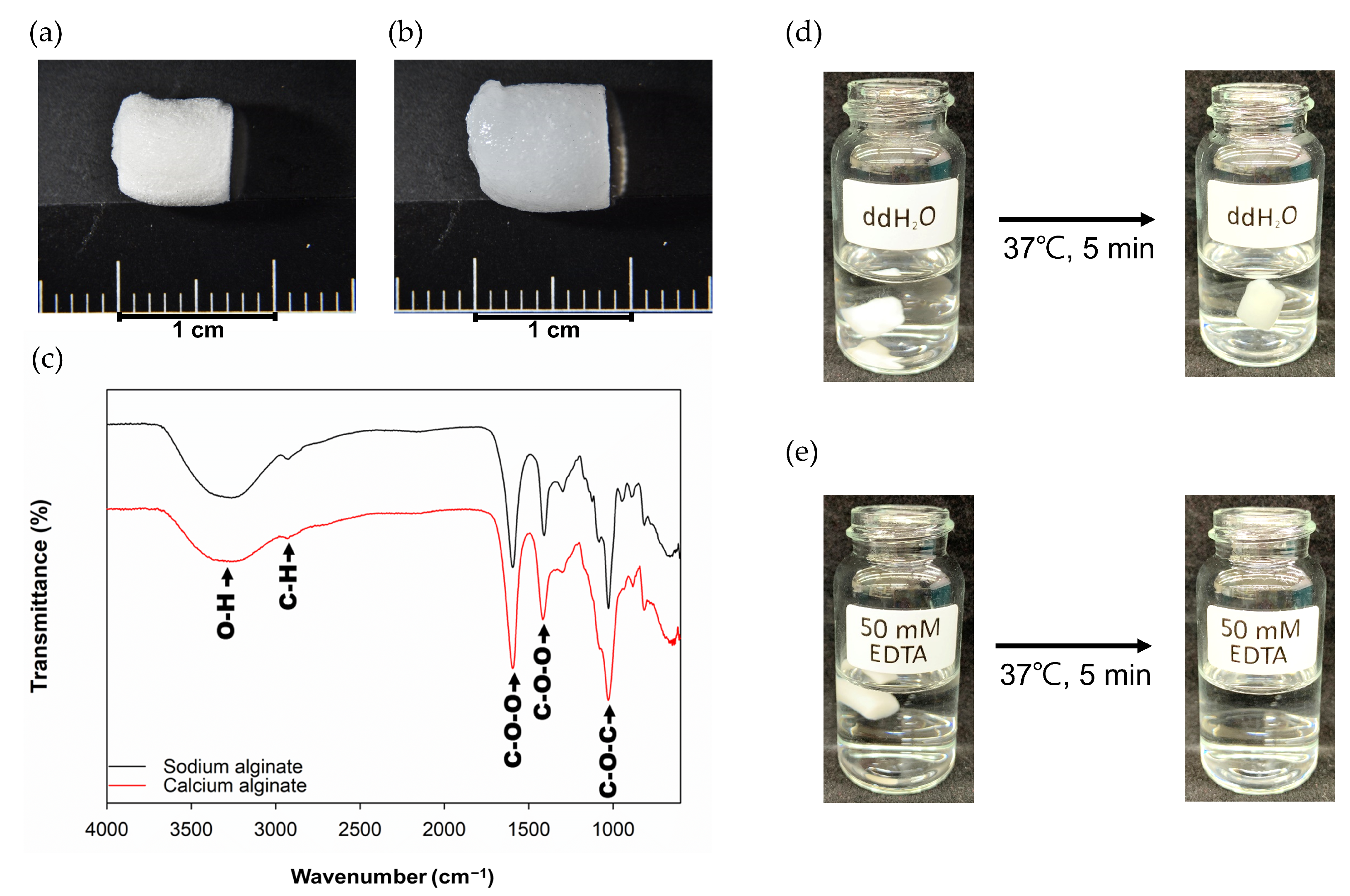
2.1. Characterization of Ca-Ag Scaffolds
2.2. Seeding Efficiency and Cell Proliferative Quantification
2.3. Gait Analysis
2.4. H&E Stain
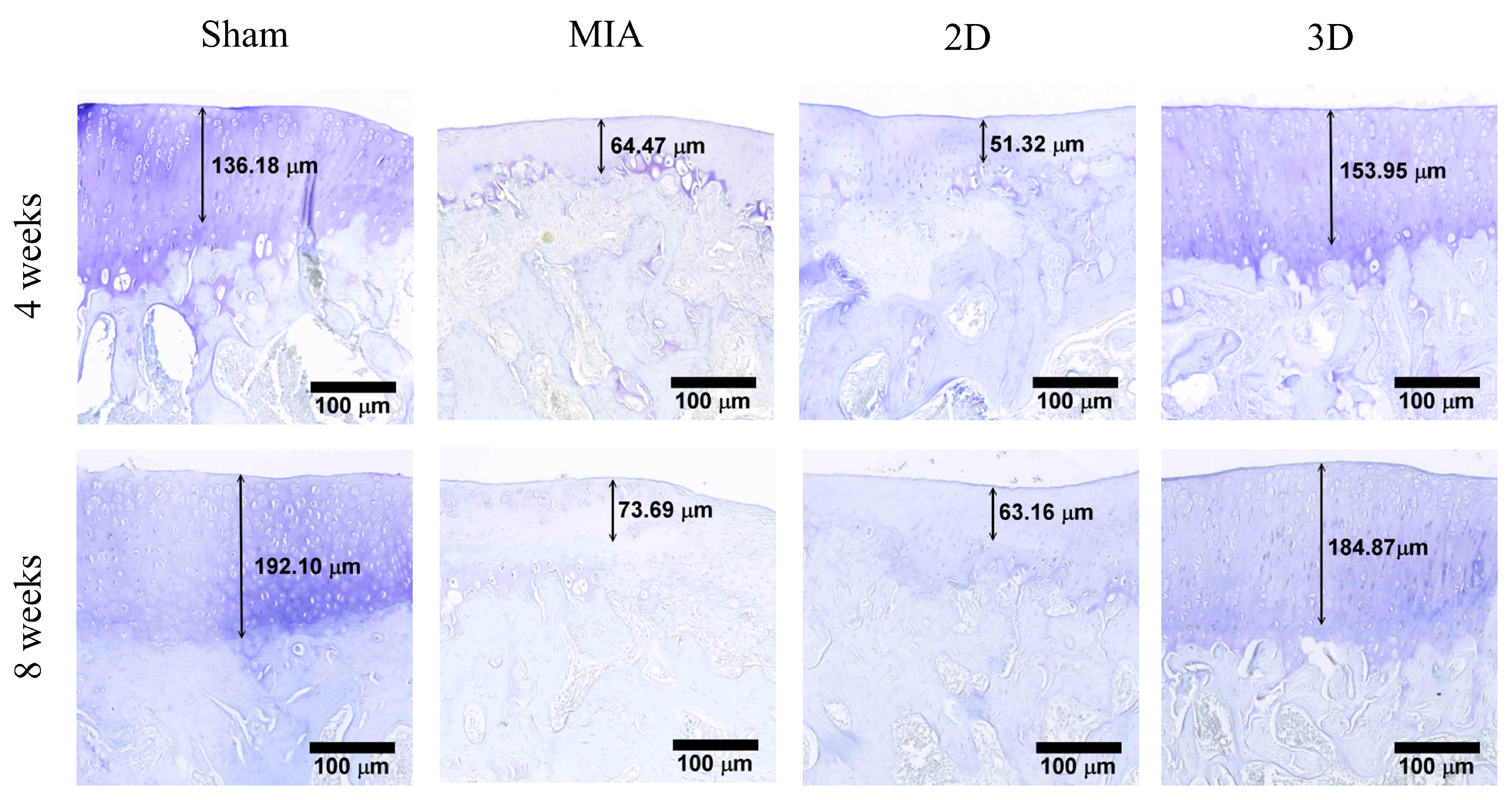
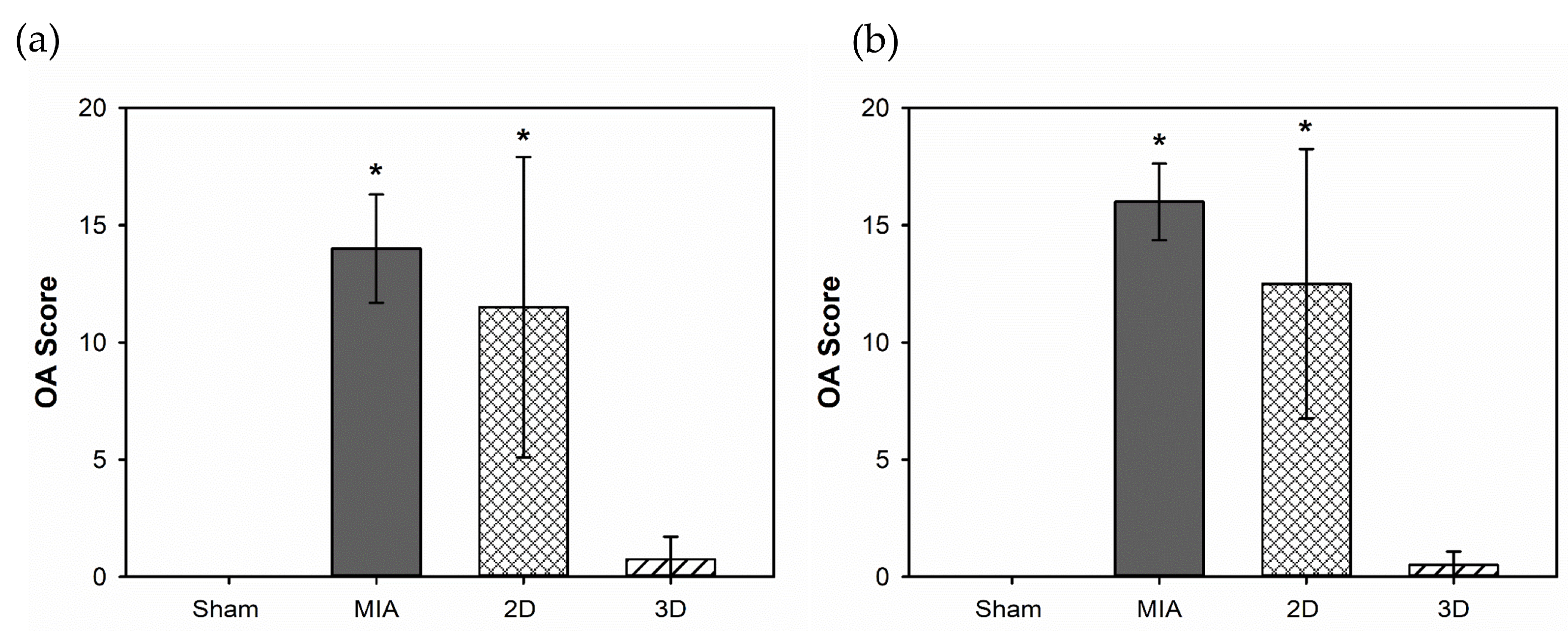
2.5. Toluidine Blue Stain and OA Score
2.6. Serological and Blood Elements Analysis
3. Discussion
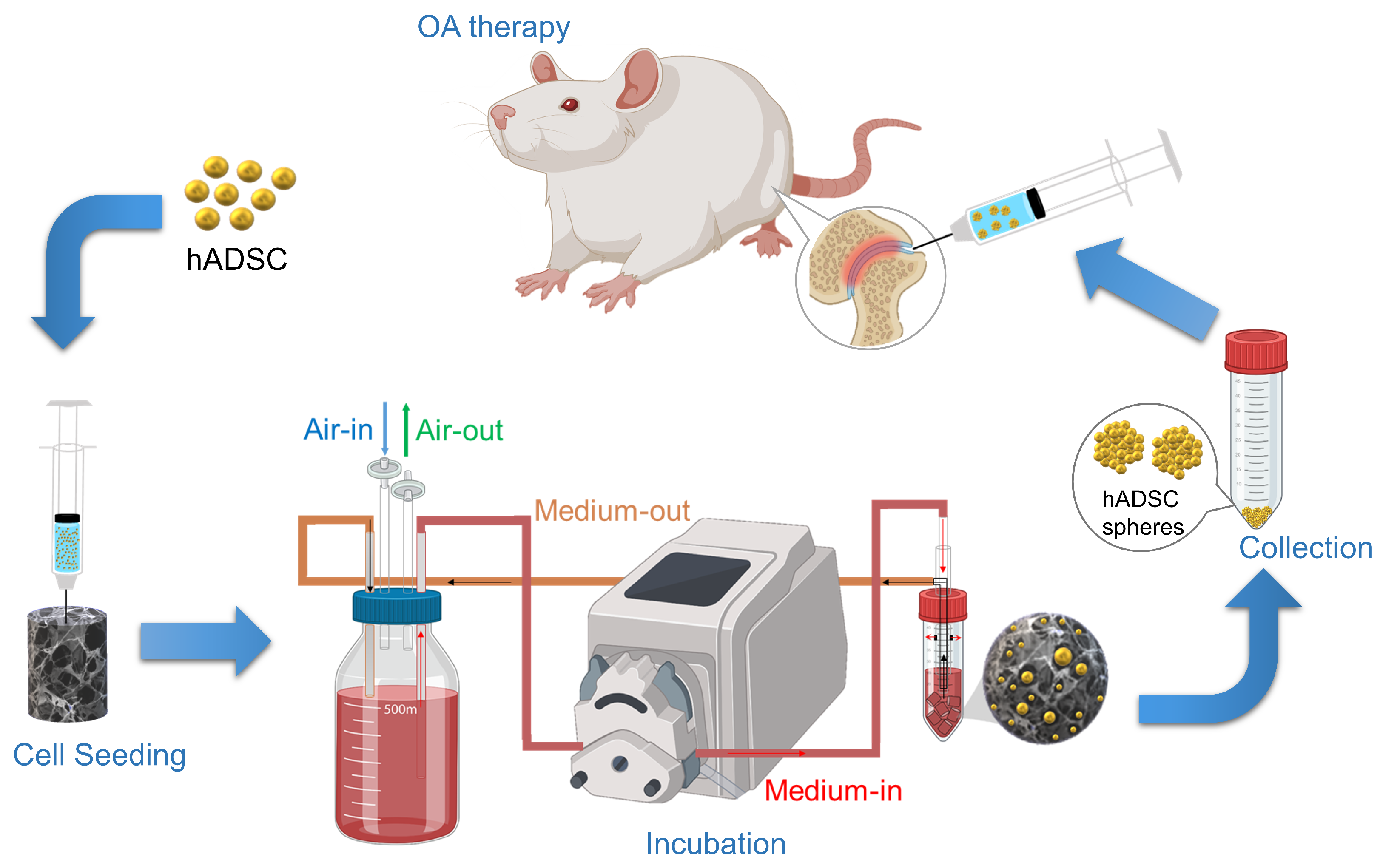
4. Materials and Methods
4.1. Materials
4.2. Preparation of Ca-Ag Scaffolds
4.3. Seeding of hADSCs on Ca-Ag Scaffolds
4.4. Seeding Efficiency and Cell Proliferative Quantification
4.5. SEM Analysis
4.6. Animal Study
4.6.1. Rat OA Model
4.6.2. Serological and Blood Elements Analysis
4.6.3. Histological Staining
4.7. Statistic Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal stem cells in osteoarthritis therapy: A review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar] [PubMed]
- Castaneda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol. 2012, 83, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Cimmino, M.A.; Scarpa, R.; Caporali, R.; Parazzini, F.; Zaninelli, A.; Atzeni, F.; Canesi, B. Osteoarthritis: An overview of the disease and its treatment strategies. Semin. Arthritis Rheum. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Soleimani, H.A.; Mohammadpour, F.; Hadizadeh, F. Synthetic Zeolites as Controlled-Release Delivery Systems for Anti-Inflammatory Drugs. Chem. Biol. Drug. Des. 2016, 87, 849–857. [Google Scholar] [CrossRef]
- Smith, C.; Patel, R.; Vannabouathong, C.; Sales, B.; Rabinovich, A.; McCormack, R.; Belzile, E.L.; Bhandari, M. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1974–1983. [Google Scholar] [CrossRef]
- Pavone, V.; Vescio, A.; Turchetta, M.; Giardina, S.M.C.; Culmone, A.; Testa, G. Injection-Based Management of Osteoarthritis of the Knee: A Systematic Review of Guidelines. Front. Pharmacol. 2021, 12, 661805. [Google Scholar] [CrossRef]
- Sucuoglu, H.; Ustunsoy, S. The short-term effect of PRP on chronic pain in knee osteoarthritis. Agri 2019, 31, 63–69. [Google Scholar] [CrossRef]
- Kraus, V.B.; Conaghan, P.G.; Aazami, H.A.; Mehra, P.; Kivitz, A.J.; Lufkin, J.; Hauben, J.; Johnson, J.R.; Bodick, N. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthr. Cartil. 2018, 26, 34–42. [Google Scholar] [CrossRef]
- Colen, S.; Haverkamp, D.; Mulier, M.; van den Bekerom, M.P. Hyaluronic acid for the treatment of osteoarthritis in all joints except the knee: What is the current evidence? BioDrugs 2012, 26, 101–112. [Google Scholar] [CrossRef]
- Andia, I.; Abate, M. Knee osteoarthritis: Hyaluronic acid, platelet-rich plasma or both in association? Expert. Opin. Biol. Ther. 2014, 14, 635–649. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Lin, Y.-Y.; Sadhasivam, S.; Kuan, C.-Y.; Chi, C.-Y.; Dong, G.-C.; Lin, F.-H. Efficacy of Bletilla striata polysaccharide on hydrogen peroxide-induced apoptosis of osteoarthritic chondrocytes. J. Polym. Res. 2018, 25, 49. [Google Scholar] [CrossRef]
- Park, I.S.; Choi, Y.J.; Kim, H.S.; Park, S.H.; Choi, B.H.; Kim, J.H.; Song, B.R.; Min, B.H. Development of three-dimensional articular cartilage construct using silica nano-patterned substrate. PLoS ONE 2019, 14, e0208291. [Google Scholar] [CrossRef]
- Brindo da Cruz, I.C.; Velosa, A.P.P.; Carrasco, S.; Dos Santos Filho, A.; Tomaz de Miranda, J.; Pompeu, E.; Fernandes, T.L.; Bueno, D.F.; Fanelli, C.; Goldenstein-Schainberg, C.; et al. Post-Adipose-Derived Stem Cells (ADSC) Stimulated by Collagen Type V (Col V) Mitigate the Progression of Osteoarthritic Rabbit Articular Cartilage. Front. Cell. Dev. Biol. 2021, 9, 606890. [Google Scholar] [CrossRef]
- Takagi, T.; Kabata, T.; Hayashi, K.; Fang, X.; Kajino, Y.; Inoue, D.; Ohmori, T.; Ueno, T.; Yoshitani, J.; Ueoka, K.; et al. Periodic injections of adipose-derived stem cell sheets attenuate osteoarthritis progression in an experimental rabbit model. BMC Musculoskelet. Disord. 2020, 21, 691. [Google Scholar] [CrossRef]
- Chen, C.Y.; Ke, C.J.; Yen, K.C.; Hsieh, H.C.; Sun, J.S.; Lin, F.H. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef]
- Joseph, J.S.; Malindisa, S.T.; Ntwasa, M. Two-dimensional (2D) and three-dimensional (3D) cell culturing in drug discovery. Cell. Culture 2018, 2, 1–22. [Google Scholar]
- Rijal, G.; Li, W. Native-mimicking in vitro microenvironment: An elusive and seductive future for tumor modeling and tissue engineering. J. Biol. Eng. 2018, 12, 20. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.B.; Rosa, G.D.S.; Rossi, M.C.; Stievani, F.C.; Pfeifer, J.P.H.; Krieck, A.M.T.; Bovolato, A.L.C.; Fonseca-Alves, C.E.; Borras, V.A.; Alves, A.L.G. In Vitro Biological Performance of Alginate Hydrogel Capsules for Stem Cell Delivery. Front. Bioeng. Biotechnol. 2021, 9, 674581. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, Y.; Chen, W.; Li, J.; Wang, X.; Chen, Y.; Yu, Y.; Shen, Z.; Zhang, Y. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J. Mater. Chem. B 2020, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Moller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef]
- Hao, Z.C.; Wang, S.Z.; Zhang, X.J.; Lu, J. Stem cell therapy: A promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell. Prolif. 2016, 49, 154–162. [Google Scholar] [CrossRef]
- Wang, L.T.; Liu, K.J.; Sytwu, H.K.; Yen, M.L.; Yen, B.L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 1288–1303. [Google Scholar] [CrossRef]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell. Regen. 2015, 4, 7. [Google Scholar] [CrossRef]
- Siregar, S.; Sasongko Noegroho, B.; Adriansjah, R.; Mustafa, A.; Wijayanti, Z. Intratesticular Human Adipose-Derived Stem Cell (hADSC) Transplantation Decreased Oxidative Stress in Testicular Torsion Model of Wistar Rat. Res. Rep. Urol. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Thysen, S.; Luyten, F.P.; Lories, R.J. Targets, models and challenges in osteoarthritis research. Dis. Model. Mech. 2015, 8, 17–30. [Google Scholar] [CrossRef]
- van der Kraan, P.M. Factors that influence outcome in experimental osteoarthritis. Osteoarthr. Cartil. 2017, 25, 369–375. [Google Scholar] [CrossRef]
- Pitcher, T.; Sousa-Valente, J.; Malcangio, M. The Monoiodoacetate Model of Osteoarthritis Pain in the Mouse. J. Vis. Exp. 2016, 111, e53746. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Csaszar, E.; Dulgar-Tulloch, A. Commercial Scale Manufacturing of Allogeneic Cell Therapy. Front. Med. 2018, 5, 233. [Google Scholar] [CrossRef]
- Nath, S.C.; Harper, L.; Rancourt, D.E. Cell-Based Therapy Manufacturing in Stirred Suspension Bioreactor: Thoughts for cGMP Compliance. Front. Bioeng. Biotechnol. 2020, 8, 599674. [Google Scholar] [CrossRef]
- Gandaglia, A.; Bagno, A.; Naso, F.; Spina, M.; Gerosa, G. Cells, scaffolds and bioreactors for tissue-engineered heart valves: A journey from basic concepts to contemporary developmental innovations. Eur. J. Cardiothorac. Surg. 2011, 39, 523–531. [Google Scholar] [CrossRef]
- Kronemberger, G.S.; Matsui, R.A.M.; Miranda, G.; Granjeiro, J.M.; Baptista, L.S. Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J. Stem Cells 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]



| Item | Unit | Sham | MIA | 2D | 3D |
|---|---|---|---|---|---|
| RBC | 106/μL | 7.67 ± 0.29 | 7.73 ± 0.88 | 7.63 ± 0.51 | 7.63 ± 0.38 |
| WBC | 103/μL | 9.08 ± 0.93 | 9.86 ± 3.39 | 9.92 ± 1.92 | 10.18 ± 0.96 |
| PLT | 103/μL | 866.50 ± 209.56 | 819.50 ± 454.21 | 906.75 ± 391.95 | 911.67 ± 198.61 |
| Monocyte | 103/μL | 0.46 ± 0.10 | 0.41 ± 0.14 | 0.45 ± 0.10 | 0.49 ± 0.07 |
| Lymphocyte | 103/μL | 6.53 ± 0.65 | 6.61 ± 3.03 | 5.96 ±2.04 | 7.15 ± 0.47 |
| GOT | U/L | 88.33 ± 9.29 | 110.00 ± 1.41 * | 108.50 ± 2.12 * | 87.50 ± 7.78 |
| GPT | U/L | 28.75 ± 2.22 | 35.75 ± 5.74 | 34.00 ± 3.46 | 30.00 ± 4.40 |
| ALP | U/L | 1127.50 ± 428.25 | 1085.00 ± 268.52 | 1082.75 ± 224.38 | 1055.00 ± 344.34 |
| BUN | mg/dL | 17.75 ± 2.00 | 19.80 ± 1.42 | 19.75 ± 1.42 | 18.65 ± 2.51 |
| CRE | mg/dL | 0.34 ± 0.04 | 0.28 ± 0.02 | 0.25 ± 0.03 * | 0.28 ± 0.05 |
| Item | Unit | Sham | MIA | 2D | 3D |
|---|---|---|---|---|---|
| RBC | 106/μL | 8.11 ± 0.54 | 7.98 ± 0.59 | 7.99 ± 0.22 | 8.32 ± 0.44 |
| WBC | 103/μL | 6.36 ± 1.50 | 6.76 ± 0.88 | 6.65 ± 0.49 | 6.54 ± 0.75 |
| PLT | 103/μL | 943.50 ± 67.18 | 939.33 ± 273.35 | 937.33 ± 290.18 | 868.50 ± 62.66 |
| Monocyte | 103/μL | 0.41 ± 0.09 | 0.32 ± 0.09 | 0.37 ± 0.11 | 0.39 ± 0.05 |
| Lymphocyte | 103/μL | 4.48 ± 0.86 | 4.03 ± 0.66 | 4.17 ± 0.52 | 4.19 ± 0.46 |
| GOT | U/L | 71.25 ± 3.40 | 67.50 ± 21.02 | 81.50 ± 19.84 | 83.50 ± 6.36 |
| GPT | U/L | 27.70 ± 2.38 | 27.75 ± 6.29 | 27.50 ± 5.92 | 32.50 ± 1.00 |
| ALP | U/L | 890.50 ± 53.03 | 850.67 ± 154.57 | 877.00 ± 150.79 | 928.00 ± 64.63 |
| BUN | mg/dL | 18.40 ± 1.14 | 19.33 ± 3.44 | 19.27 ± 4.10 | 18.20 ± 0.84 |
| CRE | mg/dL | 0.23 ± 0.02 | 0.28 ± 0.03 | 0.27 ± 0.04 | 0.26 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-Y.; Kuan, C.-Y.; Chang, C.-T.; Chuang, M.-H.; Syu, W.-S.; Zhang, K.-L.; Lee, C.-H.; Lin, P.-C.; Dong, G.-C.; Lin, F.-H. 3D-Cultured Adipose-Derived Stem Cell Spheres Using Calcium-Alginate Scaffolds for Osteoarthritis Treatment in a Mono-Iodoacetate-Induced Rat Model. Int. J. Mol. Sci. 2023, 24, 7062. https://doi.org/10.3390/ijms24087062
Lin Y-Y, Kuan C-Y, Chang C-T, Chuang M-H, Syu W-S, Zhang K-L, Lee C-H, Lin P-C, Dong G-C, Lin F-H. 3D-Cultured Adipose-Derived Stem Cell Spheres Using Calcium-Alginate Scaffolds for Osteoarthritis Treatment in a Mono-Iodoacetate-Induced Rat Model. International Journal of Molecular Sciences. 2023; 24(8):7062. https://doi.org/10.3390/ijms24087062
Chicago/Turabian StyleLin, Yu-Ying, Che-Yung Kuan, Chia-Tien Chang, Ming-Hsi Chuang, Wan-Sin Syu, Kai-Ling Zhang, Chia-Hsin Lee, Po-Cheng Lin, Guo-Chung Dong, and Feng-Huei Lin. 2023. "3D-Cultured Adipose-Derived Stem Cell Spheres Using Calcium-Alginate Scaffolds for Osteoarthritis Treatment in a Mono-Iodoacetate-Induced Rat Model" International Journal of Molecular Sciences 24, no. 8: 7062. https://doi.org/10.3390/ijms24087062
APA StyleLin, Y.-Y., Kuan, C.-Y., Chang, C.-T., Chuang, M.-H., Syu, W.-S., Zhang, K.-L., Lee, C.-H., Lin, P.-C., Dong, G.-C., & Lin, F.-H. (2023). 3D-Cultured Adipose-Derived Stem Cell Spheres Using Calcium-Alginate Scaffolds for Osteoarthritis Treatment in a Mono-Iodoacetate-Induced Rat Model. International Journal of Molecular Sciences, 24(8), 7062. https://doi.org/10.3390/ijms24087062







