CIGB-258 Exerts Potent Anti-Inflammatory Activity against Carboxymethyllysine-Induced Acute Inflammation in Hyperlipidemic Zebrafish via the Protection of Apolipoprotein A-I
Abstract
1. Introduction
2. Results
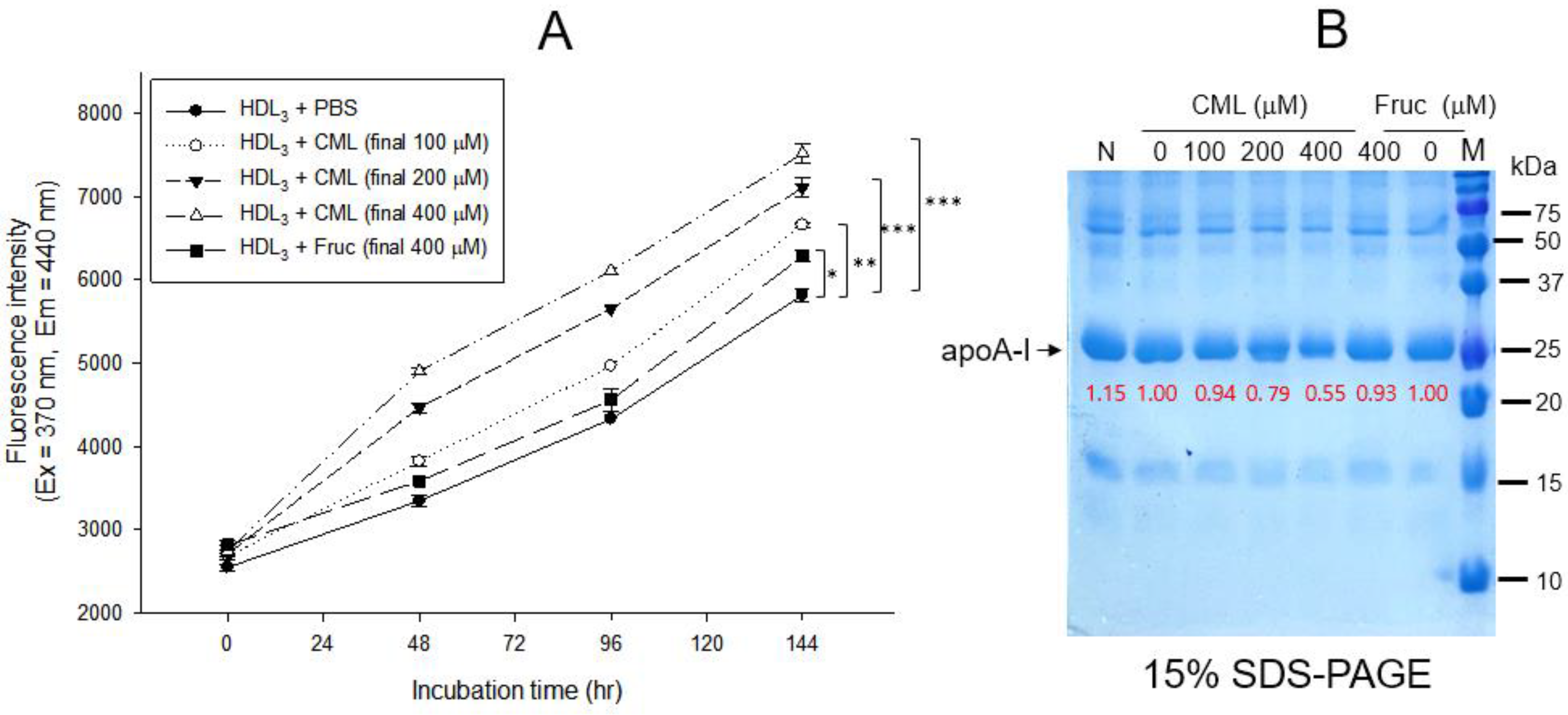
2.1. Glycation and Proteolytic Degradation of HDL by CML Treatment
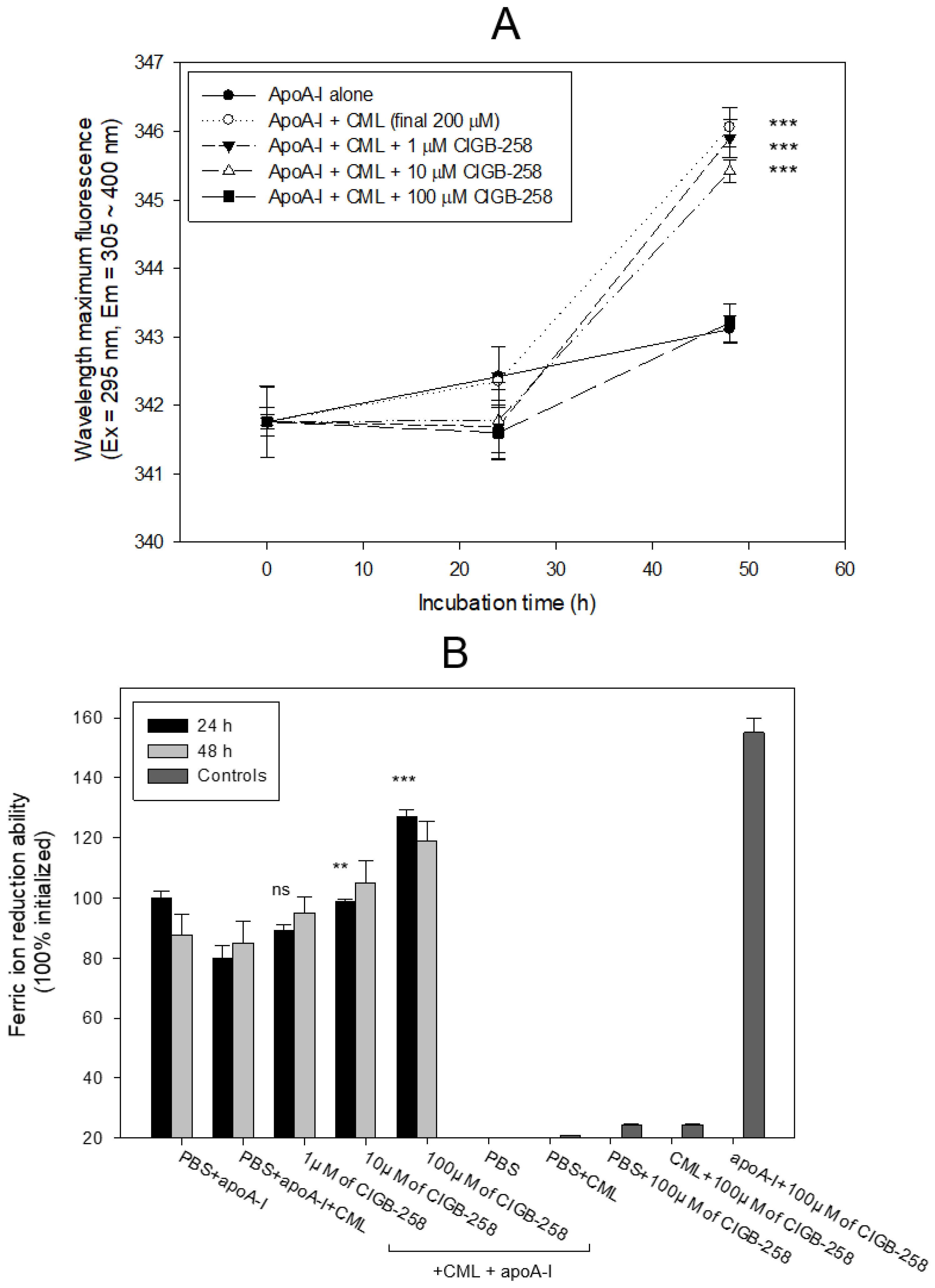
2.2. Anti-Glycation Activity of CIGB-258
2.3. Stabilization of apoA-I by CIGB-258
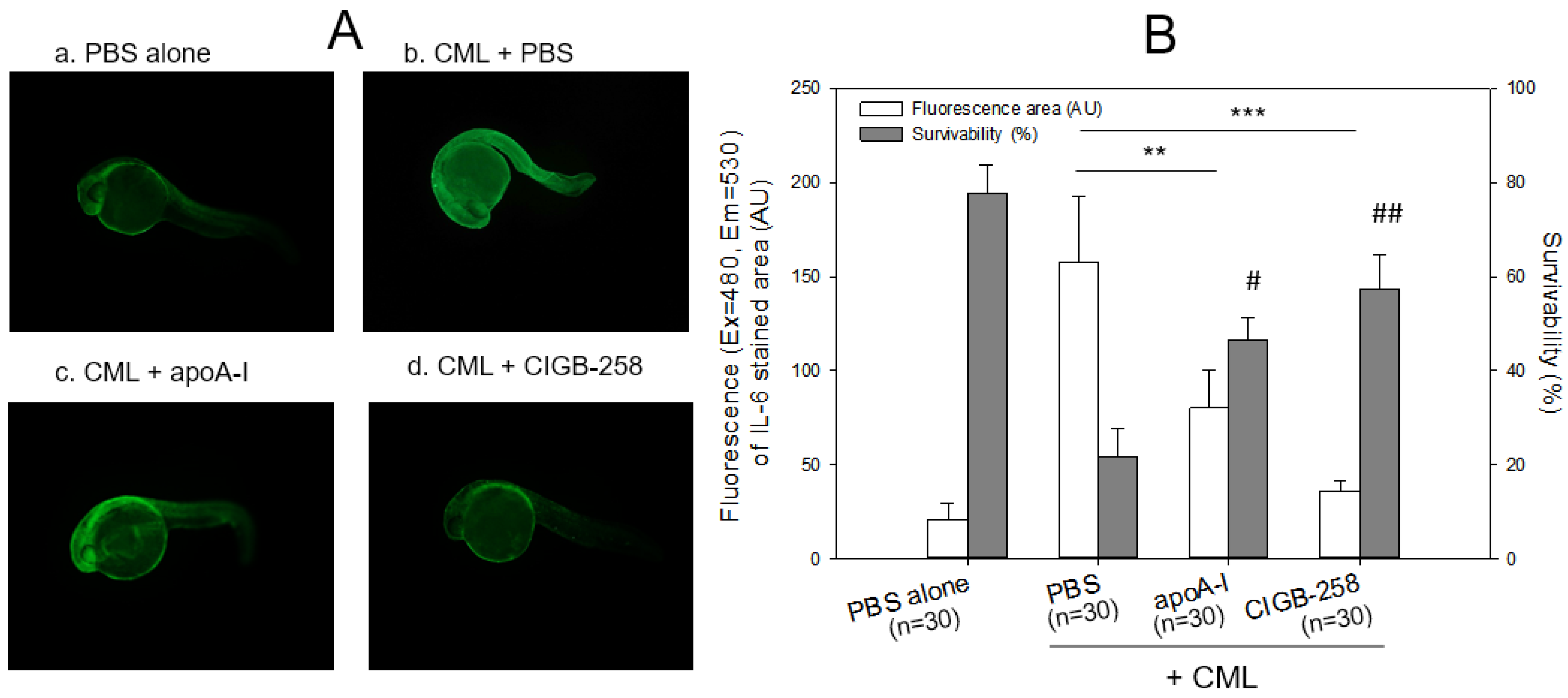
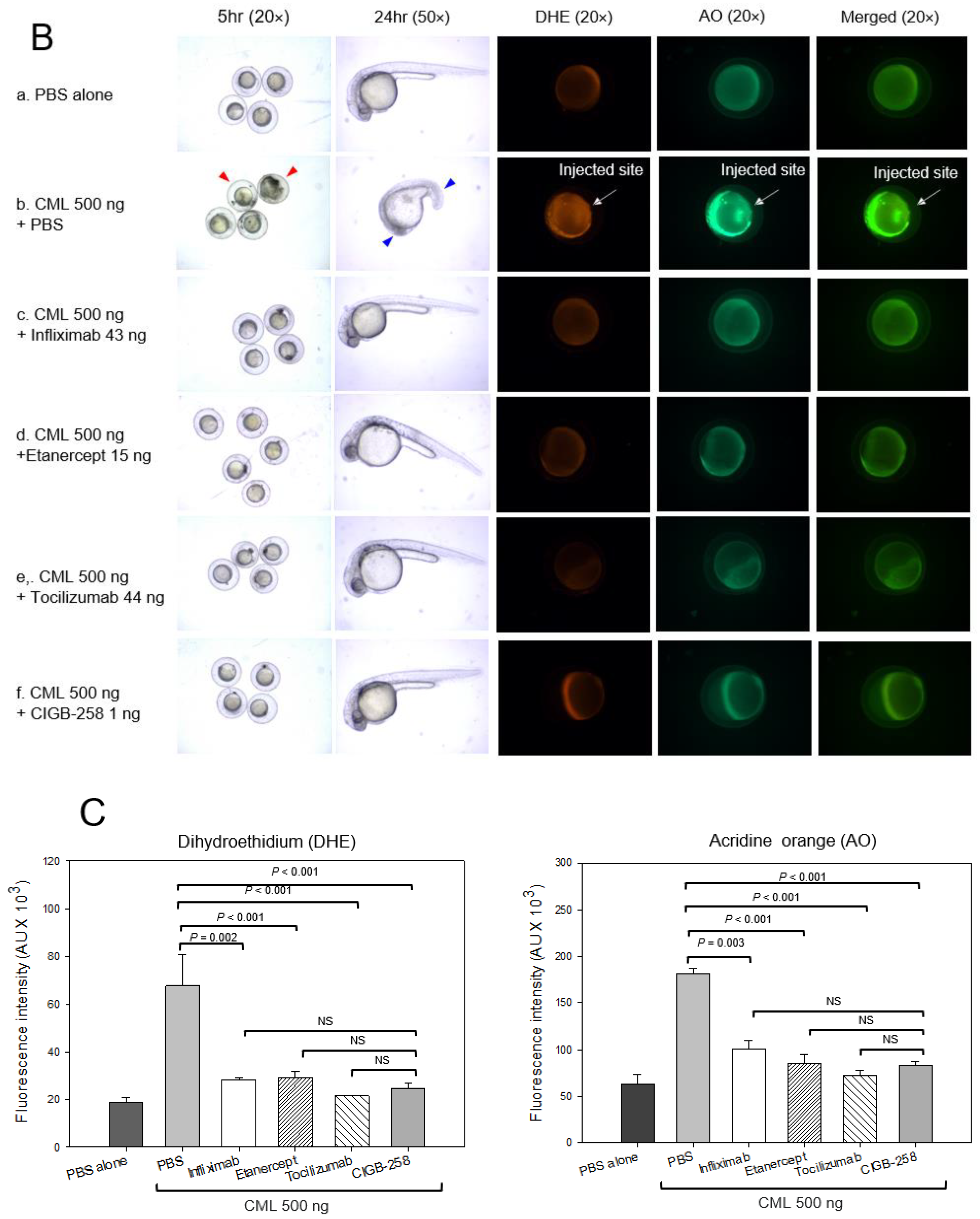
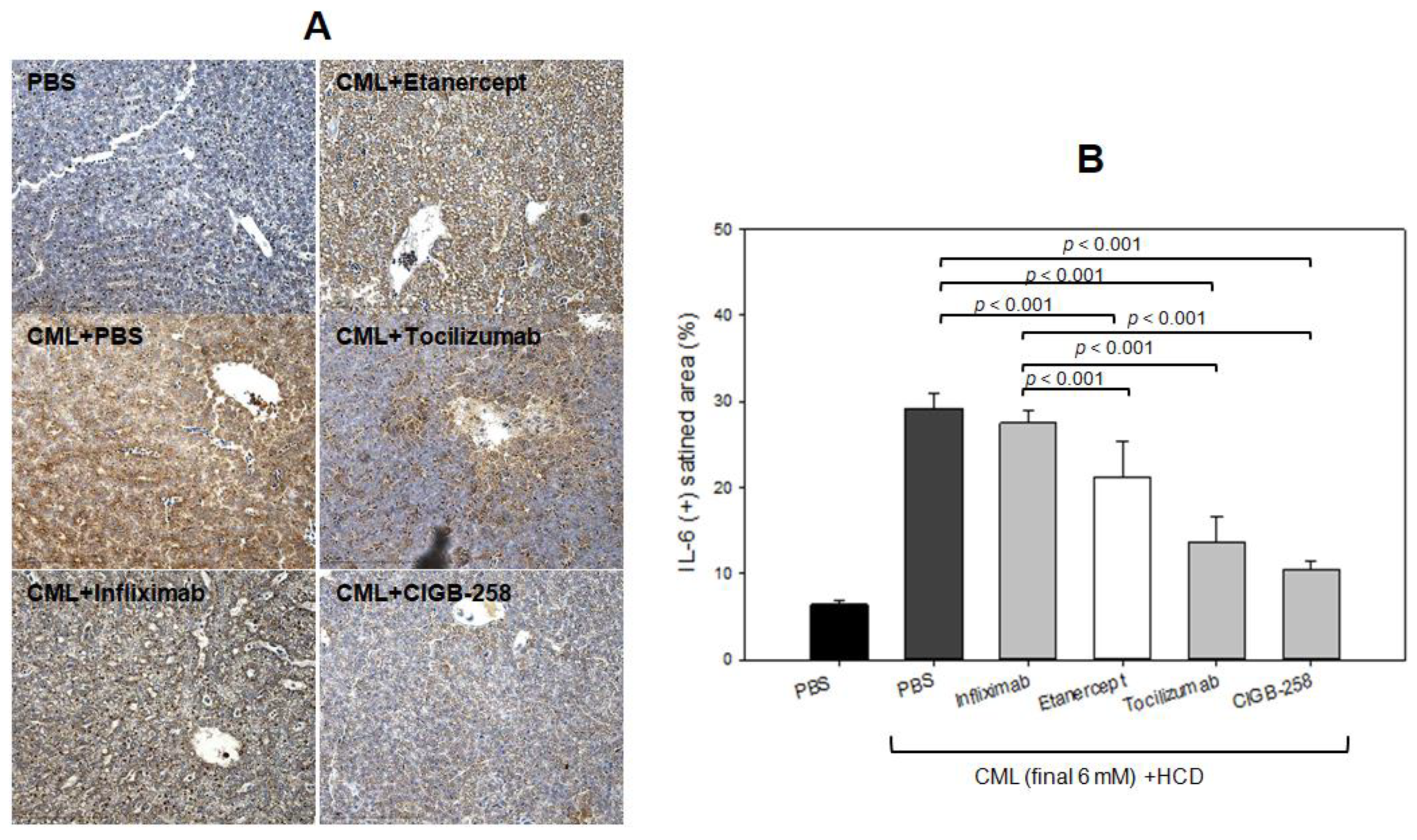
2.4. Production of IL-6 by Microinjection of CML
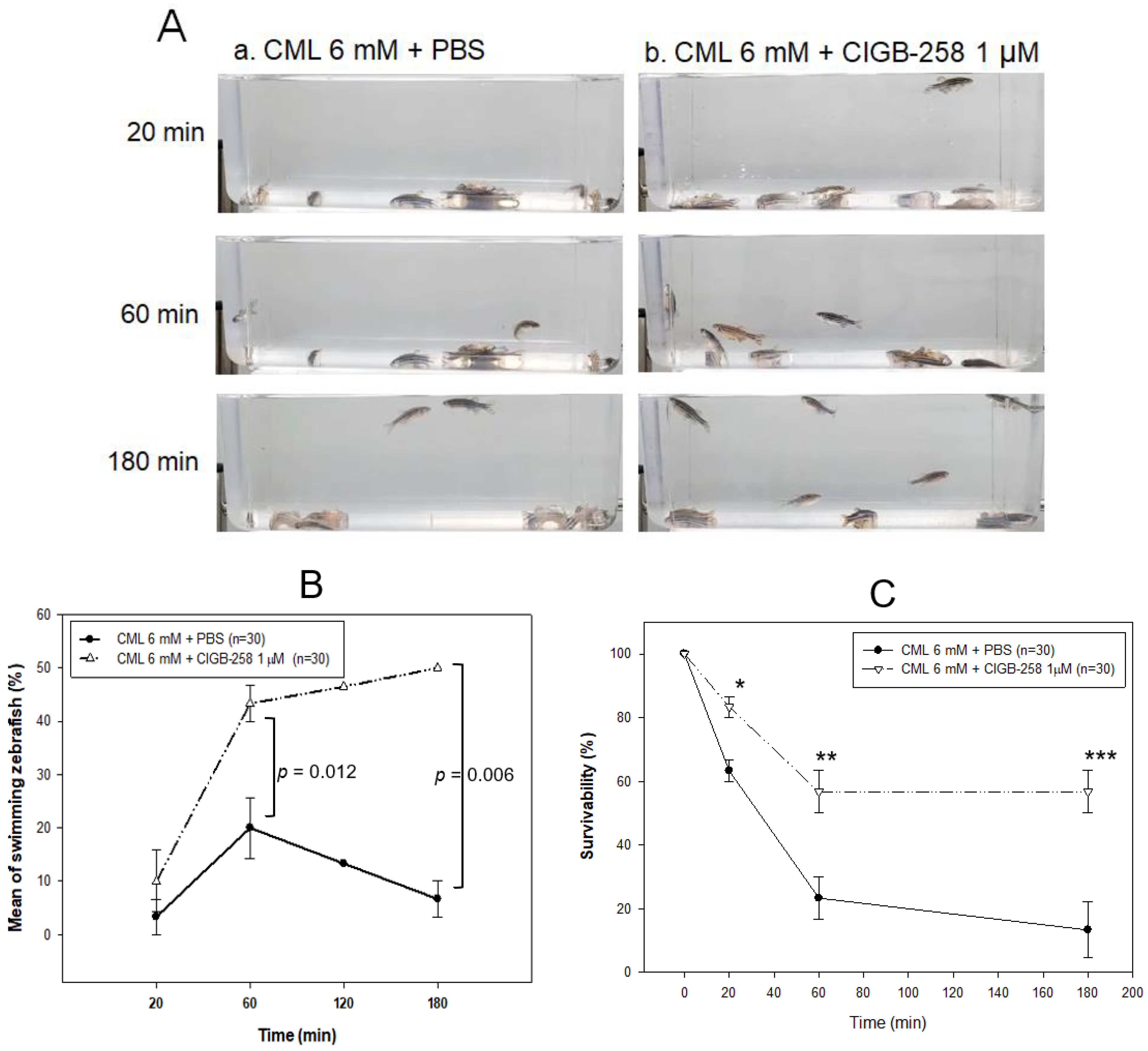
2.5. Embryo Development and Survivability
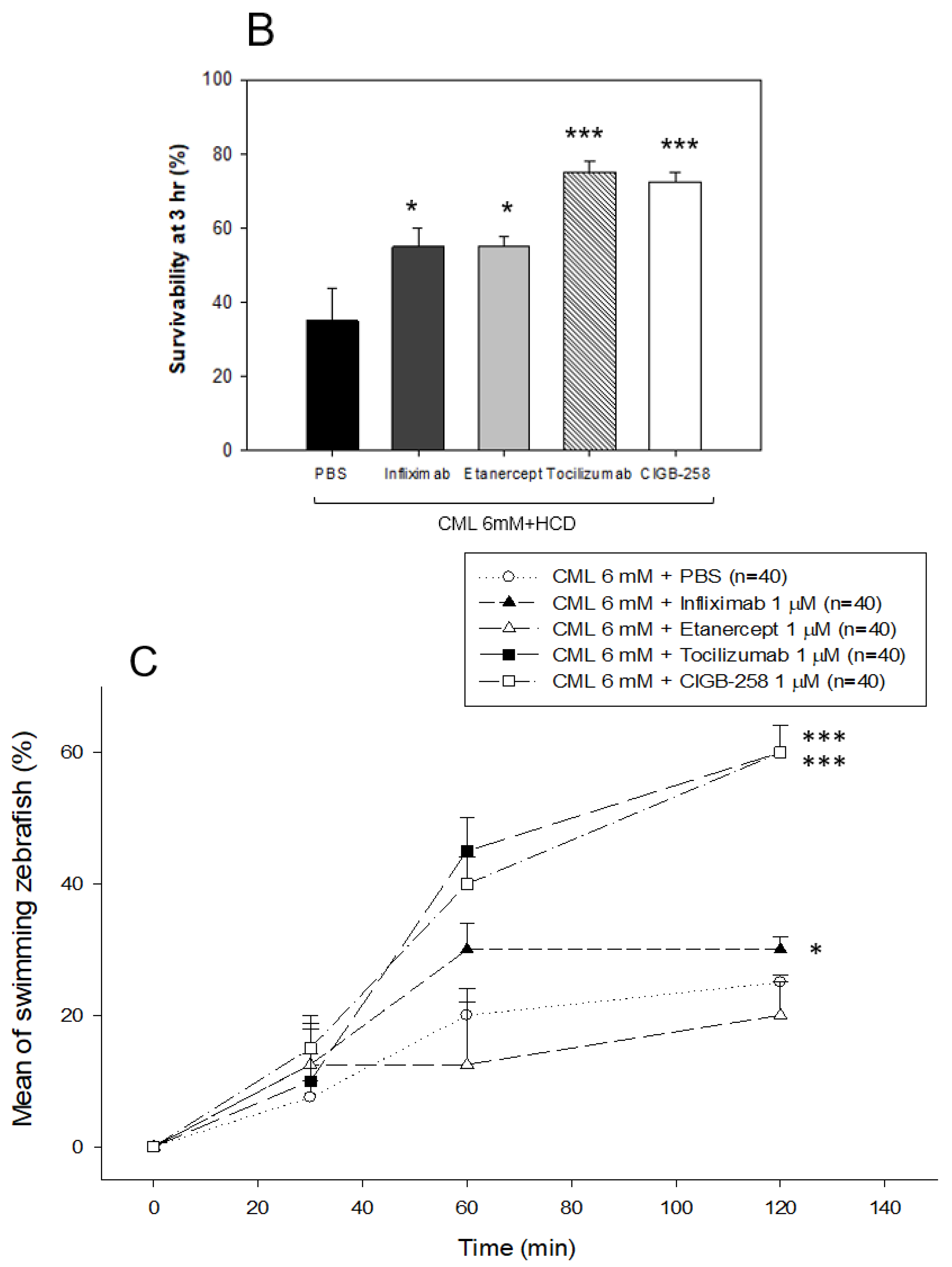
2.6. Recovery from Acute Paralysis of Hyperlipidemic Zebrafish
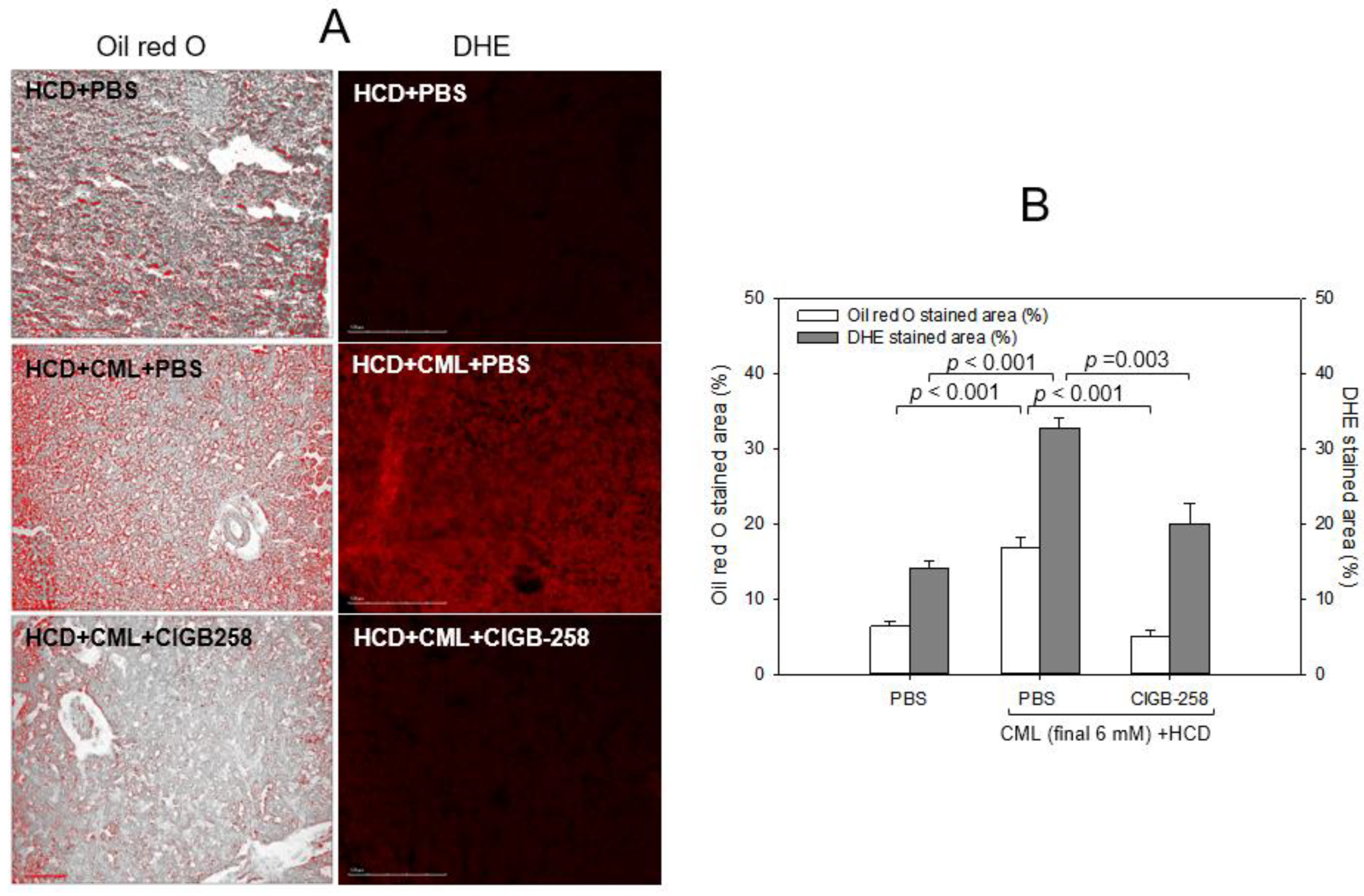
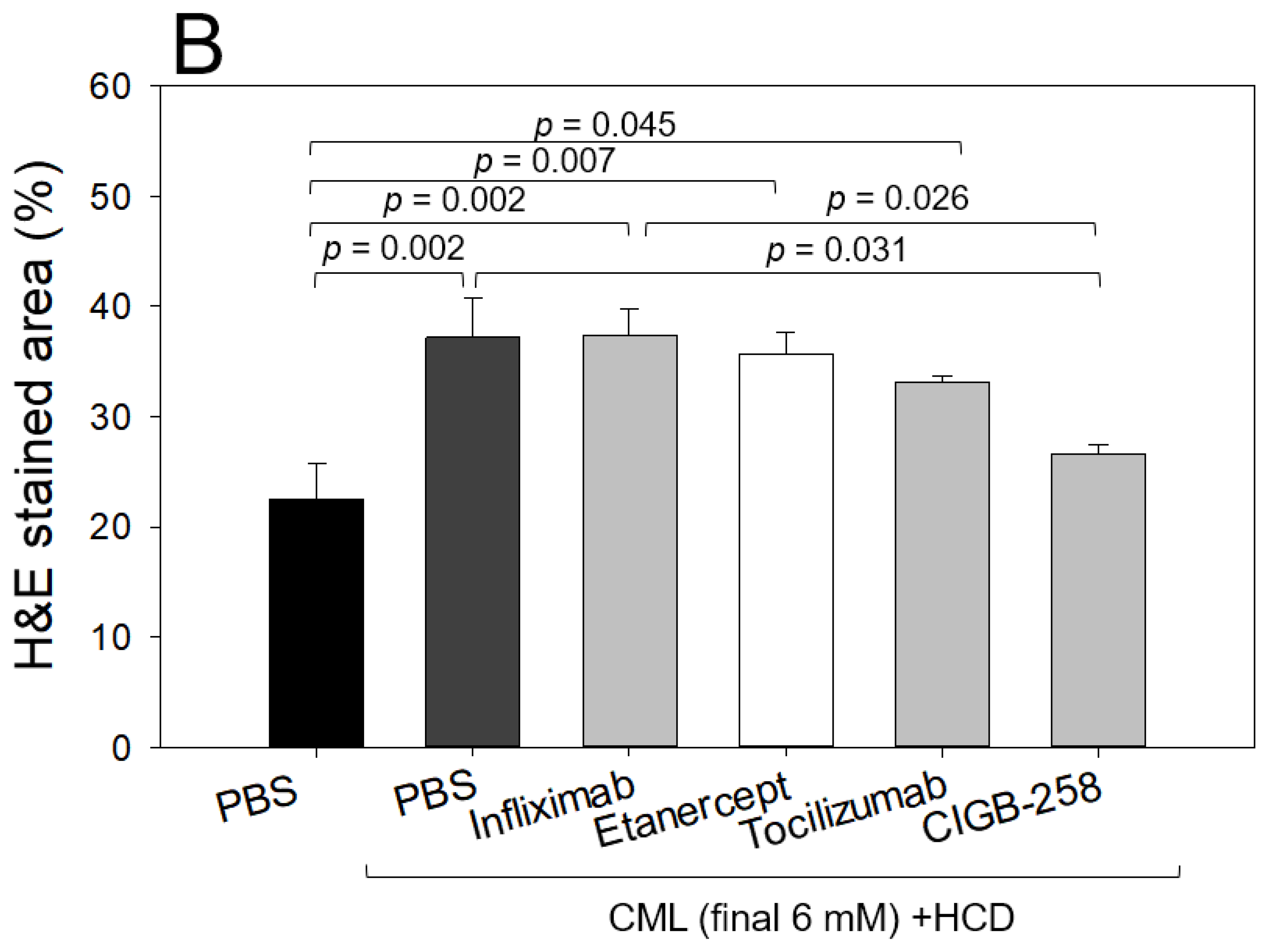
2.7. Histology Analysis
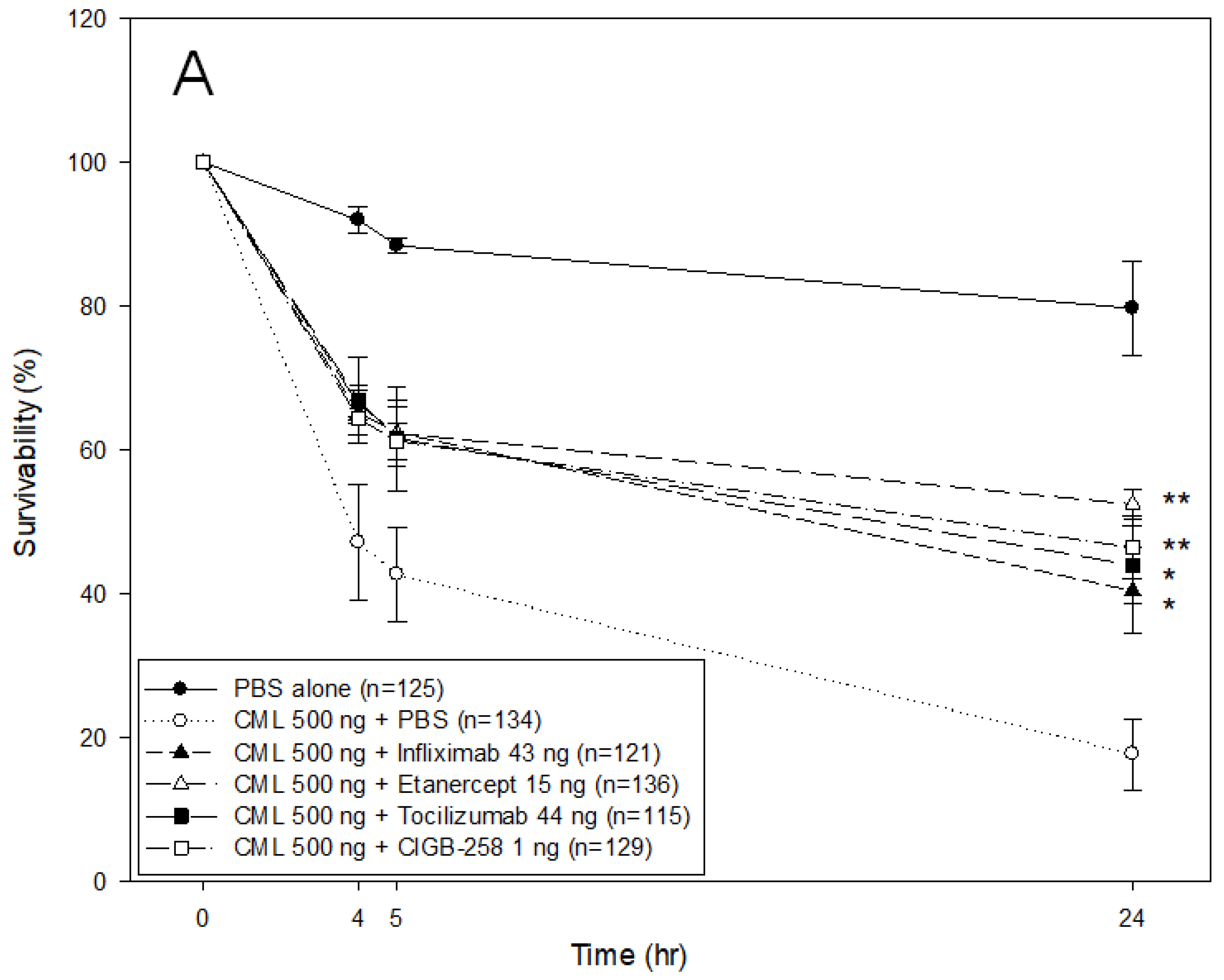
2.8. Comparison of the Anti-Inflammatory Efficacy among TNF-α Inhibitors, IL-6 Inhibitors, and CIGB-258
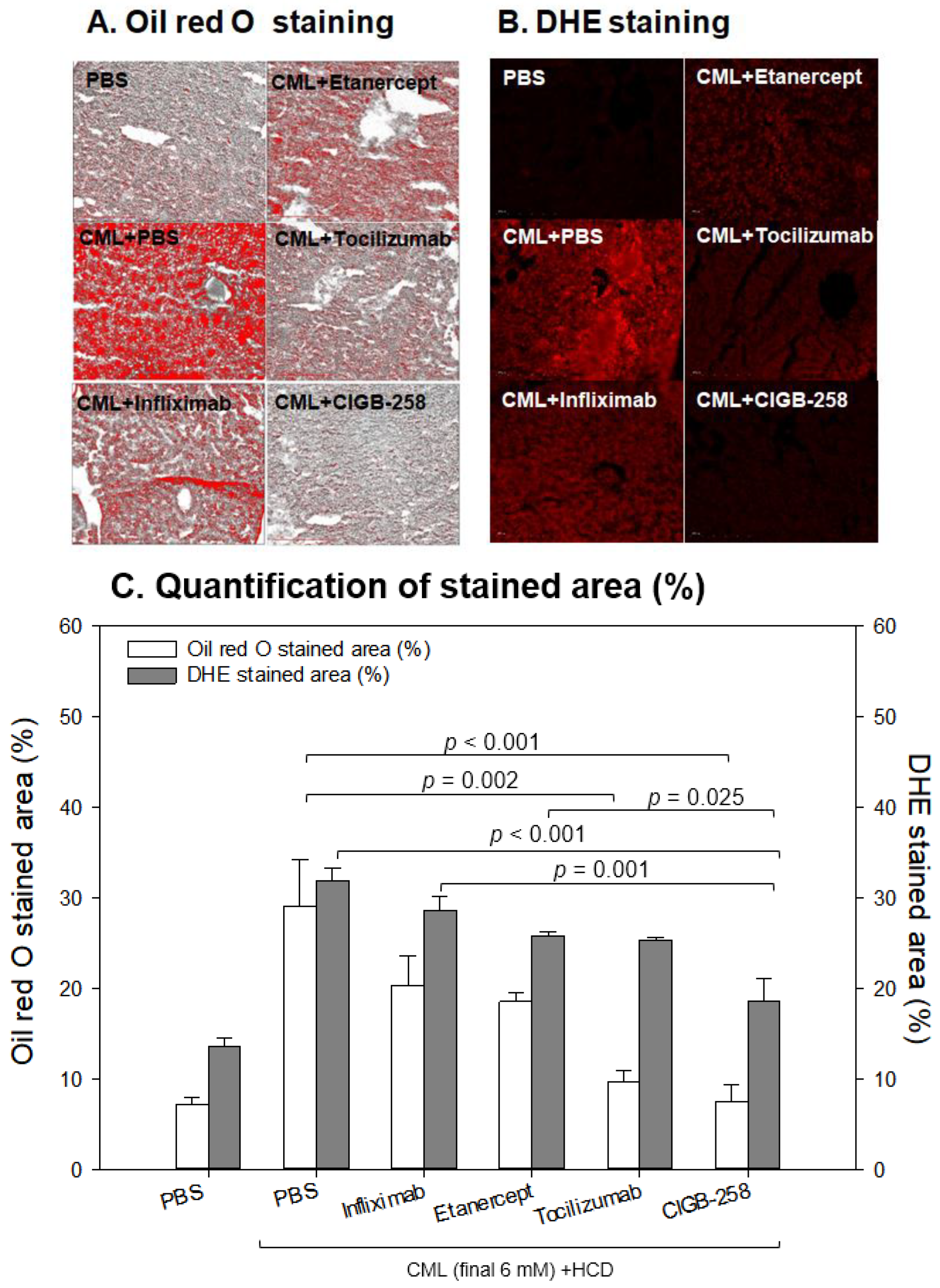
2.9. Histology and Immunohistochemistry Analyses
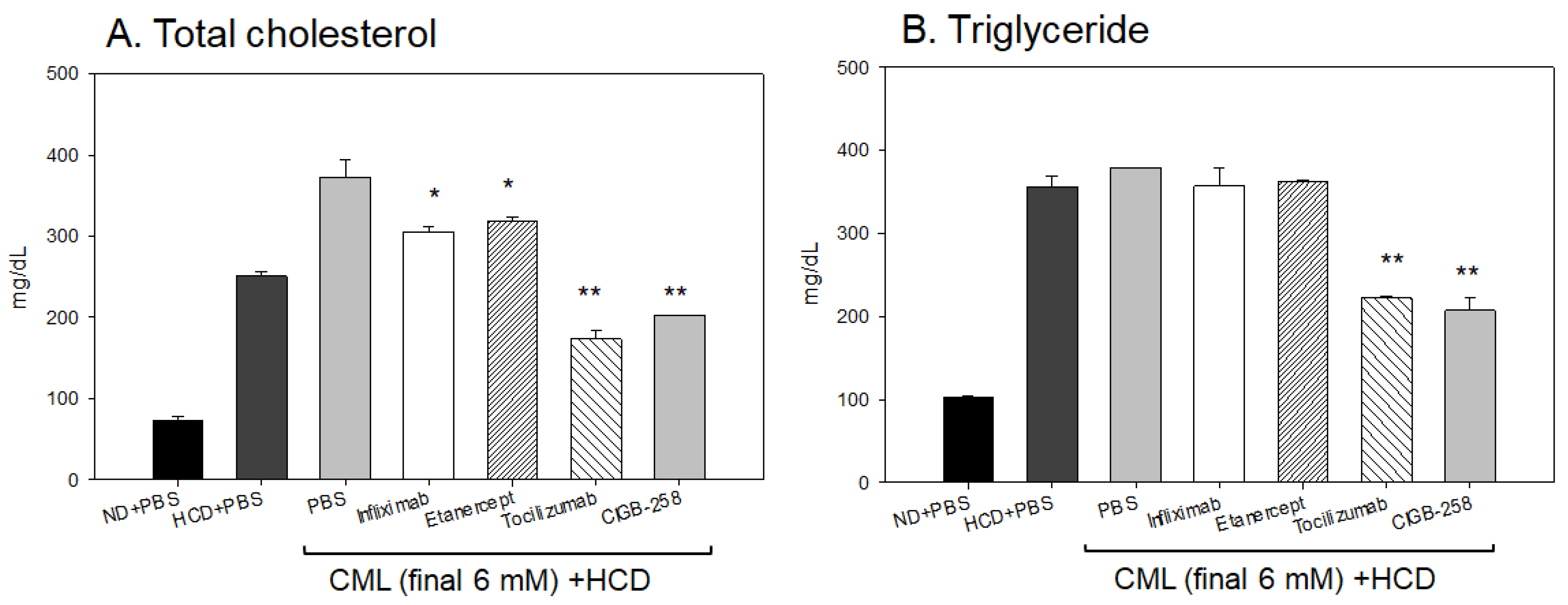
2.10. Blood Lipid Profiles
3. Discussion
4. Materials and Methods
4.1. Purification of Lipoproteins
4.2. Purification of apoA-I
4.3. Glycation of HDL and apoA-I with CML
4.4. Wavelength Maximum Fluorescence of HDL
4.5. Ferric Ion Reduction Ability
4.6. Zebrafish
4.7. Microinjection of Zebrafish Embryos
4.8. Imaging of Oxidative Stress, Apoptosis, and IL-6 in Embryo
4.9. Intraperitoneal Injection of CML and CIGB-258 into Adult Zebrafish
4.10. Plasma Analysis
4.11. Histopathology Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, T.W.; Saunders, M.E. The Immune Response: Basic and Clinical Principles; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Degan, D.; Ornello, R.; Tiseo, C.; Carolei, A.; Sacco, S.; Pistoia, F. The Role of Inflammation in Neurological Disorders. Curr. Pharm. Des. 2018, 24, 1485–1501. [Google Scholar] [CrossRef] [PubMed]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Terry, L.V.; Oo, Y.H. The Next Frontier of Regulatory T Cells: Promising Immunotherapy for Autoimmune Diseases and Organ Transplantations. Front. Immunol. 2020, 11, 565518. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Declercq, J.; Van Damme, K.F.; De Leeuw, E.; Maes, B.; Bosteels, C.; Tavernier, S.J.; De Buyser, S.; Colman, R.; Hites, M.; Verschelden, G. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): A factorial, randomised, controlled trial. Lancet Respir. Med. 2021, 9, 1427–1438. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Zhao, T.J.; Zhu, N.; Shi, Y.N.; Wang, Y.X.; Zhang, C.J.; Deng, C.F.; Liao, D.F.; Qin, L. Targeting HDL in tumor microenvironment: New hope for cancer therapy. J. Cell. Physiol. 2021, 236, 7853–7873. [Google Scholar] [CrossRef]
- Umemoto, T.; Han, C.Y.; Mitra, P.; Averill, M.M.; Tang, C.; Goodspeed, L.; Omer, M.; Subramanian, S.; Wang, S.; Den Hartigh, L.J.; et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ. Res. 2013, 112, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Pagler, T.; Gautier, E.L.; Avagyan, S.; Siry, R.L.; Han, S.; Welch, C.L.; Wang, N.; Randolph, G.J.; Snoeck, H.W.; et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010, 328, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Trakaki, A.; Marsche, G. Current Understanding of the Immunomodulatory Activities of High-Density Lipoproteins. Biomedicines 2021, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Z.; Huang, J.; Xie, Y.; Xiao, Z.; Hu, Y.; Chen, G.; Wang, M.; Li, Z.; Chen, Q.; et al. Apolipoprotein A1 Modulates Teff/Treg Balance Through Scavenger Receptor Class B Type I-Dependent Mechanisms in Experimental Autoimmune Uveitis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 23. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Wu, M.P. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine 2008, 43, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035. [Google Scholar] [CrossRef]
- Morin, E.E.; Guo, L.; Schwendeman, A.; Li, X.A. HDL in sepsis—Risk factor and therapeutic approach. Front. Pharm. 2015, 6, 244. [Google Scholar] [CrossRef]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Murphy, A.; Chin-Dusting, J.; Sviridov, D. Reconstituted HDL: A therapy for atherosclerosis and beyond. Clin. Lipidol. 2009, 4, 731–739. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Kang, D.-J.; Na, H.-J. Anti-Inflammatory Activity of CIGB-258 against Acute Toxicity of Carboxymethyllysine in Paralyzed Zebrafish via Enhancement of High-Density Lipoproteins Stability and Functionality. Int. J. Mol. Sci. 2022, 23, 10130. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zheng, L.; Wang, P.; Liu, Y.; Wu, Y.; Gong, Z. The neurotoxicity of Nε-(carboxymethyl)lysine in food processing by a study based on animal and organotypic cell culture. Ecotoxicol. Environ. Saf. 2020, 190, 110077. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, L.; Zhang, R.; Liu, G.; Xiao, S.; Qiao, X.; Wu, Y.; Gong, Z. Toxicological evaluation of advanced glycation end product Nε-(carboxymethyl)lysine: Acute and subacute oral toxicity studies. Regul. Toxicol. Pharmacol. RTP 2016, 77, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Xu, H.; Liu, X.; Liu, L.; Wu, Y.N.; Gong, Z.Y. Studies on mechanism of free Nε-(carboxymethyl)lysine-induced toxic injury in mice. J. Biochem. Mol. Toxicol. 2019, 33, e22322. [Google Scholar] [CrossRef]
- Faist, V.; Müller, C.; Drusch, S.; Erbersdobler, H.F. Selective fortification of lysinoalanine, fructoselysine and Nε-carboxymethyllysine in casein model systems. Food 2001, 45, 218–221. [Google Scholar] [CrossRef]
- Li, M.; Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Chen, J. Increased accumulation of protein-bound N(ε)-(carboxymethyl)lysine in tissues of healthy rats after chronic oral N(ε)-(carboxymethyl)lysine. J. Agric. Food Chem. 2015, 63, 1658–1663. [Google Scholar] [CrossRef]
- Buetler, T.M.; Leclerc, E.; Baumeyer, A.; Latado, H.; Newell, J.; Adolfsson, O.; Parisod, V.; Richoz, J.; Maurer, S.; Foata, F.; et al. N(epsilon)-carboxymethyllysine-modified proteins are unable to bind to RAGE and activate an inflammatory response. Mol. Nutr. Food Res. 2008, 52, 370–378. [Google Scholar] [CrossRef]
- Khatchadourian, C.; Sisliyan, C.; Nguyen, K.; Poladian, N.; Tian, Q.; Tamjidi, F.; Luong, B.; Singh, M.; Robison, J.; Venketaraman, V. Hyperlipidemia and Obesity’s Role in Immune Dysregulation Underlying the Severity of COVID-19 Infection. Clin. Pract. 2021, 11, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Bettini, S.; Bucca, G.; Sensi, C.; Dal Prà, C.; Fabris, R.; Vettor, R.; Busetto, L. Higher Levels of C-Reactive Protein and Ferritin in Patients with Overweight and Obesity and SARS-CoV-2-Related Pneumonia. Obes. Facts 2021, 14, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cedeño, M.; Venegas-Rodriguez, R.; Peña-Ruiz, R.; Bequet-Romero, M.; Santana-Sanchez, R.; Penton-Arias, E.; Martinez-Donato, G.; Guillén-Nieto, G.; Dominguez-Horta, M.d.C. CIGB-258, a peptide derived from human heat-shock protein 60, decreases hyperinflammation in COVID-19 patients. Cell Stress Chaperones 2021, 26, 515–525. [Google Scholar] [CrossRef]
- Dominguez, M.d.C.; Lorenzo, N.; Barbera, A.; Darrasse-Jeze, G.; Hernández, M.V.; Torres, A.; Hernández, I.; Gil, R.; Klatzmann, D.; Padrón, G. An altered peptide ligand corresponding to a novel epitope from heat-shock protein 60 induces regulatory T cells and suppresses pathogenic response in an animal model of adjuvant-induced arthritis. Autoimmunity 2011, 44, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, N.; Altruda, F.; Silengo, L.; Dominguez, M.d.C. APL-1, an altered peptide ligand derived from heat-shock protein, alone or combined with methotrexate attenuates murine collagen-induced arthritis. Clin. Exp. Med. 2017, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Corrales, O.; Hernández, L.; Prada, D.; Gómez, J.; Reyes, Y.; López, A.M.; González, L.J.; Horta, M.d.C.D. CIGB-814, an altered peptide ligand derived from human heat-shock protein 60, decreases anti-cyclic citrullinated peptides antibodies in patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Jang, W.; Kim, K.-Y.; Kim, J.-R.; Cho, K.-H. Fructated apolipoprotein AI showed severe structural modification and loss of beneficial functions in lipid-free and lipid-bound state with acceleration of atherosclerosis and senescence. Biochem. Biophys. Res. Commun. 2010, 392, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, J.Y.; Choi, I.; Kim, J.R.; Won, K.C.; Cho, K.H. Fructated apolipoprotein A-I exacerbates cellular senescence in human umbilical vein endothelial cells accompanied by impaired insulin secretion activity and embryo toxicity. Biochem. Cell Biol. 2016, 94, 337–345. [Google Scholar] [CrossRef]
- Yadav, D.; Kim, S.J.; Bae, M.A.; Kim, J.R.; Cho, K.H. The Ability of Different Ketohexoses to Alter Apo-A-I Structure and Function In Vitro and to Induce Hepatosteatosis, Oxidative Stress, and Impaired Plasma Lipid Profile in Hyperlipidemic Zebrafish. Oxidative Med. Cell Longev. 2018, 2018, 3124364. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Di Bartolo, B.A.; Nakhla, S.; Heather, A.K.; Mitchell, T.W.; Jessup, W.; Celermajer, D.S.; Barter, P.J.; Rye, K.A. Anti-inflammatory effects of apolipoprotein A-I in the rabbit. Atherosclerosis 2010, 212, 392–397. [Google Scholar] [CrossRef]
- Nobécourt, E.; Tabet, F.; Lambert, G.; Puranik, R.; Bao, S.; Yan, L.; Davies, M.J.; Brown, B.E.; Jenkins, A.J.; Dusting, G.J.; et al. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arter. Thromb. Vasc. Biol. 2010, 30, 766–772. [Google Scholar] [CrossRef]
- Hellwig, M.; Nitschke, J.; Henle, T. Glycation of N-ε-carboxymethyllysine. Eur. Food Res. Technol. 2022, 248, 825–837. [Google Scholar] [CrossRef]
- Hellwig, M.; Auerbach, C.; Muller, N.; Samuel, P.; Kammann, S.; Beer, F.; Gunzer, F.; Henle, T. Metabolization of the advanced glycation end product N-ε-carboxymethyllysine (CML) by different probiotic E. coli strains. J. Agric. Food Chem. 2019, 67, 1963–1972. [Google Scholar] [CrossRef]
- Toader, M.P.; Taranu, T.; Constantin, M.M.; Olinici, D.; Mocanu, M.; Costan, V.V.; Toader, S. High serum level of interleukin-6 is linked with dyslipidemia in oral lichen planus. Exp. Med. 2021, 22, 987. [Google Scholar] [CrossRef]
- Qiao, Q.; Bouwman, F.G.; van Baak, M.A.; Roumans, N.J.T.; Vink, R.G.; Mariman, E.C.M. Plasma Levels of Triglycerides and IL-6 Are Associated with Weight Regain and Fat Mass Expansion. J. Clin. Endocrinol. Metab 2022, 107, 1920–1929. [Google Scholar] [CrossRef]
- Nonogaki, K.; Fuller, G.M.; Fuentes, N.L.; Moser, A.H.; Staprans, I.; Grunfeld, C.; Feingold, K.R. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 1995, 136, 2143–2149. [Google Scholar] [CrossRef]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Pietrzak, D.; Kandzierski, G.; Wawrzycki, B.O.; Roliñski, J.; Gawêda, K.; Krasowska, D. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postep. Derm. Alergol. 2020, 37, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, G.; Volpato, S.; Blè, A.; Bandinelli, S.; Corsi, A.M.; Lauretani, F.; Paolisso, G.; Fellin, R.; Ferrucci, L. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: The InChianti study. Atherosclerosis 2007, 192, 384–390. [Google Scholar] [CrossRef]
- Gomaraschi, M.; Basilico, N.; Sisto, F.; Taramelli, D.; Eligini, S.; Colli, S.; Sirtori, C.R.; Franceschini, G.; Calabresi, L. High-density lipoproteins attenuate interleukin-6 production in endothelial cells exposed to pro-inflammatory stimuli. Biochim. Biophys. Acta 2005, 1736, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Bushinsky, D.A.; Becker, M.A. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res. Ther. 2006, 8 (Suppl. S1), S4. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S2. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, D.G.; Kim, J.R.; Cho, K.H. Senescence-related truncation and multimerization of apolipoprotein A-I in high-density lipoprotein with an elevated level of advanced glycated end products and cholesteryl ester transfer activity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 600–610. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Brewer, H.B., Jr.; Ronan, R.; Meng, M.; Bishop, C. Isolation and characterization of apolipoproteins AI, A-II, and A-IV. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1986; Volume 128, pp. 223–246. [Google Scholar]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Park, M.-H.; Kim, J.-H. Long-Term Alcohol Consumption Caused a Significant Decrease in Serum High-Density Lipoprotein (HDL)-Cholesterol and Apolipoprotein AI with the Atherogenic Changes of HDL in Middle-Aged Korean Women. Int. J. Mol. Sci. 2022, 23, 8623. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kang, D.-J.; Nam, H.-S.; Kim, J.-H.; Kim, S.-Y.; Lee, J.-O.; Kim, B.-J. Ozonated sunflower oil exerted protective effect for embryo and cell survival via potent reduction power and antioxidant activity in HDL with strong antimicrobial activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2010.
- Park, K.-H.; Cho, K.-H. A zebrafish model for the rapid evaluation of pro-oxidative and inflammatory death by lipopolysaccharide, oxidized low-density lipoproteins, and glycated high-density lipoproteins. Fish Shellfish Immunol. 2011, 31, 904–910. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Shull, L.C.; Sen, R.; Menzel, J.; Goyama, S.; Kurokawa, M.; Artinger, K.B. The conserved and divergent roles of Prdm3 and Prdm16 in zebrafish and mouse craniofacial development. Dev. Biol. 2020, 461, 132–144. [Google Scholar] [CrossRef]
- Burris, B.; Jensen, N.; Mokalled, M.H. Assessment of Swim Endurance and Swim Behavior in Adult Zebrafish. J. Vis. Exp. 2021, 177, e63240. [Google Scholar] [CrossRef]
- OECD. Test No. 203: Fish, Acute Toxicity Testing; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Cho, K.H.; Yadav, D.; Kim, S.J.; Kim, J.R. Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules 2018, 23, 1080. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Nam, H.-S.; Kim, J.-E.; Na, H.-J.; del Carmen Dominguez-Horta, M.; Martinez-Donato, G. CIGB-258 Exerts Potent Anti-Inflammatory Activity against Carboxymethyllysine-Induced Acute Inflammation in Hyperlipidemic Zebrafish via the Protection of Apolipoprotein A-I. Int. J. Mol. Sci. 2023, 24, 7044. https://doi.org/10.3390/ijms24087044
Cho K-H, Nam H-S, Kim J-E, Na H-J, del Carmen Dominguez-Horta M, Martinez-Donato G. CIGB-258 Exerts Potent Anti-Inflammatory Activity against Carboxymethyllysine-Induced Acute Inflammation in Hyperlipidemic Zebrafish via the Protection of Apolipoprotein A-I. International Journal of Molecular Sciences. 2023; 24(8):7044. https://doi.org/10.3390/ijms24087044
Chicago/Turabian StyleCho, Kyung-Hyun, Hyo-Seon Nam, Ji-Eun Kim, Hye-Jee Na, Maria del Carmen Dominguez-Horta, and Gillian Martinez-Donato. 2023. "CIGB-258 Exerts Potent Anti-Inflammatory Activity against Carboxymethyllysine-Induced Acute Inflammation in Hyperlipidemic Zebrafish via the Protection of Apolipoprotein A-I" International Journal of Molecular Sciences 24, no. 8: 7044. https://doi.org/10.3390/ijms24087044
APA StyleCho, K.-H., Nam, H.-S., Kim, J.-E., Na, H.-J., del Carmen Dominguez-Horta, M., & Martinez-Donato, G. (2023). CIGB-258 Exerts Potent Anti-Inflammatory Activity against Carboxymethyllysine-Induced Acute Inflammation in Hyperlipidemic Zebrafish via the Protection of Apolipoprotein A-I. International Journal of Molecular Sciences, 24(8), 7044. https://doi.org/10.3390/ijms24087044






