Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance
Abstract
1. Introduction
2. Friction Reduction Coatings
2.1. Inorganic Fullerene-like Nanoparticles of Tungsten Disulfide Coatings
2.2. Zinc and Zinc Compound Coatings
2.3. Zirconia Compound Coatings
2.4. Titanium and Titanium Compound Coatings
2.5. Silver and Silver Compound Coatings
2.6. Aluminum and Aluminum Compound Coatings
2.7. Other Metal and Metallic Compound Coatings
2.8. Carbon-Based Coatings
2.9. Polymeric and Bioactive Coatings
| Ref. | Coating Materials | Coating Technique | Substrate | Study Type | Roughness | Friction Reduction Effectiveness | Wear Mechanism | Wear Resistance | Other Effectiveness |
|---|---|---|---|---|---|---|---|---|---|
| [67] | Ag | Direct current sputtering | SS archwires | In vitro (dry condition) | NA | Compared to uncoated archwires, the friction force of 0.019 × 0.025-inch SS archwires reduced 1 N after coating (p = 0.032), but no significant difference between coated and uncoated 0.017 × 0.025-inch SS archwires (p = 0.854). | NA | NA | NA |
| [30] | Al2O3 | Radio frequency magnetron sputtering | SS brackets, NiTi archwires, SS archwires | In vitro (dry condition) | Surface roughness decreased with the coating process. Ra = 331.53 nm, Rq = 426.17 nm (coated brackets); Ra = 361.64 nm, Rq = 466.01 nm (coated NiTi archwires); Ra = 95.86 nm, Rq = 128.01 nm (coated SS archwires) | NiTi archwires: When both brackets and archwires were coated and only brackets were coated, CoF reduced from 0.316 to 0.238 and 0.251, respectively. CoF increased from 0.316 to 0.400 when only archwires were coated. SS archwires: When both brackets and archwires were coated, only brackets were coated, and only archwires were coated, CoF reduced from 0.552 to 0.227, 0.2,35, and 0.445, respectively. | No peeling off | The coatings did not peel off after friction, thermal, and brushing tests. | NA |
| [70] | Al-SiO2 | Magnetron sputtering | NiTi and SS archwires | In vitro (in artificial saliva) | NA | CoF of NiTi archwires and SS archwires reduced from 0.68 to 0.46 and from 0.58 to 0.45, respectively. | NA | Corrosion-resistant | Corrosion-resistant effectiveness Biocompatibility |
| [40] | BG | Electrophoretic deposition | SS archwires | In vitro (dry condition) | Sa increased from 0 to 0.46–0.79. | Friction forces showed no significant reduction. | NA | None of the coatings were damaged after three-point bending and they sustained good interfacial adhesion. | Aesthetic effect |
| [84] | CF4 | Plasma-based ion implantation and deposition | NiTi archwires | In vitro (dry condition) | NA | Friction forces reduced from 129.48 to 104.97 gf, which showed no significant reduction. | NA | NA | NA |
| [84] | CH4 | Plasma-based ion implantation and deposition | NiTi archwires | In vitro (dry condition) | NA | Friction forces reduced from 129.48 to 87.30 gf, which showed no significant reduction. | NA | NA | NA |
| [29] | CNx | Ion beam-assisted deposition | 304L SS disks | In vitro (friction test: NA; antibacterial test: in bacterial suspension) | The films slightly reduce the surface roughness parameter. Ra reduced from 0.181 to 0.140 μm | CoF reduced from 0.431 to 0.188 (p < 0.05). | NA | NA | Antibacterial Effectiveness Biocompatibility |
| [30] | CrN | Radio frequency magnetron sputtering | SS brackets, NiTi archwires, SS archwires | In vitro (dry condition) | Surface roughness decreased with the coating process. Ra = 276.85 nm, Rq = 360.24 nm (coated brackets); Ra = 354.35 nm, Rq = 454.66 nm (coated NiTi archwires); Ra = 190.28 nm, Rq = 229.26 nm (coated SS archwires) | NiTi archwires: When both brackets and archwires were coated, only brackets were coated, and only archwires were coated, CoF increased from 0.316 to 0.443, 0.324, and 0.505, respectively. SS archwires: When both brackets and archwires were coated and only archwires were coated, CoF increased from 0.552 to 0.598 and 0.586, respectively. CoF reduced from 0.552 to 0.410 when only brackets were coated. | Peeling off | Large areas of peeling could be seen after friction, thermal, and brushing tests. | NA |
| [24] | CTS (NPs) | Sol-gel dip coating | SS archwires and SS brackets | In vitro | NA | Friction force decreased by ~53%. | NA | NA | NA |
| [84] | DLC | Plasma-based ion implantation and deposition | NiTi archwires | In vitro (dry condition) | NA | The friction force reduced from 129.48 to 86.13 gf (p = 0.039). | NA | NA | NA |
| [87] | DLC | Plasma-based ion implantation and deposition | SS archwires | In vitro (in artificial saliva and dry condition) | NA | When coated with DLC-2, the static friction reduced from 2.39 to 2.09 N in artificial saliva and from 2.49 to 2.25 N under dry conditions, and the kinetic friction reduced from 2.37 to 1.99 N in artificial saliva and from 2.55 to 2.21 N under dry conditions, which showed a significantly lower frictional force than the uncoated archwires, while DCL-1 showed no significant difference compared with uncoated samples. | Rupture | No rupture was observed for the DLC-2 condition after the drawing-friction testing. | NA |
| [97] | Epoxy | NA (commercial) | SS archwires | In vitro (in distilled-deionized water) | NA | The average resistance under 0° bracket-wire angle reduced from 1.63 to 1.13 N immediately after being coated. The average resistance under a 3° bracket-wire angle reduced from 5.12 to 4.27 N immediately after being coated. | NA | NA | Durability (>4 weeks) |
| [94] | Epoxy | NA (commercial) | SS archwires | In vitro (in artificial saliva and dry condition) | NA | The friction force increased from 3.00–9.00 N to 16.00–20.50 N in both wet and dry conditions. | NA | NA | NA |
| [93] | Epoxy | NA (commercia) | NiTi archwires | In vitro (dry condition) | NA | Friction forces increased from 49.287 to 53.316 gf. | NA | NA | NA |
| [89] | GO | Silane coupling | NiTi archwires | In vitro (friction test: NA; antibacterial test: in bacterial suspension; corrosion test: artificial saliva) | The surface of the samples coated with 2 mg/mL GO concentrations was smooth with a uniformly coated area. | CoF reduced from ~0.9 to 0.2–0.4. | Grooves in the same direction as the gliding | The samples coated with 0.5 mg/mL GO concentrations had fewer grooves, but a small amount of wear debris was present. | Antibacterial effectiveness Corrosion-resistant effectiveness Biocompatibility |
| [88] | GO/Ag (NPs) | Electrophoretic deposition | NiTi alloy | In vitro (dry condition) | Ra ranged from 50.72–69.93 nm. | CoF reduced from 0.060 to 0.006, and increased coating time led to lower CoF. | NA | NA | NA |
| [46,90] | GSEC | Electron cyclotron resonance plasma sputtering | SS archwires | In vitro (in artificial saliva) | NA | CoF reduced to ~0.10 and remained under 0.30 after 30 days. | Peeling off | The corresponding wear rate was strongly decreased from 4.84 × 10−6 to 0.11 × 10−6 mm3/Nm. | NA |
| [21] | HCCP | Electroplating | SS archwires | In vitro (in PBS solution and dry condition) | There were very small protrusions on the surfaces of the coated archwires, while the surfaces of uncoated archwires were smooth. | The friction force reduced from 147.15 to 124.61 gf (p = 0.0076) and from 143.55 to 121.41 gf (p = 0.04) under dry and wet conditions, respectively. | Scratches | After the friction test, scratches were seen on the surfaces on the coated surfaces. | Aesthetic effect |
| [18] | Ni+MoS2 (NPs) | Electrochemical co-deposition | SS archwires | In vitro (in artificial saliva and dry condition) | NA | Dry conditions: CoF reduced from 0.58–1.43 to 0.50–1.19. In artificial saliva: CoF reduced from 0.95–2.52 to 0.94–2.35. | NA | NA | NA |
| [18] | Ni+WS2 (NPs) | Electrochemical co-deposition | SS archwires | In vitro (in artificial saliva and dry condition) | NA | Dry conditions: CoF reduced from 0.58–1.43 to 0.42–1.06. In artificial saliva: CoF reduced from 0.95–2.52 to 0.66–1.46. | NA | NA | NA |
| [57] | Ni-Ti-Cr | Chronopotentiometry | NiTi archwires | In vitro (in artificial saliva) | NA | CoF showed no significant reduction. | NA | NA | Corrosion-resistant effectiveness Durability |
| [57] | Ni-Ti-Mo | Chronopotentiometry | NiTi archwires | In vitro (in artificial saliva) | NA | CoF reduced from 0.288 to 0.252–0.265. | NA | NA | Corrosion-resistant effectiveness Durability (>60 days) |
| [97] | Parylene | NA (commercial) | SS archwires | In vitro (in distilled-deionized water) | NA | The average resistance under 0° bracket-wire angle increased from 1.63 to 5.39 N immediately after being coated. The average resistance under the 3° bracket-wire angle increased from 5.12 to 11.38 N immediately after being coated. | NA | NA | Durability |
| [97] | PTFE | NA (commercial) | SS archwires | In vitro (in distilled-deionized water) | NA | The average resistance under 0° bracket-wire angle reduced from 1.63 to 1.15 N immediately after being coated. The average resistance under a 3° bracket-wire angle showed no significant change immediately after being coated. | NA | NA | Durability |
| [95] | PTFE | Spraying | SS, Ni-Ti, and β-titanium archwires | In vitro | Ra of coated archwires increased from 0.02–0.21 to 0.53–0.58 μm. | The friction force of coated archwires reduced from 123.94–152.61 to 102.98–124.40 gf compared to uncoated archwires and 200 °C coating resulted in less friction against brackets than did the conventional 380 °C coating. | Scratches | PTFE-coating at 200 °C resulted in good microbial adhesion and tolerance of wear. | Durability (>3 months) Aesthetic effect |
| [94] | PTFE | NA (commercial) | SS archwires | In vitro (in artificial saliva and dry condition) | NA | The friction force was higher than uncoated archwires but lower than epoxy and rhodium-coated archwires in wet and dry conditions. Dry conditions: The friction force increased from 3.80–9.00 to 5.50–12.80 N. In artificial saliva: The friction force increased from 3.00–8.20 to 3.80–11.60 N. | NA | NA | NA |
| [93] | PTFE | NA (commercial) | NiTi archwires | In vitro (dry condition) | NA | Friction forces increased from 49.287 to 61.427 gf. | NA | NA | NA |
| [92] | PTFE | NA (commercial) | SS archwires | In vitro | The surface roughness increased after the friction test. | When compared with ceramic with a metal slot, the friction force reduced from 1.61 to 1.03 N after the archwires were coated with PTFE. | NA | NA | NA |
| [21] | Rhodium | NA (commercial) | SS archwires | In vitro (in PBS and dry condition) | There were protrusions on the surfaces of the coated archwires, while the surfaces of uncoated archwires were smooth. | The friction force increased from 147.15 to 216.29 gf (p < 0.001) and from 143.55 to 210.21 gf (p < 0.001) under dry and wet conditions, respectively. | Scratches | After the friction test, scratches were seen on the surfaces on the coated surfaces. | Aesthetic effect |
| [94] | Rhodium | NA (commercial) | SS archwires | In vitro (in artificial saliva and dry condition) | NA | The friction force increased from 3.00–9.00 N to 17.30–21.70 N in both wet and dry conditions. | NA | NA | NA |
| [71] | Rhodium | NA (commercial) | SS archwires | In vitro (in artificial saliva and 0.05% NaF mouthwash) | Surface roughness showed no significant reduction with the coating process. | The friction force was reduced from 2.22 to 1.49 N in artificial saliva and reduced from 2.72 to 2.17 N in 0.05% sodium fluoride mouthwash. | NA | NA | NA |
| [60] | SiO2 (NPs) | NA (commercial) | Cr-Ni archwires | In vitro (in artificial saliva and dry condition) | Surface roughness decreased with the coating process. Ra reduced from 0.673 to 0.040 μm. | Friction force reduced from 0.51 to 0.38 N and from 0.56 to 0.44 N under wet and dry conditions, respectively. | NA | NA | NA |
| [68] | TaAgB | Magnetron sputtering with Ag-doping | 304 SS sheets | In vitro (in artificial saliva) | NA | CoF reduced from 0.38 to 0.08. | No serious wear phenomenon | The wear rate was 6.51 × 10−15 m3/Nm. | Antibacterial effectiveness Biocompatibility |
| [32] | TiCN | PVD | 316L SS plates | In vitro (in artificial saliva and dry condition) | Ra = 29.86 nm; root mean square roughness: Rq = 39.09 nm | CoF reduced from 0.20 to less than 0.06 under dry conditions with loads of 5 N. | NA | NA | NA |
| [31] | TiCN | Direct current reactive magnetron sputtering | 316L SS and (100)-oriented Si substrates | In vitro (in artificial saliva) | NA | CoF reduced to less than 0.30, and the addition of N2 and C in the Ti matrix further lowered the CoF. | NA | The lowest wear rate was 5.6 × 10−6 mm3/Nm | Corrosion-resistant effectiveness Aesthetic effect |
| [32] | Ti-DLC | PVD | 316L SS plates | In vitro (in artificial saliva and dry condition) | The surface of the Ti-DLC film has many nanocrystal clusters that cause substantial pitting and layer defects. Ra = 10.40 nm; Rq = 13.43 nm | CoF remained less than 0.04 under both dry and artificial saliva conditions. | NA | NA | NA |
| [29] | TiN | Ion beam-assisted deposition | 304L SS disks | In vitro (friction test: NA; antibacterial test: in bacterial suspension) | The films slightly reduce the surface roughness parameter. Ra reduced from 0.181 to 0.162 μm. | CoF increased from 0.431 to 0.469 (p < 0.05). | NA | NA | Antibacterial Effectiveness Biocompatibility |
| [58] | TiN | Ion plating | SS and NiTi archwires | In vitro (friction test: NA; corrosion test: in 0.9% NaCl solution) | Ra of TiN-coated SS archwire increased from 0.023 to 0.046 µm. Ra of TiN-coated NiTi archwire remained at 0.001 µm. | At angles of 0 degrees, the friction forces of the TiN-coated NiTi archwires reduced, but SS archwires showed no significant difference. At angles of 10 degrees, the friction forces of the TiN-coated SS and NiTi archwires were both reduced. | NA | NA | Corrosion-resistant effectiveness |
| [32] | TiN | PVD | 316L SS plates | In vitro (in artificial saliva and dry condition) | The surface of the TiN film has some particles and pinholes. Ra = 26.59 nm; Rq = 32.62 nm | CoF reduced from 0.08 to less than 0.03 under dry conditions with loads of 5 N. | NA | NA | NA |
| [30] | TiN | Radio frequency magnetron sputtering | SS brackets, NiTi, and SS archwires | In vitro (dry condition) | Ra = 354.12 nm, Rq = 458.74 nm (coated brackets); Ra = 391.99 nm, Rq = 534.36 nm (coated NiTi archwires); Ra = 105.15 nm, Rq = 132.67 nm (coated SS archwires) | NiTi archwires: When both brackets and archwires were coated, only brackets were coated, and only archwires were coated, CoF increased from 0.316 to 0.399, 0.331, and 0.446, respectively. SS archwires: When both brackets and archwires were coated and only brackets were coated, CoF reduced from 0.552 to 0.372 and 0.237, respectively. CoF increased from 0.552 to 0.818 when only archwires were coated. | Peeling off | After friction, thermal, and brushing tests, the coatings peeled off in some small areas. | NA |
| [31] | TiN | Direct current reactive magnetron sputtering | 316 L SS and (100)-oriented Si substrates | In vitro (in artificial saliva) | NA | CoF was reduced to less than 0.30, and the addition of N2 in the Ti matrix further lowered the CoF. | NA | The lowest wear rate was 7.8 × 10−6 mm3/Nm | Corrosion-resistant effectiveness |
| [29] | TiO2 | Ion beam-assisted deposition | 304L SS disks | In vitro (friction test: NA; antibacterial test: in bacterial suspension) | NA | NA | NA | The TiO2 film wore out in a few seconds. | Antibacterial effectiveness Biocompatibility |
| [60] | TiO2 (NPs) | NA (commercial) | Cr-Ni archwires | In vitro (in artificial saliva and dry condition) | Surface roughness decreased with the coating process. Ra reduced from 0.673 to 0.042 μm. | Friction force showed no significant reduction or even increased from 0.56 to 0.61 N compared to uncoated archwires. | NA | NA | NA |
| [48] | Zn | PVD | SS archwires | In vitro | NA | The friction force reduced from 2.98 to 2.03 N (p = 0.001) and from 3.51 to 1.72 N (p < 0.0001) at 0° and 10° angles, respectively. | Cracks and scratches | The scratches and cracks could be seen clearly. | NA |
| [46] | ZnO (NPs) | Sol-gel dip coating | SS archwires and SS brackets | In vitro | NA | Friction force decreased by ~64%. | NA | NA | NA |
| [27] | ZnO (NPs) | Electrochemical deposition | NiTi archwires | In vitro (friction test: NA; antibacterial test: on nutrient agar plates) | NA | Friction force decreased by 34%. | NA | NA | Antibacterial effectiveness |
| [75] | Niobium (NPs) | Plasma sputtering | 316L SS | In vitro | Ra reduced from 157.8 to 133.3–156.1 nm after being coated (p > 0.05). | Potential friction reduction effectiveness | NA | NA | NA |
| [75] | Tantalum (NPs) | Plasma sputtering | 316L SS | In vitro | Ra reduced from 157.8 to 110–130.6 nm after being coated (p > 0.05). | Potential friction reduction effectiveness | NA | NA | NA |
| [75] | Vanadium (NPs) | Plasma sputtering | 316L SS | In vitro | Ra reduced from 157.8 to 83.4–96.8 nm after being coated (p = 0.002). | Potential friction reduction effectiveness | NA | NA | NA |
3. Antibacterial Coatings
3.1. Silver and Silver Compound Coatings
3.2. Titanium and Titanium Compound Coatings
3.3. Zinc and Zinc Compound Coatings
3.4. Other Metal and Metallic Compound Coatings
3.5. Polymeric and Bioactive Materials
| Ref. | Coating Materials | Coating Technique | Substrate | Study Type | Roughness | Antibacterial Effectiveness | Other Effectiveness |
|---|---|---|---|---|---|---|---|
| [109] | Ag | Galvanic, PVD, PIIID, and deposition | SS brackets | Clinical trial | Ra for the untreated bracket material was 0.04 µm, Ra for the galvanic coating was 0.12 µm, Ra for the PVD coating was 0.08 µm, and Ra for the PIIID procedure coating was 0.06 µm. | The biofilm volume per test specimen for the control was 7.24 × 108 µm3. For the galvanically applied silver coating, the biofilm volume decreased to 2.62 × 107 µm3, for the PVD coating to 4.44 × 107 µm3, and the PIIID procedure to 3.82 × 107 µm3. The reduction of the biofilm volume compared to the control was statistically significant for all surface modifications. The percentage surface coverage per test specimen was 64.40% for the unmodified control and decreased to 16.97% for the galvanic silver surface, 23.81% for the PVD coating, and 23.63% for the PIIID-modified surface. | NA |
| [112] | Ag (NPs) | PVD | SS brackets | In vitro (in artificial saliva) | NA | The inhibition percent of Streptococcus mutans and Lactobacillus acidophilus were 27.60% and 62.02%, respectively. | Durability (>3 months) |
| [106] | Ag (NPs) | NA (commercial) | NA | In vitro (in suspensions of microorganisms) | NA | The silver coating decreased the adhesion of both Streptococcus mutans and Streptococcus sanguinis to the orthodontic brackets | NA |
| [104] | Ag (NPs) | Hydrothermal synthesis | SS archwires | In vitro (in suspensions of Streptococcus mutans and Staphylococcus aureus) | It was not possible to observe changes in roughness after coating. | Microbial adhesion and biofilm formation of Staphylococcus aureus and Streptococcus mutans reduced. | Aesthetic effect |
| [107] | Ag (NPs) | Synthesized in situ | SS and ceramic brackets | In vitro (on Mueller–Hinton agar plates) | NA | The inhibitory halos obtained by the in vitro evaluation of the antibacterial effect, in terms of brackets with silver nanoparticles with Staphylococcus aureus and Escherichia coli, showed an excellent inhibition of microbial growth compared to the bracket control, with a diameter between 9 and 10 mm. | NA |
| [140] | Ag (NPs) | Aqueous reduction | NiTi, CuNiTi, SS archwires, and SS brackets | In vitro (in suspensions of Streptococcus mutans) | Roughness values were increased in SS wires (7.094 × 103 + 1 nm), followed by NiTi wires (6.234 × 103 + 1 nm), and the lowest roughness value for CuNiTi wires (3.116 × 103 + 1 nm). | Smaller Ag nanoparticles (16.7 μg/mL) had consistently better antimicrobial inhibition effects against the Streptococcus mutans strain compared to larger Ag nanoparticles (66.8 μg/mL), showing significant differences between them. The coated brackets had significantly better antiadherence activity (4.3 CFU/mL for smaller particles and 5 CFU/mL for larger particles) than the uncoated brackets (356 CFU/mL). Both sizes of Ag nanoparticles had statistically good adherence inhibition of the Streptococcus mutans strain for all types of orthodontic wires (SS = 26.1–52.6 CFU/mL, NiTi = 15.1–49.6 CFU/mL, and CuNiTi = 89.1–287.8 CFU/mL) compared with the control groups (SS = 346.7 CFU/mL, NiTi = 342.3 CFU/mL, and CuNiT = 376.2 CFU/mL). | NA |
| [112] | Ag/ZnO (NPs) | PVD | SS brackets | In vitro (in artificial saliva) | NA | The inhibition percent of Streptococcus mutans and Lactobacillus acidophilus were 45.32% and 80.29%, respectively. | Durability (>3 months) |
| [113] | Ag + polymer | NA (commercial) | NiTi archwires | In vitro (in suspensions of microorganisms + sucrose) | NA | No significant reduction in bacterial adhesion (0% sucrose) or biofilm accumulation (3% sucrose) was found when assessed by colony counting of Streptococcus mutans. | NA |
| [157] | AgNP/PTFE | Layer-by-layer deposition | 316L SS plates | In vitro (in suspensions of Escherichia coli) | The surface roughness increased from 59.4 ± 6.1 nm to 158.1 ± 2.7 nm (deposition time of 6 h) and 177.3 ± 5.1 nm (deposition time of 12 h), respectively. | Coatings with 6 and 12 h deposition time could inhibit by ~75% and ~90% bacterial growth over the initial 3 days, respectively. After 7 days, coatings with 6 and 12 h deposition time still exhibited significant antibacterial activity, reducing by ~40% and ~50% of bacterial growth, respectively. | Corrosion-resistant effectiveness Biocompatibility |
| [158] | Al2O3 (NPs) | Atomic layer deposition | 316L SS | In vitro (in simulation body fluid solution) | NA | The mean colonies forming units per milliliter were reduced by 20%; the diffusion zone was ~4 mm. | Corrosion-resistant effectiveness Biocompatibility |
| [158] | Al2O3/TiO2 Multilayer | Atomic layer deposition | 316L SS | In vitro (in simulation body fluid solution) | NA | The mean colonies forming units per milliliter were reduced by 40%; the diffusion zone was >6 mm. | Corrosion-resistant effectiveness |
| [155] | BSA | Chemical deposition | SS, ceramic, and resin brackets; SS archwires | In vitro (in suspensions of Streptococcus mutans) | The adsorbed BSA molecule was not uniform on the surface, thereby leading to slight surface roughness. | After integrating BSA molecules, the three kinds of brackets all showed more than 95.0% reduction in Streptococcus mutans adhesion (i.e., 98.3% for SS, 96.3% for ceramic, and 95.2% for resin). Compared with bare archwires, only a few bacteria (~7.5%) could be found on the BSA-coated archwires’ surface, even after incubation in bacterial suspension for 300 min; and the optimal BSA concentration was 10 mg/mL. | NA |
| [156] | CTS/PEG hydrogel | Combining silane chemistry and subsequent copolymerization | SS archwires | In vitro (in suspensions of Streptococcus mutans) | Rq of CTS/PEG-coated SS archwires increased from 0.26 to 1.57 nm. | This biointerface showed superior activity in early-stage adhesion inhibition (98.8%, 5 h) and displayed remarkably long-lasting colony-suppression activity (93.3%, 7 d). | Durability (>7 days) Wear resistance Biocompatibility |
| [29] | CNx | Ion beam-assisted deposition | 304L SS disks | In vitro (friction test: NA; antibacterial test: in bacterial suspension) | The films slightly reduce the surface roughness parameter. Ra reduced from 0.181 to 0.140 μm | Bacteria density reduced from 13.002 × 103 /mm2 to 4.030 × 103 /mm2 (p < 0.05). | Friction reduction effectiveness Biocompatibility |
| [113] | Epoxy | NA (commercial) | NiTi archwires | In vitro (in suspensions of microorganisms + sucrose) | NA | No significant reduction in bacterial adhesion (0% sucrose) or biofilm accumulation (3% sucrose) was found when assessed by colony counting of Streptococcus mutans. | NA |
| [142] | Epoxy | NA (commercial) | NiTi archwires | In vitro (in suspensions of Streptococcus mutans and Streptococcus sobrinus) | Ra = 1.29 μm (higher than uncoated archwires) | Epoxy-coated wires demonstrated an increased adhesion of Streptococcus mutans (5.55 CFU/cm2) and Streptococcus sobrinus (4.64 CFU/cm2). | NA |
| [136] | F-O-Ti | Plasma-enhanced fluorine and oxygen mono/dual CVD | Commercially available pure titanium with 99.9% purity | In vitro (in suspensions of Staphylococcus aureus and artificial saliva) | A large amount of convex texture with 100–200 nm size distributes uniformly all over the surface. | The antibacterial rates were higher than 90%. | Corrosion-resistant effectiveness Durability (~7 days) Biocompatibility |
| [89] | GO | Silane coupling | NiTi archwires | In vitro (friction test: NA; antibacterial test: in bacterial suspension; corrosion test: artificial saliva) | The surface of the samples coated with 2 mg/mL GO concentrations was smooth with a uniformly coated area. | The bacterial CFU values for Streptococcus mutans were 0.77 (samples coated with 0.5 mg/mL GO concentrations), 0.40 (samples coated with 2 mg/mL GO concentrations), and 0.23 (samples coated with 5 mg/mL GO concentrations) relative to that on bare NiTi (1.00). The number of live bacteria decreased, and the number of dead bacteria increased, indicating that GO coating could effectively resist adherent bacteria. This effect had a positive correlation with GO concentration, indicating the concentration-dependent antibacterial ability of these GO-coated surfaces. | Friction reduction effectiveness Corrosion-resistant effectiveness Wear resistance Biocompatibility |
| [154] | Lysozyme | Liquid phase deposition | CAWs | In vitro (in suspensions of Staphylococcus aureus and artificial saliva) | The surface roughness increased after being coated according to two- and three-dimensional atomic force micrographs. | When coated with 20, 40, and 60 g/L lysozyme, Live/dead bacteria staining of Staphylococcus aureus reduced from 90% to 82%, 59%, and 61%, respectively. | Corrosion-resistant effectiveness Durability (~2 weeks) Wear resistance Biocompatibility |
| [134] | N-doped TiO2 | Radio frequency magnetron sputtering | SS brackets | Clinical trial | NA | Coated archwires (38.54 and 36.84) showed greater Ct values than uncoated wires (34.71 and 31.89) at 30 d and 60 d, respectively. Greater Ct values indicate lower Streptococcus mutans adhesion. Therefore, coated wires demonstrated lower Streptococcus mutans adhesion when compared with uncoated wires. | Durability |
| [132] | N-doped TiO2 | Radio frequency magnetron sputtering | SS brackets | In vitro (in suspensions of Streptococcus mutans) | NA | The coating decreased Streptococcus mutans colonies from 401.21 to 37.82 CFU/mL. | Durability (>90 days) |
| [114] | PEDOT/Ag | Layer-by-layer deposition | 316L SS plates | In vitro (in suspensions of Streptococcus mutans and Escherichia coli) | NA | The antiadhesive and antibacterial activity against Streptococcus mutans and Escherichia coli significantly increased. | Biocompatibility |
| [20] | PDA and HCDs | Electrostatic adsorption | SS brackets | In vitro (in the soaking solution of the archwires) | NA | After soaking in artificial saliva for 7 and 14 days, the coatings formed by 50 μL and 100 μL of HCDs showed antibacterial rates against Escherichia coli and Streptococcus mutans that could still reach more than 50%, but the antibacterial properties of the coatings formed by 150 μL of HCDs were much weaker than those of the above two groups. | Durability (>7 days) Biocompatibility |
| [113] | PTFE | NA (commercial) | NiTi archwires | In vitro (in suspensions of microorganisms + sucrose) | NA | No significant reduction in bacterial adhesion (0% sucrose) or biofilm accumulation (3% sucrose) was found when assessed by colony counting of Streptococcus mutans. | NA |
| [142] | PTFE | NA (commercial) | NiTi archwires | In vitro (in suspensions of Streptococcus mutans and Streptococcus sobrinus) | Ra = 0.74 μm (higher than uncoated archwires) | PTFE-coated wires demonstrated an increased adhesion of Streptococcus mutans (4.76 CFU/cm2) and Streptococcus sobrinus (3.73 CFU/cm2). | NA |
| [157] | PTFE | NA | 316L SS plates | In vitro (in suspensions of Escherichia coli) | The surface roughness increased from 59.4 ± 6.1 nm to 134.7 ± 3.9 nm after being coated. | The PTFE coating only demonstrated short-term antiadhesive activity, reducing by ~45% biomass adhesion on the first day as compared with 316L SS, while coatings with 6 h and 12 h deposition time inhibited by ~65% and ~80% of biomass formation. | Corrosion-resistant effectiveness Biocompatibility |
| [113] | Rhodium | NA (commercial) | NiTi archwires | In vitro (in suspensions of microorganisms + sucrose) | NA | No significant reduction in bacterial adhesion (0% sucrose) or biofilm accumulation (3% sucrose) was found when assessed by colony counting of Streptococcus mutans. | NA |
| [142] | Rhodium | NA (commercial) | NiTi archwires | In vitro (in suspensions of Streptococcus mutans and Streptococcus sobrinus) | Ra = 0.34 μm (higher than uncoated archwires) | Rhodium-coated wires demonstrated an increased adhesion of Streptococcus mutans (3.85 CFU/cm2) and Streptococcus sobrinus (2.79 CFU/cm2). | NA |
| [68] | TaAgB | Magnetron sputtering with Ag-doping | 304 SS sheets | In vitro (in artificial saliva) | NA | The amount of Streptococcus mutans adhesion on the TaAgB surface was significantly reduced and the morphology changed according to the SEM image. | Friction reduction effectiveness Biocompatibility |
| [29] | TiN | Ion beam-assisted deposition | 304L SS disks | In vitro (friction test: NA; antibacterial test: in bacterial suspension) | The films slightly reduce the surface roughness parameter. Ra reduced from 0.181 to 0.162 μm. | Bacteria density reduced from 13.002 × 103 /mm2 to 3.888 × 103 /mm2 (p < 0.05). | Friction reduction effectiveness Biocompatibility |
| [135] | TiN | Cathodic cage deposition | SS brackets | In vitro (in suspensions of Streptococcus mutans) | NA | The presence of coatings did not influence the formation of the Streptococcus mutans biofilm (p = 0.06). | NA |
| [29] | TiO2 | Ion beam-assisted deposition | 304L SS disks | In vitro (friction test: NA; antibacterial test: in bacterial suspension) | NA | Bacteria density reduced from 13.002 × 103 /mm2 to 1.368 × 103 /mm2 (p < 0.05). | Wear resistance Biocompatibility |
| [123] | TiO2 | Radio frequency magnetron sputtering | SS brackets | In vitro (in suspensions of Streptococcus mutans) | NA | The “weight increase” reduced from 1.0100 × 103 mg to 0.6750 × 103 mg, which showed increased antiadhesive activity against Streptococcus mutans. The survival rate reduced from 3548.3350 to 2895.0000 CFU/mL, which showed increased antibacterial activity against Streptococcus mutans. | NA |
| [127] | TiO2 | Radio frequency magnetron sputtering | NiTi archwires | Clinical trial | Ra reduced from 1591.08 to 746.14 nm. | Coated archwires (30.97) showed greater Ct values than uncoated wires (37.00). Greater Ct values indicate lower Streptococcus mutans adhesion. Therefore, coated wires demonstrated lower Streptococcus mutans adhesion when compared with uncoated wires. This difference was statistically significant (p = 0.0005). | Durability (~1 month) |
| [128] | TiO2 | PVD | SS archwires | Clinical trial | NA | The coating decreased Streptococcus mutans colonies from 5122.0 to 1400.0 and from 1141.8 to 297.7 CFU/mL in the first and the third week, respectively. | Durability (>3 weeks) Biocompatibility |
| [158] | TiO2 (NPs) | Atomic layer deposition | 316L SS | In vitro (in simulation body fluid solution) | NA | The mean colonies forming units per milliliter were reduced by 15%; the diffusion zone was ~2 mm. | Corrosion-resistant effectiveness Biocompatibility |
| [135] | TNCP | Cathodic cage deposition | SS brackets | In vitro (in suspensions of Streptococcus mutans) | NA | The presence of coatings did not influence the formation of the Streptococcus mutans biofilm (p = 0.06). | NA |
| [123] | ZnO | Radio frequency magnetron sputtering | SS brackets | In vitro (in suspensions of Streptococcus mutans) | NA | The antiadhesive and antibacterial activity against Streptococcus mutans showed no significant increase. | NA |
| [112] | ZnO (NPs) | PVD | SS brackets | In vitro (in artificial saliva) | NA | The inhibition percent of Streptococcus mutans and Lactobacillus acidophilus were 17.54% and 28.85%, respectively. | Durability (>3 months) |
| [139] | ZnO (NPs) | CVD, chemical precipitation method, polymer composite coating, sol-gel synthesis, the electrospinning process | NiTi archwires | In vitro (in suspensions of Streptococcus mutans) | The sizes of the NPs were 59–61 nm, 30–150 nm, and 28 nm when coated during CVD, chemical precipitation method, and sol-gel synthesis process, respectively. Electrospinning gave a branch of fibers gathered together in a network, and polymer composite coating showed a nonuniform and excursive surface. | The highest Streptococcus mutans antibacterial effect with 98%, 96%, and 93% microbial cell reduction belonged to CVD, precipitation method, and sol-gel synthesis, respectively, and the lowest cell reduction was seen in the electrospinning method (72%) | NA |
| [27] | ZnO (NPs) | Electrochemical deposition | NiTi archwires | In vitro (friction test: NA; antibacterial test: on nutrient agar plates) | NA | The inhibition zone of Staphylococcus aureus, Streptococcus pyogenes, and Escherichia coli was formed around all ZnO nanoparticles coated archwires, with diameters of 4.25, 6.25, and 3.57 mm, respectively. | Friction reduction effectiveness |
| [159] | ZrO2 | EBPVD | 316L SS | In vitro (in artificial saliva and artificial saliva containing NaF) | Ra reduced from 10 to 3 nm after being coated. | The bacterial adhesion reduced from 8 to 5.5 × 103 CFU/cm2. | Corrosion-resistant effectiveness Wear resistance Biocompatibility |
4. Corrosion-Resistant Coatings
4.1. Titanium and Titanium Compound Coatings
4.2. Carbon-Based Coatings
4.3. Other Metal and Metallic Compound Coatings
4.4. Polymeric and Bioactive Coatings
4.5. Ion Injection Coatings
| Ref. | Coating Materials | Coating Technique | Substrate | Study Type | Roughness | Corrosion Resistant Effectiveness | Other Effectiveness |
|---|---|---|---|---|---|---|---|
| [182] | aC:H | Reactive magnetron sputtering | SS brackets, tubes, and bands | In vitro (in Fusayama-Meyer artificial saliva) | The coating presented a low Ra of ~7 nm. | Right after 7 days of immersion, the Cr release rate from coated samples was ~40% higher than that from uncoated samples, but was similar at day 30. Ni release from coatings was ~10% lower than uncoated samples after 7 days but ~55% higher after 30 days. Finally, Fe release was ~15% lower and similar to uncoated samples after 7 and 30 days, respectively. However, there were no segregation, metallic inclusion, delamination, or detachments on coated samples after 30 days of immersion. | Wear resistance Durability (>30 days) |
| [157] | AgNP/PTFE | Layer-by-layer deposition | 316L SS plates | In vitro (in suspensions of Escherichia coli) | The surface roughness increased from 59.4 ± 6.1 nm to 158.1 ± 2.7 nm (deposition time of 6 h) and 177.3 ± 5.1 nm (deposition time of 12 h). | The Icorr values reduced from 2.01 × 10−6 to 1.58–2.51 × 10−7 A/cm2, indicating enhanced corrosion protection. However, the corrosion protection was still lower than the PTFE coatings. | Antibacterial effectiveness Durability (>7 days) Biocompatibility |
| [158] | Al2O3 (NPs) | Atomic layer deposition | 316L SS | In vitro (in simulation body fluid solution) | NA | The corrosion rate calculated by current density was 1.913 mpy for an uncoated sample and reduced after coated to 0.203 mpy for titania film, 0.174 mpy for alumina film, and 0.164 mpy for multilayer. The corrosion resistance was effectively enhanced by thin films, multilayer proved to be more corrosion protection than single layers, and Al2O3 had better corrosion resistance than TiO2. | Antibacterial effectiveness Biocompatibility |
| [158] | Al2O3/TiO2 Multilayer | Atomic layer deposition | 316L SS | In vitro (in simulation body fluid solution) | NA | The corrosion rate calculated by current density was 1.913 mpy for an uncoated sample and reduced after coated to 0.203 mpy for titania film, 0.174 mpy for alumina film, and 0.164 mpy for multilayer. The corrosion resistance was effectively enhanced by thin films, multilayer proved to be more corrosion protection than single layers, and Al2O3 had better corrosion resistance than TiO2. | Antibacterial effectiveness |
| [70] | Al-SiO2 | Magnetron sputtering | NiTi and SS archwires | In vitro (in artificial saliva) | NA | The Icorr values decreased from 23.72 to 1.21 μA/cm2 and from 0.22 to 0.06 μA/cm2 after coating with Al-SiO2 on the NiTi archwires and SS archwires, respectively. | Friction reduction effectiveness Wear resistance Biocompatibility |
| [188] | B-HAp | Electrophoretic deposition | NiTi alloy | In vitro (in simulated body fluid) | NA | The corrosion rate of 10 wt.% B-HAp and 15 wt.% B-Hap coatings reduced from 0.055 to 0.046 and 0.036 mpy, respectively, which showed better corrosion-resistant effectiveness than other concentrations and only-HAp coatings. | Adhesion strength (up to 20–30 Mpa) |
| [184] | Epoxy | NA (commercial) | NiTi archwires | In vitro (in artificial saliva) | NA | The Ni release was reduced from 8.36 to 0.57 mg/L. | Durability (>30 days) |
| [136] | F-O-Ti | Plasma-enhanced fluorine and oxygen mono/dual chemical vapor deposition | Commercially available pure titanium with 99.9% purity | In vitro (in suspensions of Staphylococcus aureus and artificial saliva) | A large amount of convex texture with 100–200 nm size distributes uniformly all over the surface. | Icorr reduced from 0.22 to 0.09 μA/cm2 | Antibacterial effectiveness Durability (~7 days) Biocompatibility |
| [193] | GB-PP | Pulsed-direct current plasma-enhanced chemical vapor deposition | AISI 304 SS | In vitro (in modified Fusayama artificial saliva) | Ra increased from 0.062 to 10.64 μm after being coated. | The coating particles were retained on surfaces even after being worn 500 times by a toothbrush, peanut, and nougat, while the uncoated substrates showed some scratching and surface pitting or cavity after the wear tests, especially by peanut and nougat. | Wear resistance Durability |
| [89] | GO | Silane coupling | NiTi archwires | In vitro (friction test: NA; antibacterial test: in bacterial suspension; corrosion test: artificial saliva) | The surface of the samples coated with 2 mg/mL GO concentrations was smooth with a uniformly coated area. | When coated with 0.5, 2, or 5 mg/mL GO concentrations, nickel ion release decreased from 20.75 to 19.75, 18.00, and 17.75 μg/L cm2, weight loss decreased from 0.31% to 0.29%, 0.25%, and 0.23%, Icorr decreased from 0.696 to 0.547, 0.381 and 0.504 μA/cm2, upon application of 4 mm dislocation, respectively. | Friction reduction effectiveness Antibacterial effectiveness Wear resistance Biocompatibility |
| [181] | GO | Electrophoretic deposition | NiTi alloy | In vitro (in 3.5% NaCl solution) | Ra increased from 7.55 to 11.67 nm after being coated. | Icorr reduced from 0.158 to 0.017 μA/cm2, and Ecorr increased from −0.170 to 0.031 V vs. SCE, which exhibited good corrosion resistance. | Biocompatibility |
| [181] | GO/Ag | Electrophoretic deposition | NiTi alloy | In vitro (in 3.5% NaCl solution) | Ra increased from 7.55 to 18.43 nm after being coated. | Icorr reduced from 0.158 to 0.002 μA/cm2, and Ecorr increased from −0.170 to 0.008 V vs. SCE, which exhibited good corrosion resistance. | Biocompatibility |
| [154] | Lysozyme | Liquid phase deposition | CAWs | In vitro (in suspensions of Staphylococcus aureus and artificial saliva) | The surface roughness increased after being coated according to two- and three-dimensional atomic force micrographs. | When coated with 20, 40, and 60 g/L lysozyme, the copper ion release of the archwires reduced from 0.225 to 0.20, 0.1,5, and 0.125 μg, respectively. | Antibacterial effectiveness Wear resistance Durability (>2 weeks) Biocompatibility |
| [174] | Ni-free oxide layer | Pulsed anodization | NiTi plate | In vitro (in PBS solution) | Ra of the anodized surfaces, calculated from the SPM image, was 1.78 nm for the current-anodized surface and 1.16 nm for the pulse-anodized surface. The current-anodized surface included tiny pores (~10 nm), where the depth was determined as ~10 nm; these pores did not appear on the pulse-anodized surface. | The pores on the surface reduced, and Ni release of a pulse-anodized surface at 168 h reduced from ~0.55 to ~0.2 μg·cm−2; however, Ni release of a current-anodized surface at 168 h increased from ~0.55 to ~1.0 μg·cm−2. | Durability (>168 h) Biocompatibility |
| [57] | Ni-Ti-Cr | Chronopotentiometry | NiTi archwires | In vitro (in artificial saliva) | NA | Ecorr of electrodes of coated archwires (−427–328 mV) were higher than the uncoated archwires (−447 mV), which represented the corrosion-resistant effectiveness of the coatings. | Friction reduction effectiveness Durability (>60 days) |
| [57] | Ni-Ti-Mo | Chronopotentiometry | NiTi archwires | In vitro (in artificial saliva) | NA | Ecorr of electrodes of coated archwires (−395−366 mV) were higher than the uncoated archwires (−447 mV), which represented the corrosion-resistant effectiveness of the coatings. | Friction reduction effectiveness Durability (>60 days) |
| [157] | PTFE | NA | 316L SS plates | In vitro (in suspensions of Escherichia coli) | The surface roughness increased from 59.4 ± 6.1 nm to 134.7 ± 3.9 nm after being coated. | The PTFE coating exhibited the best substrate protection as the Icorr parameter was over one order of magnitude lower in value than the 316L SS substrate. | Antibacterial effectiveness Biocompatibility |
| [185] | PTFE | NA (commercial) | NiTi archwires | In vitro (using before-use and after-use samples) | NA | A significant reduction (17.2%) in the percentage of Ni was observed between before- and after-use archwires, which exhibited an unsatisfactory corrosion resistance. | Wear resistance Durability (>2 months) Biocompatibility |
| [185] | Rhodium | NA (commercial) | NiTi archwires | In vitro (using before-use and after-use samples) | NA | A significant reduction (9.6%) in the percentage of Ni was observed between before- and after-use archwires, which exhibited an unsatisfactory corrosion resistance. | Wear resistance Durability (>2 months) Biocompatibility |
| [184] | Rhodium | NA (commercial) | NiTi archwires | In vitro (in artificial saliva) | NA | The Ni release was reduced from 8.36 to 1.52 mg/L. | Durability (>30 days) |
| [31] | TiCN | Direct current reactive magnetron sputtering | 316L SS and (100)-oriented Si substrates | In vitro (in artificial saliva) | NA | The corrosion resistance of TiCN increased with the nitrogen content and carbon content of the preparation process when the N2 flux was larger than 2.5 sccm. | Friction reduction effectiveness Aesthetic effect |
| [166] | TiCN | Multi-arc ion plating | 316L SS plates | In vitro (in artificial saliva) | NA | The TiCN film showed a corrosion current density of ~7 μA/cm2, which showed better corrosion resistance than TiN, but not as good as Ti-DLC. | NA |
| [167] | TiCN | Magnetron sputtering | Ni-Cr-Mo disks | In vitro (dry condition) | Ra increased from 0.33 to 0.38–0.45 μm after being coated. | There were no visible areas with an elevated content of nickel or chromium, which proved that the base had not been revealed. | Wear resistance |
| [168] | TiCN | Magnetron sputtering | Ni-Cr alloy | In vitro (in neutral salt spray and seawater acetic acid) | NA | The mass loss of the examined samples reduced from 0.37 to ~0.20 mg/mm2 × 10−4. | Durability (>30 days) |
| [176] | Ti-Cr-N | Radiofrequency reactive sputtering | 304 SS | In vitro (in artificial saliva) | The RMS roughness increased from 5 to 105–20 nm after being coated. | Ti-Cr-N coatings reduced 304 SS’s release of chromium species by ~67%, and annealed at 400 °C displayed higher corrosion resistance. | Durability (>90 days) |
| [166] | Ti-DLC | Multi-arc ion plating | 316L SS plates | In vitro (in artificial saliva) | The surface roughness was 10.4 nm after being coated. | The Ti-DLC film showed the lowest corrosion current density (~4.577 μA/cm2) and thickness reduction (~0.12 μm) in different electrolytes compared to TiN and TiCN. | Wear resistance |
| [31] | TiN | Direct current reactive magnetron sputtering | 316 L SS and (100)-oriented Si substrates | In vitro (in artificial saliva) | NA | The corrosion resistance of TiN decreased with the nitrogen content and carbon content used in the preparation process. | Friction reduction effectiveness Wear resistance |
| [58] | TiN | Ion plating | SS and NiTi archwires | In vitro (friction test: NA; corrosion test: in 0.9% NaCl solution) | Ra of TiN-coated SS archwire increased from 0.023 to 0.046 µm. Ra of TiN-coated NiTi archwire remained at 0.001 µm. | Corrosion resistance increased in coated archwires. The pitting corrosion was reduced compared to uncoated archwires. The breakdown potential of the non-coated and the TiN-coated SS wire was 0.46 and 0.61 V (p < 0.05), and that of the non-coated and the TiN-coated Ni-Ti wire was 1.20 V and more than 2.0 V, respectively. | Friction reduction effectiveness |
| [166] | TiN | Multi-arc ion plating | 316L SS plates | In vitro (in artificial saliva) | NA | The TiN film showed a corrosion current density of ~8.5 μA/cm2, which showed lower corrosion resistance than TiCN and Ti-DLC. | NA |
| [19] | TiN | Hollow cathode discharge | SS and NiTi archwires | In vitro (in physiological saline, sterile water, 35% hydrochloric acid, and 88% lactic acid) | When SS archwires were coated, Ra increased from ~0.025 to ~0.04 μm; when NiTi archwires were coated, Ra increased from ~0.14 to ~0.15 μm. | The acid-mediated corrosion and the elution of Ni ions from the wire surface reduced from 2.38, 3.37, 215, 499.84, and 2.29 μg/L to 1.35, 4.16, 13536.28, and 1.44 μg/L after being immersed for 30 min in sterile water, physiological saline, 35% hydrochloric acid, and 88% lactic acid, respectively. | NA |
| [33] | TiN/Ti | High-power magnetron sputtering deposition | Ti6Al4V plate | In vitro | The average grain size was 9.1–11.6 μm after being coated. | The 1-layer, 2-layer, and 4-layer TiN/Ti multilayer coatings had similar wear rates which were less than 1/20 of the wear rate of the Ti6Al4V substrate, while the wear rates of the 8-layer and 12-layer TiN/Ti multilayer coatings were higher than that of the Ti6Al4V substrate. | Wear resistant (1-layer, 2-layer, and 4-layer TiN/Ti multilayer coatings) |
| [177] | Ti-Nb | Laser cladding | Cold-rolled NiTi alloy | In vitro (in simulated body fluid) | NA | Icorr reduced from 272.4 to 163.7 nA/cm2 and Ecorr increased from −0.184 to −0.128 V, which exhibited a good corrosion resistance. | NA |
| [173] | TiO2 | Sol-gel dip-coating | SS archwires | In vitro (in Ringer’s solution) | NA | Icorr increased from 0.007 to 39.9 μA/cm2 and Ecorr reduced from −162 to −300 mV, which exhibited an unsatisfactory corrosion resistance. | NA |
| [158] | TiO2 (NPs) | Atomic layer deposition | 316L SS | In vitro (in simulation body fluid solution) | NA | The corrosion rate calculated by current density was 1.913 mpy for an uncoated sample and reduced after coated to 0.203 mpy for titania film, 0.174 mpy for alumina film, and 0.164 mpy for multilayer. The corrosion resistance was effectively enhanced by thin films, multilayer proved to be more corrosion protection than single layers, and Al2O3 had better corrosion resistance than TiO2. | Antibacterial effectiveness Biocompatibility |
| [173] | TiO2/Ag | Sol-gel dip-coating | SS archwires | In vitro (in Ringer’s solution) | NA | Icorr increased from 0.007 to 30.0 μA/cm2 and Ecorr reduced from −162 to −285 mV, which exhibited an unsatisfactory corrosion resistance. | Wear resistance |
| [159] | ZrO2 | EBPVD | 316L SS | In vitro (in artificial saliva and artificial saliva containing 0.2% or 2% of NaF) | Ra reduced from 10 to 3 nm after being coated. | Icorr reduced from 0.34–9.27 to 0.04–0.46 μA/cm2; Ecorr increased from −0.08775 to −0.0739, from −0.2122 to −0.1267, and from −0.2831 to −0.1714 mV when immersed in artificial saliva, artificial saliva containing 0.2% NaF, and artificial saliva containing 2% of NaF, respectively, which exhibited a good corrosion resistance. | Antibacterial effectiveness Wear resistance Biocompatibility |
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and Surface Treatments on Orthodontic Metallic Materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Lee, S.M.; Hwang, C.-J. A comparative study of frictional force in self-ligating brackets according to the bracket-archwire angulation, bracket material, and wire type. Korean J. Orthod. 2015, 45, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Bluestein, M.; Moore, B.K.; Benson, G. Friction in perspective. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Julien, K.C.; Buschang, P.H.; Campbell, P.M. Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthod. 2013, 83, 641–647. [Google Scholar] [CrossRef]
- Øgaard, B.; Rølla, G.; Arends, J. Orthodontic appliances and enamel demineralization: Part 1. Lesion development. Am. J. Orthod. Dentofac. Orthop. 1988, 94, 68–73. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. The Continuum of Dental Caries—Evidence for a Dynamic Disease Process. J. Dent. Res. 2004, 83, 39–42. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Cong, J. The periodontal status of removable appliances vs fixed appliances: A comparative meta-analysis. Medicine 2020, 99, e23165. [Google Scholar] [CrossRef]
- Beberhold, K.; Sachse-Kulp, A.; Schwestka-Polly, R.; Hornecker, E.; Ziebolz, D. The Orthodontic Plaque Index: An oral hygiene index for patients with multibracket appliances. Orthod. Art Pract. Dentofac. Enhanc. 2012, 13, 94–99. [Google Scholar]
- House, K.; Sernetz, F.; Dymock, D.; Sandy, J.R.; Ireland, A.J. Corrosion of orthodontic appliances—Should we care? Am. J. Orthod. Dentofac. Orthop. 2008, 133, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, T.P.; Upadhayay, S.N. An overview of orthodontic material degradation in oral cavity. Indian J. Dent. Res. 2010, 21, 275–284. [Google Scholar] [CrossRef]
- Eliades, T.; Athanasiou, A.E. In vivo aging of orthodontic alloys: Implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002, 72, 222–237. [Google Scholar]
- Chaturvedi, T.P. Corrosion Behaviour of Orthodontic Alloys—A Review; Banaras Hindu University: Varanasi, India, 2008. [Google Scholar]
- Pulikkottil, V.J.; Chidambaram, S.; Bejoy, P.U.; Femin, P.K.; Paul, P.; Rishad, M. Corrosion resistance of stainless steel, nickel-titanium, titanium molybdenum alloy, and ion-implanted titanium molybdenum alloy archwires in acidic fluoride-containing artificial saliva: An in vitro study. J. Pharm. Bioallied Sci. 2016, 8, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cameán, A.; Jos, A.; Puerto, M.; Calleja, A.; Iglesias-Linares, A.; Solano, E.; Cameán, A.M. In vivo determination of aluminum, cobalt, chromium, copper, nickel, titanium and vanadium in oral mucosa cells from orthodontic patients with mini-implants by Inductively coupled plasma-mass spectrometry (ICP-MS). J. Trace Elem. Med. Biol. 2015, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kovac, V.; Poljsak, B.; Bergant, M.; Scancar, J.; Mezeg, U.; Primozic, J. Differences in Metal Ions Released from Orthodontic Appliances in an In Vitro and In Vivo Setting. Coatings 2022, 12, 190. [Google Scholar] [CrossRef]
- Rahilly, G.; Price, N. Nickel allergy and orthodontics. J. Orthod. 2003, 30, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Ahing, S.I.; Karaiskos, N.E.; Wiltshire, W.A. Nickel allergy and orthodontics, a review and report of two cases. Br. Dent. J. 2008, 204, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Gracco, A.; Dandrea, M.; Deflorian, F.; Zanella, C.; De Stefani, A.; Bruno, G.; Stellini, E. Application of a Molybdenum and Tungsten Disulfide Coating to Improve Tribological Properties of Orthodontic Archwires. Nanomaterials 2019, 9, 753. [Google Scholar] [CrossRef]
- Ito, A.; Kitaura, H.; Sugisawa, H.; Noguchi, T.; Ohori, F.; Mizoguchi, I. Titanium Nitride Plating Reduces Nickel Ion Release from Orthodontic Wire. Appl. Sci. 2021, 11, 9745. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, C.; Ge, Z.; Li, Z.; Chen, L.; Guo, X.; Dong, G.; Zhou, P. A novel antibacterial and fluorescent coating composed of polydopamine and carbon dots on the surface of orthodontic brackets. J. Mater. Sci. Mater. Med. 2023, 34, 10. [Google Scholar] [CrossRef]
- Usui, T.; Iwata, T.; Miyake, S.; Otsuka, T.; Koizumi, S.; Shirakawa, N.; Kawata, T. Mechanical and frictional properties of aesthetic orthodontic wires obtained by hard chrome carbide plating. J. Dent. Sci. 2018, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Redlich, M.; Rapoport, L.; Wagner, H.D.; Tenne, R. Self-lubricating coatings containing fullerene-like WS2 nanoparticles for orthodontic wires and other possible medical applications. Tribol. Lett. 2006, 21, 135–139. [Google Scholar] [CrossRef]
- Kachoei, M.; Eskandarinejad, F.; Divband, B.; Khatamian, M. The effect of zinc oxide nanoparticles deposition for friction reduction on orthodontic wires. Dent. Res. J. 2013, 10, 499–505. [Google Scholar]
- Elhelbawy, N.; Ellaithy, M. Comparative evaluation of Stainless-steel wires and brackets coated with nanoparticles of Chitosan or Zinc oxide upon friction: An in vitro study. Int. Orthod. 2021, 19, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Kachoei, M.; Nourian, A.; Divband, B.; Kachoei, Z.; Shirazi, S. Zinc-oxide nanocoating for improvement of the antibacterial and frictional behavior of nickel-titanium alloy. Nanomedicine 2016, 11, 2511–2527. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, A.; Kachoei, M.; Khatamian, M.; Divband, B. The effect of ZnO nanoparticle coating on the frictional resistance between orthodontic wires and ceramic brackets. J. Dent. Res. Dent. Clin. Dent. Prospect. 2016, 10, 106. [Google Scholar] [CrossRef]
- Hammad, S.M.; El-Wassefy, N.A.; Shamaa, M.S.; Fathy, A. Evaluation of zinc-oxide nanocoating on the characteristics and antibacterial behavior of nickel-titanium alloy. Dent. Press J. Orthod. 2020, 25, 51–58. [Google Scholar] [CrossRef]
- Kao, C.-T.; Guo, J.-U.; Huang, T.-H. Comparison of friction force between corroded and noncorroded titanium nitride plating of metal brackets. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 594–600. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Shang, H.; Lin, J. Comparison of TiN and CNx coatings on orthodontic stainless steel: Tribological and biological evaluation. Surf. Coat. Technol. 2019, 362, 381–387. [Google Scholar] [CrossRef]
- Arici, N.; Akdeniz, B.S.; Oz, A.A.; Gencer, Y.; Tarakci, M.; Arici, S. Effectiveness of medical coating materials in decreasing friction between orthodontic brackets and archwires. Korean J. Orthod. 2021, 51, 270–281. [Google Scholar] [CrossRef]
- Suciu, V.; Ferreira, A.; Correa, M.A.; Vaz, F.; Munteanu, D. Structural, Mechanical, and Decorative Properties of Sputtered TiN and Ti (N, C) Films for Orthodontic Applications; an In Vitro Study. Materials 2021, 14, 5175. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, J.; He, H.; Xie, Y. Comparative Investigation on the Tribological Performances of TiN, TiCN, and Ti-DLC Film-Coated Stainless Steel. JOM 2019, 71, 4872–4879. [Google Scholar] [CrossRef]
- Sheng, L.; Xiao, Y.; Jiao, C.; Du, B.; Li, Y.; Wu, Z.; Shao, L. Influence of layer number on microstructure, mechanical properties and wear behavior of the TiN/Ti multilayer coatings fabricated by high-power magnetron sputtering deposition. J. Manuf. Process. 2021, 70, 529–542. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Husmann, P.; Bourauel, C.; Wessinger, M.; Jäger, A. The frictional behavior of coated guiding archwires. J. Orofac. Orthop. /Fortschr. Kieferorthopädie 2002, 63, 199–211. [Google Scholar] [CrossRef]
- Farronato, G.; Maijer, R.; Carìa, M.P.; Esposito, L.; Alberzoni, D.; Cacciatore, G. The effect of Teflon coating on the resistance to sliding of orthodontic archwires. Eur. J. Orthod. 2012, 34, 410–417. [Google Scholar] [CrossRef]
- Katta, A.; Vannala, V.; Navaneethakrishnan, K.K.; Kandasamy, R.; Venkatachalam, B.; Arafath, M. Coated, uncoated stainless steel ligatures versus Self ligation-In the perspective of friction. Pak. Orthod. J. 2013, 5, 60–66. [Google Scholar]
- Sukh, R.; Singh, G.K.; Tandon, P.; Singh, G.P.; Singh, A. A comparative study of frictional resistance during simulated canine retraction on typodont model. J. Orthod. Sci. 2013, 2, 61–66. [Google Scholar] [CrossRef]
- Abbas, A.A.; Alhuwaizi, A.F. The effect of wire dimension, type and thickness of coating layer on friction of coated stainless-steel arch wires. Int. J. Med. Res. Health Sci. 2018, 7, 115–121. [Google Scholar]
- Kawaguchi, K.; Iijima, M.; Muguruma, T.; Endo, K.; Mizoguchi, I. Effects of bioactive glass coating by electrophoretic deposition on esthetical, bending, and frictional performance of orthodontic stainless steel wire. Dent. Mater. J. 2020, 39, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, A.R.; Shetty, P.C.; Bhat, N.S.; Ramachandra, C.S.; Laxmikanth, S.M.; Nagarahalli, K.; Tekale, P.D. Antiadherent and antibacterial properties of stainless steel and NiTi orthodontic wires coated with silver against Lactobacillus acidophilus—An in vitro study. Prog. Orthod. 2015, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Redlich, M.; Katz, A.; Rapoport, L.; Wagner, H.D.; Feldman, Y.; Tenne, R. Improved orthodontic stainless steel wires coated with inorganic fullerene-like nanoparticles of WS2 impregnated in electroless nickel–phosphorous film. Dent. Mater. 2008, 24, 1640–1646. [Google Scholar] [CrossRef]
- Chow, P.K.; Singh, E.; Viana, B.C.; Gao, J.; Luo, J.; Li, J.; Lin, Z.; Elías, A.L.; Shi, Y.; Wang, Z.; et al. Wetting of Mono and Few-Layered WS2 and MoS2 Films Supported on Si/SiO2 Substrates. ACS Nano 2015, 9, 3023–3031. [Google Scholar] [CrossRef]
- Kozbial, A.; Gong, X.; Liu, H.; Li, L. Understanding the Intrinsic Water Wettability of Molybdenum Disulfide (MoS2). Langmuir 2015, 31, 8429–8435. [Google Scholar] [CrossRef]
- Wang, L.; Jibin, P.U.; Wang, H.; Zeng, C.; Xue, Q. Tantalum-Doped Molybdenum Disulfide/Tungsten Disulfide Multi-Layer Film as Well as Preparation Method and Use Thereof. U.S. Patent Application US20220341023A1, 27 October 2022. [Google Scholar]
- Wang, P.; Luo, X.; Qin, J.; Pan, Z.; Zhou, K. Effect of Graphene Sheets Embedded Carbon Films on the Fretting Wear Behaviors of Orthodontic Archwire–Bracket Contacts. Nanomaterials 2022, 12, 3430. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.; Bradford, B.; Castelli, D.; Dufour, E.; Nash, J.F.; Pape, W.; Schulte, S.; Tooley, I.; van den Bosch, J.; Schellauf, F. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem. Photobiol. Sci. 2010, 9, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Karandish, M.; Pakshir, M.; Moghimi, M.; Jafarpour, D. Evaluating the Mechanical Properties of Zinc-Coated Stainless Steel Orthodontic Wires Using Physical Vapor Deposition. Int. J. Dent. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.B.; Bari, R.H. Nanostructured ZrO2 thin films deposited by spray pyrolysis techniques for ammonia gas sensing application. Int. Lett. Chem. Phys. Astron. 2015, 56, 120–130. [Google Scholar] [CrossRef]
- Sollazzo, V.; Pezzetti, F.; Scarano, A.; Piattelli, A.; Bignozzi, C.A.; Massari, L.; Brunelli, G.; Carinci, F. Zirconium oxide coating improves implant osseointegration in vivo. Dent. Mater. 2008, 24, 357–361. [Google Scholar] [CrossRef]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Golshah, A.; Feyli, S.A. Effect of zirconium oxide nano-coating on frictional resistance of orthodontic wires. J. Orthod. Sci. 2022, 11, 35. [Google Scholar] [CrossRef]
- Davidson, J.A. Zirconium Oxide Coated Prosthesis for Wear and Corrosion Resistance. U.S. Patent Application US5037438A, 6 August 1991. [Google Scholar]
- Zhang, Z.X.; Fu, B.F.; Zhang, D.Y.; Cheng, Y.; Sheng, L.Y.; Lai, C.; Xi, T.F. Safety and efficacy of nano lamellar TiN coatings on nitinol atrial septal defect occluders in vivo. Mater. Sci. Eng. C 2013, 33, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, C.Y.; Kim, D.W.; Lee, I.S.; Lee, G.H.; Park, J.C.; Lee, S.J.; Lee, K.Y. Wear performance of self-mating contact pairs of TiN and TiAlN coatings on orthopedic grade Ti-6Al-4V. Biomed. Mater. 2010, 5, 044108. [Google Scholar] [CrossRef]
- Steele, J.G.; McCabe, J.F.; Barnes, I.E. Properties of a titanium nitride coating for dental instruments. J. Dent. 1991, 19, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Zortuk, F.B.; Özyılmaz, A.T. Evaluation of the surface properties of modified Ni-Ti arch wires. Int. Dent. Res. 2021, 11, 167–175. [Google Scholar] [CrossRef]
- Sugisawa, H.; Kitaura, H.; Ueda, K.; Kimura, K.; Ishida, M.; Ochi, Y.; Kishikawa, A.; Ogawa, S.; Takano-Yamamoto, T. Corrosion resistance and mechanical properties of titanium nitride plating on orthodontic wires. Dent. Mater. J. 2018, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Krishnan, A.; Remya, R.; Ravikumar, K.K.; Nair, S.A.; Shibli, S.M.A.; Varma, H.K.; Sukumaran, K.; Kumar, K.J. Development and evaluation of two PVD-coated β-titanium orthodontic archwires for fluoride-induced corrosion protection. Acta Biomater. 2011, 7, 1913–1927. [Google Scholar] [CrossRef]
- da Silveira, R.E.; Elias, C.N.; do Amaral, F.L.B. Assessment of frictional resistance and surface roughness in orthodontic wires coated with two different nanoparticles. Microsc. Res. Tech. 2022, 85, 1884–1890. [Google Scholar] [CrossRef]
- Maliael, M.T.; Jain, R.K.; Srirengalakshmi, M. Effect of Nanoparticle Coatings on Frictional Resistance of Orthodontic Archwires: A Systematic Review and Meta-analysis. World J. Dent. 2022, 13, 417–424. [Google Scholar]
- Syed, S.S.; Kulkarni, D.; Todkar, R.; Bagul, R.S.; Parekh, K.; Bhujbal, N. A novel method of coating orthodontic archwires with nanoparticles. J. Int. Oral Health JIOH 2015, 7, 30. [Google Scholar]
- Ghasemi, T.; Arash, V.; Rabiee, S.M.; Rajabnia, R.; Pourzare, A.; Rakhshan, V. Antimicrobial effect, frictional resistance, and surface roughness of stainless steel orthodontic brackets coated with nanofilms of silver and titanium oxide: A preliminary study. Microsc. Res. Tech. 2017, 80, 599–607. [Google Scholar] [CrossRef]
- Jung, S.-C.; Kim, S.-J.; Imaishi, N.; Cho, Y.-I. Effect of TiO2 thin film thickness and specific surface area by low-pressure metal–organic chemical vapor deposition on photocatalytic activities. Appl. Catal. B Environ. 2005, 55, 253–257. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Attar, H. Selective Laser Melting of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Review. Adv. Eng. Mater. 2015, 18, 463–475. [Google Scholar] [CrossRef]
- Krishnan, M.; Seema, S.; Tiwari, B.; Sharma, H.S.; Londhe, S.; Arora, V. Surface characterization of nickel titanium orthodontic arch wires. Med. J. Armed Forces India 2015, 71, S340–S345. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Sharma, P.; Goje, S.K. Comparative Evaluation of Frictional Resistance of Silver-Coated Stainless Steel Wires with Uncoated Stainless Steel Wires: An In vitro Study. Contemp. Clin. Dent. 2018, 9, S331–S336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, D.; Li, Y.; Miao, K.; Bao, X.; Hu, M.; Zhang, K. Tribological and biological assessments of TaAgB solid solution coatings for orthodontic treatment. Appl. Surf. Sci. 2022, 597, 153704. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Xiaoyi, L.; Shaoqin, Z.; Horváth, P.G.; Bak, M.; Bejó, L.; Sipos, G.; Alpár, T. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023—A review on game changing materials. Heliyon 2022, 8, e12322. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Yan, Y.; Zheng, B.; Algahefi, A.L.; Ma, S.; Liu, Y. Study of Al–SiO2 Aesthetic Composite Coating on Orthodontic Metal Archwire. Coatings 2022, 12, 746. [Google Scholar] [CrossRef]
- Nik, T.H.; Ghadirian, H.; Hooshmand, T.; Kharazifard, M.J.; Nasiri, M.; Mahd, M.J. Effect of 0.05% Sodium Fluoride Mouthwash on Surface Roughness and Friction between Ceramic Brackets and Rhodium-Coated and Uncoated Stainless Steel Wires. Front. Dent. 2019, 16, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hwang, E.Y.; Park, H.K.; Park, Y.G. Correlation between frictional force and surface roughness of orthodontic archwires. Scanning 2015, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kusy, R.P.; Whitley, J.Q.; Mayhew, M.J.; Buckthal, J.E. Surface roughness of orthodontic archwires via laser spectroscopy. Angle Orthod. 1988, 58, 33–45. [Google Scholar] [PubMed]
- Kusy, R.P.; Whitley, J.Q. Effects of surface roughness on the coefficients of friction in model orthodontic systems. J. Biomech. 1990, 23, 913–925. [Google Scholar] [CrossRef]
- Aldabagh, D.J.; Alzubaydi, T.L.; Alhuwaizi, A.F. Surface Characterization of Stainless Steel 316L Coated with Various Nanoparticle Types. Int. J. Biomater. 2023, 2023, 3997281. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohgoe, Y.; Ozeki, K.; Sato, K.; Sumiya, T.; Hirakuri, K.K.; Aoki, H. Diamond-like carbon coatings on orthodontic archwires. Diam. Relat. Mater. 2005, 14, 1094–1097. [Google Scholar] [CrossRef]
- Akaike, S.; Hayakawa, T.; Kobayashi, D.; Aono, Y.; Hirata, A.; Hiratsuka, M.; Nakamura, Y. Reduction in static friction by deposition of a homogeneous diamond-like carbon (DLC) coating on orthodontic brackets. Dent. Mater. J. 2015, 34, 888–895. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Nagano-Takebe, F.; Endo, K.; Mizoguchi, I. Frictional Properties and Characterization of a Diamond-Like Carbon Coating Formed on Orthodontic Stainless Steel. J. Biomater. Tissue Eng. 2017, 7, 119–126. [Google Scholar] [CrossRef]
- Huang, T.-H.; Guo, J.-U.; Kao, C.-T. A comparison of the friction associated with diamond-like carbon (DLC) or titanium nitride (TiN) plating metal brackets. Surf. Coat. Technol. 2010, 205, 1917–1921. [Google Scholar] [CrossRef]
- Kang, T.; Huang, S.-Y.; Huang, J.-J.; Li, Q.-H.; Diao, D.-F.; Duan, Y.-Z. The effects of diamond-like carbon films on fretting wear behavior of orthodontic archwire-bracket contacts. J. Nanosci. Nanotechnol. 2015, 15, 4641–4647. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Brantley, W.A.; Mizoguchi, I. Effects of a diamond-like carbon coating on the frictional properties of orthodontic wires. Angle Orthod. 2011, 81, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, S.; Wang, D.; Zhou, T.; Wang, L.; Ma, J. Effects of nanostructured, diamondlike, carbon coating and nitrocarburizing on the frictional properties and biocompatibility of orthodontic stainless steel wires. Angle Orthod. 2016, 86, 782–788. [Google Scholar] [CrossRef]
- Huang, S.Y.; Huang, J.J.; Kang, T.; Diao, D.F.; Duan, Y.Z. Coating NiTi archwires with diamond-like carbon films: Reducing fluoride-induced corrosion and improving frictional properties. J. Mater. Sci. Mater. Med. 2013, 24, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Tantiwinyupong, N.; Chintavalakorn, R.; Santiwong, P.; Khantachawana, A. Frictional and Mechanical Properties of Surface Modified Nickel-Titanium Orthodontic Archwires. Key Eng. Mater. 2019, 801, 39–43. [Google Scholar] [CrossRef]
- Akaike, S.; Kobayashi, D.; Aono, Y.; Hiratsuka, M.; Hirata, A.; Hayakawa, T.; Nakamura, Y. Relationship between static friction and surface wettability of orthodontic brackets coated with diamond-like carbon (DLC), fluorine-or silicone-doped DLC coatings. Diam. Relat. Mater. 2016, 61, 109–114. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Brantley, W.A.; Nakagaki, S.; Endo, K.; Mizoguchi, I. Frictional and mechanical properties of diamond-like carbon-coated orthodontic brackets. Eur. J. Orthod. 2011, 35, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, T.; Iijima, M.; Kawaguchi, M.; Mizoguchi, I. Effects of sp2/sp3 Ratio and Hydrogen Content on In Vitro Bending and Frictional Performance of DLC-Coated Orthodontic Stainless Steels. Coatings 2018, 8, 199. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Graphene oxide/silver nanoparticle coating produced by electrophoretic deposition improved the mechanical and tribological properties of NiTi alloy for biomedical applications. J. Nanosci. Nanotechnol. 2019, 19, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Zhou, D.; He, L.; Wang, C.; Zhang, C. Graphene oxide nanocoating for enhanced corrosion resistance, wear resistance and antibacterial activity of nickel-titanium shape memory alloy. Surf. Coat. Technol. 2022, 431, 128012. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, Q.; Wang, P.; Diao, D. Robust low friction performance of graphene sheets embedded carbon films coated orthodontic stainless steel archwires. Friction 2021, 10, 142–158. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Shamohammadi, M.; Rastegaar, Z.; Skini, M.; Rakhshan, V. Effect of esthetic coating on surface roughness of orthodontic archwires. Int. Orthod. 2017, 15, 312–321. [Google Scholar] [CrossRef]
- Bhat, K.R.R.; Ahmed, N.; Joseph, R.; Abrar, A.Y. Comparative Evaluation of Frictional Resistance between Different Types of Ceramic Brackets and Stainless Steel Brackets with Teflon-Coated Stainless Steel and Stainless Steel Archwires: An In-Vitro Study. Cureus 2022, 14, e24161. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghroosh, D.H.; Basim, A.; Nahidh, M.; Ghazi, A. Assessment of Static Friction Generated from Different Aesthetic Archwires (In-Vitro Study). J. Pharm. Sci. Res. 2018, 10, 3310. [Google Scholar]
- Joseph, J.N.; Ammayappan, P.; Sankar, H.; Yashwant, V.A.; Alexander, L. Comparison of frictional resistance of different esthetic archwires in different esthetic brackets in dry and wet fields: An in vitro study. J. Dent. Res. Rev. 2021, 8, 176. [Google Scholar] [CrossRef]
- Kameda, T.; Sato, H.; Oka, S.; Miyazaki, A.; Ohkuma, K.; Terada, K. Low temperature polytetrafluoroethylene (PTFE) coating improves the appearance of orthodontic wires without changing their mechanical properties. Dent. Mater. J. 2020, 39, 721–734. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Tsuka, Y.; Nakajima, K.; Sumi, K.; Yoshimi, Y.; Kado, I.; Inada, A.; Kiritoshi, Y.; Tanimoto, K. The Influence of 2-Methacryloyloxyethyl Phosphorylcholine Polymer Materials on Orthodontic Friction and Attachment of Oral Bacteria. Materials 2022, 15, 5770. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Kim, J.S.; Lin, E.T.; Lin, E.T. Prolonged water immersion alters resistance to sliding of aesthetic orthodontic coated wires. Orthod. Craniofac. Res. 2021, 24, 111–120. [Google Scholar] [CrossRef]
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; de Camargo, E.R.; Barbosa, D.B. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Zamarreño, C.R.; Arregui, F.J.; Matías, I.R. An antibacterial coating based on a polymer/sol-gel hybrid matrix loaded with silver nanoparticles. Nanoscale Res. Lett. 2011, 6, 305. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, L.; Bai, R.; Zhuang, Z.; Zhang, Y.; Yu, T.; Peng, L.; Xin, T.; Chen, S.; Han, B. Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers 2021, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.S.; Viale, A.B.; Sormani, N.N.; Pizzol, K.E.D.C.; Araujo-Nobre, A.R.d.; Oliveira, P.C.S.d.; Barud, H.G.d.O.; Antonio, S.G.; Barud, H.d.S. Antimicrobial orthodontic wires coated with silver nanoparticles. Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Arash, V.; Keikhaee, F.; Rabiee, S.M.; Rajabnia, R.; Khafri, S.; Tavanafar, S. Evaluation of antibacterial effects of silver-coated stainless steel orthodontic brackets. J. Dent. Tehran Univ. Med. Sci. 2016, 13, 49–54. [Google Scholar]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Sawada, T.; Yamaguchi, R. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: In vitro study. Prog. Orthod. 2020, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Toral-Rizo, V.H.; López-Castañares, R.; Morales-Luckie, R.A. Synthesis and characterization of silver nanoparticles on orthodontic brackets: A new alternative in the prevention of white spots. Coatings 2019, 9, 480. [Google Scholar] [CrossRef]
- Metin-Gürsoy, G.; Taner, L.; Akca, G. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur. J. Orthod. 2017, 39, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Kobbe, V.; Doll, K.; Stiesch, M.; Schwestka-Polly, R.; Demling, A. Comparison of intraoral biofilm reduction on silver-coated and silver ion-implanted stainless steel bracket material: Biofilm reduction on silver ion-implanted bracket material. J. Orofac. Orthop./Fortschr. Kieferorthopädie 2018, 80, 32–43. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Bae, I.-H.; Lee, K.-G.; Hwang, H.-S.; Lee, K.-H.; Koh, J.-T.; Cho, J.-H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2012, 82, 151–157. [Google Scholar] [CrossRef]
- Fatani, E.J.; Almutairi, H.H.; Alharbi, A.O.; Alnakhli, Y.O.; Divakar, D.D.; Muzaheed; Alkheraif, A.A.; Khan, A.A. In vitro assessment of stainless steel orthodontic brackets coated with titanium oxide mixed Ag for anti-adherent and antibacterial properties against Streptococcus mutans and Porphyromonas gingivalis. Microb. Pathog. 2017, 112, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, N.K.; Enany, N.M.; Mohamed, G.G.; Marzouk, E.S. The antibacterial effect of silver, zinc-oxide and combination of silver/zinc oxide nanoparticles coating of orthodontic brackets (an in vitro study). BMC Oral Health 2022, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.C.; Thomson, J.J.; Alhabeil, J.A.; Toma, J.M.; Plecha, S.C.; Pacheco, R.R.; Cuevas-Suárez, C.E.; Piva, E.; Lund, R.G. In vitro Streptococcus mutans adhesion and biofilm formation on different esthetic orthodontic archwires. Angle Orthod. 2021, 91, 786–793. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lin, Y.-C.; Hsu, W.-C.; Hou, C.-H.; Shyue, J.-J.; Hsiao, S.-Y.; Wu, P.-J.; Lee, Y.-T.; Luo, S.-C. Engineering Antifouling and Antibacterial Stainless Steel for Orthodontic Appliances through Layer-by-Layer Deposition of Nanocomposite Coatings. ACS Appl. Bio Mater. 2020, 3, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.; Yu, T.; Song, G.; Xu, T.; Xin, T.; Lin, Y.; Han, B. Nano-Based Drug Delivery Systems for Periodontal Tissue Regeneration. Pharmaceutics 2022, 14, 2250. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Peng, L.; Sun, Q.; Zhang, Y.; Zhang, L.; Wei, Y.; Han, B. Metallic Antibacterial Surface Treatments of Dental and Orthopedic Materials. Materials 2020, 13, 4594. [Google Scholar] [CrossRef]
- Chhattani, S.; Shetty, P.C.; Laxmikant, S.M.; Ramachandra, C.S. In vitro assessment of photocatalytic titanium oxide surface-modified stainless steel and nickel titanium orthodontic wires for its antiadherent and antibacterial properties against Streptococcus mutans. J. Indian Orthod. Soc. 2014, 48, 82–87. [Google Scholar] [CrossRef]
- Chun, M.-J.; Shim, E.; Kho, E.-H.; Park, K.-J.; Jung, J.; Kim, J.-M.; Kim, B.; Lee, K.-H.; Cho, D.-L.; Bai, D.-H.; et al. Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod. 2007, 77, 483–488. [Google Scholar] [CrossRef]
- Özyildiz, F.; Uzel, A.; Hazar, A.S.; Güden, M.; Ölmez, S.; Aras, I.; Karaboz, I. Photocatalytic antimicrobial effect of TiO2 anatase thin-film-coated orthodontic arch wires on 3 oral pathogens. Turk. J. Biol. 2014, 38, 289–295. [Google Scholar] [CrossRef]
- Liu, J.; Lou, Y.; Zhang, C.; Yin, S.; Li, H.; Sun, D.; Sun, X. Improved corrosion resistance and antibacterial properties of composite arch-wires by N-doped TiO2 coating. RSC Adv. 2017, 7, 43938–43949. [Google Scholar] [CrossRef]
- Özyıldız, F.; Güden, M.; Uzel, A.; Karaboz, I.; Akil, O.; Bulut, H. Antimicrobial activity of TiO2-coated orthodontic ceramic brackets against Streptococcus mutans and Candida albicans. Biotechnol. Bioprocess Eng. 2010, 15, 680–685. [Google Scholar] [CrossRef]
- Math, M.; Shah, A.G.; Gangurde, P.; Karandikar, A.G.; Gheware, A.; Jadhav, B.S. In-vitro Comparative Assessment of Antibacterial and Anti-adherent Effect of Two Types of Surface Modificants on Stainless Steel Orthodontic Brackets against Streptococcus mutans. J. Indian Orthod. Soc. 2021, 56, 282–289. [Google Scholar] [CrossRef]
- Shah, A.G.; Shetty, P.C.; Ramachandra, C.S.; Bhat, N.S.; Laxmikanth, S.M. In vitro assessment of photocatalytic titanium oxide surface modified stainless steel orthodontic brackets for antiadherent and antibacterial properties against Lactobacillus acidophilus. Angle Orthod. 2011, 81, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Chung, C.J.; Oh, K.-T.; Choi, Y.-J.; Kim, K.-H. Photocatalytic antibacterial effect of TiO2 film of TiAg on Streptococcus mutans. Angle Orthod. 2009, 79, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.D.; Subramaniam, S.; Arumugam, I.; Padmanabhan, S. Assessment of antibacterial and cytotoxic effects of orthodontic stainless steel brackets coated with different phases of titanium oxide: An in-vitro study. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 678–684. [Google Scholar] [CrossRef]
- Venkatesan, K.; Kailasam, V.; Padmanabhan, S. Evaluation of titanium dioxide coating on surface roughness of nickel-titanium archwires and its influence on Streptococcus mutans adhesion and enamel mineralization: A prospective clinical study. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mollabashi, V.; Farmany, A.; Alikhani, M.Y.; Sattari, M.; Soltanian, A.R.; Kahvand, P.; Banisafar, Z. Effects of TiO2-coated stainless steel orthodontic wires on Streptococcus mutans bacteria: A clinical study. Int. J. Nanomed. 2020, 15, 8759–8766. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, K.H.; Choy, K.C.; Oh, K.T.; Kim, K.N. Photocatalytic antibacterial effect of TiO2 film formed on Ti and TiAg exposed to Lactobacillus acidophilus. J. Biomed. Mater. Res. 2006, 80, 353–359. [Google Scholar]
- Domínguez-Espíndola, R.B.; Bruguera-Casamada, C.; Silva-Martínez, S.; Araujo, R.M.; Brillas, E.; Sirés, I. Photoelectrocatalytic inactivation of Pseudomonas aeruginosa using an Ag-decorated TiO2 photoanode. Sep. Purif. Technol. 2019, 208, 83–91. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Li, N.; Liu, B.; Zhang, Y. Preparation of an orthodontic bracket coated with an nitrogen-doped TiO2-xNy thin film and examination of its antimicrobial performance. Dent. Mater. J. 2013, 32, 311–316. [Google Scholar] [CrossRef]
- Salehi, P.; Babanouri, N.; Roein-Peikar, M.; Zare, F. Long-term antimicrobial assessment of orthodontic brackets coated with nitrogen-doped titanium dioxide against Streptococcus mutans. Prog. Orthod. 2018, 19, 35. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Fan, L.; Yue, Z.; Liu, B.; Cao, B. Highly antibacterial activity of N-doped TiO2 thin films coated on stainless steel brackets under visible light irradiation. Appl. Surf. Sci. 2014, 309, 119–127. [Google Scholar] [CrossRef]
- Monica, A.; Padmanabhan, S. The effect of nitrogen-doped titanium dioxide–modified stainless steel brackets on Streptococcus mutans: A randomized clinical trial. Angle Orthod. 2022, 92, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.P.; Gontijo, L.C.; Franco Júnior, A.R.; Pereira, M.F.; Schuenck, R.P.; Malacarne-Zanon, J. Evaluation of antimicrobial potential and surface morphology in thin films of titanium nitride and calcium phosphate on orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, H.; Wang, X.; Qin, G.; Zhang, E. Improvement in antibacterial properties and cytocompatibility of titanium by fluorine and oxygen dual plasma-based surface modification. Appl. Surf. Sci. 2019, 463, 261–274. [Google Scholar] [CrossRef]
- Mobeen, N.; Duraisamy, S.; Ravi, K. Evaluation of the Ion release from nanoparticles coated orthodontic brackets-In vitro Study. Int. J. Orthod. Rehabil. 2022, 13, 10–21. [Google Scholar] [CrossRef]
- Ramazanzadeh, B.; Jahanbin, A.; Yaghoubi, M.; Shahtahmassbi, N.; Ghazvini, K.; Shakeri, M.; Shafaee, H. Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against Streptococcus mutans. J. Dent. 2015, 16, 200–205. [Google Scholar]
- Gholami, M.; Esmaeilzadeh, M.; Kachoei, Z.; Kachoei, M.; Divband, B. Influence of Physical Dimension and Morphological-Dependent Antibacterial Characteristics of ZnO Nanoparticles Coated on Orthodontic NiTi Wires. BioMed Res. Int. 2021, 2021, 6397698. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrillo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and Antimicrobial Properties of Silver Nanoparticles against Streptococcus mutans on Brackets and Wires Used for Orthodontic Treatments. J. Nanomater. 2018, 2018, 9248527. [Google Scholar] [CrossRef]
- Kim, I.-H.; Park, H.-S.; Kim, Y.K.; Kim, K.-H.; Kwon, T.-Y. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014, 84, 680–686. [Google Scholar] [CrossRef]
- Asiry, M.A.; AlShahrani, I.; Almoammar, S.; Durgesh, B.H.; Al Kheraif, A.A.; Hashem, M.I. Influence of epoxy, polytetrafluoroethylene (PTFE) and rhodium surface coatings on surface roughness, nano-mechanical properties and biofilm adhesion of nickel titanium (Ni-Ti) archwires. Mater. Res. Express 2018, 5, 026511. [Google Scholar] [CrossRef]
- Vellwock, A.E.; Yao, H. Biomimetic and bioinspired surface topographies as a green strategy for combating biofouling: A review. Bioinspir. Biomim. 2021, 16, 041003. [Google Scholar] [CrossRef]
- Vellwock, A.E.; Su, P.; Zhang, Z.; Feng, D.; Yao, H. Reconciling the Conflict between Optical Transparency and Fouling Resistance with a Nanowrinkled Surface Inspired by Zebrafish’s Cornea. ACS Appl. Mater. Interfaces 2022, 14, 7617–7625. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Xie, Y.; Zhang, Q.; Zhang, W.; Jiang, X.; Lin, J. Biological safe gold nanoparticle-modified dental aligner prevents the Porphyromonas gingivalis biofilm formation. ACS Omega 2020, 5, 18685–18692. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, M.; Zhang, W.; Liu, X.; Zheng, W.; Jiang, X. Gold nanoclusters-coated orthodontic devices can inhibit the formation of Streptococcus mutans biofilm. ACS Biomater. Sci. Eng. 2020, 6, 1239–1246. [Google Scholar] [CrossRef]
- Raji, S.H.; Shojaei, H.; Ghorani, P.S.; Rafiei, E. Bacterial colonization on coated and uncoated orthodontic wires: A prospective clinical trial. Dent. Res. J. 2014, 11, 680–683. [Google Scholar]
- Al-Lami, A.A.; Al-Sheakli, I.I. Quantitative assessment of Mutans Streptococci adhesion to coated and uncoated orthodontic archwires: In vitro study. J. Baghdad Coll. Dent. 2014, 26, 156–162. [Google Scholar]
- Demling, A.; Elter, C.; Heidenblut, T.; Bach, F.-W.; Hahn, A.; Schwestka-Polly, R.; Stiesch, M.; Heuer, W. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur. J. Orthod. 2010, 32, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Fuchslocher Hellemann, C.; Grade, S.; Heuer, W.; Dittmer, M.P.; Stiesch, M.; Schwestka-Polly, R.; Demling, A.P. Three-dimensional analysis of initial biofilm formation on polytetrafluoroethylene in the oral cavity. J. Orofac. Orthop./Fortschr. Kieferorthopädie 2013, 74, 458–467. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Kaizer, M.R.; Azevedo, M.S.; Ogliari, F.A.; Cenci, M.S.; Moraes, R.R. (Super) hydrophobic coating of orthodontic dental devices and reduction of early oral biofilm retention. Biomed. Mater. 2015, 10, 065004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, R.; Sun, Q.; Zhuang, Z.; Zhang, Y.; Chen, S.; Han, B. Bio-inspired special wettability in oral antibacterial applications. Front. Bioeng. Biotechnol. 2022, 10, 1001616. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Chang, L.; Liu, X.; Lin, J.; Liu, H.; Han, B.; Wang, S. Antibacterial property of a polyethylene glycol-grafted dental material. ACS Appl. Mater. Interfaces 2017, 9, 17688–17692. [Google Scholar] [CrossRef]
- He, L.; Cui, Y.; Zhang, C. The corrosion resistance, cytotoxicity, and antibacterial properties of lysozyme coatings on orthodontic composite arch wires. RSC Adv. 2020, 10, 18131–18137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, L.; Meng, J.; Zhu, Z.; Han, B.; Wang, S. Protein-mediated anti-adhesion surface against oral bacteria. Nanoscale 2018, 10, 2711–2714. [Google Scholar] [CrossRef]
- Peng, L.; Chang, L.; Si, M.; Lin, J.; Wei, Y.; Wang, S.; Liu, H.; Han, B.; Jiang, L. Hydrogel-Coated Dental Device with Adhesion-Inhibiting and Colony-Suppressing Properties. ACS Appl. Mater. Interfaces 2020, 12, 9718–9725. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol–gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]
- Abbass, M.K.; Ajeel, S.A.; Wadullah, H.M. Biocompatibility, Bioactivity and Corrosion Resistance of Stainless Steel 316L Nanocoated with TiO2 and Al2O3 by Atomic Layer Deposition Method. J. Phys. Conf. Ser. 2018, 1032, 012017. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Kirubaharan, K.; Dharini, T.; Ramachandran, D.; Muthaiah, B. Corrosion and biocompatibility behaviour of zirconia coating by EBPVD for biomedical applications. Surf. Coat. Technol. 2018, 334, 336–343. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Han, E.-h.; Ke, W. Influence of fluoride and chloride on corrosion behavior of NiTi orthodontic wires. Acta Biomater. 2007, 3, 807–815. [Google Scholar] [CrossRef]
- da Silva, D.L.; Mattos, C.T.; Simão, R.A.; de Oliveira Ruellas, A.C. Coating stability and surface characteristics of esthetic orthodontic coated archwires. Angle Orthod. 2013, 83, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-T.; Ding, S.-J.; Chen, Y.-C.; Huang, T.-H. The anticorrosion ability of titanium nitride (TiN) plating on an orthodontic metal bracket and its biocompatibility. J. Biomed. Mater. Res. 2002, 63, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Xie, Y.; Zhang, J.; Wei, Q.; Zhou, B.; Luo, J.; Wang, Y.; Yu, Z.M.; Tang, Z.G. TiN coated stainless steel bracket: Tribological, corrosion resistance, biocompatibility and mechanical performance. Surf. Coat. Technol. 2015, 277, 227–233. [Google Scholar] [CrossRef]
- Yuan, X.-S.; Wang, Y.; Cao, L.; Cao, B.-C.; Liang, J. Effects of N-Doped TiO2 Thin Films on Corrosion Resistance of Stainless Steel Orthodontic Brackets in Artificial Saliva. Corrosion 2015, 71, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kitaura, H.; Noguchi, T.; Ohori, F.; Mizoguchi, I. Analysis of Coating Loss from Coated Stainless Steel Orthodontic Wire. Appl. Sci. 2022, 12, 9497. [Google Scholar] [CrossRef]
- Lou, J.; Gao, Z.; Zhang, J.; He, H.; Wang, X. Comparative Investigation on Corrosion Resistance of Stainless Steels Coated with Titanium Nitride, Nitrogen Titanium Carbide and Titanium-Diamond-like Carbon Films. Coatings 2021, 11, 1543. [Google Scholar] [CrossRef]
- Banaszek, K.; Klimek, L.; Dąbrowski, J.R.; Jastrzębski, W. Fretting Wear in Orthodontic and Prosthetic Alloys with Ti(C,N) Coatings. Processes 2019, 7, 874. [Google Scholar] [CrossRef]
- Banaszek, K.; Maślanka, M.; Semenov, M.; Klimek, L. Corrosive Studies of a Prosthetic Ni-Cr Alloy Coated with Ti(C,N) Type Layers. Materials 2022, 15, 2471. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, P.K.; Lin, G.; Yang, D. Effects of Ti/TiN multilayer on corrosion resistance of nickel–titanium orthodontic brackets in artificial saliva. Corros. Sci. 2007, 49, 3783–3796. [Google Scholar] [CrossRef]
- Katić, V.; Ćurković, H.O.; Semenski, D.; Baršić, G.; Marušić, K.; Špalj, S. Influence of surface layer on mechanical and corrosion properties of nickel-titanium orthodontic wires. Angle Orthod. 2014, 84, 1041–1048. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Mendoza, A.; Peña, J. Inhibition of Ni Release from NiTi and NiTiCu Orthodontic Archwires by Nitrogen Diffusion Treatment. J. Appl. Biomater. Biomech. 2004, 2, 151–155. [Google Scholar]
- Liu, J.-K.; Liu, I.-H.; Liu, C.; Chang, C.-J.; Kung, K.-C.; Liu, Y.-T.; Lee, T.-M.; Jou, J.-L. Effect of titanium nitride/titanium coatings on the stress corrosion of nickel–titanium orthodontic archwires in artificial saliva. Appl. Surf. Sci. 2014, 317, 974–981. [Google Scholar] [CrossRef]
- Kielan-Grabowska, Z.; Bącela, J.; Zięty, A.; Seremak, W.; Gawlik-Maj, M.; Kawala, B.; Borak, B.; Detyna, J.; Sarul, M. Improvement of Properties of Stainless Steel Orthodontic Archwire Using TiO2:Ag Coating. Symmetry 2021, 13, 1734. [Google Scholar] [CrossRef]
- Ohtsu, N.; Yamasaki, K.; Taniho, H.; Konaka, Y.; Tate, K. Pulsed anodization of NiTi alloy to form a biofunctional Ni-free oxide layer for corrosion protection and hydrophilicity. Surf. Coat. Technol. 2021, 412, 127039. [Google Scholar] [CrossRef]
- Anuradha, P.; Varma, N.K.S.; Balakrishnan, A. Reliability performance of titanium sputter coated Ni–Ti arch wires: Mechanical performance and nickel release evaluation. Bio-Med. Mater. Eng. 2015, 26, 67–77. [Google Scholar] [CrossRef]
- Kosari Mehr, A.; Kosari Mehr, A.; Babaei, R. Performance of reactively co-sputtered titanium chromium nitride films in artificial saliva: Corrosion protection and reduction in the release of potentially toxic elements. Surf. Coat. Technol. 2021, 427, 127855. [Google Scholar] [CrossRef]
- Hu, J.; Ren, Y.; Huang, Q.; He, H.; Liang, L.; Liu, J.; Li, R.; Wu, H. Microstructure and Corrosion Behavior of Ti-Nb Coatings on NiTi Substrate Fabricated by Laser Cladding. Coatings 2021, 11, 597. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Kobayashi, S.; Ozeki, K.; Aoki, H.; Nakamori, H.; Hirakuri, K.K.; Miyashita, O. Reduction effect of nickel ion release on a diamond-like carbon film coated onto an orthodontic archwire. Thin Solid Film. 2006, 497, 218–222. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohgoe, Y.; Ozeki, K.; Hirakuri, K.; Aoki, H. Dissolution effect and cytotoxicity of diamond-like carbon coatings on orthodontic archwires. J. Mater. Sci. Mater. Med. 2007, 18, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liao, J.; Peng, Y.; Xiao, Y.; Huang, J.; Li, L. Enhancement of sp3 C Fraction in Diamond-like Carbon Coatings by Cryogenic Treatment. Coatings 2022, 12, 42. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Rokaya, D.; Thunyakitpisal, P.; Qin, J.; Saengkiettiyut, K. Corrosion Resistance of Graphene oxide/Silver Coatings on Ni–Ti alloy and Expression of IL-6 and IL-8 in Human Oral Fibroblasts. Sci. Rep. 2020, 10, 3247. [Google Scholar] [CrossRef]
- Fróis, A.; Evaristo, M.; Santos, A.C.; Louro, C.S. Salivary pH Effect on Orthodontic Appliances: In Vitro Study of the SS/DLC System. Coatings 2021, 11, 1302. [Google Scholar] [CrossRef]
- Redlich, M.; Gorodnev, A.; Feldman, Y.; Kaplan-Ashiri, I.; Tenne, R.; Fleischer, N.; Genut, M.; Feuerstein, N. Friction reduction and wear resistance of electro-co-deposited inorganic fullerene-like WS2 coating for improved stainless steel orthodontic wires. J. Mater. Res. 2008, 23, 2909–2915. [Google Scholar] [CrossRef]
- de Amorim, M.C.; da Rocha Gomes, S.; da Silva, B.P.; Aoki, I.V.; Basting, R.T. Surface Micromorphology, Ion Release and Resistance to Corrosion of Orthodontic Wires Aesthetic Coating Subject to Degradation. J. Bio- Tribo-Corros. 2021, 8, 22. [Google Scholar] [CrossRef]
- Escobar, L.M.; Rivera, J.R.; Arbelaez, E.; Torres, L.F.; Villafañe, A.; Díaz-Báez, D.; Mora, I.; Lafaurie, G.I.; Tanaka, M. Comparison of Cell Viability and Chemical Composition of Six Latest Generation Orthodontic Wires. Int. J. Biomater. 2021, 2021, 8885290. [Google Scholar] [CrossRef] [PubMed]
- Khaneh Masjedi, M.; Niknam, O.; Haghighat Jahromi, N.; Javidi, P.; Rakhshan, V. Effects of Fixed Orthodontic Treatment Using Conventional, Copper-Included, and Epoxy-Coated Nickel-Titanium Archwires on Salivary Nickel Levels: A Double-Blind Randomized Clinical Trial. Biol. Trace Elem. Res. 2016, 174, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Trino, L.D.; Dias, L.F.G.; Albano, L.G.S.; Bronze-Uhle, E.S.; Rangel, E.C.; Graeff, C.F.O.; Lisboa-Filho, P.N. Zinc oxide surface functionalization and related effects on corrosion resistance of titanium implants. Ceram. Int. 2018, 44, 4000–4008. [Google Scholar] [CrossRef]
- Aksoy, M.E.; Aksakal, B.; Aslan, N.; Dikici, B. Boron-Doped Hydroxyapatite Coatings on NiTi Alloys Using the Electrophoretic Deposition Method: Enhanced Corrosion and Adhesion Performances. J. Mater. Eng. Perform. 2021, 30, 7365–7375. [Google Scholar] [CrossRef]
- Mehrvarz, A.; Khalil-Allafi, J.; Etminanfar, M.; Mahdavi, S. The study of morphological evolution, biocorrosion resistance, and bioactivity of pulse electrochemically deposited Hydroxyapatite/ZnO composite on NiTi superelastic alloy. Surf. Coat. Technol. 2021, 423, 127628. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Bononi, M.; Colombini, E.; Giovanardi, R.; Veronesi, P.; Tranquillo, E. Coating of Titanium Substrates with ZrO2 and ZrO2-SiO2 Composites by Sol-Gel Synthesis for Biomedical Applications: Structural Characterization, Mechanical and Corrosive Behavior. Coatings 2019, 9, 200. [Google Scholar] [CrossRef]
- Krishnan, M.; Seema, S.; Kumar, A.V.; Varthini, N.P.; Sukumaran, K.; Pawar, V.R.; Arora, V. Corrosion resistance of surface modified nickel titanium archwires. Angle Orthod. 2014, 84, 358–367. [Google Scholar] [CrossRef]
- Mareci, D.; Earar, K.; Zetu, I.; Bolat, G.; Crimu, C.; Istrate, B.; Munteanu, C.; Matei, M.N. Comparative electrochemical behaviour, of uncoated and coated Ni Ti, for dental orthodontic wires. Mater. Plast. 2015, 52, 150–153. [Google Scholar]
- Lin, C.-W.; Chung, C.-J.; Chou, C.-M.; He, J.-L. In vitro wear tests of the dual-layer grid blasting-plasma polymerized superhydrophobic coatings on stainless steel orthodontic substrates. Thin Solid Film. 2019, 687, 137464. [Google Scholar] [CrossRef]
- Ahmed, R.A.; Farghali, R.A.; Alshahrani, W.A. Influence of Fluoride and/or Bovine Albumin on Electrochemical Properties of Bare and ionic liquid-Coated Ni47Ti49Co4 Orthodontic archwires in artificial saliva solution. Int. J. Electrochem. Sci. 2021, 16, 2. [Google Scholar] [CrossRef]
- Yu, D.; Miao, K.; Li, Y.; Bao, X.; Hu, M.; Zhang, K. Sputter-deposited TaCuN films: Structure, tribological and biomedical properties. Appl. Surf. Sci. 2021, 567, 150796. [Google Scholar] [CrossRef]
- Il-Jeong, P.; Kim, M.-S.; Suk-Kyu, L.E.E. Highly Corrosion-Resistant Plated Steel Sheet Having Excellent Plating Adhesion and Resistance to Liquid Metal Embrittlement. U.S. Patent Application US11530470B2, 27 September 2018. [Google Scholar]
- Cui, H.-Z.; Zhang, G.-S.; Tian, S.-C.; Wei, N.; Tian, J.; Wang, X.-Z. A Kind of Layered Bionic Wear-and Corrosion-Resistant Anti-Friction Coating and Preparation Method and Application. CN Patent Application CN109208044B, 9 June 2020. [Google Scholar]
- Singh, V.P.; Pratap, K.; Sinha, J.; Desiraju, K.; Bahal, D.; Kukreti, R. Critical evaluation of challenges and future use of animals in experimentation for biomedical research. Int. J. Immunopathol. Pharmacol. 2016, 29, 551–561. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Chen, L.; Yu, T.; Sun, J.; Guan, C. Microstructure and mechanical properties of Ti–C–TiN-reinforced Ni204-based laser-cladding composite coating. Ceram. Int. 2021, 47, 5918–5928. [Google Scholar] [CrossRef]
- Bahrami, R.; Pourhajibagher, M.; Badiei, A.; Masaeli, R.; Tanbakuchi, B. Evaluation of the cell viability and antimicrobial effects of orthodontic bands coated with silver or zinc oxide nanoparticles: An in vitro study. Korean J. Orthod. 2023, 53, 16–25. [Google Scholar] [CrossRef] [PubMed]
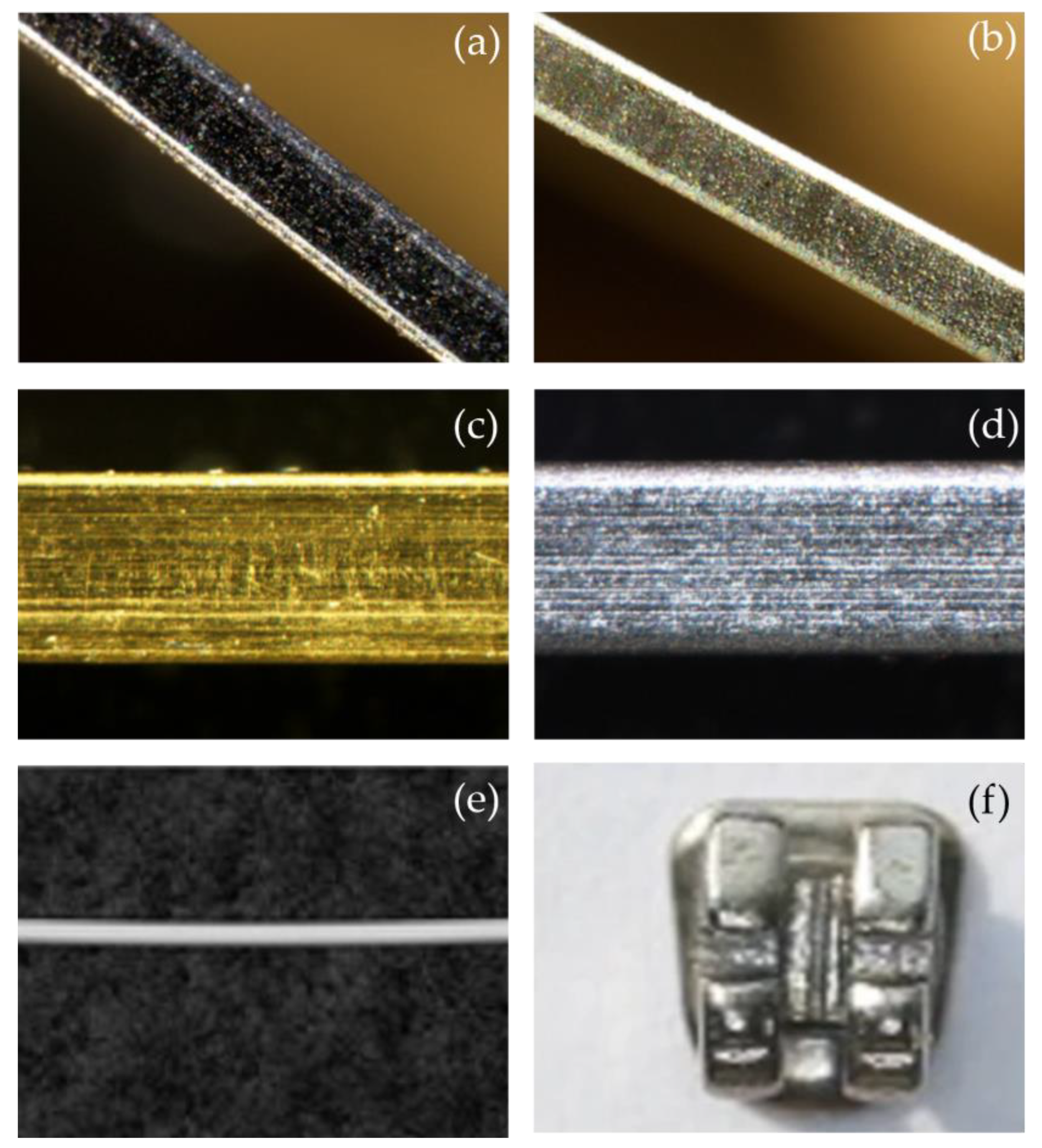
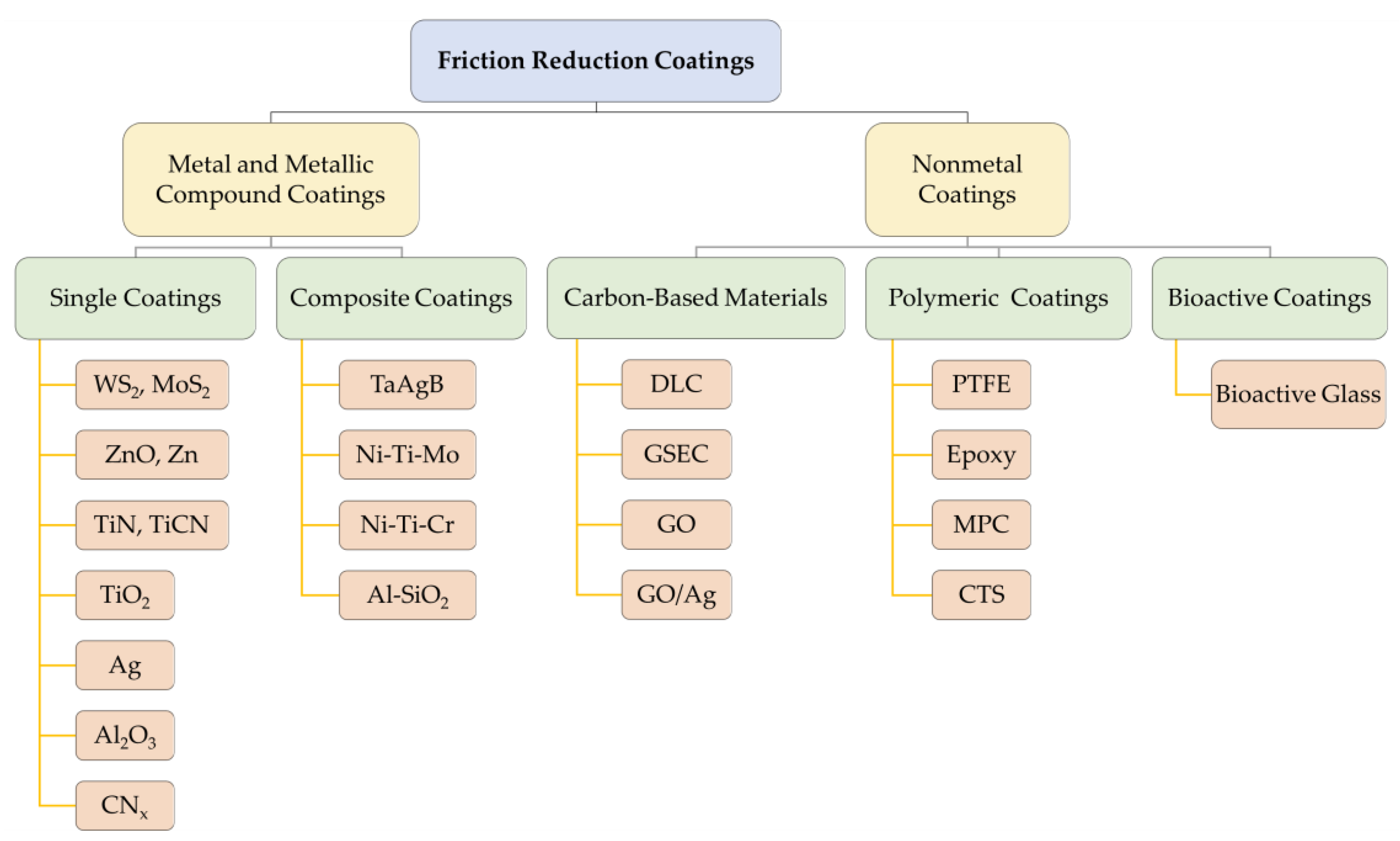
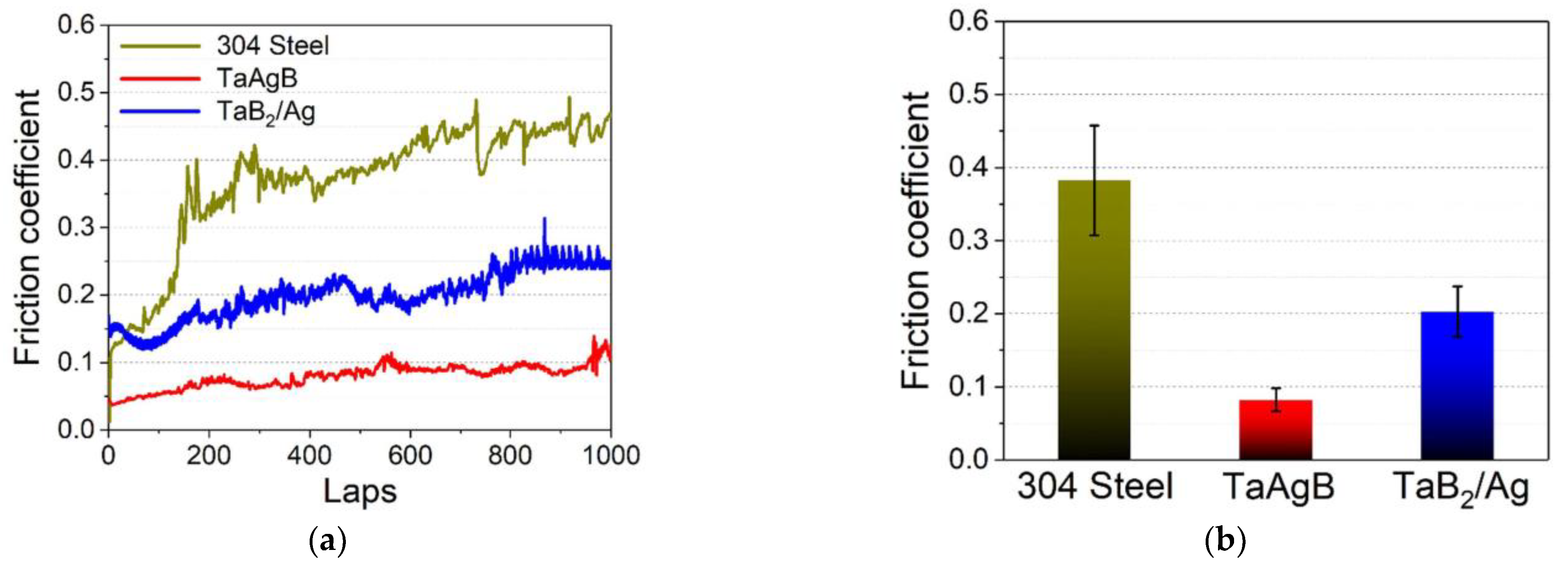
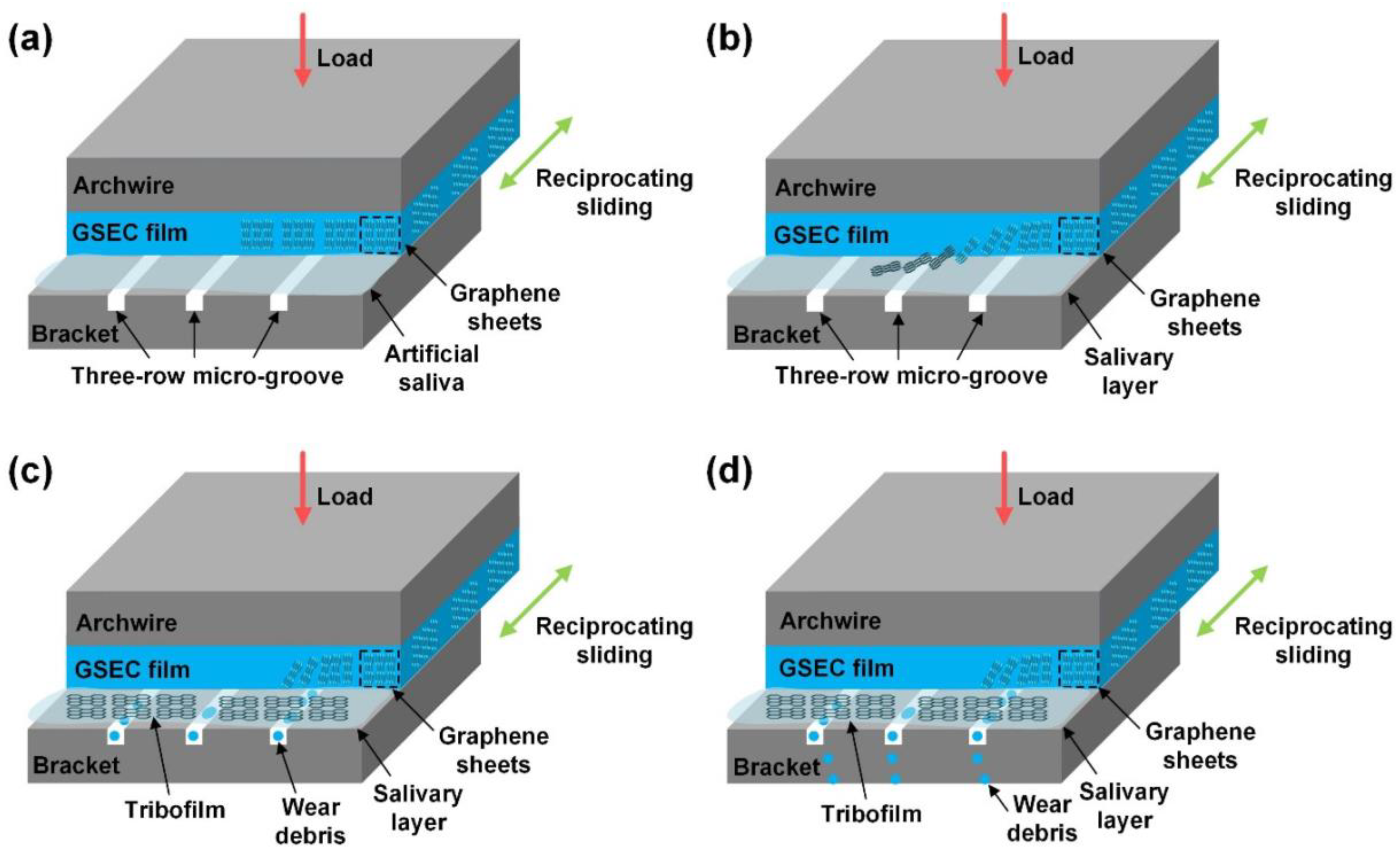
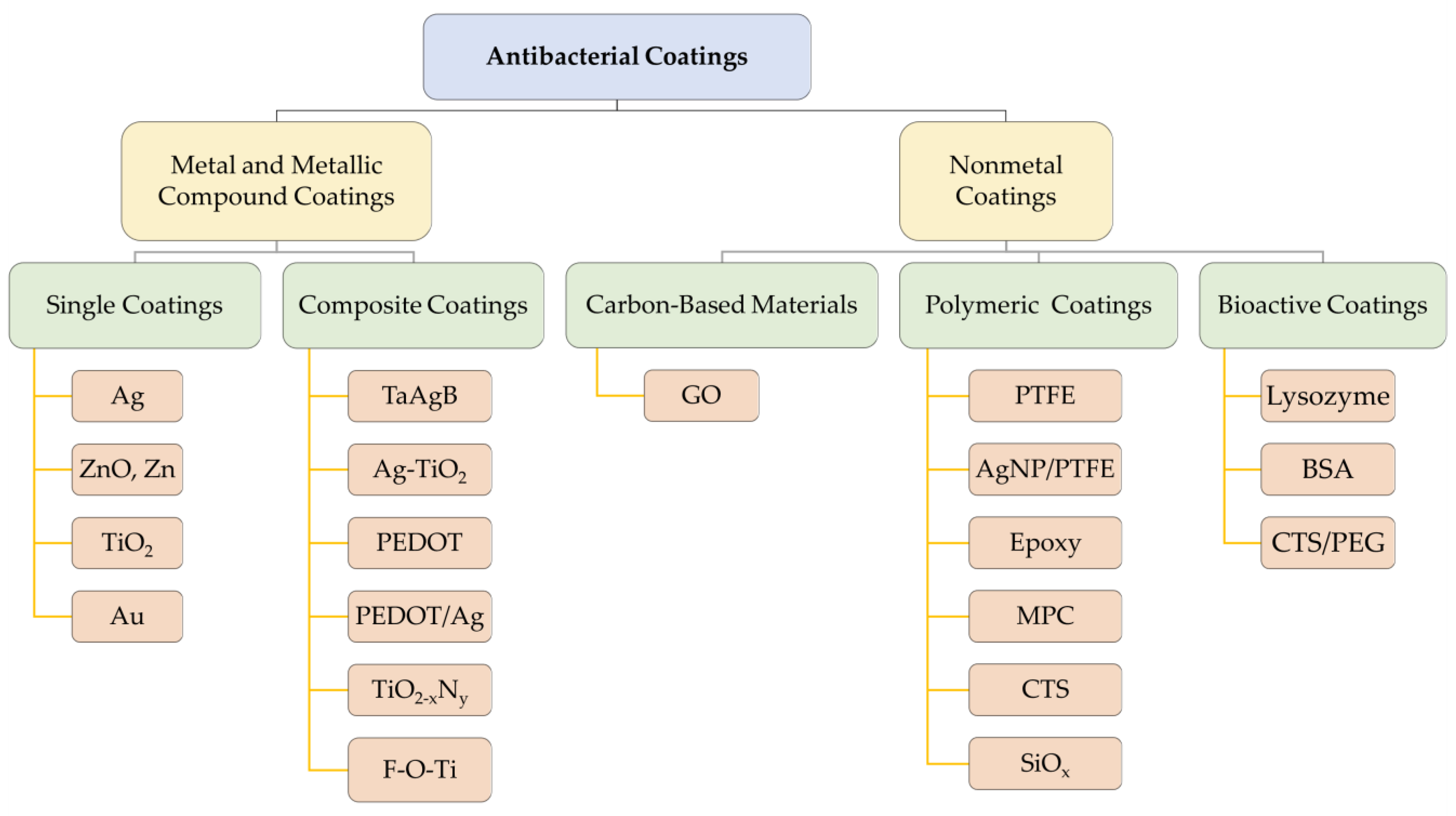
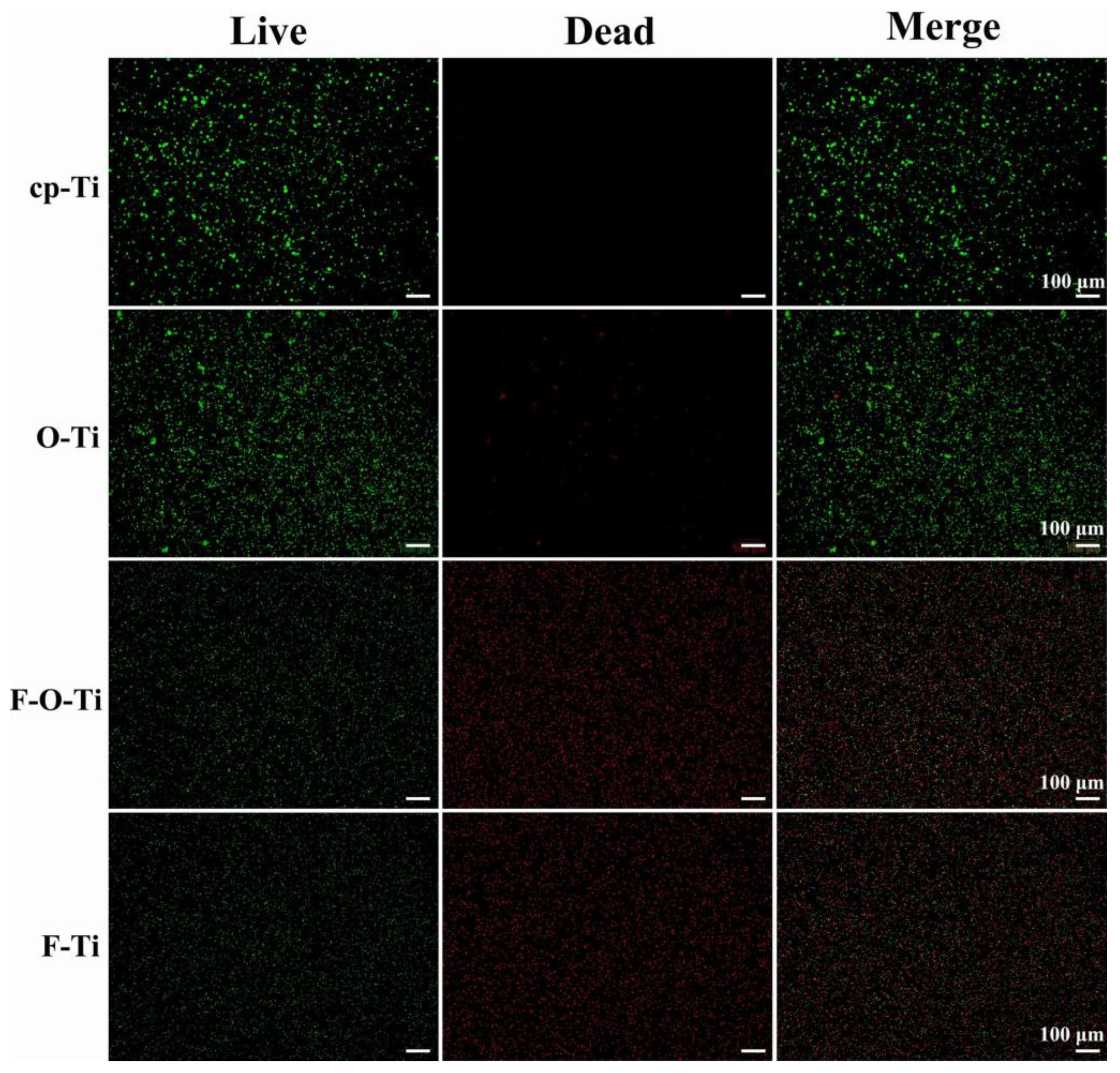
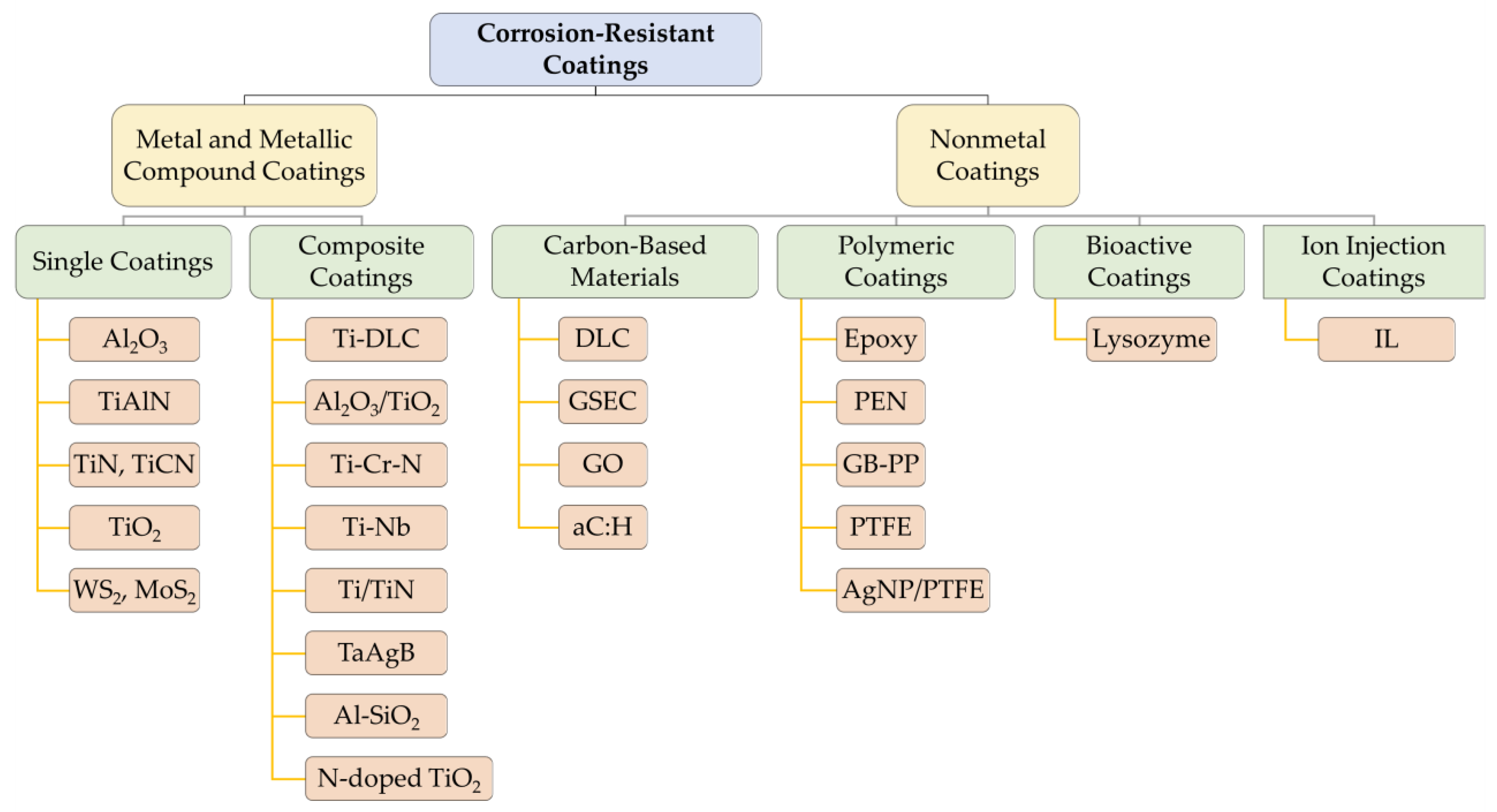
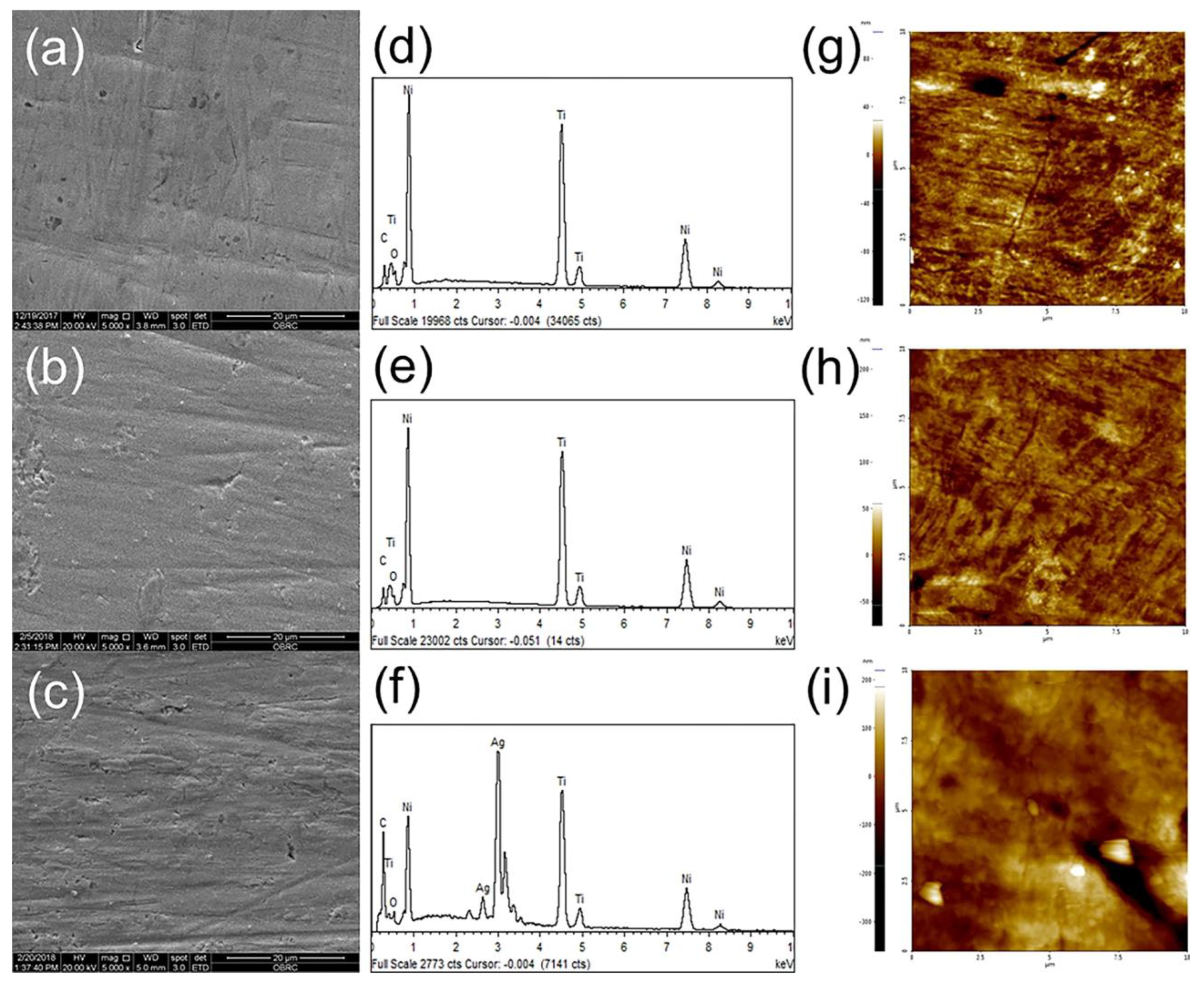
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Han, B.; Liu, X. Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance. Int. J. Mol. Sci. 2023, 24, 6919. https://doi.org/10.3390/ijms24086919
Zhang R, Han B, Liu X. Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance. International Journal of Molecular Sciences. 2023; 24(8):6919. https://doi.org/10.3390/ijms24086919
Chicago/Turabian StyleZhang, Ruichu, Bing Han, and Xiaomo Liu. 2023. "Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance" International Journal of Molecular Sciences 24, no. 8: 6919. https://doi.org/10.3390/ijms24086919
APA StyleZhang, R., Han, B., & Liu, X. (2023). Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance. International Journal of Molecular Sciences, 24(8), 6919. https://doi.org/10.3390/ijms24086919






