RNA Transcripts in Human Ovarian Cells: Two-Time Cryopreservation Does Not Affect Developmental Potential
Abstract
1. Introduction
2. Results
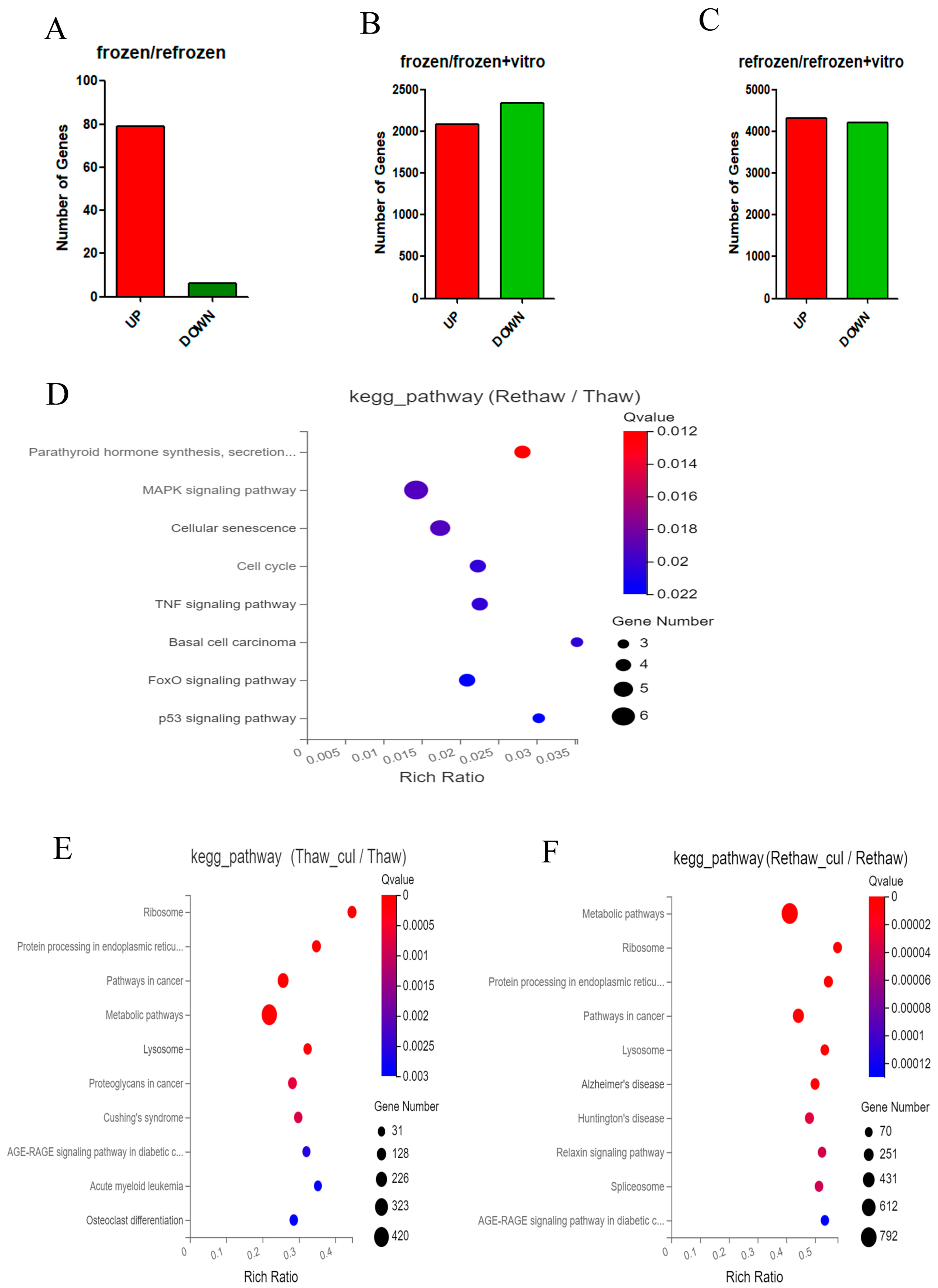
2.1. Differential Expression Genes (DEGs)
2.2. Kyoto Encyclopedia of Genes and Genomes (KEGG)
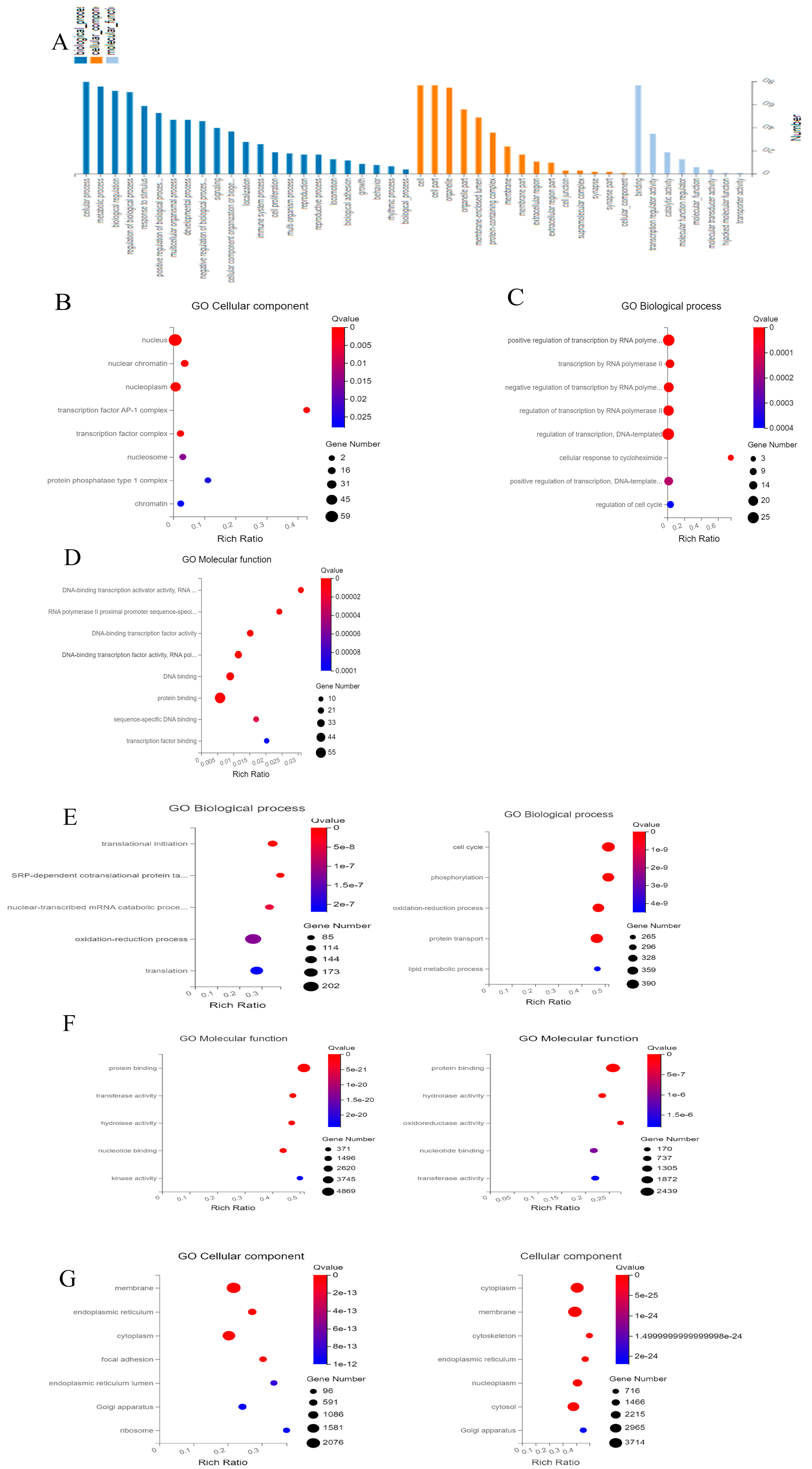
2.3. Gene Ontology
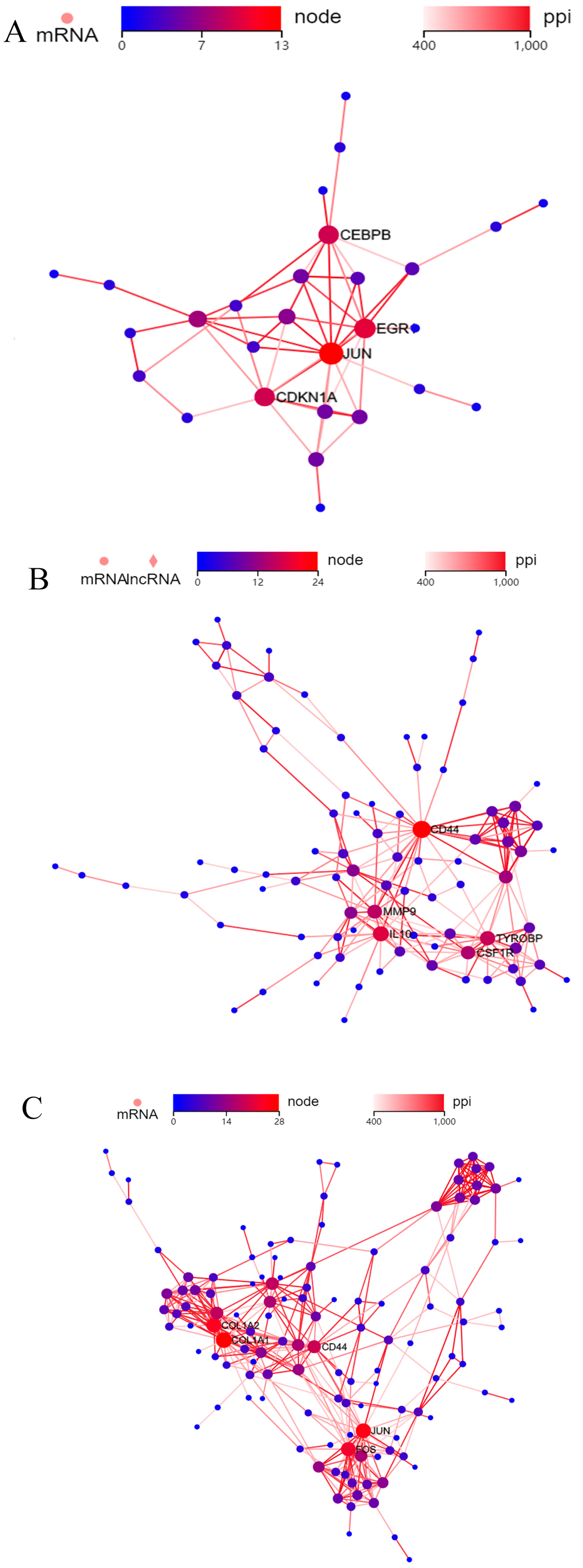
2.4. Protein–Protein Interactions (PPIs)
2.5. Intact and Active Follicles from Two-Time Cryopresrved Ovarian Tissues Can Be Used for the Formation of Artificial Ovaries
3. Discussion
3.1. Differential Expressed Genes (DEGs)
3.2. GO Terms Analysis
3.3. What Is KEGG Pathways Analysis?
3.4. PPI Analysis
4. Materials and Methods
4.1. Design of Experiments
4.2. Cell Collection and Cryopreservation (Freezing and Thawing)
4.3. Confocal Microscopy
4.4. Artificial Ovary and Imaging
4.5. Sequencing and Data Extraction
4.6. Differential Gene Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulou, M.; Boostandoost, E.; Papaxoinis, G.; de La Motte Rouge, T.; Khayat, D.; Psyrri, A. Anticancer Treatment and Fertility: Effect of Therapeutic Modalities on Reproductive System and Functions. Crit. Rev. Oncol. Hematol. 2016, 97, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Drakeley, A.; Homburg, R.; Bambang, K. Fertility Preservation in Female Patients with Cancer. Clin. Oncol. 2022, 34, 508–513. [Google Scholar] [CrossRef]
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of Cryopreserved Ovarian Tissue in a Series of 285 Women: A Review of Five Leading European Centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjorn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of Frozen-Thawed Ovarian Tissue: An Update on Worldwide Activity Published in Peer-Reviewed Papers and on the Danish Cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Donnez, J.; Cacciottola, L. Fertility Preservation: The Challenge of Freezing and Transplanting Ovarian Tissue. Trends Mol. Med. 2021, 27, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.; Smith, A.G.; Kelsey, T.W.; Edgar, A.E.; Anderson, R.A. Fertility Preservation for Girls and Young Women with Cancer: Population-Based Validation of Criteria for Ovarian Tissue Cryopreservation. Lancet Oncol. 2014, 15, 1129–1136. [Google Scholar] [CrossRef]
- Kolibianaki, E.E.; Goulis, D.G.; Kolibianakis, E.M. Ovarian Tissue Cryopreservation and Transplantation to Delay Menopause: Facts and Fiction. Maturitas 2020, 142, 64–67. [Google Scholar] [CrossRef]
- Van Leer, P. The Risk of Cardiovascular Disease, Fracture, Dementia, and Cancer after Long-Term Hormone Therapy in Perimenopausal and Postmenopausal Women. Am. Fam. Physician 2018, 98, 117–118. [Google Scholar]
- Marjoribanks, J.; Farquhar, C.M.; Roberts, H.; Lethaby, A. Cochrane Corner: Long-Term Hormone Therapy for Perimenopausal and Postmenopausal Women. Heart 2018, 104, 93–95. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Manavella, D.D. Recent Advances in Fertility Preservation. J. Obstet. Gynaecol. Res. 2019, 45, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Masciangelo, R. Risk of Transplanting Malignant Cells in Cryopreserved Ovarian Tissue. Minerva Ginecol. 2018, 70, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Zhang, D.; Shi, J.; Wu, Y.J. Comparison of Vitrification and Conventional Slow Freezing for Cryopreservation of Ovarian Tissue with Respect to the Number of Intact Primordial Follicles: A Meta-Analysis. Medicine 2016, 95, e4095. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Isachenko, E.; Reinsberg, J.; Montag, M.; van der Ven, K.; Dorn, C.; Roesing, B.; van der Ven, H. Cryopreservation of Human Ovarian Tissue: Comparison of Rapid and Conventional Freezing. Cryobiology 2007, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Isachenko, V.; Kreienberg, R.; Sauer, H.; Todorov, P.; Tawadros, S.; Mallmann, P.; Nawroth, F.; Isachenko, E. Re-Vascularisation in Human Ovarian Tissue after Conventional Freezing or Vitrification and Xenotransplantation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 149, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, C.E.; Brady, B.M.; McLaughlin, M.; Telfer, E.E.; White, J.; Cowie, F.; Zahra, S.; Wallace, W.H.; Anderson, R.A. Re-Implantation of Cryopreserved Ovarian Cortex Resulting in Restoration of Ovarian Function, Natural Conception and Successful Pregnancy after Haematopoietic Stem Cell Transplantation for Wilms Tumour. J. Assist. Reprod. Genet. 2016, 33, 1615–1620. [Google Scholar] [CrossRef]
- Sanfilippo, S.; Canis, M.; Romero, S.; Sion, B.; Dechelotte, P.; Pouly, J.L.; Janny, L.; Smitz, J.; Brugnon, F. Quality and Functionality of Human Ovarian Tissue after Cryopreservation Using an Original Slow Freezing Procedure. J. Assist. Reprod. Genet. 2013, 30, 25–34. [Google Scholar] [CrossRef]
- Westphal, J.R.; Gerritse, R.; Braat, D.D.M.; Beerendonk, C.C.M.; Peek, R. Complete Protection against Cryodamage of Cryopreserved Whole Bovine and Human Ovaries Using Dmso as a Cryoprotectant. J. Assist. Reprod. Genet. 2017, 34, 1217–1229. [Google Scholar] [CrossRef]
- Hossay, C.; Donnez, J.; Dolmans, M.M. Whole Ovary Cryopreservation and Transplantation: A Systematic Review of Challenges and Research Developments in Animal Experiments and Humans. J. Clin. Med. 2020, 9, 3196. [Google Scholar] [CrossRef]
- Hossay, C.; Camboni, A.; Cacciottola, L.; Nguyen, T.Y.T.; Masciangelo, R.; Donnez, J.; Dolmans, M.M. Can Frozen-Thawed Human Ovary Withstand Refreezing-Rethawing in the Form of Cortical Strips? J. Assist. Reprod. Genet. 2020, 37, 3077–3087. [Google Scholar] [CrossRef]
- Jochumsen, K.M.; Tan, Q.; Dahlgaard, J.; Kruse, T.A.; Mogensen, O. Rna Quality and Gene Expression Analysis of Ovarian Tumor Tissue Undergoing Repeated Thaw-Freezing. Exp. Mol. Pathol. 2007, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bjarkadottir, B.D.; Walker, C.A.; Fatum, M.; Lane, S.; Williams, S.A. Analysing Culture Methods of Frozen Human Ovarian Tissue to Improve Follicle Survival. Reprod. Fertil. 2021, 2, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, Y.; Zheng, X.; Song, X.; Yang, R.; Yan, J.; Feng, H.; Qiao, J. Current Perspectives on in Vitro Maturation and Its Effects on Oocyte Genetic and Epigenetic Profiles. Sci. China Life Sci. 2018, 61, 633–643. [Google Scholar] [CrossRef]
- He, Z.Y.; Wang, H.Y.; Zhou, X.; Liang, X.Y.; Yan, B.; Wang, R.; Ma, L.H.; Wang, Y.L. Evaluation of Vitrification Protocol of Mouse Ovarian Tissue by Effect of DNA Methyltransferase-1 and Paternal Imprinted Growth Factor Receptor-Binding Protein 10 on Signaling Pathways. Cryobiology 2018, 80, 89–95. [Google Scholar] [CrossRef]
- Long, H.; Yu, W.; Yu, S.; Yin, M.; Wu, L.; Chen, Q.; Cai, R.; Suo, L.; Wang, L.; Lyu, Q.; et al. Progesterone Affects Clinic Oocyte Yields by Coordinating with Follicle Stimulating Hormone Via Pi3k/Akt and Mapk Pathways. J. Adv. Res. 2021, 33, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Ryu, K.J.; Lee, S.; Kim, T. Changes in Telomere Length and Senescence Markers During Human Ovarian Tissue Cryopreservation. Sci. Rep. 2021, 11, 2238. [Google Scholar] [CrossRef]
- Yoshida, O.; Yamane, M.; Yamamoto, S.; Okazaki, M.; Toyooka, S.; Oto, T.; Sano, Y.; Miyoshi, S. Impact of Prolonged Cold Preservation on the Graft Function and Gene Expression Levels in an Experimental Lung Transplantation Model. Surg. Today 2013, 43, 81–87. [Google Scholar] [CrossRef]
- Sperandio, S.; Fortin, J.; Sasik, R.; Robitaille, L.; Corbeil, J.; de Belle, I. The Transcription Factor Egr1 Regulates the Hif-1alpha Gene During Hypoxia. Mol. Carcinog. 2009, 48, 38–44. [Google Scholar] [CrossRef]
- Yenuganti, V.R.; Ravinder; Singh, D. Endotoxin Induced Tlr4 Signaling Downregulates Cyp19a1 Expression through Cebpb in Buffalo Granulosa Cells. Toxicol. In Vitro 2017, 42, 93–100. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsuda, Y.; Naito, Z.; Ishiwata, T. Cd44 in Human Glioma Correlates with Histopathological Grade and Cell Migration. Pathol. Int. 2012, 62, 463–470. [Google Scholar] [CrossRef]
- Fedorchenko, O.; Stiefelhagen, M.; Peer-Zada, A.A.; Barthel, R.; Mayer, P.; Eckei, L.; Breuer, A.; Crispatzu, G.; Rosen, N.; Landwehr, T.; et al. Cd44 Regulates the Apoptotic Response and Promotes Disease Development in Chronic Lymphocytic Leukemia. Blood 2013, 121, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of Cd44 and Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 31–42. [Google Scholar] [PubMed]
- Leng, Y.; Abdullah, A.; Wendt, M.K.; Calve, S. Hyaluronic Acid, Cd44 and Rhamm Regulate Myoblast Behavior During Embryogenesis. Matrix Biol. 2019, 78–79, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pei, C.; Isachenko, E.; Zhou, Y.; Wang, M.; Rahimi, G.; Liu, W.; Mallmann, P.; Isachenko, V. Automatic Evaluation for Bioengineering of Human Artificial Ovary: A Model for Fertility Preservation for Prepubertal Female Patients with a Malignant Tumor. Int. J. Mol. Sci. 2022, 23, 12419. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, W.; Todorov, P.; Pei, C.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Nawroth, F.; Isachenko, V. RNA Transcripts in Human Ovarian Cells: Two-Time Cryopreservation Does Not Affect Developmental Potential. Int. J. Mol. Sci. 2023, 24, 6880. https://doi.org/10.3390/ijms24086880
Zhou Y, Wang W, Todorov P, Pei C, Isachenko E, Rahimi G, Mallmann P, Nawroth F, Isachenko V. RNA Transcripts in Human Ovarian Cells: Two-Time Cryopreservation Does Not Affect Developmental Potential. International Journal of Molecular Sciences. 2023; 24(8):6880. https://doi.org/10.3390/ijms24086880
Chicago/Turabian StyleZhou, Yang, Wanxue Wang, Plamen Todorov, Cheng Pei, Evgenia Isachenko, Gohar Rahimi, Peter Mallmann, Frank Nawroth, and Volodimir Isachenko. 2023. "RNA Transcripts in Human Ovarian Cells: Two-Time Cryopreservation Does Not Affect Developmental Potential" International Journal of Molecular Sciences 24, no. 8: 6880. https://doi.org/10.3390/ijms24086880
APA StyleZhou, Y., Wang, W., Todorov, P., Pei, C., Isachenko, E., Rahimi, G., Mallmann, P., Nawroth, F., & Isachenko, V. (2023). RNA Transcripts in Human Ovarian Cells: Two-Time Cryopreservation Does Not Affect Developmental Potential. International Journal of Molecular Sciences, 24(8), 6880. https://doi.org/10.3390/ijms24086880