Molecular Dynamics Simulations Reveal the Conformational Transition of GH33 Sialidases
Abstract
1. Introduction
2. Results
2.1. Overall Structure of GH33 Sialidases
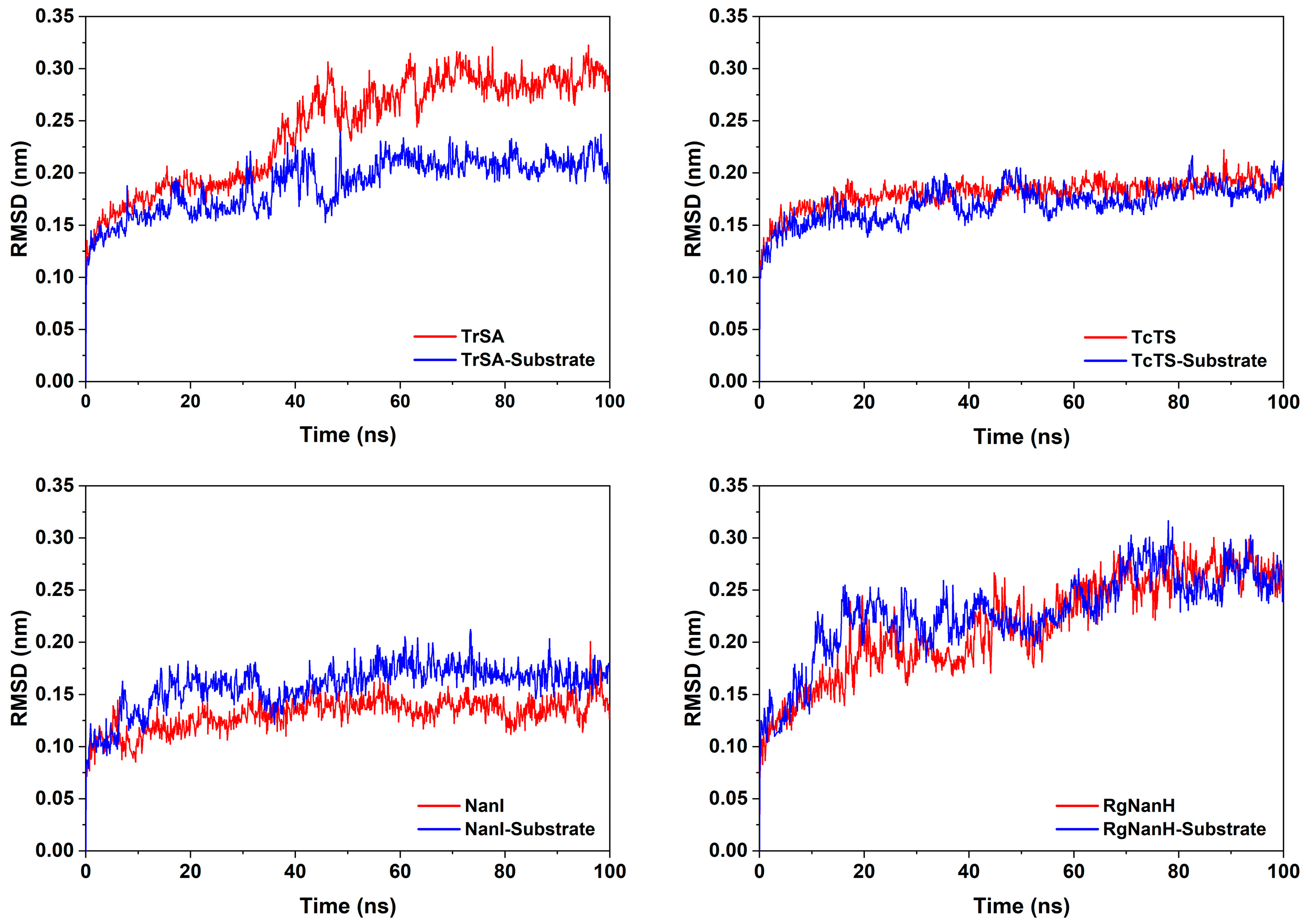
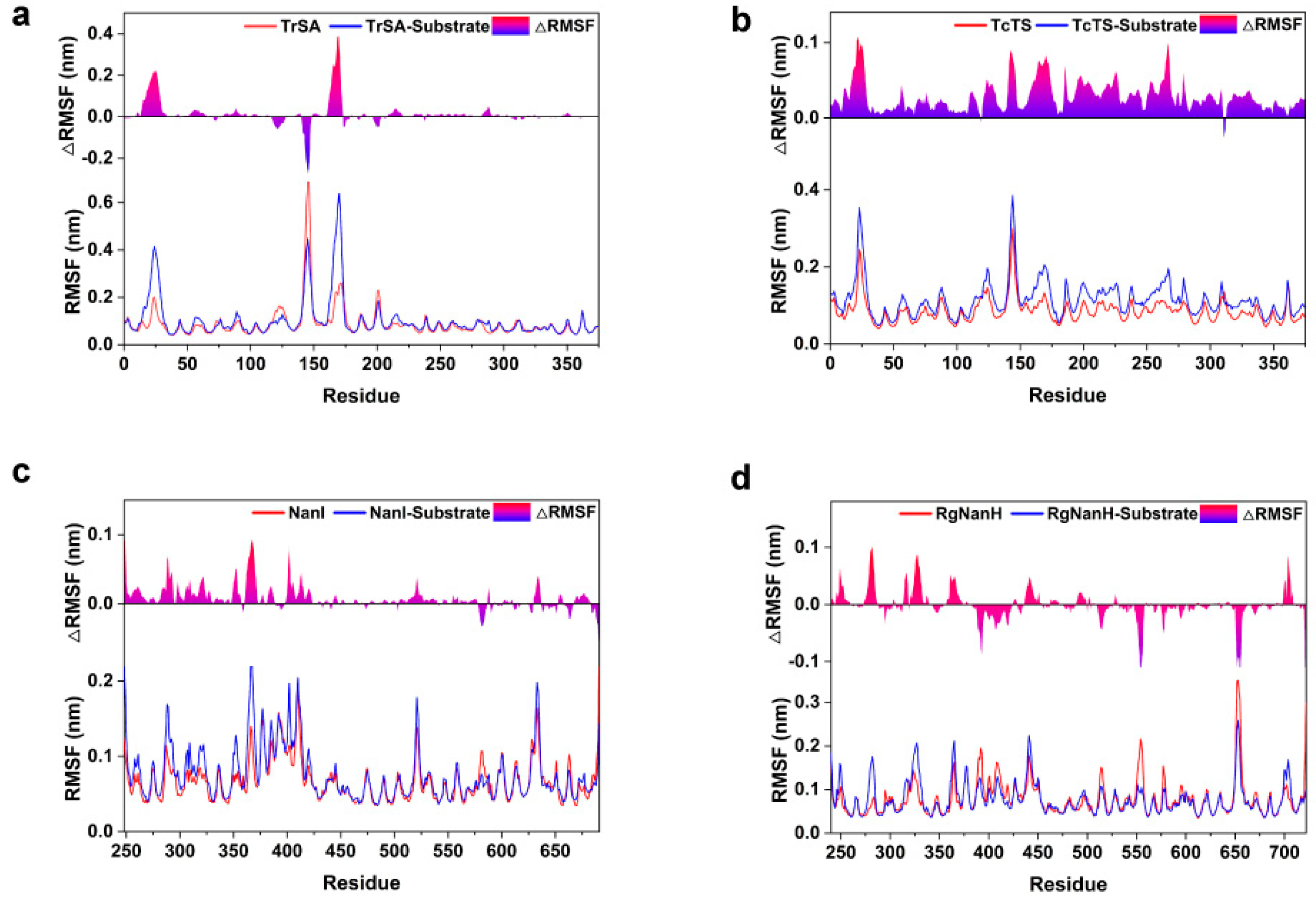
2.2. Structural Flexibility of GH33 Sialidases
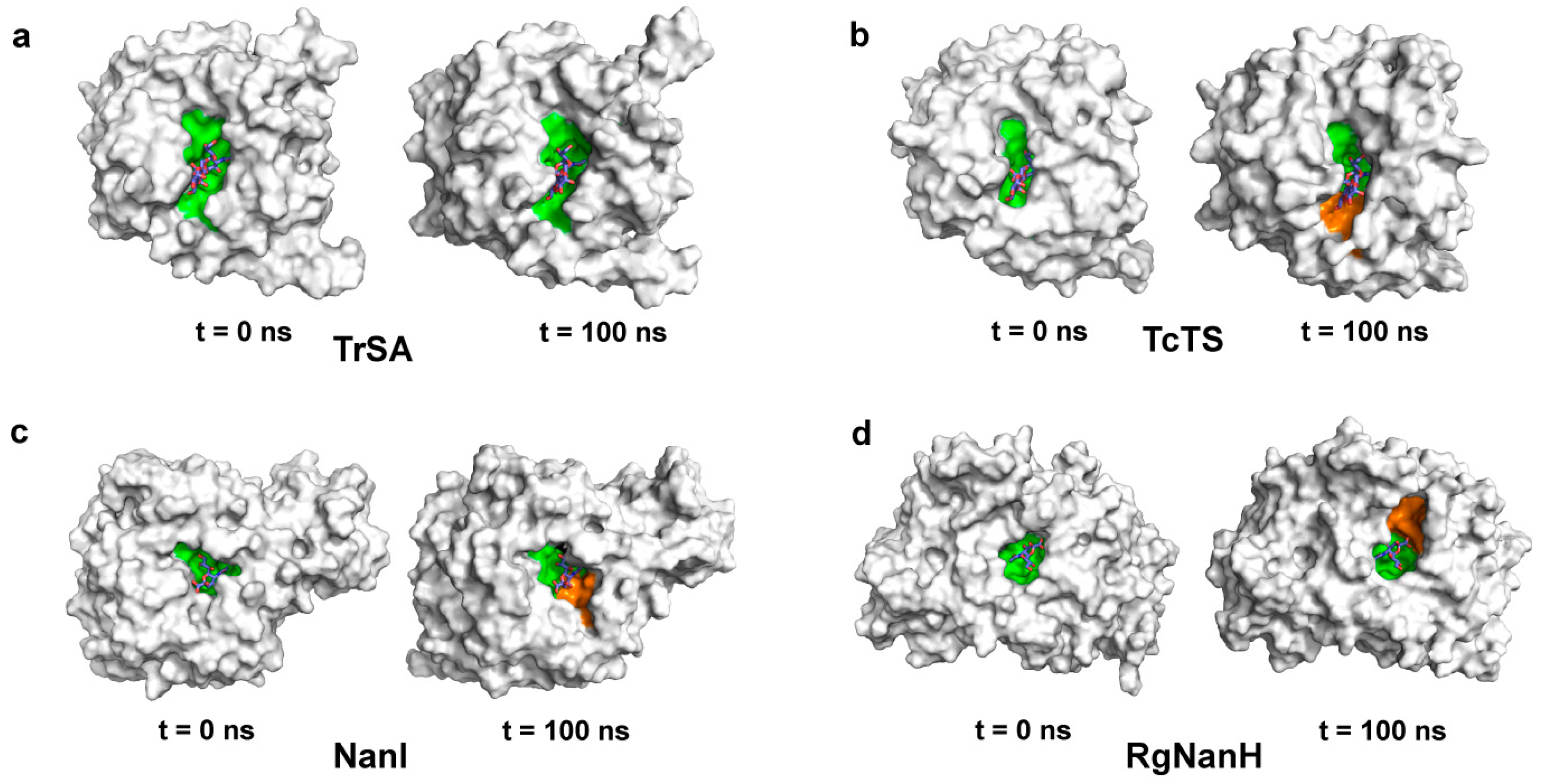
2.3. The Conformational Transition of GH33 Sialidases
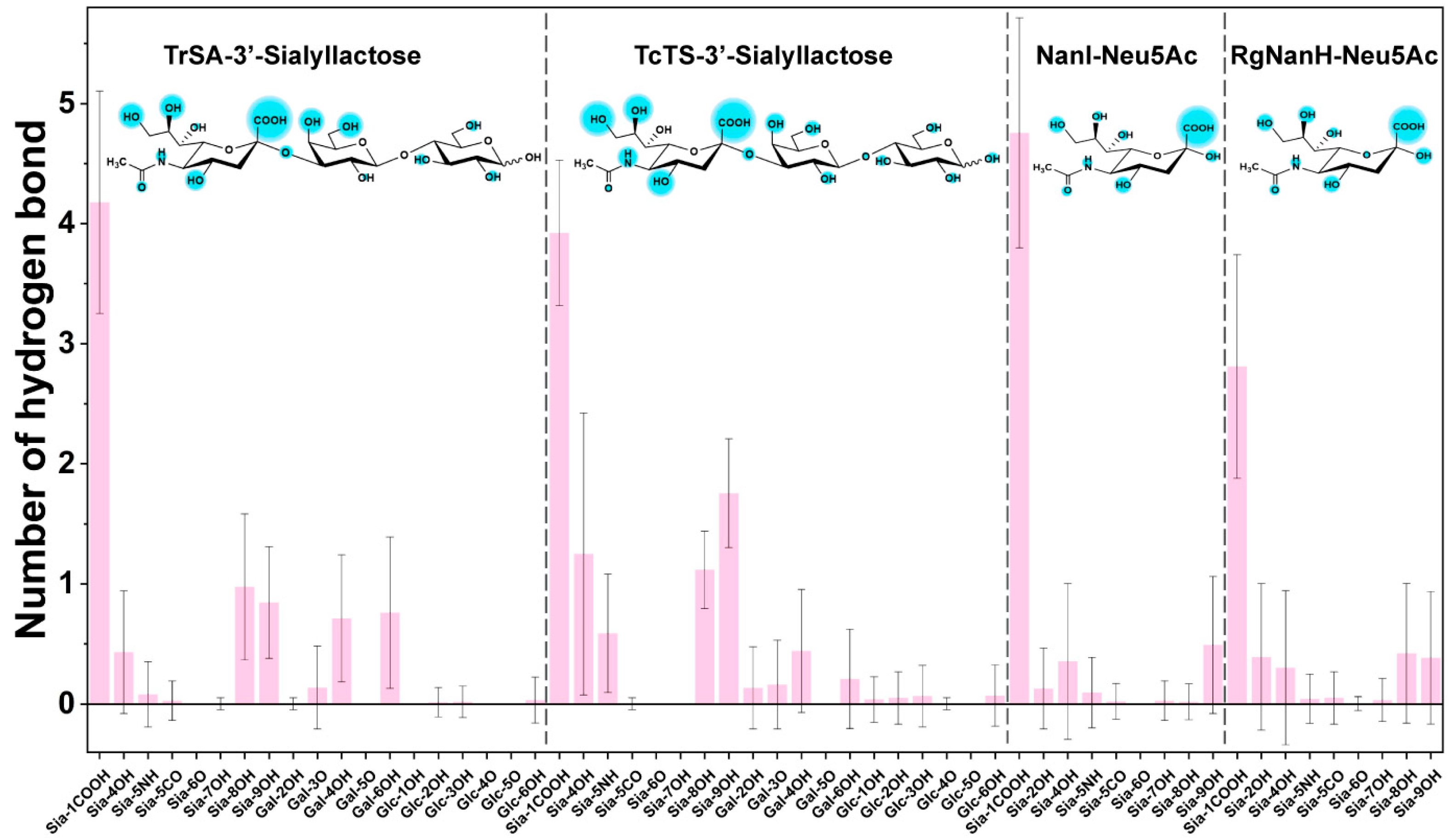
2.4. Key Structural Moieties of the Substrate Recognized by GH33 Sialidases
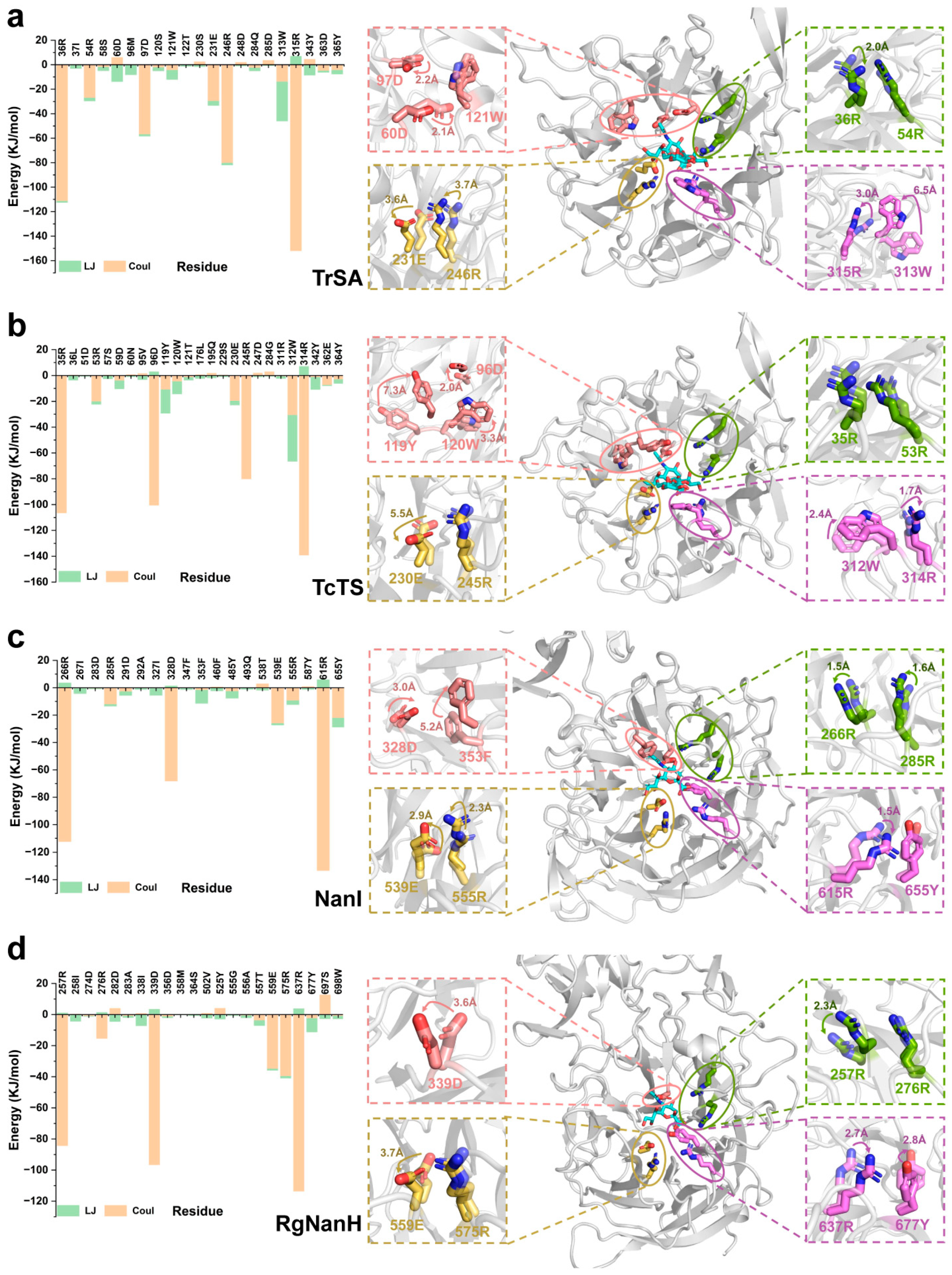
2.5. Key Residues Responsible for Interacting with the Substrates
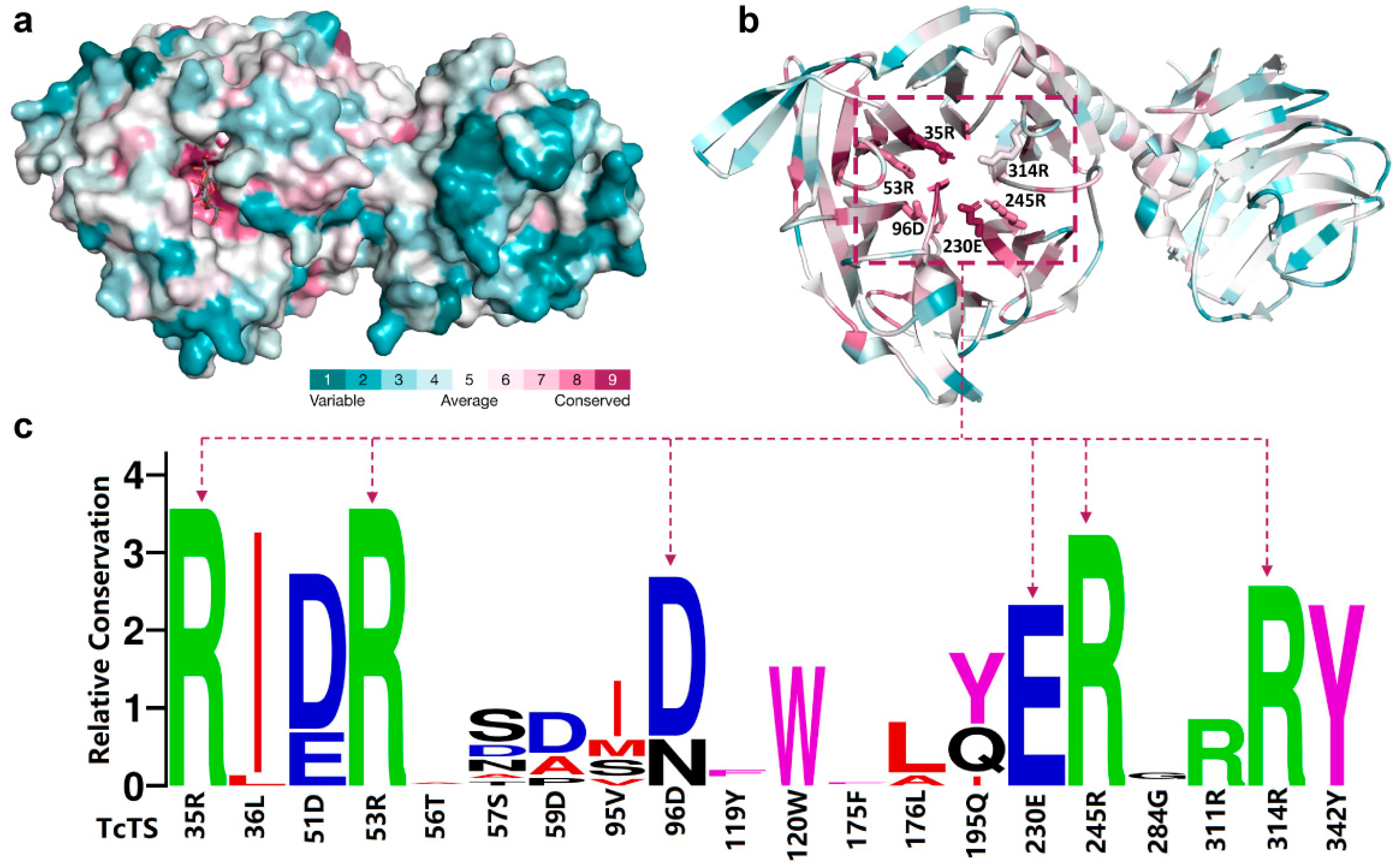
2.6. Conservation Analysis of GH33 Sialidases
3. Discussion
4. Methods
4.1. Protein Preparation
4.2. Molecular Dynamics Simulations
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pham, H.T.T.; Ten Kate, G.A.; Dijkhuizen, L.; van Leeuwen, S.S. Synthesis and characterization of sialylated lactose- and lactulose-derived oligosaccharides by Trypanosoma cruzi trans-sialidase. J. Agric. Food. Chem. 2019, 67, 3469–3479. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.-Q.; Liu, F.; Wu, J.-Y. Human milk oligosaccharides and infant gut microbiota: Molecular structures, utilization strategies and immune function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef]
- German, J.B.; Freeman, S.L.; Lebrilla, C.B.; Mills, D.A. Human milk oligosaccharides: Evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr. Workshop Ser. Pediatr. Program 2008, 62, 205–218; discussion 218–222. [Google Scholar]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, A.; Sprenger, N.; Kurakevich, E.; Borsig, L.; Chassard, C.; Hennet, T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J. Exp. Med. 2010, 207, 2843–2854. [Google Scholar] [CrossRef]
- Kurakevich, E.; Hennet, T.; Hausmann, M.; Rogler, G.; Borsig, L. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl. Acad. Sci. USA 2013, 110, 17444–17449. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. The role of milk sialyllactose in intestinal bacterial colonization. Adv. Nutr. 2012, 3, 483S–488S. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Varki, A. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Ando, H. Synthetic approach toward complexity of sialic acid-containing glycans. Biosci. Biotechnol. Biochem. 2015, 79, 343–346. [Google Scholar] [CrossRef]
- Schelch, S.; Zhong, C.; Petschacher, B.; Nidetzky, B. Bacterial sialyltransferases and their use in biocatalytic cascades for sialo-oligosaccharide production. Biotechnol. Adv. 2020, 44, 107613. [Google Scholar] [CrossRef]
- de Lederkremer, R.M.; Giorgi, M.E.; Agusti, R. Trans-sialylation: A strategy used to incorporate sialic acid into oligosaccharides. RSC Chem. Biol. 2022, 3, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, D.B.; Kang, H.A.; Kwon, O. Features and applications of bacterial sialidases. Appl. Microbiol. Biotechnol. 2011, 91, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morrone-Pozzuto, P.; Uhrig, M.L.; Agusti, R. Synthesis of oligosaccharides containing the S-Galp(α1→3)Galp unit, glycomimetic of the epitope recognized by lytic antibodies. J. Org. Chem. 2022, 87, 13455–13468. [Google Scholar] [CrossRef]
- Guo, L.; Chen, X.; Xu, L.; Xiao, M.; Lu, L. Enzymatic synthesis of 6’-sialyllactose, a dominant sialylated human milk oligosaccharide, by a novel exo-α-sialidase from Bacteroides fragilis NCTC9343. Appl. Environ. Microbiol. 2018, 84, e00071-18. [Google Scholar] [CrossRef] [PubMed]
- Wilbrink, M.H.; ten Kate, G.A.; van Leeuwen, S.S.; Sanders, P.; Sallomons, E.; Hage, J.A.; Dijkhuizen, L.; Kamerling, J.P. Galactosyl-lactose sialylation using Trypanosoma cruzi trans-sialidase as the biocatalyst and bovine κ-casein-derived glycomacropeptide as the donor substrate. Appl. Environ. Microbiol. 2014, 80, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Nyffenegger, C.; Nordvang, R.T.; Jers, C.; Meyer, A.S.; Mikkelsen, J.D. Design of Trypanosoma rangeli sialidase mutants with improved trans-sialidase activity. PLoS ONE 2017, 12, e0171585. [Google Scholar] [CrossRef]
- Perna, V.N.; Dehlholm, C.; Meyer, A.S. Enzymatic production of 3’-sialyllactose in milk. Enzyme Microb. Technol. 2021, 148, 109829. [Google Scholar] [CrossRef]
- Lipnicanova, S.; Chmelova, D.; Ondrejovic, M.; Frecer, V.; Miertus, S. Diversity of sialidases found in the human body-A review. Int. J. Biol. Macromol. 2020, 148, 857–868. [Google Scholar] [CrossRef]
- Owen, C.D.; Tailford, L.E.; Monaco, S.; Suligoj, T.; Vaux, L.; Lallement, R.; Khedri, Z.; Yu, H.; Lecointe, K.; Walshaw, J.; et al. Unravelling the specificity and mechanism of sialic acid recognition by the gut symbiont Ruminococcus gnavus. Nat. Commun. 2017, 8, 2196. [Google Scholar] [CrossRef]
- Agusti, R.; Gallo-Rodriguez, C.; de Lederkremer, R.M. Trypanosoma cruzi trans-sialidase. A tool for the synthesis of sialylated oligosaccharides. Carbohydr. Res. 2019, 479, 48–58. [Google Scholar] [CrossRef]
- Paris, G.; Ratier, L.; Amaya, M.F.; Nguyen, T.; Alzari, P.M.; Frasch, A.C. A sialidase mutant displaying trans-sialidase activity. J. Mol. Biol. 2005, 345, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Newstead, S.L.; Potter, J.A.; Wilson, J.C.; Xu, G.; Chien, C.H.; Watts, A.G.; Withers, S.G.; Taylor, G.L. The structure of Clostridium perfringens NanI sialidase and its catalytic intermediates. J. Biol. Chem. 2008, 283, 9080–9088. [Google Scholar] [CrossRef] [PubMed]
- Crost, E.H.; Tailford, L.E.; Monestier, M.; Swarbreck, D.; Henrissat, B.; Crossman, L.C.; Juge, N. The mucin-degradation strategy of Ruminococcus gnavus: The importance of intramolecular trans-sialidases. Gut Microbes 2016, 7, 302–312. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, H.; Fang, J.; Zhang, X. Enzymatic and chemoenzymatic synthesis of human milk oligosaccharides and derivatives. Carbohydr. Polym. 2022, 291, 119564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ze, X.; Rui, B.; Li, X.; Zeng, N.; Yuan, J.; Li, W.; Yan, J.; Li, M. Studies and application of sialylated milk components on regulating neonatal gut microbiota and health. Front. Nutr. 2021, 8, 766606. [Google Scholar] [CrossRef] [PubMed]
- Jers, C.; Michalak, M.; Larsen, D.M.; Kepp, K.P.; Li, H.; Guo, Y.; Kirpekar, F.; Meyer, A.S.; Mikkelsen, J.D. Rational design of a new Trypanosoma rangeli trans-sialidase for efficient sialylation of glycans. PLoS ONE 2014, 9, e83902. [Google Scholar] [CrossRef]
- Oliveira, I.A.; Goncalves, A.S.; Neves, J.L.; von Itzstein, M.; Todeschini, A.R. Evidence of ternary complex formation in Trypanosoma cruzi trans-sialidase catalysis. J. Biol. Chem. 2014, 289, 423–436. [Google Scholar] [CrossRef]
- Todeschini, A.R.; Dias, W.B.; Girard, M.F.; Wieruszeski, J.M.; Mendonca-Previato, L.; Previato, J.O. Enzymatically inactive trans-sialidase from Trypanosoma cruzi binds sialyl and β-galactopyranosyl residues in a sequential ordered mechanism. J. Biol. Chem. 2004, 279, 5323–5328. [Google Scholar] [CrossRef] [PubMed]
- Buschiazzo, A.A.M.; Cremona, M.L.; Frasch, A.C.; Alzari, P.M. The crystal structure and mode of action of trans-sialidase, a key enzyme in Trypanosoma cruzi pathogenesis. Mol. Cell 2002, 10, 757–768. [Google Scholar] [CrossRef]
- Watson, J.N.; Newstead, S.; Dookhun, V.; Taylor, G.; Bennet, A.J. Contribution of the active site aspartic acid to catalysis in the bacterial neuraminidase from Micromonospora viridifaciens. FEBS Lett. 2004, 577, 265–269. [Google Scholar] [CrossRef]
- Durrant, J.D.; Bush, R.M.; Amaro, R.E. Microsecond molecular dynamics simulations of influenza neuraminidase suggest a mechanism for the increased virulence of stalk-deletion mutants. J. Phys. Chem. B 2016, 120, 8590–8599. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.F.; Watts, A.G.; Damager, I.; Wehenkel, A.; Nguyen, T.; Buschiazzo, A.; Paris, G.; Frasch, A.C.; Withers, S.G.; Alzari, P.M. Structural insights into the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Structure 2004, 12, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Nordvang, R.T.; Nyffenegger, C.; Holck, J.; Jers, C.; Zeuner, B.; Sundekilde, U.K.; Meyer, A.S.; Mikkelsen, J.D. It all starts with a sandwich: Identification of sialidases with trans-glycosylation activity. PLoS ONE 2016, 11, e0158434. [Google Scholar] [CrossRef]
- Demir, O.; Roitberg, A.E. Modulation of catalytic function by differential plasticity of the active site: Case study of Trypanosoma cruzi trans-sialidase and Trypanosoma rangeli sialidase. Biochemistry 2009, 48, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, F.L.; Miles, S.M.; Neres, J.; Bichenkova, E.V.; Bryce, R.A. Tryptophan as a molecular shovel in the glycosyl transfer activity of Trypanosoma cruzi trans-sialidase. Biophys. J. 2010, 98, L38–L40. [Google Scholar] [CrossRef]
- Nemec, T. Nucleation parameters of SPC/E and TIP4P/2005 water vapor measured in NPT molecular dynamics simulations. J. Mol. Model. 2022, 28, 174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.C.; Cheng, H.H.; Zeng, X.; Zhang, X.L.; Sang, P.; Chen, B.H.; Yang, L.Q. Deciphering gp120 sequence variation and structural dynamics in HIV neutralization phenotype by molecular dynamics simulations and graph machine learning. Proteins 2022, 90, 1413–1424. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, G.; Wang, L. Structural and dynamic evolution of the amphipathic N-terminus diversifies enzyme thermostability in the glycoside hydrolase family 12. Phys. Chem. Chem. Phys. 2016, 18, 21340–21350. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, F.; Niu, H.; Yuan, L.; Tian, J.; Cai, S.; Bi, X.; Zhou, L. Structural studies and molecular dynamic simulations of polyphenol oxidase treated by high pressure processing. Food Chem. 2022, 372, 131243. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Yu, X.W. A phenylalanine dynamic switch controls the interfacial activation of Rhizopus chinensis lipase. Int. J. Biol. Macromol. 2021, 173, 1–12. [Google Scholar] [CrossRef]
- Braun, E.; Moosavi, S.M.; Smit, B. Anomalous effects of velocity rescaling algorithms: The flying ice cube effect revisited. J. Chem. Theory Comput. 2018, 14, 5262–5272. [Google Scholar] [CrossRef]
- Martonak, R.; Laio, A.; Parrinello, M. Predicting crystal structures: The Parrinello-Rahman method revisited. Phys. Rev. Lett. 2003, 90, 075503. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. SETTLE: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Simmonett, A.C.; Brooks, B.R. Analytical Hessians for Ewald and Particle Mesh Ewald electrostatics. J. Chem. Phys. 2021, 154, 104101. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Wu, X.U.E.; Xiu, Z.; Wang, J.; Yin, L.I.U.; Li, G. Understanding the molecular mechanism of binding modes of Aurora a inhibitors by long time scale GPU dynamics. J. Theor. Comput. Chem. 2013, 12, 1341003. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, W.; Han, L.; Shen, W.; Chen, X.; Yang, H. Significant improvement of both catalytic efficiency and stability of fructosyltransferase from Aspergillus niger by structure-guided engineering of key residues in the conserved sequence of the catalytic domain. J. Agric. Food Chem. 2022, 70, 7202–7210. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kardam, V.; Tripathi, A.; TG, S.; Dubey, K.D. The performance of different water models on the structure and function of cytochrome P450 enzymes. J. Chem. Inf. Model. 2022, 62, 6679–6690. [Google Scholar] [CrossRef]
- Li, Z.L.; Buck, M. Modified Potential Functions Result in Enhanced Predictions of a Protein Complex by All-Atom Molecular Dynamics Simulations, Confirming a Stepwise Association Process for Native Protein-Protein Interactions. J. Chem. Theory Comput. 2019, 15, 4318–4331. [Google Scholar] [CrossRef]
- Li, S.; Cao, L.; Yang, X.; Wu, X.; Xu, S.; Liu, Y. Simultaneously optimizing multiple properties of beta-glucosidase Bgl6 using combined (semi-)rational design strategies and investigation of the underlying mechanisms. Bioresour. Technol. 2023, 374, 128792. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, G.; Liu, R.; Wei, J.; Zhang-Negrerie, D.; Jian, X.; Gao, Q. Mechanistic Study of Human Glucose Transport Mediated by GLUT1. J. Chem. Inf. Model. 2016, 56, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Liu, S.; Wang, S.; Wang, L. Ligand-binding specificity and promiscuity of the main lignocellulolytic enzyme families as revealed by active-site architecture analysis. Sci. Rep. 2016, 6, 23605. [Google Scholar] [CrossRef] [PubMed]
| System | PDB ID | Number of Water | Number of Na+ | Number of Cl− |
|---|---|---|---|---|
| TrSA | 1MZ5 | 51,135 | 145 | 150 |
| TrSA-3′-Sialyllactose | 51,178 | 145 | 146 | |
| TcTS | 1S0I | 47,222 | 133 | 139 |
| TcTS-3′-Sialyllactose | 47,212 | 133 | 138 | |
| NanI | 2BF6 | 27,279 | 89 | 77 |
| NanI-Neu5Ac | 27,283 | 90 | 77 | |
| RgNanH | 4X47 | 23,393 | 86 | 66 |
| RgNanH-Neu5Ac | 23,373 | 87 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Yang, X.; Xiao, M.; Jiang, X. Molecular Dynamics Simulations Reveal the Conformational Transition of GH33 Sialidases. Int. J. Mol. Sci. 2023, 24, 6830. https://doi.org/10.3390/ijms24076830
Cao X, Yang X, Xiao M, Jiang X. Molecular Dynamics Simulations Reveal the Conformational Transition of GH33 Sialidases. International Journal of Molecular Sciences. 2023; 24(7):6830. https://doi.org/10.3390/ijms24076830
Chicago/Turabian StyleCao, Xueting, Xiao Yang, Min Xiao, and Xukai Jiang. 2023. "Molecular Dynamics Simulations Reveal the Conformational Transition of GH33 Sialidases" International Journal of Molecular Sciences 24, no. 7: 6830. https://doi.org/10.3390/ijms24076830
APA StyleCao, X., Yang, X., Xiao, M., & Jiang, X. (2023). Molecular Dynamics Simulations Reveal the Conformational Transition of GH33 Sialidases. International Journal of Molecular Sciences, 24(7), 6830. https://doi.org/10.3390/ijms24076830





