Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines
Abstract
1. Introduction
2. Results
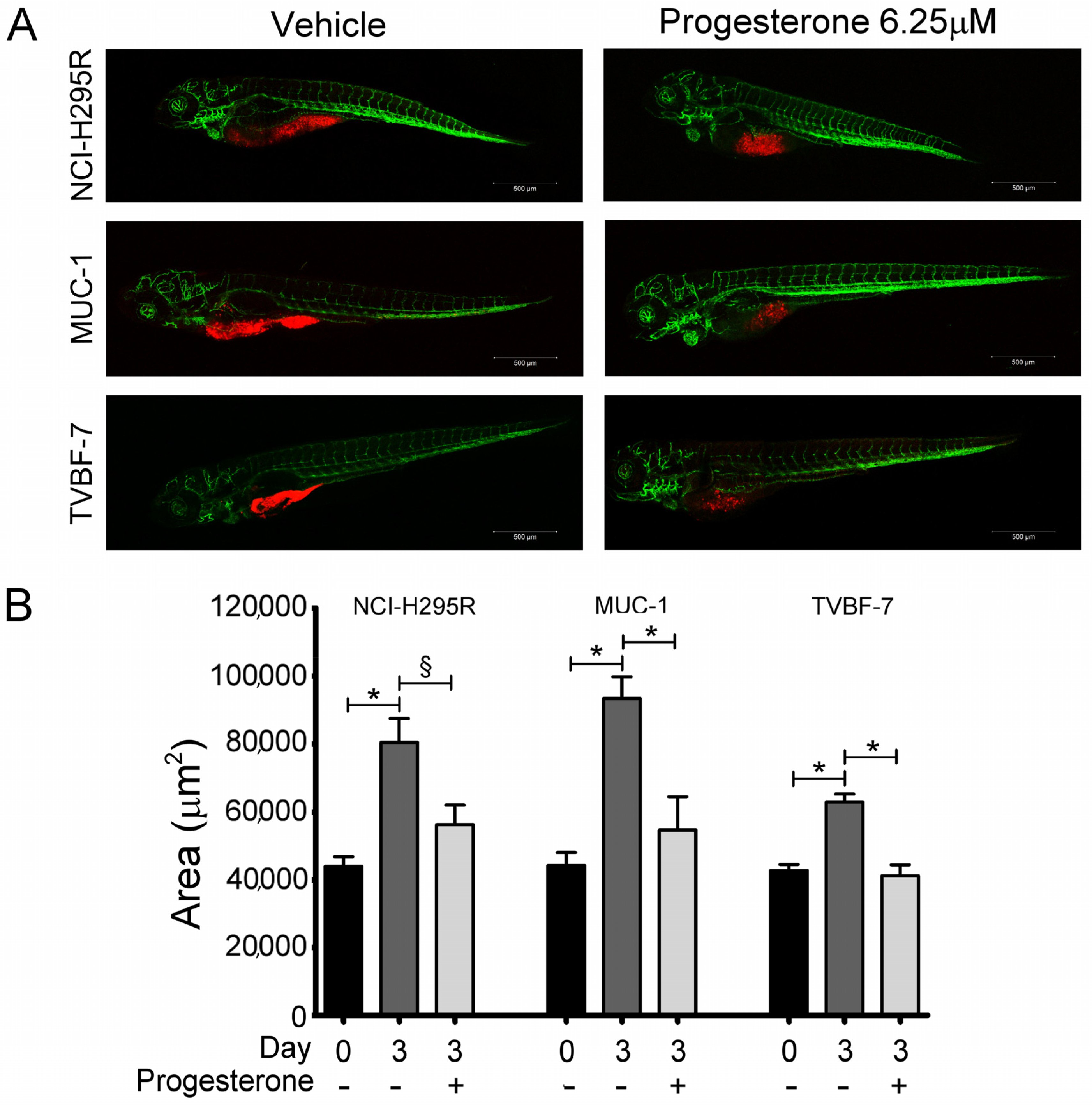
2.1. Pg Inhibited Tumor Growth and Metastases Formation in the Zebrafish/Tumor Xenograft Model
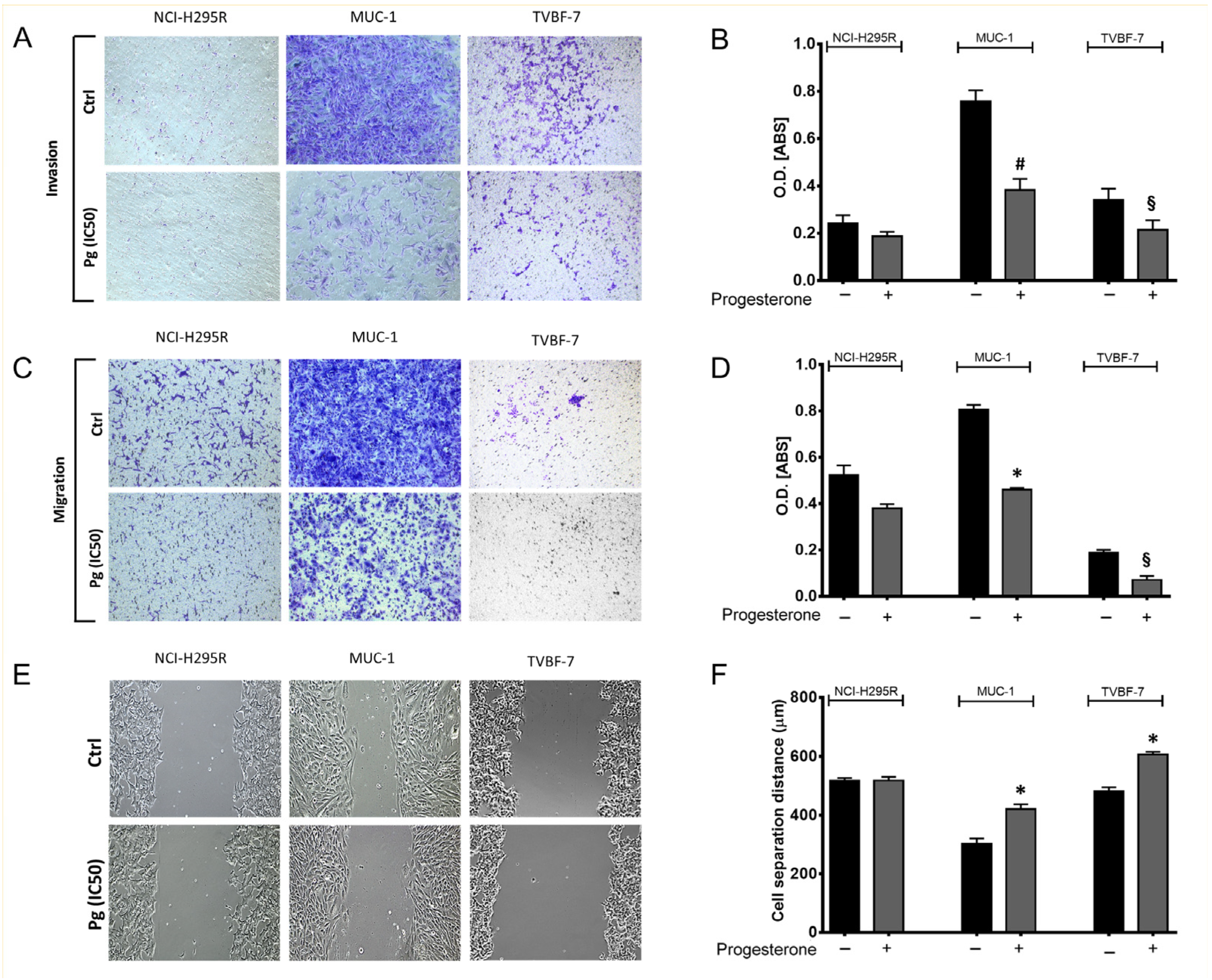
2.2. Pg Suppressed the Migration and Invasion Ability of ACC Cells
2.3. Pg Interfered with Metalloprotease 2 (MMP-2) Activity
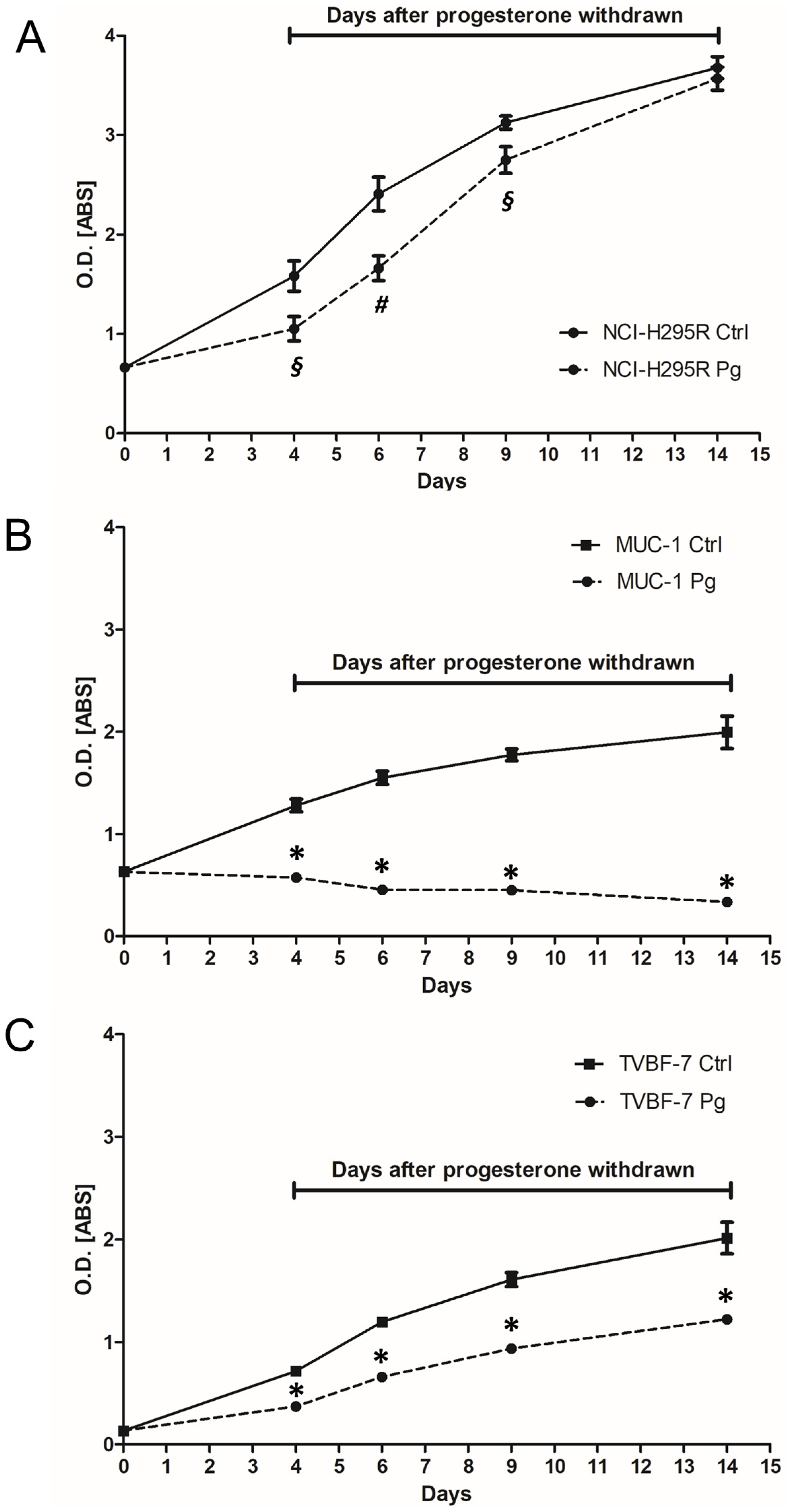
2.4. Pg Exerted a Long-Lasting Inhibitory Effect after Treatment
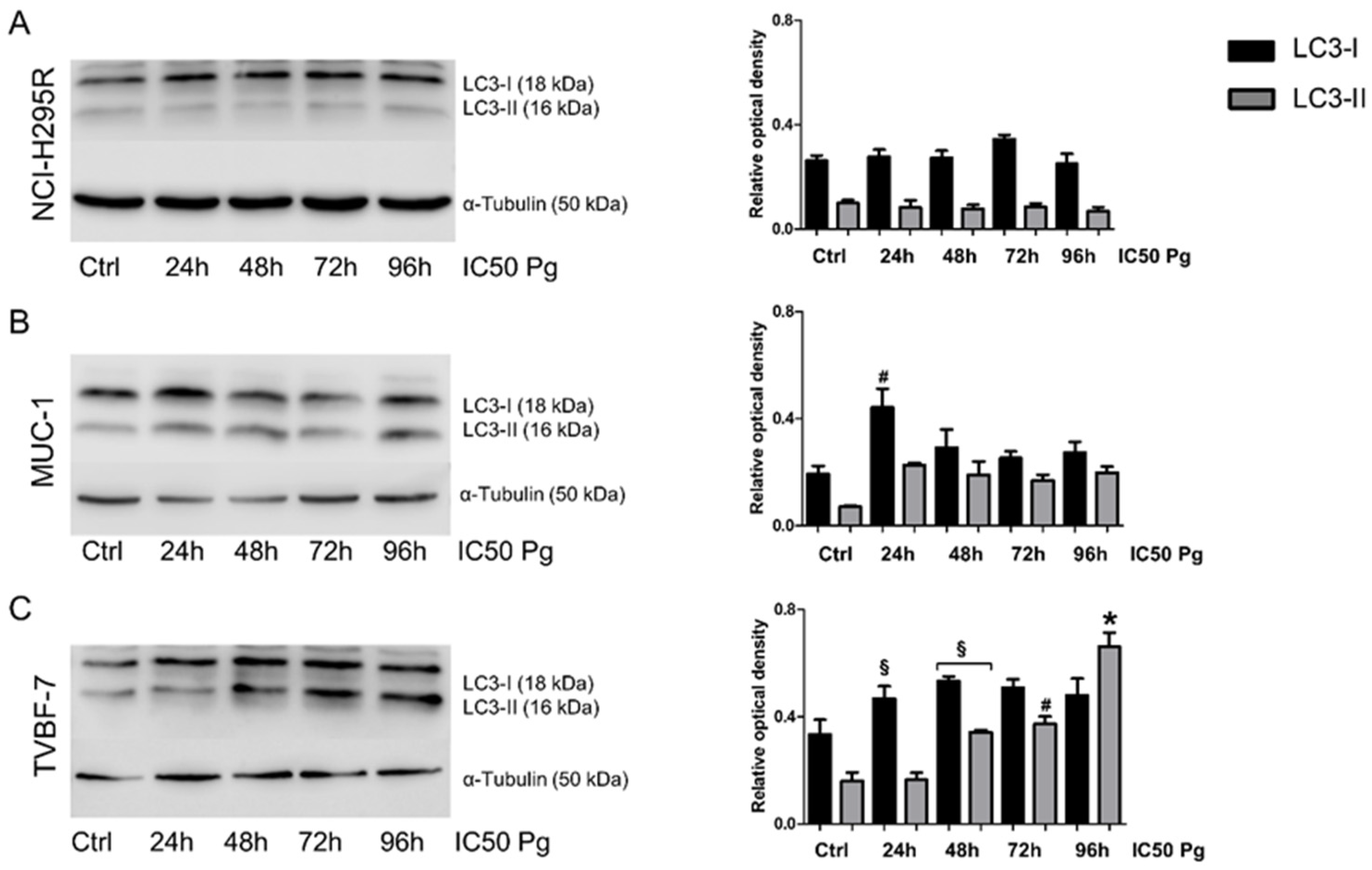
2.5. Pg Exerted Cytotoxic Effects on ACC Cells through Autophagy-Related Apoptosis
2.6. Pg Induced Changes in the Cell-Cycle Distribution
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Cell Treatments
4.3. Measurement of Cell Apoptosis
4.4. Cell-Cycle Analysis
4.5. Drug Withdrawal Experiments
4.6. Fish and Embryos Maintenance
4.7. Tumor Xenograft
4.8. Migration and Invasion Assay
4.9. Wound Healing Assay
4.10. Western Blot
4.11. Zymography
4.12. Gene Expression
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Terzolo, M.; Sperone, P.; Pia, A.; Della Casa, S.; Gross, D.J.; Carnaghi, C.; Casali, P.; Porpiglia, F.; Mantero, F.; et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: A large prospective phase II trial. Endocr. Relat. Cancer 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef]
- Laganà, M.; Grisanti, S.; Cosentini, D.; Ferrari, V.D.; Lazzari, B.; Ambrosini, R.; Sardini, C.; Volta, A.D.; Palumbo, C.; Poliani, P.L.; et al. Efficacy of the EDP-M Scheme Plus Adjunctive Surgery in the Management of Patients with Advanced Adrenocortical Carcinoma: The Brescia Experience. Cancers 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Cosentini, D.; Laganà, M.; Abate, A.; Rossini, E.; Sigala, S.; Berruti, A. Are we failing in treatment of adrenocortical carcinoma? Lights and shadows of molecular signatures. Curr. Opin. Endocr. Metab. Res. 2019, 8, 80–87. [Google Scholar] [CrossRef]
- Grisanti, S.; Cosentini, D.; Laganà, M.; Volta, A.D.; Palumbo, C.; Massimo Tiberio, G.A.; Sigala, S.; Berruti, A. The long and winding road to effective immunotherapy in patients with adrenocortical carcinoma. Future Oncol. 2020, 16, 3017–3020. [Google Scholar] [CrossRef] [PubMed]
- Cremaschi, V.; Abate, A.; Cosentini, D.; Grisanti, S.; Rossini, E.; Laganà, M.; Tamburello, M.; Turla, A.; Sigala, S.; Berruti, A. Advances in adrenocortical carcinoma pharmacotherapy: What is the current state of the art? Expert Opin. Pharm. 2022, 23, 1413–1424. [Google Scholar] [CrossRef]
- Rossouw, J.; Anderson, G.; Prentice, R.; LaCroix, A.; Kooperberg, C.; Stefanick, M.; Jackson, M.L.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc. 2002, 288, 321–333. [Google Scholar]
- Lieberman, A.; Curtis, L. In Defense of Progesterone: A Review of the Literature. Altern. Ther. Health Med. 2017, 23, 24–32. [Google Scholar] [PubMed]
- Núñez, C.; Capelo, J.L.; Igrejas, G.; Alfonso, A.; Botana, L.M.; Lodeiro, C. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials 2016, 97, 34–50. [Google Scholar] [CrossRef]
- Motamed, H.R.; Shariati, M.; Ahmadi, R.; Khatamsaz, S.; Mokhtari, M. The apoptotic effects of progesterone on breast cancer (MCF-7) and human osteosarcoma (MG-636) cells. Physiol. Int. 2020, 107, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Manson, J.E.; Kraft, P.; Cochrane, B.B.; Gunter, M.J.; Chlebowski, R.T.; Zhang, S.M. Estrogen and progesterone-related gene variants and colorectal cancer risk in women. BMC Med. Genet. 2011, 12, 78. [Google Scholar] [CrossRef]
- Schürmann, R.; Cronin, M.; Meyer, J.U. Estrogen plus progestin and colorectal cancer in postmenopausal women. N. Engl. J. Med. 2004, 350, 2417–2419. [Google Scholar] [PubMed]
- Simon, M.S.; Chlebowski, R.T.; Wactawski-Wende, J.; Johnson, K.C.; Muskovitz, A.; Kato, I.; Young, A.; Hubbell, F.A.; Prentice, R.L. Estrogen plus progestin and colorectal cancer incidence and mortality. J. Clin. Oncol. 2012, 30, 3983–3990. [Google Scholar] [CrossRef]
- Motylewska, E.; Mełeń-Mucha, G. Estrone and progesterone inhibit the growth of murine MC38 colon cancer line. J. Steroid. Biochem. Mol. Biol. 2009, 113, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, M.; Shao, K.; Yang, Y.; Wang, Q.; Cai, M. Progesterone induces cell apoptosis via the CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer. Oncol. Rep. 2020, 43, 121–132. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, L.B.; Yang, H.Y.; Zhang, H.P. Effects of estradiol and progesterone on the growth of HeLa cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3959–3965. [Google Scholar]
- Hong, Y.; Liu, Y.; Zhang, G.; Wu, H.; Hou, Y. Progesterone suppresses Aβ(42)-induced neuroinflammation by enhancing autophagy in astrocytes. Int. Immunopharmacol. 2018, 54, 336–343. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, S.J.; Koh, J.Y. The neurosteroids, allopregnanolone and progesterone, induce autophagy in cultured astrocytes. Neurochem. Int. 2012, 60, 125–133. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.Y.; Cho, K.S.; Kim, H.N.; Koh, J.Y. Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 59, 80–85. [Google Scholar] [CrossRef]
- Godbole, M.; Tiwary, K.; Badwe, R.; Gupta, S.; Dutt, A. Progesterone suppresses the invasion and migration of breast cancer cells irrespective of their progesterone receptor status—A short report. Cell. Oncol. 2017, 40, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Silva, S.V.; Jaeger, R.G.; Freitas, V.M. Progesterone decreases ovarian cancer cells migration and invasion. Steroids 2020, 161, 108680. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Dorjbal, B.; Hamilton, C.A.; Maxwell, G.L.; Rodriguez, G.C.; Syed, V. Progesterone and calcitriol reduce invasive potential of endometrial cancer cells by targeting ARF6, NEDD9 and MT1-MMP. Oncotarget 2017, 8, 113583–113597. [Google Scholar] [CrossRef]
- Fiorentini, C.; Fragni, M.; Perego, P.; Vezzoli, S.; Bonini, S.A.; Tortoreto, M.; Galli, D.; Claps, M.; Tiberio, G.A.; Terzolo, M.; et al. Antisecretive and Antitumor Activity of Abiraterone Acetate in Human Adrenocortical Cancer: A Preclinical Study. J. Clin. Endocrinol. Metab. 2016, 101, 4594–4602. [Google Scholar] [CrossRef] [PubMed]
- Fragni, M.; Fiorentini, C.; Rossini, E.; Fisogni, S.; Vezzoli, S.; Bonini, S.A.; Dalmiglio, C.; Grisanti, S.; Tiberio, G.A.M.; Claps, M.; et al. In vitro antitumor activity of progesterone in human adrenocortical carcinoma. Endocrine 2019, 63, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Rossini, E.; Tamburello, M.; Laganà, M.; Cosentini, D.; Grisanti, S.; Fiorentini, C.; Tiberio, G.A.M.; Scatolini, M.; Grosso, E.; et al. Ribociclib Cytotoxicity Alone or Combined With Progesterone and/or Mitotane in in Vitro Adrenocortical Carcinoma Cells. Endocrinology 2022, 163, bqab248. [Google Scholar] [CrossRef]
- Rossini, E.; Tamburello, M.; Abate, A.; Beretta, S.; Fragni, M.; Cominelli, M.; Cosentini, D.; Hantel, C.; Bono, F.; Grisanti, S.; et al. Cytotoxic Effect of Progesterone, Tamoxifen and Their Combination in Experimental Cell Models of Human Adrenocortical Cancer. Front. Endocrinol. 2021, 12, 669426. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Volante, M.; Sperone, P.; Bollito, E.; Frangipane, E.; Rosas, R.; Daffara, F.; Martinez-Avila, N.; Martinez-Fierro, M.L. Matrix metalloproteinase type 2 expression in malignant adrenocortical tumors: Diagnostic and prognostic significance in a series of 50 adrenocortical carcinomas. Mod Pathol. 2006, 19, 1563–1569. [Google Scholar] [CrossRef]
- González-Polo, R.A.; Boya, P.; Pauleau, A.L.; Jalil, A.; Larochette, N.; Souquère, S.; Eskelinen, E.-L.; Pierron, G.; Saftig, P.; Kroemer, G. The apoptosis/autophagy paradox: Autophagic vacuolization before apoptotic death. J. Cell Sci. 2005, 118 Pt 14, 3091–3102. [Google Scholar] [CrossRef]
- De Cremoux, P.; Rosenberg, D.; Goussard, J.; Brémont-Weil, C.; Tissier, F.; Tran-Perennou, C.; Groussin, L.; Bertagna, X.; Bertherat, J.; Raffin-Sanson, M.L. Expression of progesterone and estradiol receptors in normal adrenal cortex, adrenocortical tumors, and primary pigmented nodular adrenocortical disease. Endocr. Relat. Cancer 2008, 15, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Letrado, P.; de Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding Up the Cancer Drug Discovery Process. Cancer Res. 2018, 78, 6048–6058. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Bothou, C.; Sharma, A.; Oo, A.; Kim, B.; Perge, P.; Igaz, P.; Ronchi, C.L.; Shapiro, I.; Hantel, C. Novel Insights into the Molecular Regulation of Ribonucleotide Reductase in Adrenocortical Carcinoma Treatment. Cancers 2021, 13, 4200. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, L.; Kuang, Y.; Li, X.; Deng, X.; Liang, X.; Li, L.; Yang, H.; Huang, Z.; Lu, D.; et al. Tauroursodeoxycholic acid mediates endoplasmic reticulum stress and autophagy in adrenocortical carcinoma cells. Oncol. Lett. 2019, 18, 6475–6482. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Pereira, S.S.; Pignatelli, D. Modulation of Autophagy in Adrenal Tumors. Front. Endocrinol. 2022, 13, 937367. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.; Towers, C.; Kar, A.; Zhang, Y.; Kiseljak-Vassiliades, K.; Thorburn, A.; Wierman, M. SAT-LB064 Mitotane Induces Autophagy: A Mechanism to Promote Chemoresistance in Adrenocortical Carcinoma. J. Endocr. Soc. 2019, 3 (Suppl. S1), SAT-LB064. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F. Autophagy modulation for cancer therapy. Cancer Biol. Ther. 2011, 11, 169–176. [Google Scholar] [CrossRef]
- Atif, F.; Yousuf, S.; Stein, D.G. Anti-tumor effects of progesterone in human glioblastoma multiforme: Role of PI3K/Akt/mTOR signaling. J. Steroid. Biochem. Mol. Biol. 2015, 146, 62–73. [Google Scholar] [CrossRef]
- Syed, V.; Ho, S.M. Progesterone-induced apoptosis in immortalized normal and malignant human ovarian surface epithelial cells involves enhanced expression of FasL. Oncogene 2003, 22, 6883–6890. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progestins as Anticancer Drugs and Chemosensitizers, New Targets and Applications. Pharmaceutics 2021, 13, 1616. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Dey, S.; Nath, S. Steroid Hormone Receptors: Links With Cell Cycle Machinery and Breast Cancer Progression. Front. Oncol. 2021, 11, 620214. [Google Scholar] [CrossRef] [PubMed]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rainey, W.E.; Saner, K.; Schimmer, B.P. Adrenocortical cell lines. Mol. Cell. Endocrinol. 2004, 228, 23–38. [Google Scholar] [CrossRef]
- Hantel, C.; Shapiro, I.; Poli, G.; Chiapponi, C.; Bidlingmaier, M.; Reincke, M.; Luconi, M.; Jung, S.; Beuschlein, F. Targeting heterogeneity of adrenocortical carcinoma: Evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget 2016, 7, 79292–79304. [Google Scholar] [CrossRef]
- Sigala, S.; Bothou, C.; Penton, D.; Abate, A.; Peitzsch, M.; Cosentini, D.; Tiberio, G.A.M.; Bornstein, S.R.; Berruti, A.; Hantel, C. A Comprehensive Investigation of Steroidogenic Signaling in Classical and New Experimental Cell Models of Adrenocortical Carcinoma. Cells 2022, 11, 1439. [Google Scholar] [CrossRef]
- Sigala, S.; Rossini, E.; Abate, A.; Tamburello, M.; Bornstein, S.R.; Hantel, C. An update on adrenocortical cell lines of human origin. Endocrine 2022, 77, 432–437. [Google Scholar] [CrossRef]
- Rossini, E.; Bosatta, V.; Abate, A.; Fragni, M.; Salvi, V.; Basnet, R.M.; Zizioli, D.; Bosisio, D.; Piovani, G.; Valcamonico, F.; et al. Cisplatin Cytotoxicity in Human Testicular Germ Cell Tumor Cell Lines Is Enhanced by the CDK4/6 Inhibitor Palbociclib. Clin. Genitourin. Cancer 2021, 19, 316–324. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Muscò, A.; Finazzi, D.; Sigala, S.; Rossini, E.; Tobia, C.; Guerra, J.; Presta, M.; Memo, M. Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos. Int. J. Mol. Sci. 2021, 22, 4856. [Google Scholar] [CrossRef]
- Abate, A.; Tamburello, M.; Rossini, E.; Basnet, R.M.; Ribaudo, G.; Gianoncelli, A.; Hantel, C.; Cosentini, D.; Laganà, M.; Grisanti, S.; et al. Trabectedin impairs invasiveness and metastasis in adrenocortical carcinoma preclinical models. Endocr. Relat. Cancer 2023, 30, e220273. [Google Scholar] [CrossRef]
- Fiorentini, C.; Bodei, S.; Bedussi, F.; Fragni, M.; Bonini, S.A.; Simeone, C.; Zani, D.; Berruti, A.; Missale, C.; Memo, M.; et al. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp. Cell Res. 2014, 323, 100–111. [Google Scholar] [CrossRef] [PubMed]




| NCI-H295R | MUC-1 | TVBF-7 | ||||
|---|---|---|---|---|---|---|
| Pg | − | + | − | + | − | + |
| 24 h | 14.66 ± 4.44 | 23.45 ± 1.52 | 2.16 ± 0.45 | 5.43 ± 1.19 | 16.07 ± 2.73 | 12.95 ± 1.18 |
| 48 h | 11.28 ± 4.76 | 27.95 ± 3.02 § | 1.90 ± 0.14 | 3.68 ± 1.09 | 4.63 ± 1.14 | 3.73 ± 1.58 |
| 72 h | 17.98 ± 1.93 | 23.23 ± 0.13 | 3.42 ± 0.86 | 15.47 ± 2.21 * | 8.07 ± 1.15 | 7.50 ± 2.48 |
| 96 h | 16.72 ± 2.84 | 22.57 ± 1.83 | 6.81 ± 1.13 | 21.08 ± 0.64 * | 10.64 ± 3.55 | 3.51 ± 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburello, M.; Abate, A.; Rossini, E.; Basnet, R.M.; Zizioli, D.; Cosentini, D.; Hantel, C.; Laganà, M.; Tiberio, G.A.M.; Grisanti, S.; et al. Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines. Int. J. Mol. Sci. 2023, 24, 6829. https://doi.org/10.3390/ijms24076829
Tamburello M, Abate A, Rossini E, Basnet RM, Zizioli D, Cosentini D, Hantel C, Laganà M, Tiberio GAM, Grisanti S, et al. Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines. International Journal of Molecular Sciences. 2023; 24(7):6829. https://doi.org/10.3390/ijms24076829
Chicago/Turabian StyleTamburello, Mariangela, Andrea Abate, Elisa Rossini, Ram Manohar Basnet, Daniela Zizioli, Deborah Cosentini, Constanze Hantel, Marta Laganà, Guido Alberto Massimo Tiberio, Salvatore Grisanti, and et al. 2023. "Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines" International Journal of Molecular Sciences 24, no. 7: 6829. https://doi.org/10.3390/ijms24076829
APA StyleTamburello, M., Abate, A., Rossini, E., Basnet, R. M., Zizioli, D., Cosentini, D., Hantel, C., Laganà, M., Tiberio, G. A. M., Grisanti, S., Memo, M., Berruti, A., & Sigala, S. (2023). Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines. International Journal of Molecular Sciences, 24(7), 6829. https://doi.org/10.3390/ijms24076829