Role of Orai3-Mediated Store-Operated Calcium Entry in Radiation-Induced Brain Microvascular Endothelial Cell Injury
Abstract
1. Introduction
2. Results
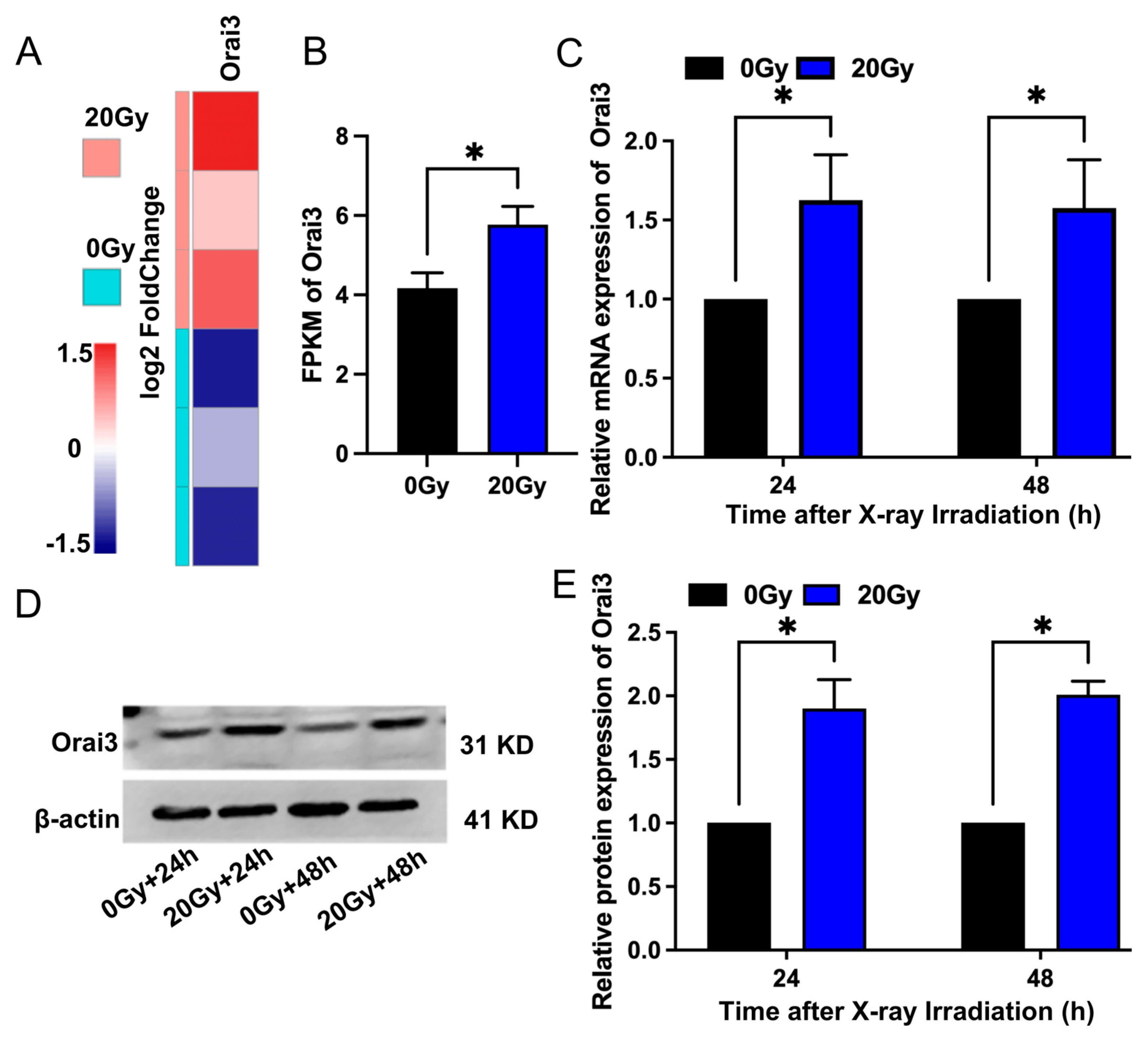
2.1. X-ray Irradiation Increases Orai3 Expression in rBMECs
2.2. Role of Orai3 in X-ray Irradiation-Induced Enhancement of SOCE in rBMECs
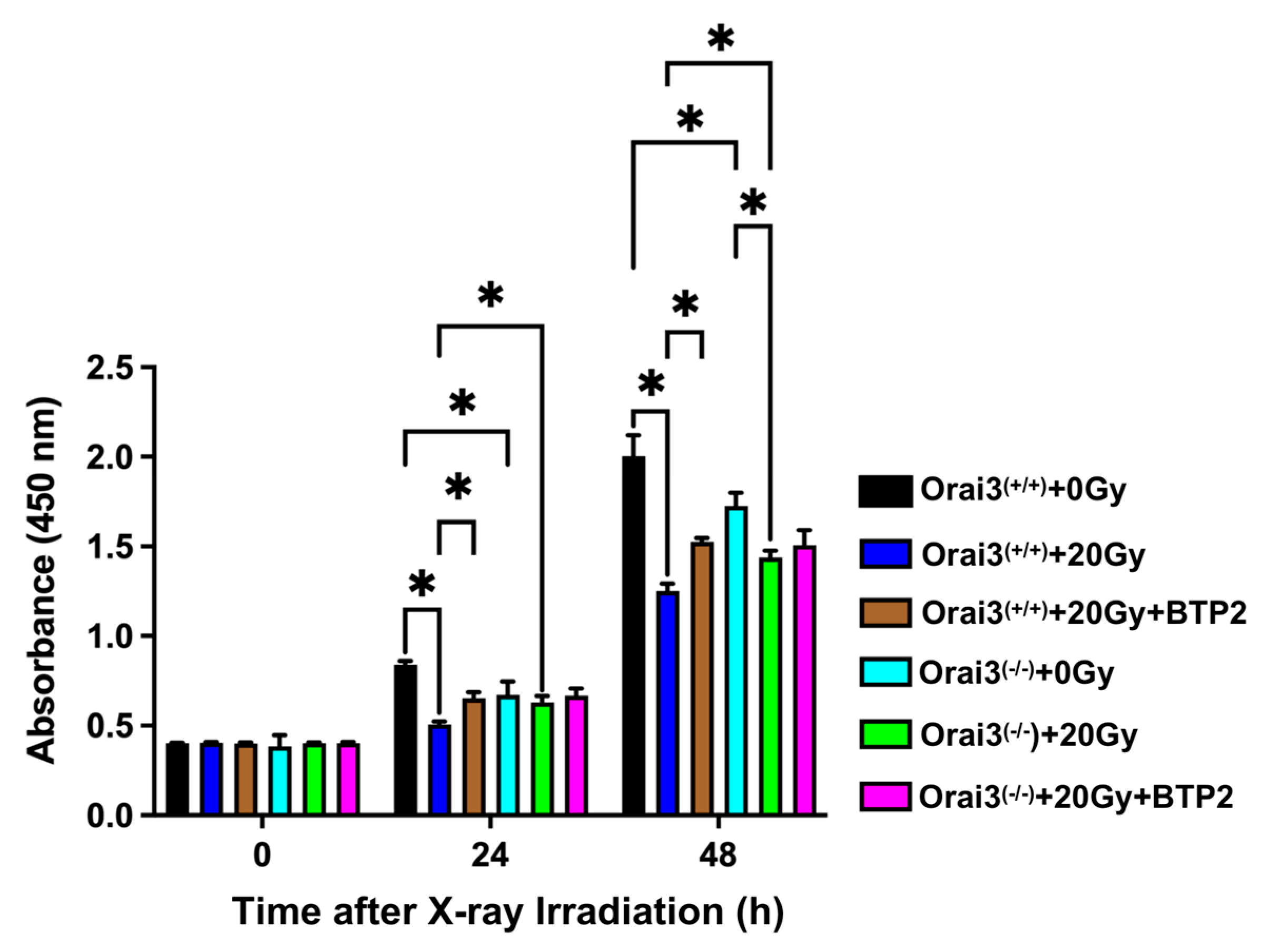
2.3. Role of Orai3 in X-ray Irradiation-Induced Inhibition of rBMEC Proliferation
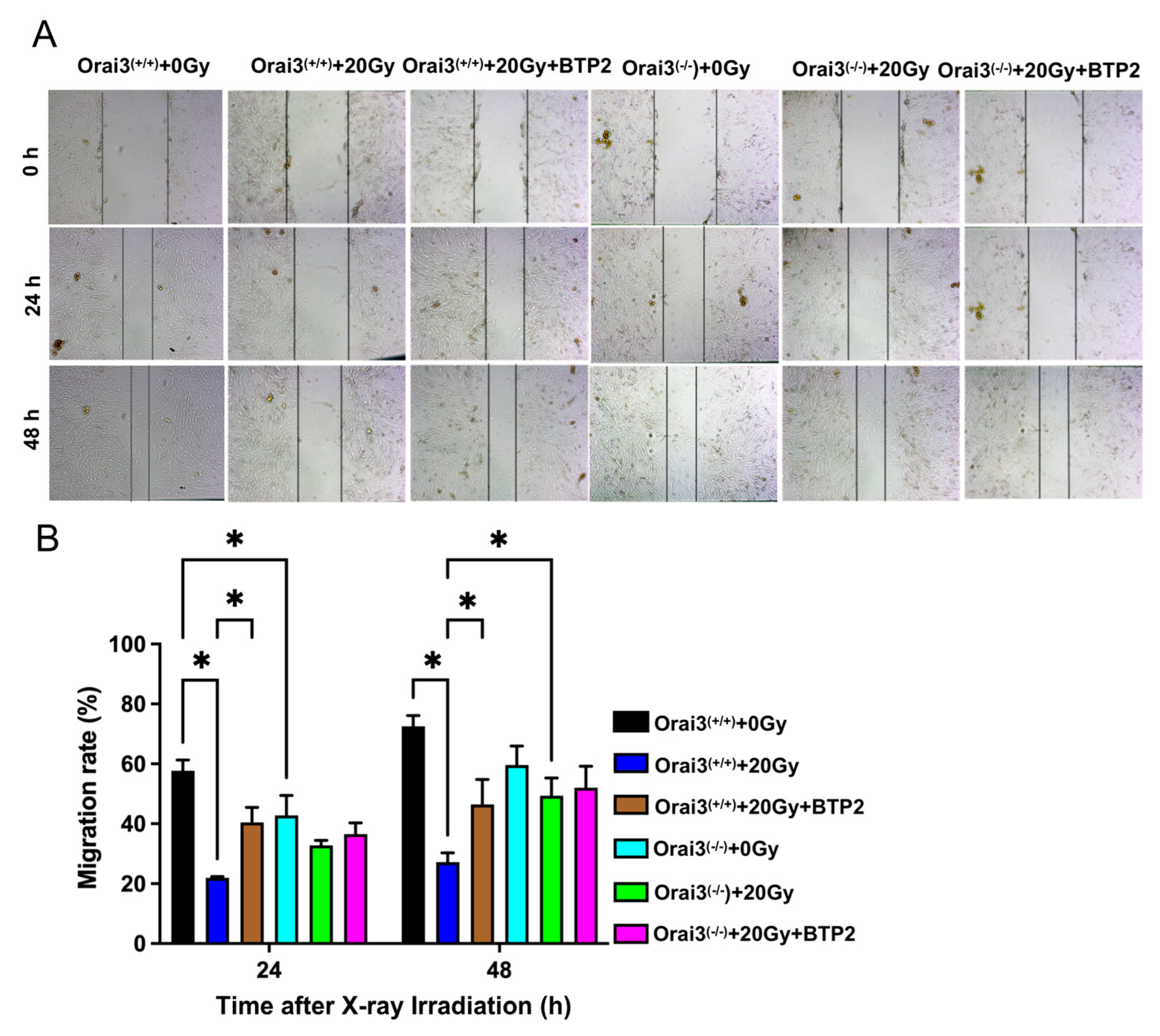
2.4. Role of Orai3 in X-ray Irradiation-Induced Inhibition of rBMEC Migration
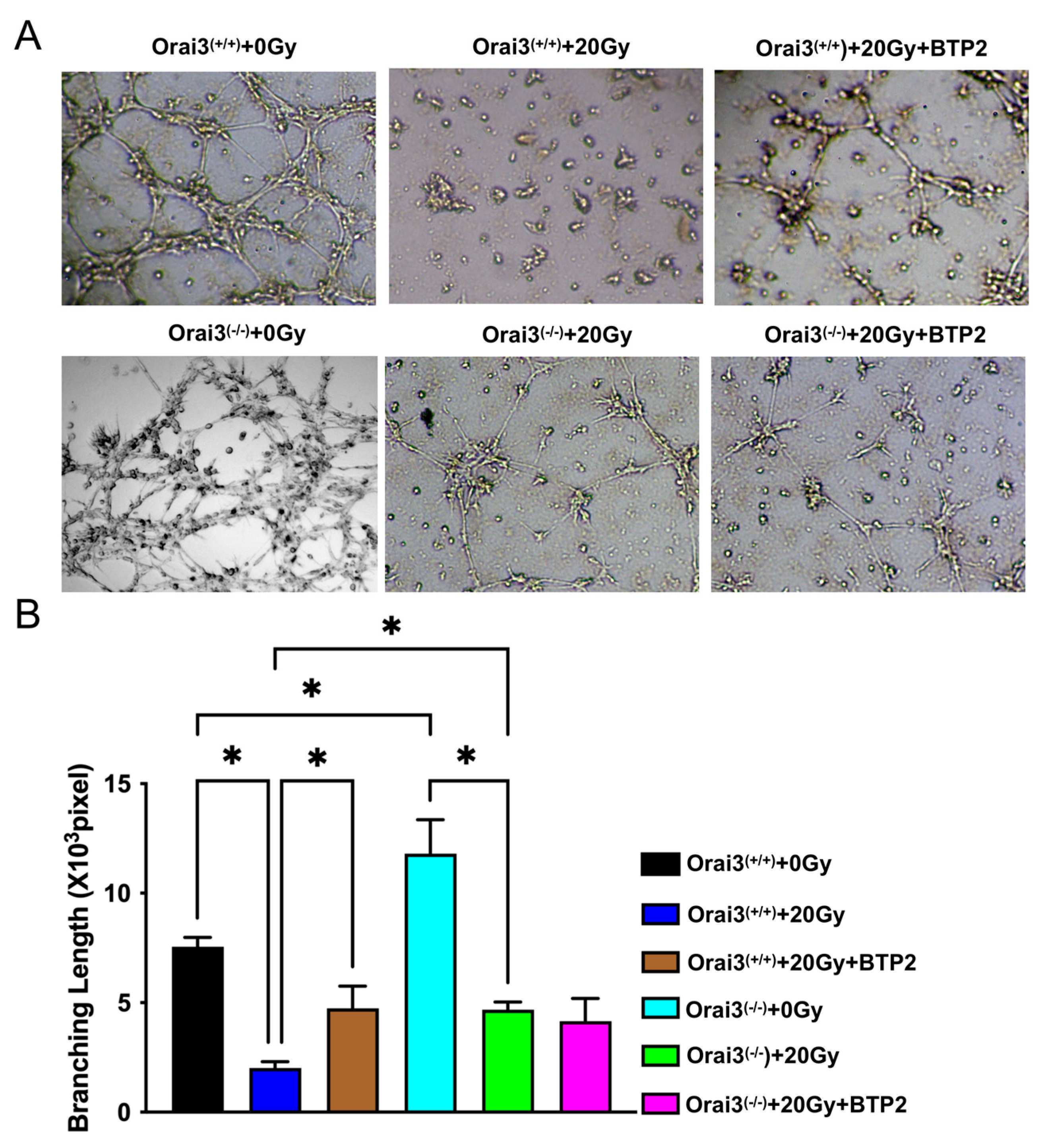
2.5. Role of Orai3 in X-ray Irradiation-Induced Inhibition of rBMEC Tube Formation
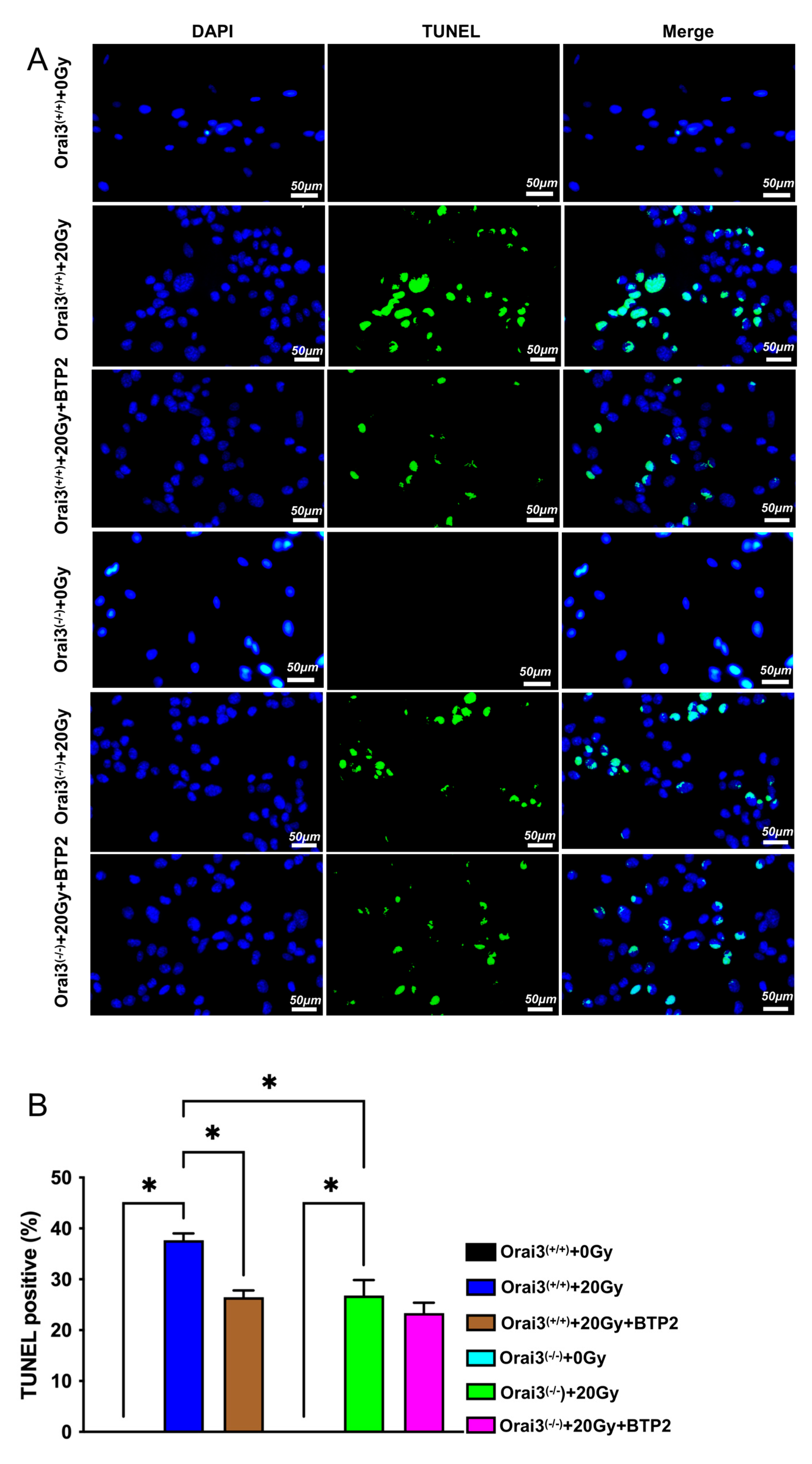
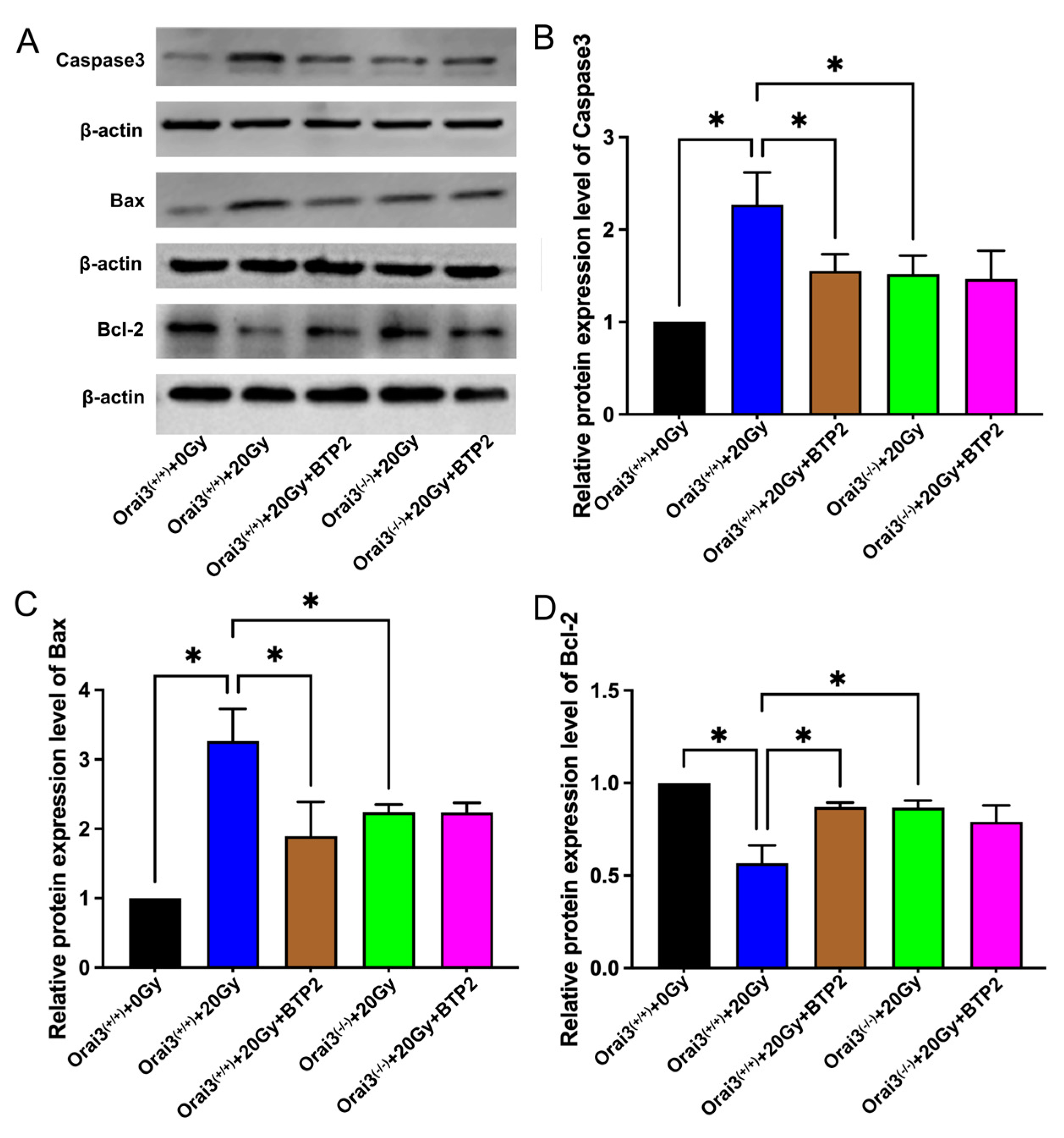
2.6. Role of Orai3 in X-ray Irradiation-Induced Apoptosis of rBMECs
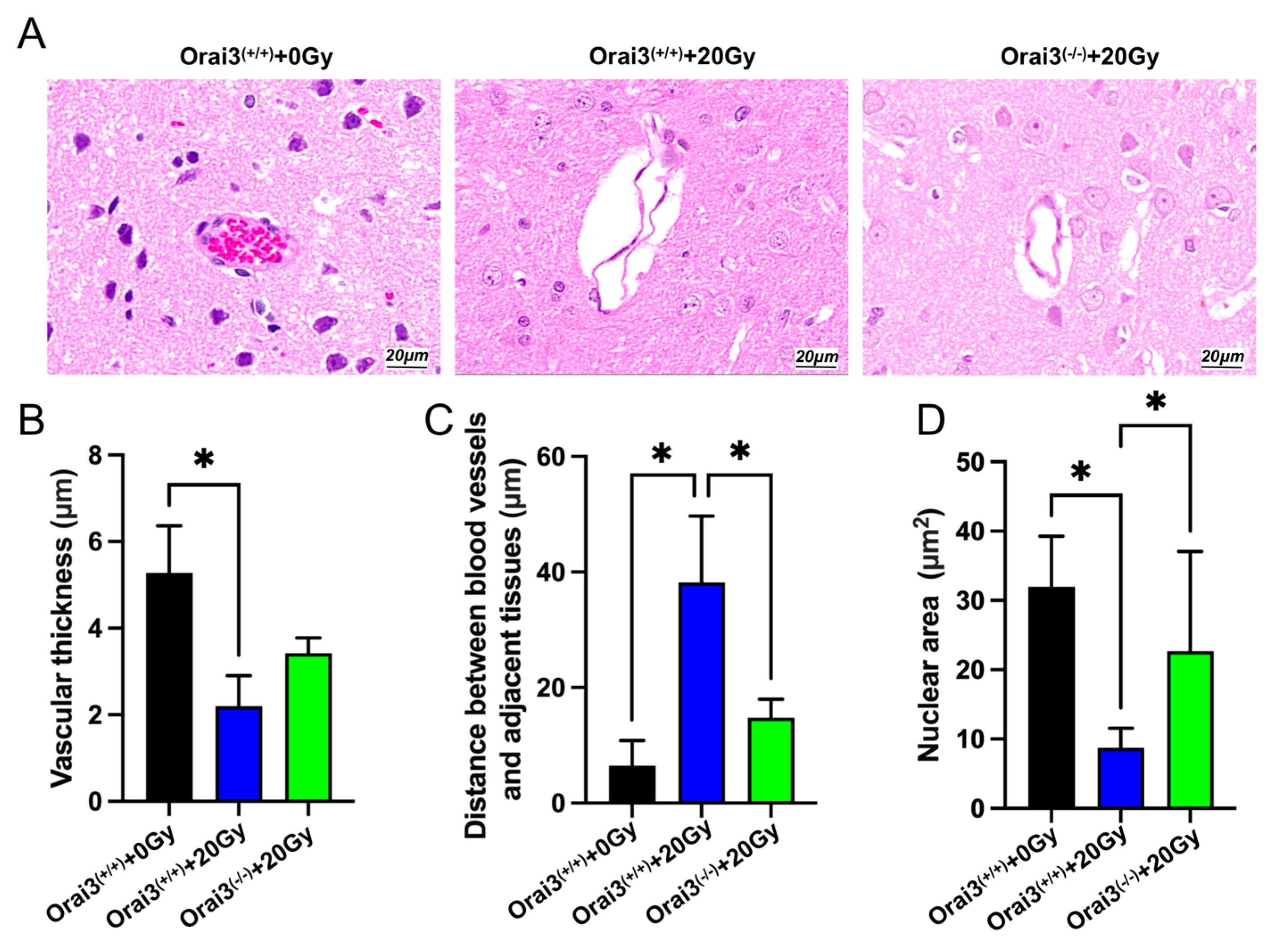
2.7. Role of Orai3 in X-ray Irradiation-Induced Rat Brain Tissue Injury
3. Discussion
4. Materials and Methods
4.1. Animals and Cells
4.2. X-ray Irradiation
4.3. Reverse-Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Assay
4.4. Western Blotting
4.5. Wound Healing Assay
4.6. Cell Viability Assay
4.7. Tube Formation Assay
4.8. TUNEL Assay
4.9. [Ca2+]i Measurement
4.10. Hematoxylin and Eosin Staining Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern radiotherapy for head and neck cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef]
- Caudell, J.J.; Torres-Roca, J.F.; Gillies, R.J.; Enderling, H.; Kim, S.; Rishi, A.; Moros, E.G.; Harrison, L.B. The future of personalised radiotherapy for head and neck cancer. Lancet Oncol. 2017, 18, e266–e273. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, N.V.; Kiang, J.G. Brain Damage and Patterns of Neurovascular Disorder after Ionizing Irradiation. Complications in Radiotherapy and Radiation Combined Injury. Radiat. Res. 2021, 196, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.N.; Caudell, D.L.; Metheny-Barlow, L.J.; Peiffer, A.M.; Tooze, J.A.; Bourland, J.D.; Hampson, R.E.; Deadwyler, S.A.; Cline, J.M. Fibronectin Produced by Cerebral Endothelial and Vascular Smooth Muscle Cells Contributes to Perivascular Extracellular Matrix in Late-Delayed Radiation-Induced Brain Injury. Radiat. Res. 2018, 190, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, M.; Kalm, M.; Eriksson, Y.; Bull, C.; Stahlberg, A.; Bjork-Eriksson, T.; Hellstrom Erkenstam, N.; Blomgren, K. A role for endothelial cells in radiation-induced inflammation. Int. J. Radiat. Biol. 2018, 94, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.I.; Kim, K.I.; Woo, S.K.; Kim, J.S.; Choi, K.J.; Lee, H.J.; Kim, K.S. Stromal Cell-Derived Factor 1 Protects Brain Vascular Endothelial Cells from Radiation-Induced Brain Damage. Cells 2019, 8, 1230. [Google Scholar] [CrossRef]
- Wood, K.; Jawahar, A.; Smelley, C.; Mullapudi, S.; DeLaune, A.; Nanda, A.; Granger, D.N. Exposure of brain to high-dose, focused gamma rays irradiation produces increase in leukocytes-adhesion and pavementing in small intracerebral blood vessels. Neurosurgery 2005, 57, 1282–1288, discussion 1282–1288. [Google Scholar] [CrossRef]
- Wu, Q.; Fang, Y.; Zhang, X.; Song, F.; Wang, Y.; Chen, H.; Du, J.; Zheng, C.B.; Shen, B. Effect of X-rays on transcript expression of rat brain microvascular endothelial cells: Role of calcium signaling in X-ray-induced endothelium damage. Biosci. Rep. 2020, 40, BSR20193760. [Google Scholar] [CrossRef]
- Pan, Z.; Ma, J. Open Sesame: Treasure in store-operated calcium entry pathway for cancer therapy. Sci. China Life Sci. 2015, 58, 48–53. [Google Scholar] [CrossRef]
- Negri, S.; Faris, P.; Moccia, F. Reactive Oxygen Species and Endothelial Ca(2+) Signaling: Brothers in Arms or Partners in Crime? Int. J. Mol. Sci. 2021, 22, 9821. [Google Scholar] [CrossRef]
- Liu, X.; Gong, B.; de Souza, L.B.; Ong, H.L.; Subedi, K.P.; Cheng, K.T.; Swaim, W.; Zheng, C.; Mori, Y.; Ambudkar, I.S. Radiation inhibits salivary gland function by promoting STIM1 cleavage by caspase-3 and loss of SOCE through a TRPM2-dependent pathway. Sci. Signal. 2017, 10, eaal4064. [Google Scholar] [CrossRef] [PubMed]
- Prishchep, S.G.; Gerasimovich, N.V.; Milyutin, A.A. The effect of low doses of gamma irradiation on the homeostasis of intracellular calcium in lymphocyte cells of rats. Radiat. Prot. Dosim. 2002, 99, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Motiani, R.K.; Stolwijk, J.A.; Newton, R.L.; Zhang, X.; Trebak, M. Emerging roles of Orai3 in pathophysiology. Channels 2013, 7, 392–401. [Google Scholar] [CrossRef]
- Xu, N.; Ayers, L.; Pastukh, V.; Alexeyev, M.; Stevens, T.; Tambe, D.T. Impact of Na+ permeation on collective migration of pulmonary arterial endothelial cells. PLoS ONE 2021, 16, e0250095. [Google Scholar] [CrossRef]
- Mirzaie-Joniani, H.; Eriksson, D.; Sheikholvaezin, A.; Johansson, A.; Lofroth, P.O.; Johansson, L.; Stigbrand, T. Apoptosis induced by low-dose and low-dose-rate radiation. Cancer 2002, 94, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liang, B.; Chen, Z.; Liu, X.; Zhang, Z. The dynamic changes of capillary permeability and upregulation of VEGF in rats following radiation-induced brain injury. Microcirculation 2014, 21, 171–177. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, P.; Haimovitz-Friedman, A.; Reilly, R.M.; Wong, C.S. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003, 63, 5950–5956. [Google Scholar]
- Yuan, H.; Gaber, M.W.; McColgan, T.; Naimark, M.D.; Kiani, M.F.; Merchant, T.E. Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: Modulation with anti-ICAM-1 antibodies. Brain Res. 2003, 969, 59–69. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Gao, H.; Zhang, X.; Hu, Y.; Han, T.; Shen, B.; Zhang, L.; Wu, Q. X-ray Causes mRNA Transcripts Change to Enhance Orai2-Mediated Ca(2+) Influx in Rat Brain Microvascular Endothelial Cells. Front. Mol. Biosci. 2021, 8, 646730. [Google Scholar] [CrossRef]
- Motiani, R.K.; Abdullaev, I.F.; Trebak, M. A novel native store-operated calcium channel encoded by Orai3: Selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J. Biol. Chem. 2010, 285, 19173–19183. [Google Scholar] [CrossRef] [PubMed]
- Saliba, Y.; Keck, M.; Marchand, A.; Atassi, F.; Ouille, A.; Cazorla, O.; Trebak, M.; Pavoine, C.; Lacampagne, A.; Hulot, J.S.; et al. Emergence of Orai3 activity during cardiac hypertrophy. Cardiovasc. Res. 2015, 105, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Pan, H.; Yao, J.; Zhou, Y.; Han, W. SOCE and cancer: Recent progress and new perspectives. Int. J. Cancer 2016, 138, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, M.; Zhou, D.; Deng, Z.; Guo, J.; Huang, H. Endothelial progenitor cell transplantation restores vascular injury in mice after whole-brain irradiation. Brain Res. 2020, 1746, 147005. [Google Scholar] [CrossRef]
- Lee, W.H.; Cho, H.J.; Sonntag, W.E.; Lee, Y.W. Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 and Ang-2 in rat brain. Radiat. Res. 2011, 176, 753–760. [Google Scholar] [CrossRef]
- Chu, C.; Gao, Y.; Lan, X.; Lin, J.; Thomas, A.M.; Li, S. Stem-Cell Therapy as a Potential Strategy for Radiation-Induced Brain Injury. Stem Cell Rev. Rep. 2020, 16, 639–649. [Google Scholar] [CrossRef]
- Guipaud, O.; Jaillet, C.; Clement-Colmou, K.; Francois, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Zhang, R.; Xia, L.; Chen, L.; Guo, J.; Dai, F.; Du, J.; Shen, B. Orai3 exacerbates apoptosis of lens epithelial cells by disrupting Ca2+ homeostasis in diabetic cataract. Clin. Transl. Med. 2021, 11, e327. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, R.; Zhou, M.; Li, J.; Yao, X.; Du, J.; Chen, J.; Shen, B. TRPP2 and STIM1 form a microdomain to regulate store-operated Ca2+ entry and blood vessel tone. Cell Commun. Signal 2020, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, N.; Li, P.; Xu, S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Fang, Y.; Huang, X.; Zheng, F.; Ma, S.; Zhang, X.; Han, T.; Gao, H.; Shen, B. Role of Orai3-Mediated Store-Operated Calcium Entry in Radiation-Induced Brain Microvascular Endothelial Cell Injury. Int. J. Mol. Sci. 2023, 24, 6818. https://doi.org/10.3390/ijms24076818
Wu Q, Fang Y, Huang X, Zheng F, Ma S, Zhang X, Han T, Gao H, Shen B. Role of Orai3-Mediated Store-Operated Calcium Entry in Radiation-Induced Brain Microvascular Endothelial Cell Injury. International Journal of Molecular Sciences. 2023; 24(7):6818. https://doi.org/10.3390/ijms24076818
Chicago/Turabian StyleWu, Qibing, Yang Fang, Xiaoyu Huang, Fan Zheng, Shaobo Ma, Xinchen Zhang, Tingting Han, Huiwen Gao, and Bing Shen. 2023. "Role of Orai3-Mediated Store-Operated Calcium Entry in Radiation-Induced Brain Microvascular Endothelial Cell Injury" International Journal of Molecular Sciences 24, no. 7: 6818. https://doi.org/10.3390/ijms24076818
APA StyleWu, Q., Fang, Y., Huang, X., Zheng, F., Ma, S., Zhang, X., Han, T., Gao, H., & Shen, B. (2023). Role of Orai3-Mediated Store-Operated Calcium Entry in Radiation-Induced Brain Microvascular Endothelial Cell Injury. International Journal of Molecular Sciences, 24(7), 6818. https://doi.org/10.3390/ijms24076818





