Assessment of BMP7, SMAD4, and CDH1 Expression Profile and Regulatory miRNA-542-3p in Eutopic and Ectopic Endometrium of Women with Endometriosis
Abstract
1. Introduction
2. Results
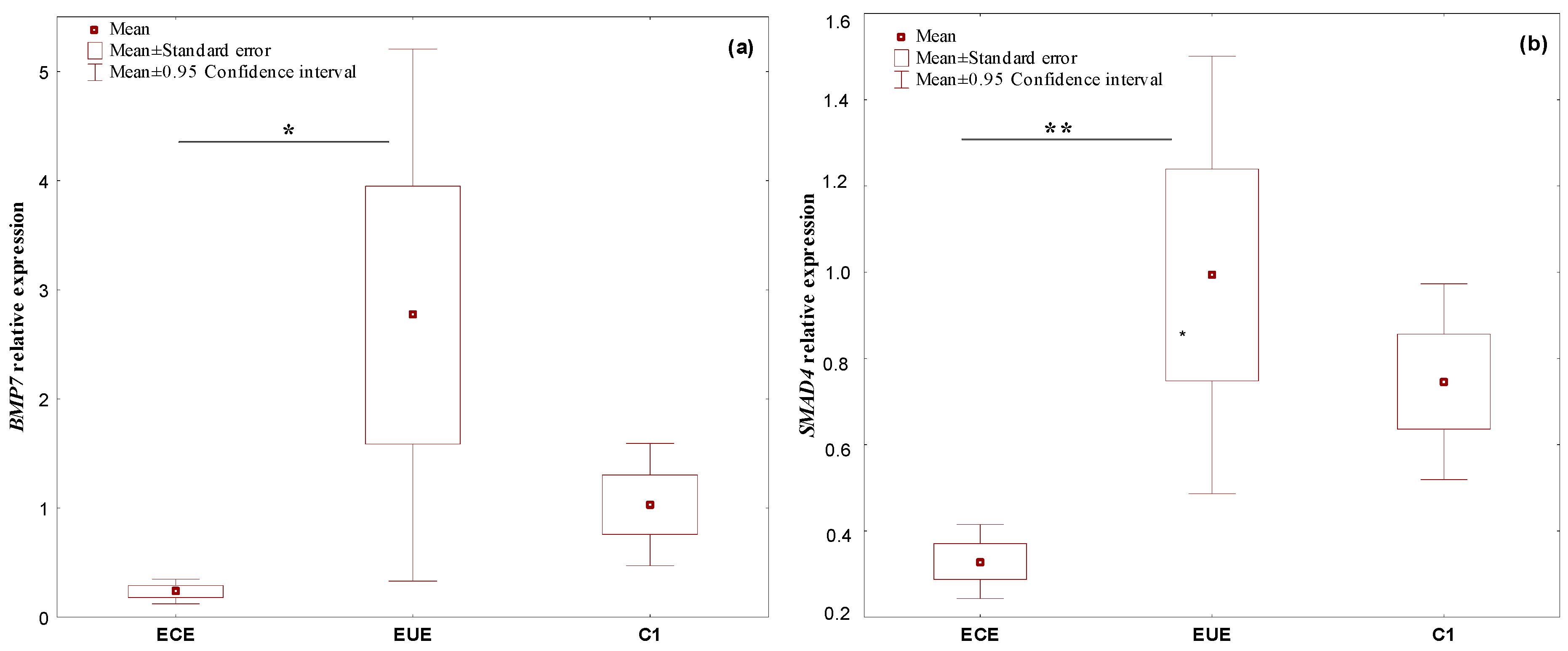
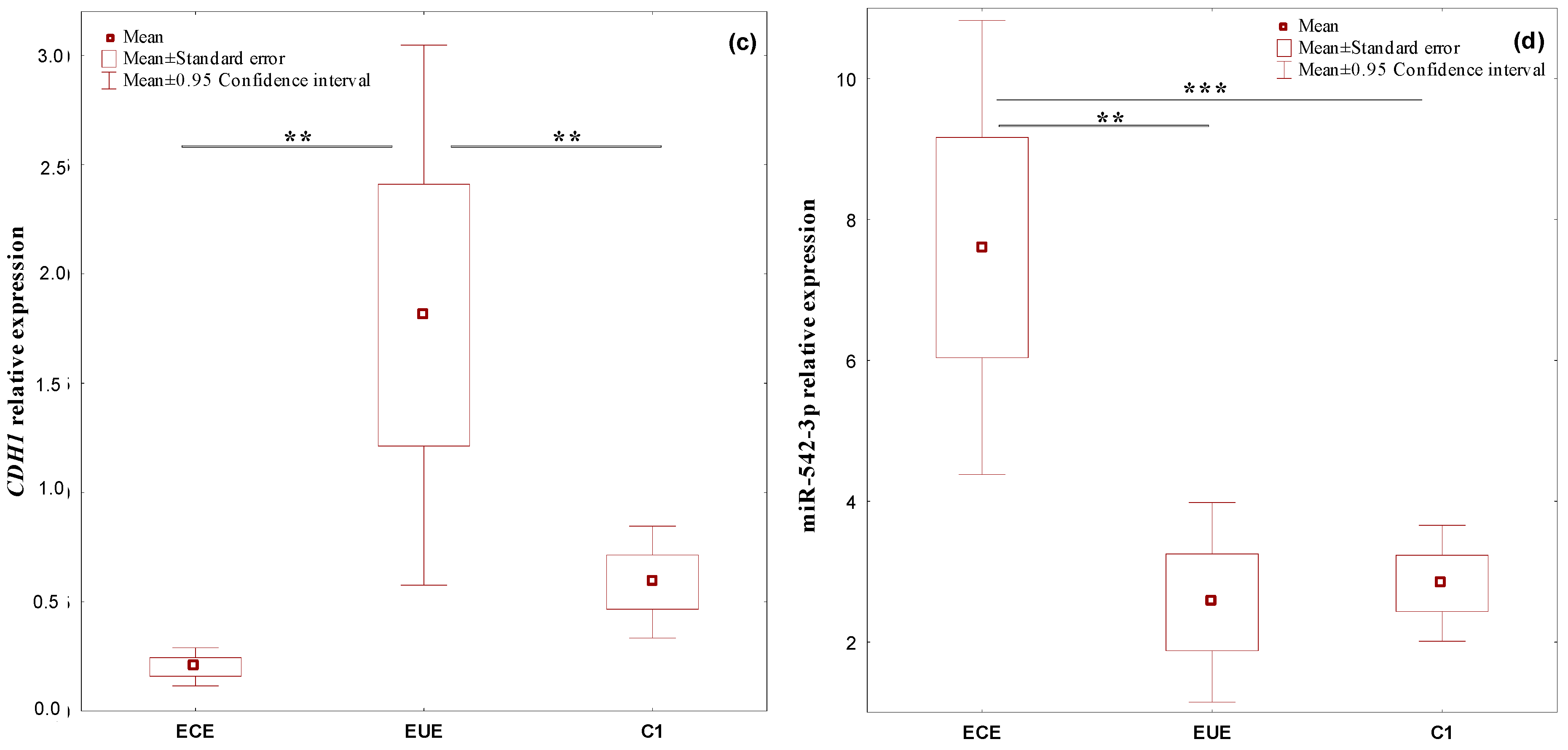
2.1. The Expression Profile of the Studied Genes, BMP7, SMAD4, CDH1, and miR-542-3p, in Ectopic Lesions (ECE) and Eutopic Endometrium (EUE) vs. Control Endometrium (C1)
2.2. Expression Profile of the Studied Genes, BMP7, SMAD4, CDH1, and miR-542-3p, in PBMCs: From Patients with Endometriosis vs. Patients without Endometriosis (C2)
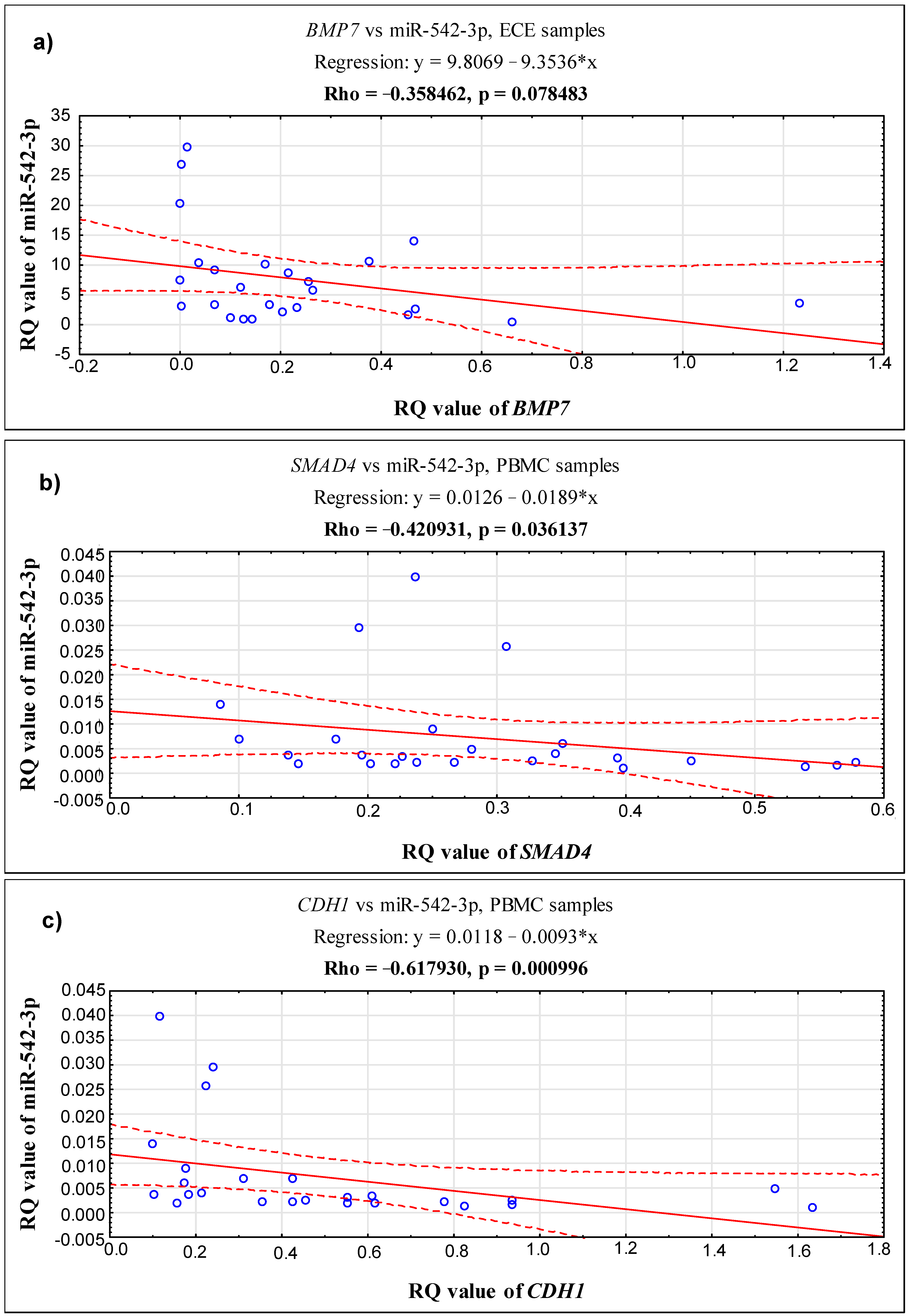
2.3. Correlations between the Expression Level of the Studied Genes, BMP7, SMAD4, CDH1 and miR-542-3p, in 3 Different Biological Materials (Ectopic Endometrium—ECE, Eutopic Endometrium—EUE and PBMCs) Obtained from the Same Patient with Endometriosis
2.4. Correlations between the Expression Levels of the Studied Genes, BMP7, SMAD4, CDH1, and miR-542-3p, in Relation to the Clinical Characteristics and Biochemical Parameters of Patients with Endometriosis
2.5. Correlations between the Expression Levels of the Studied Genes, BMP7, SMAD4, CDH1, and miR-542-3p, in Relation to the Phase of the Menstrual Cycle in Ectopic Lesion (ECE), Eutopic Endometrium (EUE), and Control Endometrium (C1) Samples
3. Discussion
4. Materials and Methods
4.1. Research Ethics
4.2. Clinical Groups
4.3. Sample Collection and RNA Isolation
4.4. Relative Gene and miRNA Expression (RQ)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef]
- Maddern, J.; Grundy, L.; Castro, J.; Brierley, S.M. Pain and endometriosis. Front. Cell Neurosci. 2020, 14, 590823. [Google Scholar] [CrossRef]
- Koninckx, P.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Rivera, E.; Ruiz, L.A.; Santiago, O.I.; Vernon, M.V.; Appleyard, C.B. Molecular profling of experimental endometriosis identifed gene expression patterns in common with human disease. Fertil. Steril. 2007, 87, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Amant, F.; Neven, P.; Vergote, I.; Moerman, P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 349–371. [Google Scholar] [CrossRef]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhang, T.; Deng, J.; Xia, X.; Fang, X. microRNA-141 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition through inhibition of the TGF-beta1/SMAD2 signalling pathway in endometriosis. Arch. Gynecol. Obstet. 2020, 301, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 10276, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial/mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yi, B.R.; Kim, N.H.; Choi, K.C. Role of the epithelial-mesenchymal transition and its effects on embryonic stem cells. Exp. Mol. Med. 2014, 46, e108. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, L.; Liu, H.; Li, N.; Du, Y.; He, H.; Zhang, Z.; Liu, Y. E2-mediated EMT by activation of β-catenin/Snail signalling during the development of ovarian endometriosis. J. Cell Mol. Med. 2019, 23, 8035–8045. [Google Scholar] [CrossRef]
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal–epithelial transition in development and reprogramming. Nat. Cell Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Chen, X.Y.; Bao, Q.F.; Yu, J.; Chen, L.H. ILK enhances migration and invasion abilities of human endometrial stromal cells by facilitating the epithelial-mesenchymal transition. Gynecol. Endocrinol. 2018, 34, 1091–1096. [Google Scholar] [CrossRef]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.H.; Leong, C.O.; Ngai, S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, O.; Coatham, M.; Jewer, M.; Postovit, L.M. Epithelial-to-Mesenchymal Transition in the Female Reproductive Tract: From Normal Functioning to Disease Pathology. Front. Oncol. 2017, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Mohamet, L.; Hawkins, K.; Ward, C.M. Loss of function of e-cadherin in embryonic stem cells and the relevance to models of tumorigenesis. J. Oncol. 2011, 2011, 352616. [Google Scholar] [CrossRef]
- Schlosshauer, P.W.; Ellenson, L.H.; Soslow, R.A. Beta-catenin and E-cadherin expression patterns in high-grade endometrial carcinoma are associated with histological subtype. Mod. Pathol. 2002, 15, 1032–1037. [Google Scholar] [CrossRef]
- Salama, E.; Eldeen, G.N.; Abdel-Rasheed, M.; Abdel Atti, S.; Elnoury, A.; Taha, T.; Azmy, O. Differentially expressed genes: OCT-4, SOX 2, STAT 3, CDH 1 and CDH 2, in cultured mesenchymal stem cells challenged with serum of women with endometriosis. J. Genet. Eng. Biotechnol. 2018, 16, 63–69. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streube, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Fei, X.; Zhang, T.; Zhou, J.; Lin, J. Roles of cell migration and invasion mediated by Twist in endometriosis. J. Obstet. Gynaecol. Res. 2019, 45, 1488–1496. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, R.; Jiang, Q.; Li, Z.; Wu, R. Expression of cellular adherent and invasive molecules in recurrent ovarian endometriosis. J. Int. Med. Res. 2020, 48, 0300060520971993. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Dai, H.; Zhao, J.; Liu, T.; Zhang, G. Silencing of Forkhead Box M1 Reverses Transforming Growth Factor-β1-Induced Invasion and Epithelial-Mesenchymal Transition of Endometriotic Epithelial Cells. Gynecol. Obstet. Investig. 2019, 84, 485–494. [Google Scholar] [CrossRef]
- Usta, C.S.; Turan, G.; Bulbul, C.B.; Usta, A.; Adali, E. Differential expression of Oct-4, CD44, and E-cadherin in eutopic and ectopic endometrium in ovarian endometriomas and their correlations with clinicopathological variables. Reprod. Biol. Endocrinol. 2020, 18, 116. [Google Scholar] [CrossRef]
- Poncelet, C.; Leblanc, M.; Walker-Combrouze, F.; Soriano, D.; Feldmann, G.; Madelenat, P.; Scoazec, J.Y.; Daraï, E. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet. Gynecol. Scand. 2002, 81, 195–203. [Google Scholar] [CrossRef]
- Bartley, J.; Julicher, A.; Hotz, B.; Mechsner, S.; Hotz, H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obstet. 2014, 289, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Shaco-Levy, R.; Charabi, S.; Beharroch, D.; Piura, B.; Sion-Vardy, N. Matrix metalloproteinases 2 and 9, E-cadherin and betacatenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Janusz, J.; Janusz, A.; Kondera-Anasz, Z.; Sikora, S.; Smycz-Kubańska, M.; Englisz, A.; Wendlocha, D.; Mielczarek-Palacz, A. Participation of Selected Soluble BMP-2 and BMP-7 Bone Morphogenetic Proteins and Their Soluble Type I ALK-1 and Type II BMPR2 Receptors in Formation and Development of Endometriosis. Biomedicines 2021, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.G.; El-Nashar, S.A.; Schoolmeester, J.K.; Keeney, G.L.; Mariani, A.; Hopkins, M.R.; Dowdy, S.C.; Daftary, G.S.; Famuyide, A.O. Abnormal uterine bleeding is associated with increased BMP7 expression in human endometrium. Reprod. Sci. 2017, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Clementi, C.; Peng, J.; Fullerton, P.T., Jr.; Prunskaite-Hyyrylainen, R.; Vainio, S.J.; Matzuk, M.M. BMP7 Induces Uterine Receptivity and Blastocyst Attachment. Endocrinology 2017, 158, 979–992. [Google Scholar] [CrossRef]
- Large, M.J.; Wetendorf, M.; Lanz, R.B.; Hartig, S.M.; Creighton, C.J.; Mancini, M.A.; Kovanci, E.; Lee, K.F.; Threadgill, D.W.; Lydon, J.P.; et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014, 10, e1004451. [Google Scholar] [CrossRef]
- Massagué, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef]
- Ping, S.; Ma, C.; Liu, P.; Yang, L.; Yang, X.; Wu, Q.; Zhao, X.; Gong, B. Molecular mechanisms underlying endometriosis pathogenesis revealed by bioinformatics analysis of microarray data. Arch. Gynecol. Obstet. 2016, 293, 797–804. [Google Scholar] [CrossRef]
- De Conto, E.; Matte, U.; Cunha-Filho, J.S. BMP-6 and SMAD4 gene expression is altered in cumulus cells from women with endometriosis-associated infertility. Acta Obstet. Gynecol. Scand. 2021, 100, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Tripurani, S.K.; Burton, J.C.; Clementi, C.; Larina, I.; Pangas, S.A. SMAD Signaling Is Required for Structural Integrity of the Female Reproductive Tract and Uterine Function During Early Pregnancy in Mice. Biol. Reprod. 2016, 95, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Feihong, J.I.; Wang, K.; Zhang, Y.; Mao, X.-L.; Huang, Q.; Wang, J.; Ye, L.; Li, Y. MiR-542-3p controls hepatic stellate cell activation and fibrosis via targeting BMP-7. J. Cell Biochem. 2019, 120, 4573–4581. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Yuan, Y.; Nie, F.; Peng, R.; Li, Q.; Lyu, Z.; Mao, Z.; Huang, L.; Zhou, L.; et al. MiR542-3p regulates the Epithelial-Mesenchymal Transition by directly targeting BMP7 in NRK52e. Int. J. Mol. Sci. 2015, 16, 27945–27955. [Google Scholar] [CrossRef] [PubMed]
- Tochigi, H.; Kajihara, T.; Mizuno, Y.; Mizuno, Y.; Tamaru, S.; Kamei, Y.; Okazaki, Y.; Brosens, J.J.; Ishihara, O. Loss of Mir-542-3P enhances Igfp-1 expression in decidualizing human endometrial stromal cells. Sci. Rep. 2017, 7, 40001. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, W.; Ren, C.; Zhao, M.; Jiang, X.; Fang, X.; Xia, X. Analysis of Serum MicroRNA Profile by Solexa Sequencing in Women with Endometriosis. Reprod. Sci. 2016, 23, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Yao, Y.; Guan, J.; Zhou, Z.-Q.; Yang, J. MicroRNA-542-3P functions as a tumor suppressor via directly targeting survivin in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 99, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kureel, J.; Dixit, M.; Tyagi, A.M.; Mansoori, M.N.; Srivastava, K.; Raghuvanshi, A.; Maurya, R.; Trivedi, R.; Goel, A.; Singh, D. Mir-542-3P suppresses osteoblast cell proliferation and diferentiation, targets Bmp-7 signaling and inhibits bone formation. Cell Death. Dis. 2014, 5, e1050. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.H.; Zhou, J.D.; Chi, Q. Upregulation of Mir-542-3P inhibits the growth and invasion of human colon cancer cells through Pi3K/Akt/survivin signaling. Oncol. Rep. 2017, 38, 3545–3553. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and Controversies in Classification, Pathogenesis, Diagnosis, and Treatment. F1000Research 2019, 8, Rev-529. [Google Scholar] [CrossRef]
- Bendifallah, S.; Dabi, Y.; Suisse, S.; Jornea, L.; Bouteiller, D.; Touboul, C.; Puchar, A.; Daraj, E. MicroRNome analysis generates a blood-based signature for endometriosis. Sci. Rep. 2022, 12, 4051. [Google Scholar] [CrossRef]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef]
- Marí-Alexandre, J.; Sanchez-Izquierdo, D.; Gilabert-Estelles, J.; Barcelo-molina, M.; Braza-Boils, A.; Sandoval, J. miRNAs regulation and its role as biomarkers in endometriosis. Int. J. Mol. Sci. 2016, 17, 93. [Google Scholar] [CrossRef]
- Monnaka, V.U.; Hernandes, C.; Heller, D.; Podgaec, S. Overview of miRNAs for the non-invasive diagnosis of endometriosis: Evidence, challenges and strategies. A systematic review. Einstein 2021, 19, eRW5704. [Google Scholar] [CrossRef]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in Endometriosis: Biological Function and Emerging Biomarker Candidates. Biol. Reprod. 2019, 100, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Tapmeier, T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.; Becker, C. The MiRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef]
- Braicu, O.-L.; Budisan, L.; Buiga, R.; Jurj, A.; Achimas-Cadariu, P.; Pop, L.A.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. miRNA expression profiling in formalin-fixed paraffin-embedded endometriosis and ovarian cancer samples. Onco. Targets Ther. 2017, 10, 4225–4238. [Google Scholar] [CrossRef]
- Gaia-Oltean, A.I.; Braicu, C.; Gulei, D.; Ciortea, R.; Mihu, D.; Roman, H.; Irimie, A.; Berindan-Neagoe, I. Ovarian endometriosis, a precursor of ovarian cancer: Histological aspects, gene expression and microRNA alterations (Review). Exp. Ther. Med. 2021, 21, 243. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and Molecular Features of the Endomyometrium in Endometriosis and Adenomyosis. Hum. Reprod. Update 2014, 20, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Eyster, K.M.; Klinkova, O.; Kennedy, V.; Hansen, K.A. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil. Steril. 2007, 88, 1505–1533. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Cope, D.I.; Vasquez, Y.M.; Monsivais, D. BMP/SMAD1/5 Signaling in the Endometrial Epithelium Is Essential for Receptivity and Early Pregnancy. Endocrinology 2022, 163, bqac043. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Nagashima, T.; Prunskaite-Hyyrylainen, R.; Nozawa, K.; Shimada, K.; Tang, S.; Hamor, C.; Agno, J.C.; Chen, F.; Masand, R.P.; et al. Endometrial receptivity and implantation require uterine BMP signaling through an ACVR2A-SMAD1/SMAD5 axis. Nat. Commun. 2021, 12, 3386. [Google Scholar] [CrossRef] [PubMed]
- Saare, M.; Rekker, K.; Laisk-Podar, T.; Rahmioglu, N.; Zondervan, K.; Salumets, A.; Götte, M.; Peters, M. Challenges in endometriosis miRNA studies from tissue heterogeneity to disease specific miRNAs. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2282–2292. [Google Scholar] [CrossRef]
- Nothnick, W.B. MicroRNAs and endometriosis: Distinguishing drivers from passengers in disease pathogenesis. Semin. Reprod. Med. 2017, 35, 173–180. [Google Scholar] [CrossRef]
- Oneyama, C.; Morii, E.; Okuzaki, D.; Takahashi, Y.; Ikeda, J.; Wakabayashi, N.; Akamatsu, H.; Tsujimoto, M.; Nishida, T.; Aozasa, K.; et al. MicroRNA-mediated upregulation of integrin-linked kinase promotes Src-induced tumor progression. Oncogene 2012, 31, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Choi, Y.C.; Lee, S.; Jeong, Y.; Yoon, J.; Baek, K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010, 584, 4048–4052. [Google Scholar] [CrossRef]
- Sükür, Y.E.; Taskin, S.; Simsir, C.; Atabekoglu, C.; Sonmezer, M. Highly elevated human epididymis protein 4 (HE4) following endometrioma rupture: A case report. J. Obstet. Gynaecol. 2018, 38, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Huhtinen, K.; Suvitie, P.; Hiissa, J.; Junnila, J.; Huvila, J.; Kujari, H.; Setala, M.; Harkki, P.; Makinen, J.; Auranen, A.; et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br. J. Cancer 2009, 100, 1315–1319. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, C.; Yu, H. Plasma microRNAs can be a potential diagnostic biomarker for endometriosis. Ginekol. Pol. 2022, 93, 450–459. [Google Scholar] [CrossRef]
- Wang, W.-T.; Zhao, Y.-N.; Han, B.-W.; Hong, S.-J.; Chen, Y.-Q. Circulating MicroRNAs Identified in a Genome-Wide Serum MicroRNA Expression Analysis as Noninvasive Biomarkers for Endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, 281–289. [Google Scholar] [CrossRef]
- Toloubeydokhti, T.; Pan, Q.; Luo, X.; Bukulmez, O.; Chegini, N. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod. Sci. 2008, 15, 993–1001. [Google Scholar] [CrossRef]
- Sultana, S.; Kajihara, T.; Mizuno, Y.; Sato, T.; Oguro, T.; Kimura, M.; Akita, M.; Ishihara, O. Overexpression of microRNA-542-3p attenuates the differentiating capacity of endometriotic stromal cells. Reprod. Med. Biol. 2017, 16, 170–178. [Google Scholar] [CrossRef]
- Klemmt, P.A.; Carver, J.G.; Kennedy, S.H.; Koninckx, P.R.; Mardon, H.J. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil. Steril. 2006, 85, 564–572. [Google Scholar] [CrossRef]
- Tsuno, A.; Nasu, K.; Yuge, A.; Matsumoto, H.; Nishida, M.; Narahara, H. Decidualization attenuates the contractility of eutopic and ectopic endometrial stromal cells: Implications for hormone therapy of endometriosis. J. Clin. Endocrinol. Metab. 2009, 94, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Fang, Y.; Zhuang, S.; Zhang, Y. Micro-RNA miR-542-3p suppresses decidualization by targeting ILK pathways in human endometrial stromal cells. Sci. Rep. 2021, 11, 7186. [Google Scholar] [CrossRef]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am. J. Pathol. 2001, 159, 1839–1852. [Google Scholar] [CrossRef]
- Li, J.; Shao, W.; Feng, H. MiR-542-3p, a microRNA targeting CDK14, suppresses cell proliferation, invasiveness, and tumorigenesis of epithelial ovarian cancer. Biomed. Pharmacother. 2019, 110, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, Y.; Wang, L.; Zhang, T.; Hu, W.; Bao, W.; Mao, L.; Chen, J.; Li, H.; Wen, Y.; et al. Hyperoside suppresses BMP-7-dependent PI3K/AKT pathway in human hepatocellular carcinoma cells. Ann. Transl. Med. 2021, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kayamori, T.; Murayama, C.; Miyamoto, A. Bone morphogenetic protein (BMP)-4 and BMP-7 suppress granulosa cell apoptos is via different pathways: BMP-4 via PI3K/PDK-1/Akt and BMP-7 via PI3K/PDK-1/PKC. Biochem. Biophys. Res. Commun. 2012, 417, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, E.; Jin, D.F.; Jelic, M.; Vukicevic, S.; Oppermann, H. Osteogenic protein-1 mRNA in the uterine endometrium. Biochem. Biophys. Res. Commun. 1997, 234, 242–246. [Google Scholar] [CrossRef]
- Paria, B.C.; Ma, W.; Tan, J.; Raja, S.; Das, S.K.; Dey, S.K.; Hogan, B.L. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. USA 2001, 98, 1047–1052. [Google Scholar] [CrossRef]
- Stoikos, C.J.; Harrison, C.A.; Salamonsen, L.A.; Dimitriadis, E. A distinct cohort of the TGFbeta superfamily members expressed in human endometrium regulate decidualization. Hum. Reprod. 2008, 23, 1447–1456. [Google Scholar] [CrossRef]
- Kodama, A.; Yoshino, O.; Osuga, Y.; Harada, M.; Hasegawa, A.; Hamasaki, K.; Takamura, M.; Koga, K.; Hirota, Y.; Hirata, T.; et al. Progesterone decreases bone morphogenetic protein (BMP) 7 expression and BMP7 inhibits decidualization and proliferation in endometrial stromal cells. Hum. Reprod. 2010, 25, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, X.; Song, H.; Fan, L.; Su, S.; Dong, B. BMP7 coordinates endometrial epithelial cell receptivity for blastocyst implantation through the endoglin pathway in cell lines and a mouse model. Exp. Ther. Med. 2019, 17, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Martinovic, S.; Latin, V.; Suchanek, E.; Stavljenic-Rukavina, A.; Sampath, K.I.; Vukicevic, S. Osteogenic protein-1 is produced by human fetal trophoblasts in vivo and regulates the synthesis of chorionic gonadotropin and progesterone by trophoblasts in vitro. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-P.; Chen, L.-M.; Chen, F.; Jiang, N.-H.; Sui, L. Smad signaling coincides with epithelial-mesenchymal transition in a rat model of intrauterine adhesion. Am. J. Transl. Res. 2019, 11, 4726–4737. [Google Scholar]
- Mabuchi, Y.; Yamoto, M.; Minami, S.; Umesaki, N. Immunohistochemical localization of inhibin and activin subunits, activin receptors and Smads in ovarian endometriosis. Int. J. Mol. Med. 2010, 25, 17–23. [Google Scholar] [CrossRef]
- Cruz, C.D.; Del Puerto, H.L.; Rocha, A.L.L.; Cavallo, I.K.; Clarizia, A.D.; Petraglia, F.; Reis, F.M. Expression of Nodal, Cripto, SMAD3, Phosphorylated SMAD3, and SMAD4 in the Proliferative Endometrium of Women with Endometriosis. Reprod. Sci. 2015, 22, 527–533. [Google Scholar] [CrossRef]
- Zhao, M.; Mishra, L.; Deng, C.X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018, 14, 111–123. [Google Scholar] [CrossRef]
- Schwarte-Waldhoff, I.; Volpert, O.V.; Bouck, N.P.; Sipos, B.; Hahn, S.A.; Klein-Scory, S.; Lüttges, J.; Klöppel, G.; Graeven, U.; Eilert-Micus, C.; et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 9624–9629. [Google Scholar] [CrossRef]
- Ioannou, M.; Kouvaras, E.; Papamichali, R.; Samara, M.; Chiotoglou, I.; Koukoulis, G. Smad4 and epithelial-mesenchymal transition proteins in colorectal carcinoma: An immunohistochemical study. J. Mol. Histol. 2018, 49, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Gaetje, R.; Kotzian, S.; Herrmann, G.; Baumann, R.; Starzinski-Powitz, A. Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am. J. Pathol. 1997, 150, 461–467. [Google Scholar]
- Zhu, X.; Li, Y.; Zhou, R.; Wang, N.; Kang, S. Knockdown of E-cadherin expression of endometrial epithelial cells may activate Wnt/β-catenin pathway in vitro. Arch. Gynecol. Obstem. 2018, 297, 117–123. [Google Scholar] [CrossRef]
- Fujimoto, J.; Ichigo, S.; Hori, M.; Tamaya, T. Expression of E-cadherin, alpha- and beta-catenin mRNAs in ovarian endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 67, 179–183. [Google Scholar] [CrossRef]
- Koczan, D.; Guthke, R.; Thiesen, H.J.; Ibrahim, S.M.; Kundt, G.; Krentz, H.; Gross, G.; Kunz, M. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur. J. Dermatol. 2005, 15, 251–257. [Google Scholar]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Migdalska-Sęk, M.; Jędrzejczyk, S.; Brzeziańska-Lasota, E. The Expression of TGF-β1, SMAD3, ILK and miRNA-21 in the Ectopic and Eutopic Endometrium of Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 2453. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
| Patients | RQ (Mean) in PBMCs | |||
|---|---|---|---|---|
| BMP7 | SMAD4 | CDH1 | miR-542-3p | |
| without endometriosis (C2) | below detection level | 0.260932 | 1.040384 | 0.005000 |
| with endometriosis | below detection level | 0.289032 | 0.507280 | 0.007132 |
| p value, Mann-Whitney U test | - | 0.396546 | 0.969326 | 0.254776 |
| Features (n) | RQ (Mean) | |||
|---|---|---|---|---|
| BMP7 | SMAD4 | CDH1 | miR-542-3p | |
| Age: years | ||||
| ≤40 (18) | 0.37679 | 0.45984 | 0.62759 | 6.94957 |
| >40 (11) | 0.27315 | 0.27777 | 0.19401 | 6.96345 |
| p value | 0.411953 | 0.203814 | 0.188094 | 0.610874 |
| rASRM | ||||
| III (16) | 0.49299 | 0.43848 | 0.34562 | 6.59889 |
| IV (10) | 0.12247 | 0.38937 | 0.70835 | 7.71903 |
| p value | 0.035602 * | 0.165427 | 0.335952 | 0.979370 |
| pelvic pain symptoms: | ||||
| mild (11) | 0.11175 | 0.35783 | 0.64395 | 7.29089 |
| severe (16) | 0.49299 | 0.43848 | 0.34562 | 6.59889 |
| p value | 0.017147 * | 0.098865 | 0.194506 | 0.942037 |
| CA-125 [U/mL]: | ||||
| ≤65 (10) | 0.232350 | 0.413860 | 0.699980 | 7.789560 |
| >65 (16) | 0.424319 | 0.423175 | 0.350850 | 6.554813 |
| p value | 0.451781 | 0.451781 | 0.586471 | 0.516940 |
| HE4 [pmol/L] | ||||
| ≤50 (8) | 0.207938 | 0.399375 | 0.170100 | 8.74418 |
| >50 (7) | 0.510286 | 0.438443 | 1.182729 | 10.72834 |
| p value | 0.231857 | 0.535820 | 0.335664 | 0.955089 |
| Endometrium | Cycle Phase | RQ (Mean) | |||
|---|---|---|---|---|---|
| BMP7 | SMAD4 | CDH1 | miR-542-3p | ||
| ECE | proliferative (7) | 0.204629 | 0.384071 | 0.194557 | 8.038829 |
| secretory (18): | 0.247628 | 0.307778 | 0.204900 | 7.433933 | |
| early (7) | 0.317100 | 0.323729 | 0.233671 | 6.082714 | |
| middle (4) | 0.345050 | 0.459325 | 0.156425 | 7.506125 | |
| late (7) | 0.122486 | 0.205229 | 0.203829 | 8.743900 | |
| p value | 0.4063 | 0.2282 | 0.8275 | 0.9088 | |
| EUE | proliferative (7) | 5.585586 | 1.281043 | 2.404900 | 3.252214 |
| secretory (18): | 1.672767 | 0.882044 | 1.580817 | 2.297389 | |
| early (7) | 1.198814 | 0.669300 | 1.266386 | 1.769357 | |
| middle (4) | 0.890000 | 0.588050 | 1.397600 | 0.699525 | |
| late (7) | 2.594014 | 1.262786 | 1.999943 | 3.738486 | |
| p value | 0.4570 | 0.8672 | 0.8823 | 0.3525 | |
| C1 | proliferative (6) | 2.675633 | 0.487233 | 0.575633 | 2.858267 |
| secretory (19): | 0.513374 | 0.828247 | 0.595095 | 2.825600 | |
| early (5) | 0.331440 | 0.707440 | 1.032660 | 3.428560 | |
| middle (5) | 0.411400 | 1.074680 | 0.461920 | 1.913120 | |
| late (9) | 0.671100 | 0.758456 | 0.425989 | 2.997556 | |
| p value | 0.0284 * | 0.2270 | 0.4436 | 0.6826 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrzycka, A.; Migdalska-Sęk, M.; Jędrzejczyk, S.; Brzeziańska-Lasota, E. Assessment of BMP7, SMAD4, and CDH1 Expression Profile and Regulatory miRNA-542-3p in Eutopic and Ectopic Endometrium of Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 6637. https://doi.org/10.3390/ijms24076637
Zubrzycka A, Migdalska-Sęk M, Jędrzejczyk S, Brzeziańska-Lasota E. Assessment of BMP7, SMAD4, and CDH1 Expression Profile and Regulatory miRNA-542-3p in Eutopic and Ectopic Endometrium of Women with Endometriosis. International Journal of Molecular Sciences. 2023; 24(7):6637. https://doi.org/10.3390/ijms24076637
Chicago/Turabian StyleZubrzycka, Anna, Monika Migdalska-Sęk, Sławomir Jędrzejczyk, and Ewa Brzeziańska-Lasota. 2023. "Assessment of BMP7, SMAD4, and CDH1 Expression Profile and Regulatory miRNA-542-3p in Eutopic and Ectopic Endometrium of Women with Endometriosis" International Journal of Molecular Sciences 24, no. 7: 6637. https://doi.org/10.3390/ijms24076637
APA StyleZubrzycka, A., Migdalska-Sęk, M., Jędrzejczyk, S., & Brzeziańska-Lasota, E. (2023). Assessment of BMP7, SMAD4, and CDH1 Expression Profile and Regulatory miRNA-542-3p in Eutopic and Ectopic Endometrium of Women with Endometriosis. International Journal of Molecular Sciences, 24(7), 6637. https://doi.org/10.3390/ijms24076637





