Screening for Diabetes Mellitus in Patients with Hidradenitis Suppurativa—A Monocentric Study in Germany
Abstract
1. Introduction
2. Results
2.1. Personal and Diabetes Mellitus-Specific Characteristics
2.2. Differences between HS Patients with and without DM
2.3. Identification of HS Patients with a High Risk for DM
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gold, D.A.; Reeder, V.J.; Mahan, M.G.; Hamzavi, I.H. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 2014, 70, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Kitala, D.; Łabuś, W.; Szyluk, K.; Niemiec, P.; Ochała-Gierek, G. Impact of Hidradenitis Suppurativa Surgical Treatment on Health-Related Life Quality. J. Clin. Med. 2022, 11, 4327. [Google Scholar] [CrossRef] [PubMed]
- Ocker, L.; Abu Rached, N.; Seifert, C.; Scheel, C.; Bechara, F.G. Current Medical and Surgical Treatment of Hidradenitis Suppurativa-A Comprehensive Review. J. Clin. Med. 2022, 11, 7240. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Ochała-Gierek, G.; Kitala, D.; Łabuś, W.; Bergler-Czop, B. Surgical management of hidradenitis suppurativa. Postep. Dermatol. Alergol. 2022, 39, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Abu Rached, N.; Gambichler, T.; Dietrich, J.W.; Ocker, L.; Seifert, C.; Stockfleth, E.; Bechara, F.G. The Role of Hormones in Hidradenitis Suppurativa: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 15250. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.-L.; Silva-Hirschberg, C.; Torres, J.; Armstrong, A.W. Hidradenitis suppurativa and diabetes mellitus: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 78, 395–402. [Google Scholar] [CrossRef]
- Phan, K.; Charlton, O.; Smith, S.D. Hidradenitis suppurativa and diabetes mellitus: Updated systematic review and adjusted meta-analysis. Clin. Exp. Dermatol. 2019, 44, e126–e132. [Google Scholar] [CrossRef]
- Garg, A.; Malviya, N.; Strunk, A.; Wright, S.; Alavi, A.; Alhusayen, R.; Alikhan, A.; Daveluy, S.D.; Delorme, I.; Goldfarb, N.; et al. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J. Am. Acad. Dermatol. 2022, 86, 1092–1101. [Google Scholar] [CrossRef]
- Vilanova, I.; Hernández, J.L.; Mata, C.; Durán, C.; García-Unzueta, M.T.; Portilla, V.; Fuentevilla, P.; Corrales, A.; González-Vela, M.C.; González-Gay, M.A.; et al. Insulin resistance in hidradenitis suppurativa: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 820–824. [Google Scholar] [CrossRef]
- Jennings, L.; Hambly, R.; Hughes, R.; Moriarty, B.; Kirby, B. Metformin use in hidradenitis suppurativa. J. Dermatol. Treat. 2020, 31, 261–263. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Birabaharan, M.; Strunk, A. Prevalence of type 2 diabetes mellitus among patients with hidradenitis suppurativa in the United States. J. Am. Acad. Dermatol. 2018, 79, 71–76. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Sauter, N.S.; Schulthess, F.T.; Galasso, R.; Castellani, L.W.; Maedler, K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 2008, 149, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Witte-Händel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mößner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef]
- Kiortsis, D.N.; Mavridis, A.K.; Vasakos, S.; Nikas, S.N.; Drosos, A.A. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 2005, 64, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; De Matias, J.M.; Gonzalez-Juanatey, C.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Martin, J.; Llorca, J. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2006, 24, 83–86. [Google Scholar]
- Marra, M.; Campanati, A.; Testa, R.; Sirolla, C.; Bonfigli, A.R.; Franceschi, C.; Marchegiani, F.; Offidani, A. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int. J. Immunopathol. Pharmacol. 2007, 20, 731–736. [Google Scholar] [CrossRef]
- Timper, K.; Hruz, P.; Beglinger, C.; Donath, M.Y. Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care 2013, 36, e90–e91. [Google Scholar] [CrossRef]
- Ofei, F.; Hurel, S.; Newkirk, J.; Sopwith, M.; Taylor, R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 1996, 45, 881–885. [Google Scholar] [CrossRef]
- Dominguez, H.; Storgaard, H.; Rask-Madsen, C.; Steffen Hermann, T.; Ihlemann, N.; Baunbjerg Nielsen, D.; Spohr, C.; Kober, L.; Vaag, A.; Torp-Pedersen, C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 2005, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Zanni, M.V.; Johnsen, S.; Rasheed, S.; Makimura, H.; Lee, H.; Khor, V.K.; Ahima, R.S.; Grinspoon, S.K. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E146–E150. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.E.; Berry, J.; Kim, S.; Canavan, B.; Grinspoon, S.K. Effects of etanercept in patients with the metabolic syndrome. Arch. Intern. Med. 2006, 166, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Maalmi, H.; Strassburger, K.; Zaharia, O.-P.; Ratter, J.M.; Karusheva, Y.; Elhadad, M.A.; Bódis, K.; Bongaerts, B.W.C.; Rathmann, W.; et al. Differences in Biomarkers of Inflammation Between Novel Subgroups of Recent-Onset Diabetes. Diabetes 2021, 70, 1198–1208. [Google Scholar] [CrossRef]
- De Vita, V.; Melnik, B.C. Activated mTORC1 signaling: The common driving force of type 2 diabetes and hidradenitis suppurativa. J. Am. Acad. Dermatol. 2018, 78, e121. [Google Scholar] [CrossRef]
- Monfrecola, G.; Balato, A.; Caiazzo, G.; De Vita, V.; Di Caprio, R.; Donnarumma, M.; Lembo, S.; Fabbrocini, G. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: A possible metabolic loop. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1631–1633. [Google Scholar] [CrossRef]
- Gray, N.; Picone, G.; Sloan, F.; Yashkin, A. Relation between BMI and diabetes mellitus and its complications among US older adults. South. Med. J. 2015, 108, 29–36. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.-P.; Yuan, J.; Cai, B.; Wang, X.-L.; Wu, X.-L.; Zhang, Y.-H.; Zhang, X.-Y.; Yin, T.; Zhu, X.-H.; et al. Association of body mass index and age with incident diabetes in Chinese adults: A population-based cohort study. BMJ Open 2018, 8, e021768. [Google Scholar] [CrossRef]
- Andersen, R.K.; Loft, I.C.; Burgdorf, K.; Erikstrup, C.; Pedersen, O.B.; Jemec, G.B.E. Risk of Hidradenitis Suppurativa Comorbidities Over Time: A Prospective Cohort Study of Danish Blood Donors. Acta Derm. Venereol. 2021, 101, adv00376. [Google Scholar] [CrossRef]
- Hauner, H.; Moss, A.; Berg, A.; Bischoff, S.C.; Colombo-Benkmann, M.; Ellrott, T.; Heintze, C.; Kanthak, U.; Kunze, D.; Stefan, N.; et al. Interdisziplinäre Leitlinie der Qualität S3 zur “Prävention und Therapie der Adipositas”. Adipositas Ursachen Folgeerkrankungen Ther. 2014, 08, 179–221. [Google Scholar] [CrossRef]
- Jørgensen, A.-H.R.; Yao, Y.; Ghazanfar, M.N.; Ring, H.C.; Thomsen, S.F. Burden, predictors and temporal relationships of comorbidities in patients with hidradenitis suppurativa: A hospital-based cohort study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Naldi, L.; Cazzaniga, S.; Zauli, S.; Atzori, L.; Borghi, A.; Capezzera, R.; Caproni, M.; Cardinali, C.; DeVita, V.; et al. Overweight, diabetes and disease duration influence clinical severity in hidradenitis suppurativa-acne inversa: Evidence from the national Italian registry. Br. J. Dermatol. 2016, 174, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Strunk, A.; Garg, A. Comparative Overall Comorbidity Burden Among Patients With Hidradenitis Suppurativa. JAMA Dermatol. 2019, 155, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Bahrmann, A.; Bahrmann, P.; Baumann, J.; Bauer, J.; Brückel, E.; Dreyer, M.; Freitag, M.; Friedl, A.; Gölz, S.; Grundke, S.; et al. S2k-Leitlinie Diagnostik, Therapie und Verlaufskontrolle des Diabetes mellitus im Alter. Diabetol. Und Stoffwechs. 2018, 13, 423–489. [Google Scholar] [CrossRef]
- Nauck, M.; Gerdes, C.; Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Freckmann, G.; Heinemann, L.; Schleicher, E.; Landgraf, R. Definition, Klassifikation und Diagnostik des Diabetes mellitus: Update 2020. Diabetol. Und Stoffwechs. 2020, 15, S9–S17. [Google Scholar] [CrossRef]
- Deutsch, A.J.; Ahlqvist, E.; Udler, M.S. Phenotypic and genetic classification of diabetes. Diabetologia 2022, 65, 1758–1769. [Google Scholar] [CrossRef]
| Parameter | Value (s) | |
|---|---|---|
| Sex, n (%) | Female | 46 (46.5) |
| Male | 53 (53.5) | |
| Age, mean (± SD), y | 41.6 (±13) | |
| Age of HS onset, median (IQR), y | 20 (17–30) | |
| Disease duration, median (IQR), y | 13 (7–25) | |
| BMI, mean (± SD), kg/m2 | 31.5 (±6.7) | |
| Family history of HS, n (%) | Positive | 31 (31.3) |
| Negative | 68 (78.7) | |
| Smoker, n (%) | Current smoker | 63 (64.9) |
| Ex-smokers | 3 (3.1) | |
| Non-smoker | 31 (32) | |
| Therapy with TNF-α inhibitor, n (%) | Current therapy | 14 (14.1) |
| Never | 77 (77.8) | |
| Discontinued therapy | 8 (8.1) | |
| Prediabetes, n (%) | Total | 23 (23.2) |
| Diabetes mellitus, n (%) | Total | 20 (20.2) |
| Male | 14 (70) | |
| Female | 6 (30) | |
| Fasting blood glucose level, median (IQR), mg/dL | No diabetes mellitus | 92 (86–96) |
| Prediabetes | 100 (88–116) | |
| Diabetes mellitus | 137 (83–182) | |
| Glycated hemoglobin, median (IQR), % | No diabetes mellitus | 5.2 (5–5.4) |
| Prediabetes | 5.8 (5.7–6) | |
| Diabetes mellitus | 7.8 (6.9–8.9) | |
| Prevalence of DM using Hurley classification, n (%) | Hurley I | 0 (0) |
| Hurley II | 6 (13) | |
| Hurley III | 14 (31.1) | |
| Type of DM, n (%) | Type 1 DM | 1 (5) |
| Type 2 DM | 19 (95) | |
| Type 3 DM | 0 (0) | |
| Type 4 DM | 0 (0) | |
| Diabetes cluster according to ANDIS [11] | Cluster 1 SAID | 0 (0) |
| Cluster 1 LADA | 1 (5) | |
| Cluster 2 SIDD | 3 (15) | |
| Cluster 3 MOD | 15 (75) | |
| Cluster 4 MARD | 0 (0) | |
| Cluster 5 SIRD | 0 (0) | |
| Insulin-dependent DM, n (%) | 3 (15) | |
| Antidiabetic medication, n (%) | No previous therapy | 4 (20) |
| No therapy necessary | 2 (10) | |
| Insulin therapy | 3 (15) | |
| Biguanides (metformin) | 8 (40) | |
| GLP-1 receptor agonists | 3 (15) | |
| SGLT-2 inhibitors | 2 (10) | |
| DPP-4 inhibitor | 1 (5) | |
| Initial diagnosis of DM, n (of all in %/DM patients in %) | 4 (4/20) |
| Parameters | All Patients | HS Patients without DM | HS Patients with DM | p Value |
|---|---|---|---|---|
| Male vs. female, n (%) | 53 (53.5) vs. 46 (46.5) | 39 (49.4) vs. 40 (50.6) | 14 (70) vs. 6 (30) | 0.1 |
| Age, mean (±SD), y | 41.6 (±13) | 39.6 (±12.7) | 49.7 (±11) | 0.001 ** |
| Age of onset, median (IQR), y | 20 (17–30) | 20 (16–28) | 23.5 (18–46.8) | 0.16 |
| Disease duration, median (IQR), y | 13 (7–25) | 13 (7–24) | 19 (6.5–29.8) | 0.3 |
| BMI, mean (±SD), kg/m2 | 31.5 (±6.7) | 30.6 (±6.6) | 34.8 (±6.1) | 0.008** |
| Positive family history of HS, n (%) | 31 (31.3) | 27 (34.2) | 4 (20) | 0.22 |
| Current smoker, n (%) | 63 (63.6) | 50 (63.3) | 13 (65) | 0.74 |
| Hurley III, n (%) | 45 (45.5) | 31 (39.2) | 14 (70) | 0.01 * |
| mHSS, median (IQR) | 40 (21–72) | 33 (21–64) | 64 (32.8–99.3) | 0.03 * |
| DLQI, median (IQR) | 13 (6.8–19.3) | 12 (5.8–18.3) | 16 (11.3–21.5) | 0.09 |
| Number of episodes in last 4 weeks, median (IQR) | 1 (0–3) | 0 (0–3) | 1.5 (0–3) | 0.18 |
| Pain during visit (NRS), median (IQR) | 2 (0–6) | 2 (0–5) | 4 (0–7.8) | 0.26 |
| Glycated hemoglobin, median, IQR, % | 5.5 (5.1–5.9) | 5.4 (5.1–5.7) | 7.6 (6.5–8.4) | <0.001 *** |
| Hypertension, n (%) | 33 (33.3) | 23 (29.1) | 10 (50) | 0.08 |
| Hypothyroidism, n (%) | 18 (18.2) | 12 (15.2) | 6 (30) | 0.13 |
| Psoriasis, n (%) | 4 (4) | 2 (2) | 2 (10) | 0.14 |
| Acne vulgaris/conglobata, n (%) | 15 (15.2) | 13 (16.5) | 2 (10) | 0.47 |
| PCOS, n (%) | 3 (3) | 3 (3.8) | 0 (0) | 0.38 |
| Current adalimumab, n (%) | 14 (14.1) | 11 (13.9) | 3 (15) | 0.9 |
| Parameters | r | p Value |
|---|---|---|
| Male | 0.31 | 0.002 ** |
| Age | 0.43 | <0.001 *** |
| Disease duration | 0.13 | 0.2 |
| BMI | 0.42 | <0.001 *** |
| Positive family history of HS | −0.003 | >0.9 |
| Current smoker | 0.09 | 0.4 |
| Hurley III | 0.3 | 0.002 ** |
| mHSS | 0.28 | 0.005 ** |
| DLQI | 0.22 | 0.03 * |
| Hypertension | 0.22 | 0.03 * |
| Hypothyroidism | −0.04 | 0.7 |
| Psoriasis | 0.13 | 0.2 |
| Acne vulgaris/conglobata | −0.06 | 0.6 |
| PCOS | −0.11 | 0.3 |
| Current adalimumab | −0.14 | 0.17 |
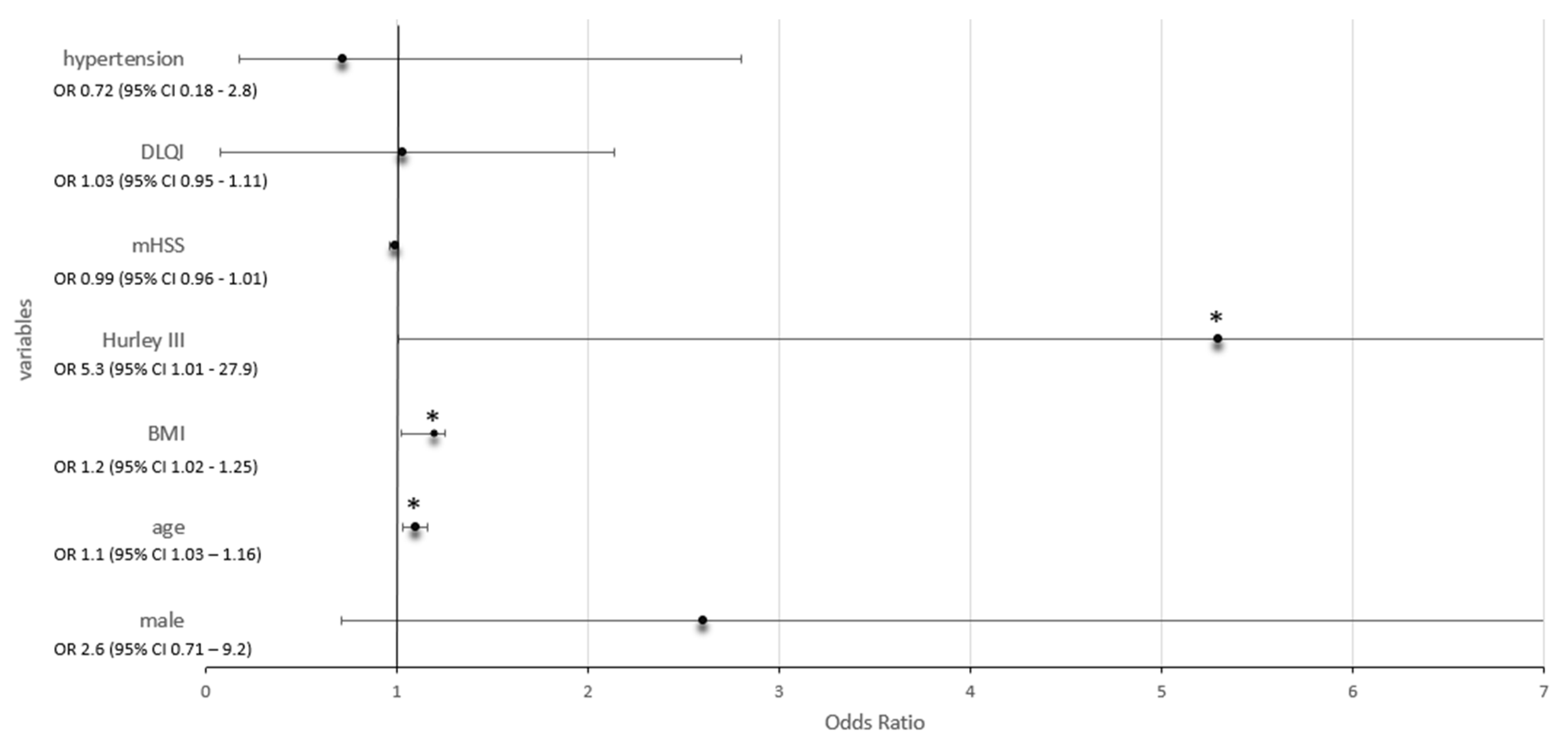
| Parameters | Odds Ratio (OR) | 95% Confidence Interval (CI) | p Value |
|---|---|---|---|
| Male | 2.6 | 0.71–9.2 | 0.2 |
| Age | 1.1 | 1.03–1.16 | 0.005 ** |
| BMI | 1.2 | 1.02–1.25 | 0.019 * |
| Hurley III | 5.3 | 1.01–27.9 | 0.048 * |
| mHSS | 0.99 | 0.96–1.01 | 0.22 |
| DLQI | 1.03 | 0.95–1.11 | 0.47 |
| Hypertension | 0.72 | 0.18–2.8 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Rached, N.; Gambichler, T.; Ocker, L.; Dietrich, J.W.; Quast, D.R.; Sieger, C.; Seifert, C.; Scheel, C.; Bechara, F.G. Screening for Diabetes Mellitus in Patients with Hidradenitis Suppurativa—A Monocentric Study in Germany. Int. J. Mol. Sci. 2023, 24, 6596. https://doi.org/10.3390/ijms24076596
Abu Rached N, Gambichler T, Ocker L, Dietrich JW, Quast DR, Sieger C, Seifert C, Scheel C, Bechara FG. Screening for Diabetes Mellitus in Patients with Hidradenitis Suppurativa—A Monocentric Study in Germany. International Journal of Molecular Sciences. 2023; 24(7):6596. https://doi.org/10.3390/ijms24076596
Chicago/Turabian StyleAbu Rached, Nessr, Thilo Gambichler, Lennart Ocker, Johannes W. Dietrich, Daniel R. Quast, Christina Sieger, Caroline Seifert, Christina Scheel, and Falk G. Bechara. 2023. "Screening for Diabetes Mellitus in Patients with Hidradenitis Suppurativa—A Monocentric Study in Germany" International Journal of Molecular Sciences 24, no. 7: 6596. https://doi.org/10.3390/ijms24076596
APA StyleAbu Rached, N., Gambichler, T., Ocker, L., Dietrich, J. W., Quast, D. R., Sieger, C., Seifert, C., Scheel, C., & Bechara, F. G. (2023). Screening for Diabetes Mellitus in Patients with Hidradenitis Suppurativa—A Monocentric Study in Germany. International Journal of Molecular Sciences, 24(7), 6596. https://doi.org/10.3390/ijms24076596









