Abstract
Multicomponent reactions (MCRs) have emerged as a powerful strategy in synthetic organic chemistry due to their widespread applications in drug discovery and development. MCRs are flexible transformations in which three or more substrates react to form structurally complex products with high atomic efficiency. They are being increasingly appreciated as a highly exploratory and evolutionary tool by the medicinal chemistry community, opening the door to more sustainable, cost-effective and rapid synthesis of biologically active molecules. In recent years, MCR-based synthetic strategies have found extensive application in the field of drug discovery, and several anticancer drugs have been synthesized through MCRs. In this review, we present an overview of representative and recent literature examples documenting different approaches and applications of MCRs in the development of new anticancer drugs.
1. Introduction: Multicomponent Reactions
Multicomponent reactions (MCRs) are one-pot processes in which at least three reagents are combined to assemble a novel complex product [1] with remarkable atom economy [2,3,4,5], in good to excellent yields, while saving resources such as time and energy [6,7]. MCRs provide a quicker and more efficient way to prepare chemical libraries than the methods used in traditional organic synthesis (Figure 1), offering great possibilities for molecular diversity and complexity in fewer steps and less time.
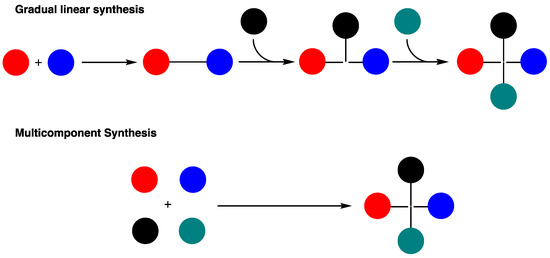
Figure 1.
Traditional linear synthesis (3 steps) vs. multicomponent assembly (1 step).
MCRs are also gaining great interest because they are generally eco-friendly and sustainable transformations [8]. Indeed, MCRs have strategic advantages over linear synthesis, being particularly appreciated by its superior atom and bond economies, its cost- and time-effective balance, and its highly exploratory nature.
Classical multicomponent reactions are well known by their proper name and scope: Ugi [4,9], Passerini [6,10], van Leusen [11,12], Strecker [13], Orru [14], Mannich [6,15], Biginelli [6,16,17] and Hantzsch syntheses [6,18] need to be mentioned. However, the discovery of new multicomponent reactions is an active research field [19,20,21,22,23,24,25,26,27], with novel exciting MCRs being discovered and incorporated to the synthetic armamentarium every year. Among these MCRs with transition metal catalysis [28,29,30,31,32,33,34], radical process [7,21,35,36] and organo-catalysis [37,38,39,40,41] should also be mentioned.
Overall, MCRs can be subcategorized into two main classes, isocyanide-based multicomponent reactions (IMCRs) and non-isocyanide-based multicomponent reactions (NIMCRs).
IMCRs are generally more versatile and often show high levels of regio- and chemo-selectivity, while the development of stereoselective IMCRs remains challenging. Isocyanides, the key component of IMCRs, have unique reactivity profiles as they can exhibit both nucleophilic and electrophilic behavior in organic reactions. Thus, isocyanides are a popular type of reactant for the progress of innovative MCRs in the rapid synthesis of valuable compounds [14,42,43,44,45,46]. Among them, the classical Passerini and Ugi reactions must be highlighted. The Passerini reaction (P-3CR) is a well-known IMCR involving a reaction between carboxylic acids and aldehydes or ketones and isocyanides to afford α-acyl carboxamides in one step [47,48] (Figure 2). The second most important IMCR is the Ugi reaction (U-3CR). This elegant four-component synthesis is a reaction between isocyanides, carboxylic acids, ketones or aldehydes and primary amines to afford dipeptide-like structures [49,50] (Figure 2).
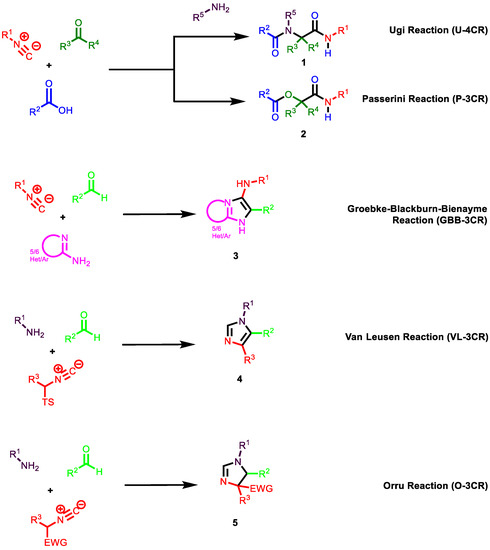
Figure 2.
Isocyanide-based multicomponent reactions.
The mechanism of the Ugi reaction involves the reaction of a Schiff base reacting with a nucleophile and an isocyanide, followed by a Mumm rearrangement. In the related Passerini reaction, lacking the amine, the isocyanide reacts directly with the carbonyl group, but the other aspects of the reaction are the same. This reaction can take place concurrently with the Ugi reaction, acting as a source of impurities. These reaction mechanisms are shown in Scheme 1.
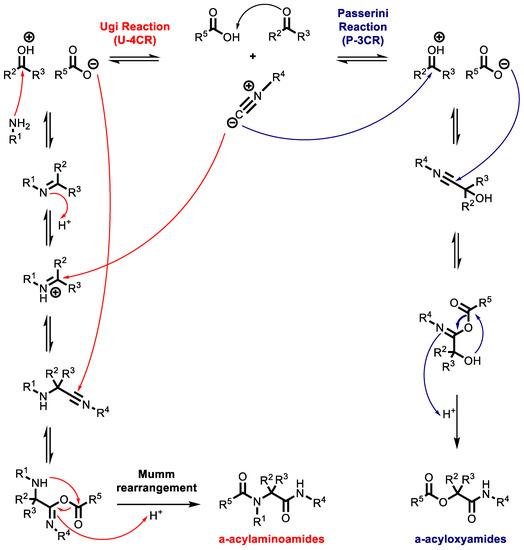
Scheme 1.
Mechanism of Ugi and Passerini reactions.
While both transformations are functional and mechanistically linked, the Ugi reaction is much more versatile than the Passerini reaction. The differences are not only based on the library size or feasibility, but mostly derive from the scaffold diversity. Both the Passerini and Ugi reactions lead to interesting peptidomimetic compounds that are potentially bioactive. Moreover, both reactions offer cost-effective and rapid access to generating large molecular libraries. The diversification of the starting compounds and the subsequent cyclization approaches provide a plethora of heterocyclic cores that have unequivocally contributed to the popularization of MCRs within the medicinal chemistry community. Other important IMCRs, depicted in Figure 3, are the Groebke–Blackburn–Bienayme reaction (GBB-3CR), Van Leusen reaction (VL-3CR), and Orru reaction (O-3CR). All of these reactions derive from further modifications of the Ugi reaction. The Groebke–Blackburn–Bienayme reaction involves the in situ formation of iminium species, followed by a non-concerted cycloaddition with an isocyanide to give the corresponding fused imidazoles. GBB-3CR is used for the one-pot synthesis of fused imidazole, bridgehead nitrogen heterocyclic compounds formaldehyde, isocyanide and amidine building blocks [51,52]. The Van Leusen-MCR (VL-3CR) is very useful for the synthesis of poly-substituted imidazoles in one pot from aryl substituted tosylmethylisocyanide (TosMIC) reagents and imines generated in situ [53]. Orru-3CR is the three-component condensation between an amine, an aldehyde and an α-acidic isocyanide, which efficiently provides highly substituted 2-imidazolines in a one-pot reaction [54,55,56].
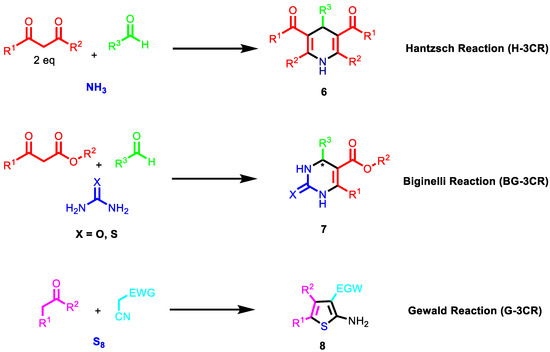
Figure 3.
Non-isocyanide-based multicomponent reactions.
Even though the classical IMCRs are not suited for generating heterocyclic compounds, new variations of the Passerini three-component reaction [48,57], Ugi three- and four-component reaction (U-4CR) [58,59], van Leusen reaction [60] and Groebke–Blackburn–Bienaymé reaction (GBBR) [61,62,63,64] have been explored and, indeed, IMCRs are leading today’s MCR chemistry [65]. For further information on isocyanide chemistry, the reader is directed toward further comprehensive review articles from Prof. Heravi’s group [66,67,68,69,70], Prof. Dömling’s group [71,72,73,74,75,76,77,78,79,80,81,82] and other extensive publications [83,84,85,86,87,88,89,90,91,92,93,94,95].
Despite its fascinating characteristics, there are several drawbacks to the use of isocyanides in organic synthesis: (1) it is harmful for the environment; (2) lability; and (3) suspected toxicity [96]. Due to these shortcomings, a practical solution was required for their wider use and further progress in isocyanide chemistry. Recently, research breakthroughs have been achieved in this direction under the title “in situ generated”, “isocyanide-less” or “odourless” isocyanide chemistry. It involves the formation of isocyanides using precursors and a subsequent reaction with other building blocks in the same pot to avoid the storage, separation and purification of isocyanides [97,98,99,100].
NIMCRs usually involve activated carbonyl compounds. An earlier example of such reactions is the Hantzsch reaction [101]. The reaction is performed with two equivalents of β-ketoesters/1,3 diketones, aldehydes and ammonia to afford symmetric dihydropyridines. Another non-isocyanide-based multicomponent reaction is the Biginelli reaction (BG-3CR) [102], which involves the synthesis of 3,4-dihydropyrimidin-2(1H)-ones from aldehydes, 1,3-dinucleophiles and β-ketoesters (Figure 4). In contrast to the previous reaction discussed, this reaction generates asymmetric products, obtaining at least two enantiomers per synthesis. In addition, depending on the 1,3-dinucleophile, more than one regioisomer can be generated. This feature allows the generation of large libraries of compounds in a short lap of time.
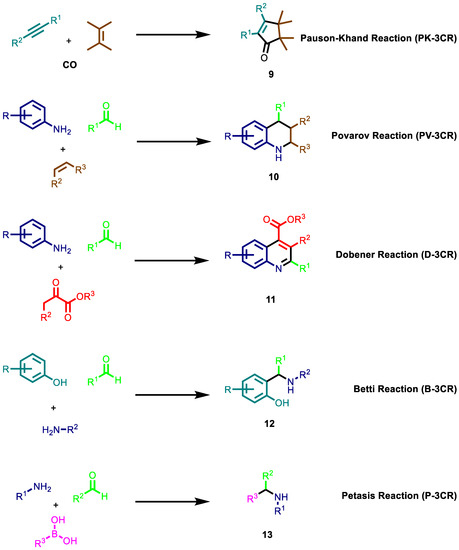
Figure 4.
Other non-isocyanide-based multicomponent reactions reactions.
Among NIMCRs, those involving cyanoacetic acid derivatives are extremely versatile, capable of affording chemically diverse scaffolds. NIMCRs usually involve Knoevenagel-type condensations of the cyanoacetic acid derivative with an aldehyde or ketone, followed by a Michael attack of a nucleophile and subsequent ring closure via a second nucleophile attack toward a nitrile. An example is the Gewald reaction (G-3CR) (Figure 4 and Scheme 2), which has recently gained ground with the usage of cyano acetamides. The G-3CR of cyanoacetic acid derivatives, active methylene carbonyls and elemental sulphur is a popular MCR often used in drug discovery yielding 2-amino-3-carbonyl thiophenes. Additionally, Gewald products can be easily transformed into more complex scaffolds by secondary transformations.
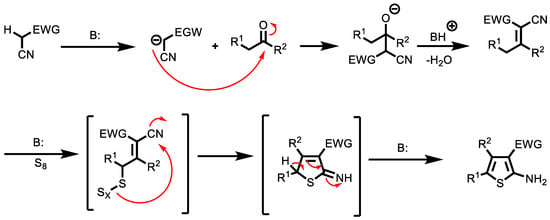
Scheme 2.
Mechanism of Gewald reaction.
The Pauson–Khand reaction (PK-3CR) is also a versatile NIMCR, enabling the rapid assembly of α,β-cyclopentenones through [2 + 2 + 1] the cycloaddition of an alkyne, an alkene and carbon monoxide. For the generation of a diverse scaffold, other NIMCRs implemented are, namely, Pavarov-3CR, Dobener-3CR, Betti-3CR, and Petasis-3CR (Figure 4). For further information on NIMCRs chemistry, the reader is directed toward comprehensive review articles and other very interesting recent publications [89,90,92,93,103,104,105,106,107,108,109,110,111,112,113].
2. Evolution of Cancer Treatment
Cancer is a process of unregulated cell growth that determines the formation of a tumor that grows abnormally (usually for years), determining first a local disease that can metastasize, thus disabling other organs or vital functions. Cancer is considered a multifactorial disease due to the interplay between numerous determinant agents, such as genetic mutations, pollution, foods contaminants, viruses, chemicals and ionizing radiation [114].
Cancer is one of the leading causes of death worldwide [115] and accounts for 8–10 million deaths each year [116]. It has been estimated that, by 2025, there will be approximately 20 million new cancer patients worldwide per year [117].
Cancer treatment has been evolving since the beginning of the 20th century. The first and most obvious solution to treat a solid tumor was surgery; however, the therapy has evolved into less invasive, non-surgical treatments focusing on the destruction of tumors by radio and chemotherapy (Figure 5).
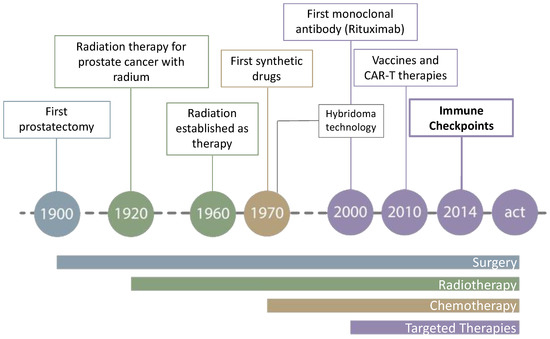
Figure 5.
Evolution of cancer treatment.
Although relatively effective, they are not highly selective and might damage healthy tissues. As a result, targeted therapies have emerged, with new therapeutic targets of a very diverse nature [118]. This, coupled with the diversity of the known cancer types, makes the rapid and effective search for anticancer agents essential.
3. Anticancer Compounds Obtained from MCRs Approaches
In this chapter, representative and recent (from 2014) examples of the exploitation of MCRs for the discovery and optimization of antineoplastic drugs are selected. The selected case studies are classified according to the MCR employed in the publication.
3.1. Passerini Reaction
In 2021, Griglio et al. [119] synthesized a series of imidazothiazole derivatives based on a compound 14 (IC50 = 77 nM) previously synthesized by Tojo et al. [120], employing the Passerini multicomponent reactions (Scheme 3) and Ugi multicomponent reaction (illustrated in the next paragraph), and studied their inhibitory activities against human Indoleamine 2,3-dioxygenase 1 (rhIDO1). The ability of the selected compounds to inhibit rhIDO1 enzyme activity was determined in an in vitro cell-based assay in order to evaluate their inhibitory effect, together with their ability to permeate the cell membrane. The human melanoma A375 cell line was selected for the cellular assay because it does not express either the rhIDO1 gene or protein under normal culture conditions.
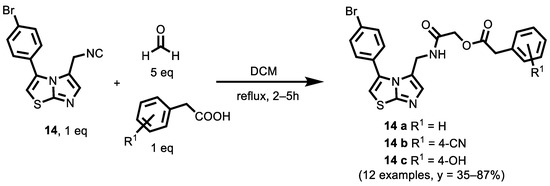
Scheme 3.
Three-Component Passerini Synthesis of α-acyloxyamides.
Acyloxyamides, in general, showed a better inhibitory activity compared to the corresponding a-acylaminoamides (illustrated in the next paragraph). Electron-withdrawing groups (R1), such as nitro and chlorine, gave rise, at any position, to a loss of activity. The cyano group or hydroxyl groups, when present at position 3, did not produce a remarkable effect on IDO1 inhibition, whereas, when displayed at position 4, they produced a significant increase in activity. Some of these compounds displayed IC50 values below 1.0 μM against the rhIDO1 enzyme; compounds 14 a, 14 b and 14 c displayed enzymatic IC50 values of 0.58 μM, 0.24 μM and 0.20 μM, respectively. This work describes the multicomponent approach as a straightforward tool to rapidly access IDO1 inhibitors, providing a new direction for their future design and development.
In 2019, Ayoup et al. [121] reported a new series of Passerini products, which were synthesized and evaluated as potent caspase-dependent apoptotic inducers. A series of α-acyloxy carboxamides was prepared from p-nitrophenyl isocyanide, cyclohexanone and various carboxylic acids, and the principal amide-based scaffold was decorated by diverse substituents in good yield (Scheme 4). All of the synthesized compounds were screened for cytotoxicity against normal human fibroblasts and for their potential anticancer activities against three human cancer cell lines: MCF-7 (breast), NFS-60 (myeloid leukemia), and HepG-2 (liver) utilizing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay.
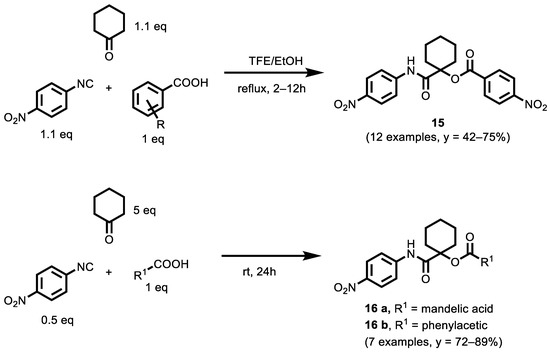
Scheme 4.
Three-Component Passerini Synthesis of α-acyloxycarboxamides.
Among the tested compounds, many have IC50 values against different cell lines that are better than the reference compound. Some of them also have a good value of selectivity index (SI) and, therefore, of safety.
Compounds 15, 16 a and 16 b were more potent than doxorubicin against the MCF-7 (IC50 = 0.0087 µM, 0.0050 µM and 0.0077 µM, respectively), NFS-60 (IC50 = 0.0077 µM, 0.0078 µM and 0.0081 µM, respectively) and HepG-2 (IC50 = 0.0141 µM, 0.0077 µM and 0.0141 µM, respectively) cancer cell lines; in addition, these compounds were the safest ones, showing the highest selectivity profiles in comparison with the other investigated compounds and doxorubicin against the NFS-60, MCF-7 and HepG-2 cell lines. The three compounds significantly induced apoptosis by caspase activation in all of the screened cancer cell lines utilizing flow cytometric analysis and a caspase 3/7 activation assay. Both compounds 15 and 16 a were more potent caspase 3/7 activators than doxorubicin, while 16 b showed comparable results. According to the physicochemical parameters, the in silico results showed that compounds 15, 16 a and 16 b may be considered as drug-like candidates.
Pyrazine-2-carboxylic acid (PA), due to its heteroaromatic ring, can be explored as an anticancer agent. In a very recent paper [122], a series of twenty novels PA derivatives were synthesized using the Passerini reaction. Their cytotoxic activity was evaluated against three different cancer cell lines, including lung (A549), breast (MCF-7) and colon (HT-29); cisplatin was used as a reference drug. The general synthetic strategy employed to synthesize the PA derivatives was based on the Passerini multicomponent reaction using isocyanide, different aldehyde derivatives and PA (Scheme 5).
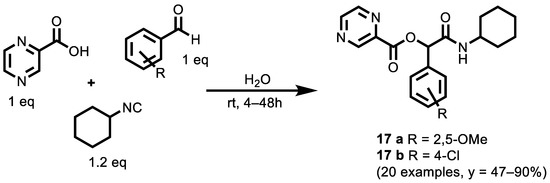
Scheme 5.
Three-Component Passerini Synthesis of α-acyloxyamides derived from pyra-zine-2-carboxylic acid (PA).
Almost all of the synthesized compounds showed higher anti-proliferative activity than PA, and resulted in a satisfying selectivity of the compounds between the cancerous cell and the non-cancerous cell line (MRC5). In all the studied cancer cell lines, the substitution of R with electron withdrawing groups such as halogens (Cl > Br > F) could affect the antitumor activity of compounds. When electron donor groups, such as methoxy (OCH3), was embedded as R, the resulting compound showed significant cytotoxic activity against the studied cancer cell lines. Among the twenty synthesized compounds, compounds 17 a and 17 b had high efficacy in suppressing cell growth and were considerably more potent in vitro cytotoxic activity than cisplatin and the others. 17 b has shown promising anticancer activity, with IC50 of 6.11, 10.64 and 14.92 μM against the A549, MCF-7 and HT-29 cell lines, respectively. Moreover, 17 a showed moderate cytotoxicity with IC50 of 14.09, 8.90 and 16.38 μM, against the A549, MCF7-7 and HT-29 cell lines, respectively (cisplatin IC50 was 9.37, 15.72 and 89.48 μM against the A549, MCF-7 and HT-29 lines, respectively). An electrophoretic mobility shift assay revealed that at higher concentrations, compound 17 b was able to initiate reactive oxygen species (ROS)-induced DNA cleavage in the presence of H2O2 (1.0 mM). Furthermore, compounds 17 a and 17 b induced apoptosis in A549 cells, and these compounds can play an essential role in the future treatment of cancer.
Natural products are often an inspiration for developing new drugs [123,124,125,126]. Moving toward this direction, Wiemann et al. [127] directed their attention to the combination of natural products and their derivatization by isocyanide-based multicomponent reactions (IMCRs). As a result, a set of 3–4 CR Ugi (illustrated in the next paragraph) and 3CR Passerini products (Scheme 6) were successfully synthesized from natural triterpenoids, i.e., oleanolic acid (OA) and maslinic acid (MA), followed by a biological evaluation of the novel α-acylamino carboxamides and the α-acyloxy carboxamides in colorimetric SRB assays to determine their cytotoxicity.
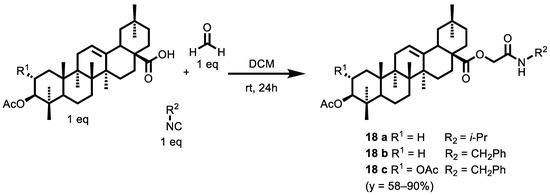
Scheme 6.
Passerini products from natural triterpenoids: oleanolic acid (OA) and maslinic acid (MA).
In comparison to U-4CR, an increased reaction rate was observed, likely due to the increased solubility of the acid component in dichloromethane (DCM). The biological activity of these compounds was examined in colorimetric sulforhodamine B (SRB) assays, and the EC50 values were determined for six human tumor cell lines: 518A2 (melanoma), A2780 (ovarian carcinoma), HT29 (colorectal carcinoma), MCF7 (breast carcinoma), A549 (lung adenocarcinoma), 8505C (thyroid carcinoma) and nonmalignant mouse fibroblasts (NIH 3T3). The EC50 values were comparable to those measured for both the Ac-OA and Ac-MA Ugi examined. Compounds 18 a, 18 b and 18 c showed a range of EC50 = 8.8–20.0 µM, EC50 = 6,4–11.3 µM and EC50 = 1.0–7.3 µM, respectively. However, a marked decline in the selectivity index was observed, thus reducing the pharmacological value of these α-acyloxy carboxamides.
In 2019, Ingold et al. [128] focused their attention on a library of NO-donor compounds through the Ugi Reaction (illustrated in the next paragraph) and the Passerini reaction with optimized yields under green chemistry conditions (Scheme 7). Nitroxyl or furoxanyl groups were incorporated into the carboxylic acid component to evaluate whether the position of the NO-donor moiety plays a fundamental role in the biological activity compared to the previous series and, thus, to explore the chemical space in search of new chemotypes with improved antitumor activity. The use of green solvents, such as water or ethanol, either at room temperature or under microwave irradiation (MW) was productive and it was also observed that the reaction could be performed under solvent-free conditions, using MW irradiation, ultrasound or conventional heating. The authors have selected as our standardized conditions: neat with MW irradiation in a closed vessel for 30 min at 60 °C.
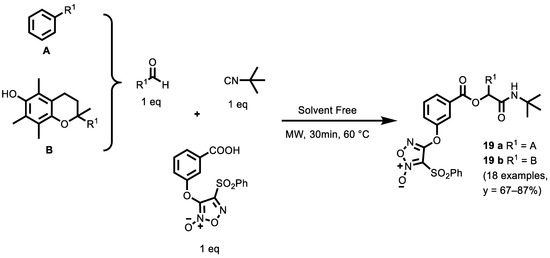
Scheme 7.
Three-Component Passerini Synthesis of NO-Donor Compounds.
Compounds have also been, in some cases, Boc-deprotected to increase the functional diversity of the library for structure–activity relationship (SAR) studies. Passerini derivatives 19 a and 19 b, each containing a furoxan system as a NO-donor, are potent anticancer agents, exhibiting GI50 values against all cells in the range 0.021–5.8 μM. Compound 19 a was almost seven times more active than cisplatin and etoposide against breast cancer cells HBL-100, and almost 30 times more potent than cisplatin and almost 140 times more potent than etoposide against SW1573 cancer cells. This compound, furthermore, exhibited lower potency against noncancerous human keratinocytes (HaCaT), indicating a selectivity against cancerous cells. In addition, compound 19 a was able to release NO in HeLa cells, and the antiproliferative activities declined with the increasing concentration of scavenger (hemoglobin), suggesting a potential role of NO release in their anticancer mechanism. Future studies should obtain a deeper insight into their antiproliferative mechanism, evaluate the antiproliferative activity of isolated isomers and determine their in vivo activity.
3.2. Ugi Reaction and Its Modifications
In parallel with the modifications of compound 20 (Scheme 8) through the Passerini reaction in order to obtain α-acyloxyamides derivatives (displayed above), Griglio et al. [119] synthesized α-acylaminoamides through the Ugi reaction. In addition, in this case, the compounds were tested for inhibitory activities against rhIDO1. In general, this type of derivative shows a lower inhibitory activity than the α-acyloxyamides derivatives, even if they express values in the micromolar range. In fact, compounds 20 a–20 e, shown in Scheme 8, displayed enzymatic IC50 value of 0.69 μM 0.45 μM, 0.81 μM, 0.63 μM and 0.73 μM, respectively (see compounds 14 a and 14 b in the previous paragraph: 0.24 μM and 0.20 μM). The effect of different substituents on the benzyl ring was investigated.
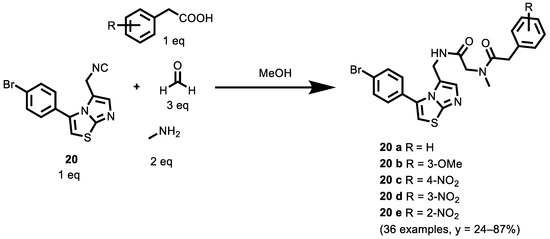
Scheme 8.
Four-Component Ugi Synthesis of α-acylaminoamides.
The cyano group produced a complete loss of activity when introduced at positions 3 and 4. The same occurred when the hydroxyl moiety was introduced at position 2; on the contrary, the hydroxyl group at position 3 gave rise to the most active compound of this series (18 b), with an IC50 value of 0.45 µM. The presence of a nitro group produced a progressive increase in the activity by moving the substituent from position 4 (20 c, IC50 = 0.81 µM) to either position 2 (20 e, IC50 = 0.73 µM) or 3 (20 d, IC50 = 0.63 µM). The presence of a chlorine at different positions on the benzylic ring did not produce any improvement in the activity.
An efficient four-component Ugi synthesis of quinoline-coumarin hybrids was described by Taheri et al. [129]. This simple access to quinoline-coumarin derivatives implicated the use of coumarin-3-carboxylic acid, diverse 2-chloroquinoline-3-carbaldehydes, aniline derivatives and aliphatic isocyanides in methanol at room temperature (Scheme 9).
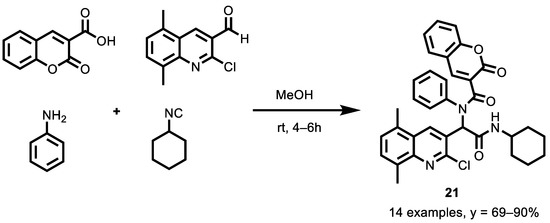
Scheme 9.
Formation of diamide Ugi reaction compounds.
The cytotoxic effects of fourteen products were investigated in A2780 human ovarian cancer cells. Several methods were also performed, including cell viability, the induction of apoptosis, a mitochondrial membrane potential (MMP) assay, the determination of intracellular ROS, caspase 9 and a 3 activity assay. Interestingly, compound 21 showed excellent anticancer activity, with an IC50 value of 25 µg/mL (0.042 µM). A2780 cells were treated with various concentrations of hybrids (0–100 μg/mL) for 24 h and the cell viability was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) growth inhibition assay; doxorubicin was chosen as a positive control (IC50 = 6.1 µM). Furthermore, the synergistic effect of compound 21 on apoptosis induction might be due to the regulation of anti-apoptotic agents, including the down-regulation of Bcl-2 and survivin and the activation of caspase 9 and 3. Further examinations indicated that compound 21 increased the ROS levels, reduced the MMP, and induced apoptosis in the A2780 cells through the intrinsic mitochondrial pathway.
In 2021, Butera et al. [130] focused their attention on novel therapeutic modalities, such as small molecules or peptides, through MCR synthesis. A small library of compounds was synthesized using the GBB-3CR, resulting in the structure–activity relationship of imidazo [1,2-a]pyridine-based inhibitors (Scheme 10). These inhibitors were tested for their biological activity using various biophysical assays, giving potent candidates with low-micromolar PD-L1 affinities.
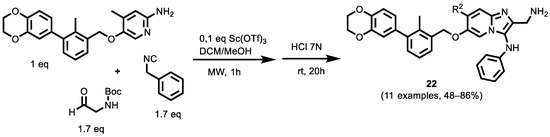
Scheme 10.
Groebke–Blackburn–Bienaymé products of imidazo[1,2-a] pyridine-based inhibitors.
The authors found the optimal reaction conditions for the used substrates in the presence of scandium triflate (10 mol%) as a catalyst, 2:1 DCM/MeOH as the solvent system, a concentration of 0.3 M concerning to the amidine, and 1.7 equivalent of the isocyanide and aldehyde components. Microwave-assisted heating for 1 h generated the corresponding GBB products in good to excellent yields (48–86%) The products were tested for their antiproliferative activity through various biological assays, exhibiting IC50 values of 16.8–1.8 μM (compound 22) at a concentration of 50 μM. These results open the door to an interesting bioactive scaffold that could lead to a new class of PD-L1 antagonists.
Wiemann and Csuk [127], in addition to the synthesis of the α-acyloxy carboxamides (illustrated in the paragraph above), also synthesized a library of 34 α-acylamino carboxamides from natural triterpene through U-4CR (Scheme 11).
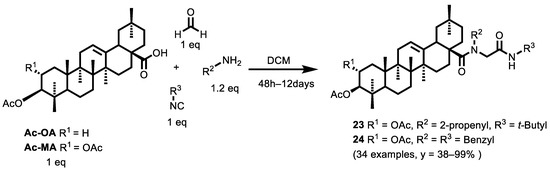
Scheme 11.
Ugi products from natural triterpenoids: oleanolic acid (OA) and maslinic acid (MA).
First, they studied the U-4CR derivation of oleanolic acid: the isocyanide used was a tert-butyl isocyanide and the amino component (R2) was varied (methyl, 2-propenyl, 3-furanyl-methyl, butyl, isopropyl, benzyl, 3-fluorobenzyl). Furthermore, different isocyanide groups were also used, such as n-butyl isocyanide or i-propyl isocyanide or benzyl isocyanide and methyl isocyanoacetate (while the amine component remained unchanged for better comparability of the biological results).
Due to the low solubility of triterpenoic acids in polar-aprotic solvents, the reactions were characterized by long reaction times and moderate conversions. However, the increase in the reaction temperature and the use of MW did not result in higher efficiencies, but the reaction time could be reduced to 2 days from 7–12 days. Secondly, they studied the U-4CR derivation of maslinic acid. Due to their better solubility, the derivatives were synthesized in one step directly from 2,3-di-O-acetyl-MA (Ac-MA). In addition, in this case, the biological activity of these compounds was investigated in SRB colorimetric assays, and the EC50 values were determined for six human tumor cell lines: 518A2 (melanoma), A2780 (ovarian carcinoma), HT29 (colorectal carcinoma), MCF7 (breast carcinoma), A549 (lung adenocarcinoma), FaDu (hypopharyngeal carcinoma with multidrug resistance) and nonmalignant mouse fibroblasts (NIH 3T3). All of the compounds showed moderate to good cytotoxicity for several human tumor cell lines. In particular, compounds 23 (R1 = OAc, R2 = 2-propenyl, R3 = t-butyl) and 24 (R1 = OAc, R2 = R3 = benzyl) gave remarkable EC50 values in the low μM range (0.3 µM and 0.4 µM, respectively, for ovarian carcinoma cells A2780) and high tumor/non-tumor cell selectivity. Further biological assays showed that treating A2780 cells with compounds 23 or 24 led to a non-necrotic, controlled cell death, in part occurring through the pathway of apoptosis. In addition, Wiemann and Csuk also highlighted how varying the substrate yielded acetylated products and compounds holding a terminal benzylamide moiety with higher selectivity, while the presence of ester groups initiated a loss of this selectivity.
In 2020, for the investigation of new drug-type G-quadruplex selective binders, Pelliccia et al. [131] used the design of nucleobases as a starting point as synthons in a multicomponent reaction. Thus, a series of multifunctional imidazo[2,1-i]purine derivatives were synthesized via a convergent GBB-3CR of amino-aza-heterocycles, benzaldehydes and isocyanide, followed by an SN2 with various aminoalkyl chlorides [R2 = 4-morpholinyl(CH2)2, pyrrolidinyl(CH2)2, Me2N(CH2)3, N-phthalamidyl(CH2)2] (Scheme 12).
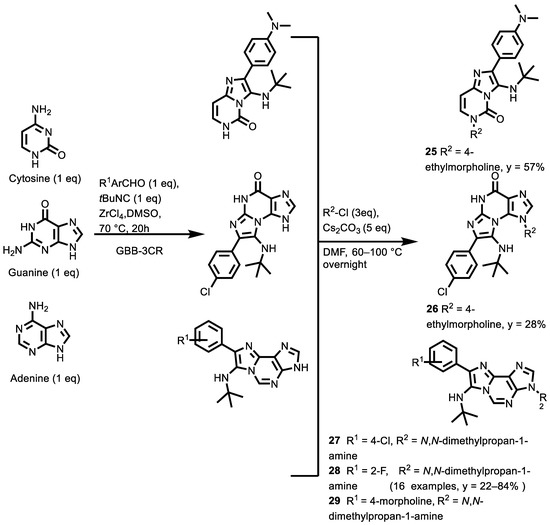
Scheme 12.
Groebke-Blackburn-Bienaymé-mediated three-component synthesis of imidazo[2,1-i]purine derivatives.
The compounds 25, 26 and 27–29 were endowed with a small molecular weight (≅500 Da), water solubility, easy functionalization, and a selectivity profile as G-quadruplex binders as safe and effective anti-cancer agents, as well as being non-toxic toward non-cancerous cells. Compounds, 27, 28 and 29, were selected as the best performing ones, both in terms of their potency and selectivity toward BCL2 and c-MYC G4s, over the other G4 topologies screened and a duplex model. Indeed, compound 27 exhibited selective antiproliferative action (IC50 = 17 µM) toward Jurkat human T lymphoblastoid cells, without showing significant cytotoxicity toward normal cells, such as skin-derived cells. Furthermore, a downregulation at both the mRNA and protein levels was observed through qPCR and Western blot analyses, in which the cells were treated with the selected compounds. On the basis of both the herein-reported biophysical and biological data and the poor availability in the literature of selective BCL2 G4 ligands, compound 27 represents a promising advancement toward the pathway of the specific targeting of cancer cell lines by means of gene promoters’ G4 binders.
Moreover, Ingold et al. [128] also obtained α-acylaminoamides derivatives (compare α-acyloxyamides derivatives in the paragraph above) under green conditions through the Ugi reaction (Scheme 13).
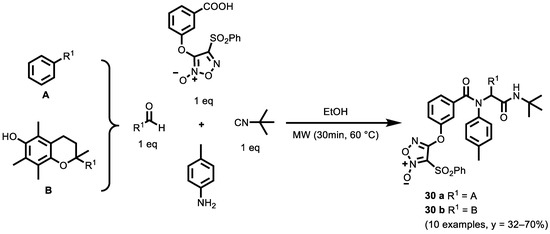
Scheme 13.
Ugi reaction for synthesis of NO-Donor Compounds.
The optimized experimental conditions for the Passerini reaction (see respective paragraph in chapter 3.1 Passerini Reaction) failed to deliver the Ugi adduct. For this reason, the IMCRs were optimized and performed (under eco-friendly conditions) in EtOH and MW irradiation (closed vessel, 30 min, 60 °C). The compounds were also, in some cases, Boc-deprotected to increase the functional diversity of the library for SAR studies. The biological activity of these compounds and their cytotoxicity against six human solid tumor cell lines (A549 (lung), HBL-100 (breast), HeLa (cervix), SW1573 (lung), T-47D (breast), WiDr (colon) have been studied. Compounds 30 a and 30 b exhibited notable activities against most of the tested cell lines, as revealed in their GI50 values being between 0.24–5.8 μM and 0.021–3.0 μM, respectively, against all of the tested cell lines, as well as their sensitivity against HBL-100 and SW1573 cell lines. Compound 30 a was almost eight times more potent than cisplatin and etoposide against the resistant cell lines, T-47D and WiDr; moreover, it was extremely potent against alveolar cell carcinoma SW1573, being 140 times more active than cisplatin and 700 times more potent than etoposide.
Furthermore, compounds 30 a and 30 b were evaluated for their ability to release NO in cancer cells as a first approximation to their mechanism of action. Compound 30 a was able to release NO in the presence of HeLa cells.
In a very recent publication, Xiong et al. [132] reported the synthesis of 4-tetrazolyl-3,4-dihydroquinazoline derivatives through a sequential Palladium-catalyzed azide-isocyanide cross-coupling/cyclization Ugi reaction, with good yields. The Ugi-azide reactions of various 2-azidobenzaldehydes (R1 = 4-F, 4-Cl, 4-CH3O and 5-Me), amines (R2 = Ph, 4-Cl-Ph, 4-Br-Ph, 4-CH3-Ph, 4-CH3O-Ph, 2-Cl-Ph, 2,4-dimethyl-Ph, n-Bu, i-Pr, t-Bu), trimethylsilyl azide and isocyanides (R3 = t-Bu, c-C6H11, 1-adamantyl, n-Bu) produced azide intermediates that were treated with diverse isocyanides (R4 = t-Bu, c-C6H11, 1-adamantyl, 4-CH3O-Ph, 4-Cl-Ph, MeO2CCH2, TsCH2) to afford compound 18 examples of dihydroquinazoline derivatives (Scheme 14).
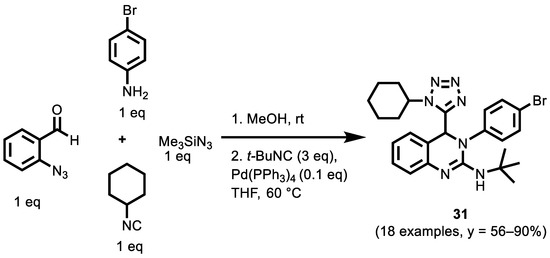
Scheme 14.
Ugi-4CR synthesis of dihydroquinazoline derivatives.
With the advantages of mild reaction conditions, good yields and the easy availability of raw materials, this strategy shows the synthetic potential of combining isocyanide-based multicomponent reactions with azide-isocyanide coupling reactions to produce structural diversity and complexity. Among the synthesized compounds, compound 31 (R1 = H, R2 = 4-Br-Ph, R3 = c-C6H11, R4 = t-Bu,) was a promising starting point for the development of a breast cancer drug. The proliferation of breast cancer cells treated with 31 at the concentration of 0.3 µM was detected using the 5-ethynyl-2′-deoxyuridine (EdU) method. The results showed that, with the increase in 31 concentrations, the number of EdU positive cells in breast cancer decreased gradually. This evidence shows that compound 31 could significantly inhibit the proliferation, and promote the apoptosis, of breast cancer cells.
In 2022, Nichugovskiy et al. [133] demonstrated for the first time that the anticancer activity of lipophilic synthetic polyamines (LPAs) with fragments of piperazine is comparable to that of aliphatic LPAs. In their publication, they demonstrated a new method for the synthesis of LPAs using the N-split Ugi multicomponent reaction and subsequent reduction in amide groups by PhSiH3 (Scheme 15). The anticancer activity was evaluated in the A-549, MCF7 and HCT116 cancer cell lines.
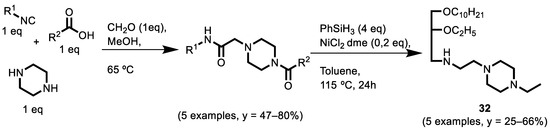
Scheme 15.
Synthesis of LPAs using N-split Ugi reaction.
With the application of this method, the authors reduced the synthetic steps and increased the total yield of the LPAs from 7% to 28%. The use of PhSiH3 and NiCl2 (ethylene glycol dimethyl ether complex) effectively permitted us to reduce several amide groups in the PA precursors and has proven to be a very reliable and efficient method. The obtained LPAs manifest excellent preliminary biological activity in cancer cell lines, and among these, compound 32 showed the highest anticancer activity within all the tested cell lines: IC50 = 3.0 ± 0.4 µM (A-549 cell line), 1.0 ± 0.1 µM (MCF7 cell line) and 3.8 ± 0.5 µM (HCT116 cell line). These IC50 values are several times higher than that of cisplatin, which is used in medical practice.
3.3. Biginelli Reaction
With the initiative to develop novel cancer immunotherapeutic agents via the modulation of the A2B adenosine receptor, Sotelo et al. [134,135,136,137,138] discovered several families of (monocyclic, bicyclic or tricyclic) pyrimidine derivatives (Scheme 16) that were readily and efficiently assembled through a three-component Biginelli reaction.
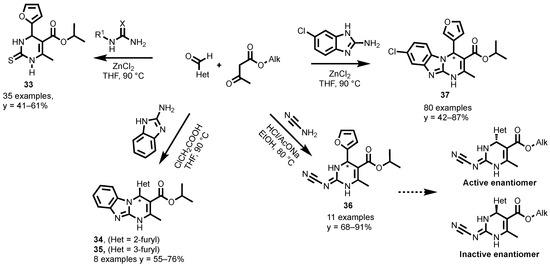
Scheme 16.
Three-Component Biginelli Synthesis of A2B antagonists.
The synthesized compounds were evaluated for their antagonistic activity toward the Adenosine Receptors. Many compounds have yielded good Ki values in A2B (Ki ≤ 25 nM) and excellent receptorial subtype selectivity profiles. The most promising for A2B are the monocyclic compounds 33 with Ki = 10.2 nM, 36 with Ki = 24.3 nM; and tricyclic compounds 34 with Ki = 3.49 nM; 35 with Ki = 11.40 nM. During the study, some A2A/A2B duals emerged, being of particular interest in cancer immunotherapy by acting synergistically on the immune system and tumor cells. The most promising is 37, with Ki = 176.2 and 6.1 nM for A2A and A2B, respectively. The differences in the affinity of both enantiomers were evaluated first on 36, and confirmed in subsequent works, showing that the affinity for A2B (and A2A) is exclusively due to one of the two enantiomers (Scheme 16).
The administration of these compounds to breast cancer patient derived cells, was first revealed through a reduced tumor spheroids growth rate [137]. The study also demonstrated a marked effect on immune system cells, recovering from adenosine-mediated immunosuppression and increasing the production of proinflammatory cytokines. This effect in the immune system has been recently verified [138].
In 2021, a new ecofriendly Biginelli reaction procedure was adapted to prepare new dihydropyrimidines using β-aroylpyruvates as synthons [139]. All of the synthesized compounds were evaluated for their anticancer activity against 59 human cancer cell lines, representing different cancer types, including leukemia and melanoma, as well as lung, colon, ovarian, renal, prostate and breast cancer. The anticancer activity was demonstrated as a percentage growth inhibition of different human cell lines exerted by a single dose of 10−5 M. Compound 38 (Scheme 17) showed marked wide spectrum anticancer activity towards most of the tested cancer cell lines, with a percentage of growth inhibition of 29.04–71.68% upon a single dose administration (10−5 M). Specifically, most of the leukemia and colon cancer cell lines were highly sensitive to the antitumor effect of compound 38, with a growth inhibiting effect exceeding 50%, as well as the colon cancer HT29 cell line (53.16%) and the leukemia cell lines, K-562 (64.97%) and SR (71.68%).
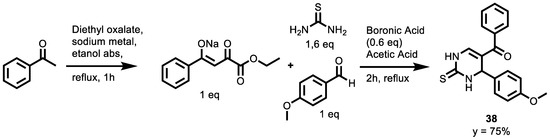
Scheme 17.
Synthesis of compound 38 of new dihydropyrimidines.
Recently, Li et al. [140] published on an interesting study ACS Macro Letters about the development of a polymer-drug strategy to explore anticancer polymers. These consist in a series of monomers containing groups with potential anti-cancer activity that were easily prepared by the Biginelli reaction. These monomers were used to produce water-soluble polymers by practical radical co-polymerization. A series of dihydropyrimidinones groups (DHPMs), similar to Monastrol, a promising anticancer agent which inhibits Eg5 kinesin protein that is highly expressed in many tumor cells and is closely related to the occurrence and development of tumors, was prepared through the Biginelli reaction (Scheme 18).
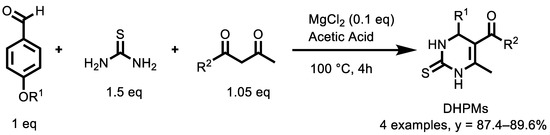
Scheme 18.
Three-component synthesis of dihydropyrimidinones group.
The obtained polymers are biocompatible and can be used directly to suppress the proliferation of various cancerous cells without the release of small molecules. In such experiments, one of the DHPMs, P4, a polymer with the best anticancer ability, effectively suppressed the proliferation of different cancer cells by inhibiting cell mitosis, similarly to Monastrol, confirming the rationale behind the theoretical calculations. The inhibition effect of P4 was investigated on different cells: a model of normal cells (L929), hepatoma cell line (SMMC-7721), human cervical cancer cell line (HeLa) and a human breast cancer cell line (MCF-7). The results suggested that P4 selectively inhibits the proliferation of cancer cells, rather than killing them. Furthermore, immunostaining was carried out to detect the inhibitory process of P4 during cellular proliferation, suggesting that P4 shares a similar anticancer mechanism of the small molecular monastrol and that it could be considered a potential anticancer polymer.
In 2020, the study by Yavuz et al. [141] aimed at developing an effective, improved and easy method to synthesize substituted pyrimidine-5-carbonitriles, without the isolation of their intermediates. In a single reaction step, pyrimidine derivatives were synthesized from the triple reaction of aromatic aldehydes, ethyl cyanoacetate and a few guanyl hydrazone derivatives (Scheme 19). In this study, the reaction conditions were optimized, monitoring the order of addition of reagents and the best base to be used. The reaction conditions were first optimized using different bases, such as NaOH, K2CO3, NaHCO3 and piperidine. When NaOH (10% mol) was added into the reaction system, the products were obtained in moderate yields in both refluxing in ethanol and at room temperature, while when piperidine was added as a catalyst to the reaction medium, higher yield compounds were obtained with the same reaction conditions. First, aromatic aldehyde, ethyl cyanoacetate and a catalytic amount of piperidine were added to obtain the best yield.
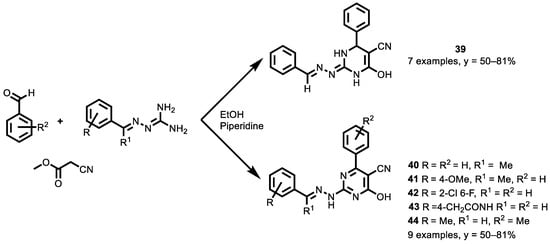
Scheme 19.
Three-component synthesis of pyrimidine derivatives.
The synthesized compounds were tested in vitro against the human colon cancerous cell line (DLD-1) and a human breast cancerous cell line (MDA-MB-231). Nearly all of those compounds showed cytotoxic activity in the tested cell lines. However, in general, the synthesized pyrimidine-based compounds exhibited better cytotoxicity against the colon cancer cell line than the tested breast cancer cell line. In particular, compounds 39, 40 and 41 have a significant effect against DLD-1. Furthermore, compounds 42, 43 and 44 exhibited lower IC50 values compared to the other synthesized compounds and were tested against MDA-MB-231.
On the other hand, in 2022, Ashma et al. [142] focused their attention on the synthesis of nicotinic acid hydrazide metal complexes and their potential anticancer and antibacterial activity, together with their ability to act as a catalyst. This work addressed newer bidentate Schiff bases, such as L5 and L6, and their metal complexes Co(II), Ni(II), Cu(II) and Zn(II) were synthesized by a one-pot method. The multicomponent reaction of Biginelli has been tested with a catalytic amount of their transition metal complexes, Co(II), Ni(II), Cu(II) and Zn(II), in the presence of a solvent. Hydrazone-substituted transition metal complexes Cu (II) showed excellent catalytic activity compared to the other derivatives. The metal complexes exhibited good anti-cancer activity, overall, in 400–800 μg for most L5 and L6 complexes, but Co, Cu and Zn showed higher activity at the nano gram scale for 24h treatment on the human colon cancer cell line HT29. The Zn(II) complex, in particular, exhibited greater efficacy, even at the 600 ng concentration, which makes it a potent candidate for further anti-cancer studies in different type of cancers cells.
3.4. Other MCRs for the Synthesis of Anticancer Drugs
In a recent paper, Alshabanah et al. [143] reported the design and synthesis of thiazoles and thiadiazoles linked to position 3 of coumarin as novel 3-azolylcoumarins as potential anticancer agents, utilizing the sonication technique and using chitosan-grafted poly(vinylpyridine) as an eco-friendly catalyst (Scheme 20).
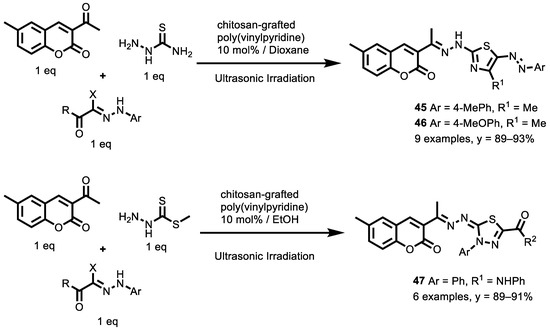
Scheme 20.
Three-component synthesis of thiazoles and thiadiazoles.
In this study, the best reaction conditions were evaluated. Regarding the base chitosan-grafted poly(vinylpyridine), 10 mol% was the best choice of a basic catalyst (respect to trietilammina and chitosan) under ultrasonic irradiation (USI). In addition, heating at 50 °C under USI was more efficient than conventional heating as it reduced the reaction time and increased the product yields for the thiazoles derivatives, but not for the thiadiazole (respect to the 25 °C). The cytotoxic activity of the newly prepared compounds was determined against the liver carcinoma cell line (HEPG2-1) using the MTT assay, and doxorubicin was used as a reference compound. Compounds 45 (Ar = 4-MePh, R1 = Me), 46 (Ar = 4-MeOPh, R2 = Me) and 47 (Ar = Ph, R2 = NHPh), with IC50 values of 0.43 µM, 0.29 µM and 0.48 µM, respectively, (doxorubicin IC50 = 0.31 µM) have promising cytotoxic activities. The 1,3-thiazole ring introduction of an electron-donating group (methyl or methoxy groups) enhanced the antitumor activity. In contrast, the introduction of the electron-withdrawing group (chlorine or bromine or nitro group) at C4 of the phenyl group at position 4 in the 1,3-thiazole ring decreased the activity. In addition, for 1,3,4-thiadiazoles, generally, on fixing the substituents at position 5, the electron-donating group (methyl) at C4 of the phenyl ring enhances the antitumor activity, while the electron-withdrawing group (chlorine) decreases the activity.
In 2017, a time-efficient three-component synthesis of a series of substituted aryl thiazoles derivatives was achieved through the reaction between different (ortho, para) substituted benzoyl hydrazides and (ortho, meta, para) aromatic isothiocyanate, followed by aromatic α-halo ketone in the presence of triethylamine under reflux in ethanol (Scheme 21) [144].
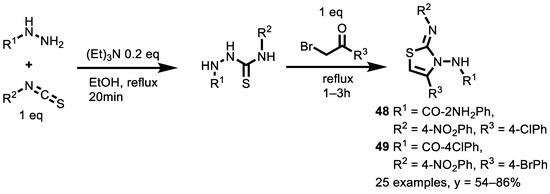
Scheme 21.
Three-component synthesis of aryl thiazoles derivatives.
In this study, twenty-five substituted aryl thiazoles were synthesized, and their in vitro cytotoxicity was evaluated against MCF-7 (ER+ve breast), MDA-MB-231 (ER−ve breast), HCT116 (colorectal) and HeLa (cervical). The activity was compared with the standard anticancer drug doxorubicin (IC50 = 1.56 ± 0.05 μM). Many of the twenty-five synthesized compounds were found to be cytotoxic for all four cancer cell lines, with IC50 values ranging between 5.37 and 46.72 μM. Compound 48 was highly toxic against the breast cancer cells, with IC50 = 5.37 ± 0.56 μM for MCF-7 (ER+ve breast), IC50 = 17.77 ± 4.32 μM for MCF-7 (ER+ve breast) and against the HCT116 (colorectal) cell line, with IC50 = 16.99 ± 0.94 μM. Nevertheless, compound 49 showed IC50 values of 10.96 ± 0.33 μM, 9.9 ± 0.59 μM and 8.28 ± 0.21 μM for MCF-7 (ER+ve breast), MDA-MB-231 (ER-ve breast) and HeLa (cervical), respectively. These compounds deserve to be further investigated in vivo as anticancer lead compounds.
In 2018, Yakaiah et al. [145] obtained novel pyrazolo-oxothiazolidine derivatives through the condensation of 1-(benzofuran-2-yl)-3-(substituted-arylprop-2-en-1-ones), thiosemicarbazide and dialkyl acetylenedicarboxylates. The reaction conditions were optimized, obtaining an efficient, one-pot multicomponent reaction (Scheme 22).
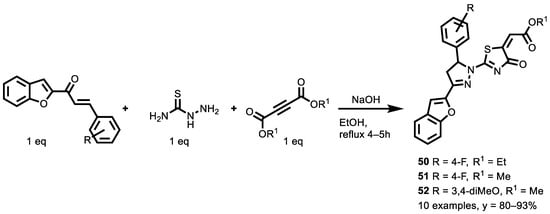
Scheme 22.
Three-component synthesis of pyrazolo-oxothiazolidine derivatives.
Initially, the reactions were performed with a variety of solvents, such as methanol, ethanol 1,4-dioxane, acetonitrile and water, to understand the solvent’s effect on the yield. To optimize the better reaction conditions, various catalysts, such as KOH, triethylamine, piperidine, H2SO4, acetic acid and NaOH, were tested. It was observed that the ethanol is the best among the tested solvents in terms of yield and NaOH was identified as a suitable catalyst, which gave the maximum isolated yield.
The synthesized compounds were evaluated for their antiproliferative activity against the A549 lung cancer cell line and the antiproliferative activity of each individual compound was compared with Sorafenib, a Food and Drug Administration (FDA) approved drug standard. Among all the tested compounds, 50 (IC50 = 0.930 μg/mL), 51 (IC50 = 0.808 μg/mL) and 52 (IC50 = 0.967 μg/mL) showed promising activity compared with the standard drug, sorafenib (IC50 = 3.779 μg/mL). From the antiproliferative activity data, it is evident that the presence of electron-rich species, such as the methoxy group, and electron withdrawing groups, such as fluoro and chloro on the pyrazoline ring, are fundamental for their antiproliferative activity. Furthermore, to gain a better understanding of the effectiveness of the ligand analogue molecules, they studied the interaction of their derivatives docked into the binding site of the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor type 2 (VEGFR2). The molecular docking studies indicated that compound 51 had the greatest affinity for the binding site of these receptors, and the docking results also give us a new direction to design new inhibitors.
To conclude this roundup of examples, the recent paper by Professor Tron’s group deserves special attention [146]. In this work, the authors developed a novel multicomponent reaction to prepare a library of 62 novel compounds, analogs of a potent antitubulin agent (TN-16), which are reported in Scheme 23. The synthesized compounds were evaluated for their possible antiproliferative activity on human neuroblastoma SH-SY5Y cells.
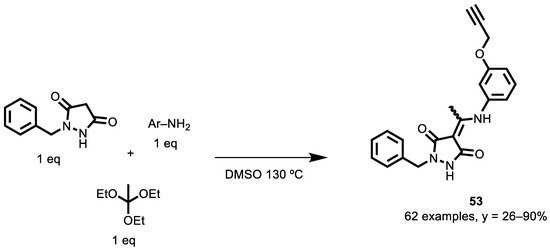
Scheme 23.
Synthetic route to prepare aza-analogs of TN-16.
62 compounds were prepared via MCR between different anilines, triethylorthoacetate and 1-benzylpyrazolidine-3,5-dione, with a yield between 26 and 90%. When the compounds were screened on SH-SY5Y cells, only compound 52 (EC50 = 102 nM) showed significant cytotoxicity. These compounds were cytotoxic on cancer cell lines and, as expected from antitubulin agents, induce G2/M cell cycle arrest. In fact, these lead to a disruption of the microtubules and an increase in α-tubulin acetylation and affect in vitro polymerization, although they have a lesser effect in cellular tubulin polymerization assays.
4. Conclusions
Relevant, recent and representative papers dealing with the exploration of small molecule anticancer compounds assembled via MCRs have been highlighted, underlining the tremendous potential of MCRs in medicinal chemistry. An advantage of MCR chemistry is the ability to explore a very large chemical space for drug discovery and medicinal chemistry purposes. In view of the great need to synthesize compounds that enrich the arsenal against cancer, still largely an unmet clinical need, we hope that this review will be useful for the researchers, worldwide, interested in this field.
Author Contributions
Conceptualization, A.S. (Angela Stefanachi) and E.S.; investigation, G.G., A.S. (Angela Stefanachi), R.P.-D. and E.S.; writing—original draft preparation, G.G., A.S. (Angela Stefanachi), R.P.-D. and E.S.; writing—review and editing, G.G., A.S. (Angela Stefanachi), R.P.-D., M.C., A.L., P.K., A.S. (Antonio Scilimati), F.L. and E.S.; supervision, A.S. (Angela Stefanachi), F.L. and E.S.; funding acquisition, F.L., E.S. and A.S. (Antonio Scilimati). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by (a) the Ministry of Economic Development (MISE) funded project “GENESI” code 092-Prog n. F/180003/03/X43 for the development of innovative radiopharmaceuticals and biomarkers for the diagnosis of cancers of the male and female reproductive system (2021–2023) financed by Ministero dello Sviluppo Economico (Rome, Italy) (Grant to A.S. (Antonio Scilimati)); (b) the Consellería de Cultura, Educación e Ordenación Universitaria of the Galician Government: (grant: ED431B 2020/43), Centro Singular de Investigación de Galicia accreditation 2019–2022 (ED431G 2019/03) and the European Regional Development Fund (ERDF); (c) Ministerio de Ciencia e Innovación—Agencia Estatal de Investigación-FEDER-UE (PID2020-118511RB-I00 and PID2021-124010OB-100); (d) Puglia region for research grant (POR PUGLIA 2/FSE/2020) (Grant to G.G.).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
MCR (Multicomponent reaction), IMCRs (Isocyanide-based multicomponent reactions), NIMCRs (non-isocyanide-based multicomponent reactions), P-3CR (Passerini three component reaction), U-4CR (Ugi four component reaction), GBB-3CR (Groebke-Blackburn-Bienayme three component reaction), VL-3CR (Van Leusen three component reaction), O-3CR (Orru three component reaction), TosMIC (Tosylmethylisocyanide), BG-3CR (Biginelli three component reaction), G-3CR (Gewald three component reaction), PK-3CR (Pauson-Kand three component reaction), rhIDOI1(Human recombinant indoleamine 2,3-dioxygenase), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide), PA (Pyrazine-2-carboxylic acid), ROS (Reactive oxygen species), OA (Oleanolic Acid), MA (Maslinic Acid), SRB (Sulforhodamine B), DCM (Dichloromethane), MW (Microwave), SAR (Structure-activity relationship), mRNA (Messenger ribonucleic acid), EC50 (Half maximal effective concentration), MMP (Mitochondria membrane potential), EdU (5-ethynyl-2′-deoxyuridine), LPA (Lipophilic synthetic polyamines), FDA (Food and Drug administration), DHPM (dihydropyrimidinone), USI (Ultrasonic irradiation), EGFR (Epidermal growth factor receptor), VEGFR2 (vascular endothelial growth factor receptor type 2).
References
- Zhi, S.; Ma, X.; Zhang, W. Consecutive Multicomponent Reactions for the Synthesis of Complex Molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of Polyheterocycles via Multicomponent Reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef]
- Keating, T.A.; Armstrong, R.W. Molecular Diversity via a Convertible Isocyanide in the Ugi Four-Component Condensation. J. Am. Chem. Soc. 1995, 117, 7842–7843. [Google Scholar] [CrossRef]
- Flores-Reyes, J.C.; Islas-Jácome, A.; González-Zamora, E. The Ugi Three-Component Reaction and Its Variants. Org. Chem. Front. 2021, 8, 5460–5515. [Google Scholar] [CrossRef]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of Biologically Active Molecules through Multicomponent Reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef] [PubMed]
- Alvim, H.G.; da Silva Júnior, E.N.; Neto, B.A. What Do We Know about Multicomponent Reactions? Mechanisms and Trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.L.; Sharma, U.K. Multicomponent Reactions and Photo/Electrochemistry Join Forces: Atom Economy Meets Energy Efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent Reactions: Advanced Tools for Sustainable Organic Synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Ahmed Fouad, M.; Abdel-Hamid, H.; Salah Ayoup, M. Two Decades of Recent Advances of Ugi Reactions: Synthetic and Pharmaceutical Applications. RSC Adv. 2020, 10, 42644–42681. [Google Scholar] [CrossRef]
- Wahby, Y.; Abdel-Hamid, H.; Ayoup, M.S. Two Decades of Recent Advances of Passerini Reactions: Synthetic and Potential Pharmaceutical Applications. New J. Chem. 2022, 46, 1445–1468. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, Z.; Zhang, D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, W.; Zhang, D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules 2020, 25, 1594. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Feng, X. Asymmetric Strecker Reactions. Chem. Rev. 2011, 111, 6947–6983. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.F.A.; Lemos, B.C.; de Souza, A.S.; Pinheiro, S.; Greco, S.J. Multicomponent Mannich Reactions: General Aspects, Methodologies and Applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar] [CrossRef]
- Wan, J.-P.; Liu, Y. Synthesis of Dihydropyrimidinones and Thiones by Multicomponent Reactions: Strategies Beyond the Classical Biginelli Reaction. Synthesis 2010, 2010, 3943–3953. [Google Scholar] [CrossRef]
- Kappe, C.O. The Generation of Dihydropyrimidine Libraries Utilizing Biginelli Multicomponent Chemistry. QSAR Comb. Sci. 2003, 22, 630–645. [Google Scholar] [CrossRef]
- Leonardi, M.; Estévez, V.; Villacampa, M.; Menéndez, J.C. The Hantzsch Pyrrole Synthesis: Non-Conventional Variations and Applications of a Neglected Classical Reaction. Synthesis 2019, 51, 816–828. [Google Scholar] [CrossRef]
- Howard, S.Y.; Di Maso, M.J.; Shimabukuro, K.; Burlow, N.P.; Tan, D.Q.; Fettinger, J.C.; Malig, T.C.; Hein, J.E.; Shaw, J.T. Mechanistic Investigation of Castagnoli–Cushman Multicomponent Reactions Leading to a Three-Component Synthesis of Dihydroisoquinolones. J. Org. Chem. 2021, 86, 11599–11607. [Google Scholar] [CrossRef]
- Li, Y.; Emge, T.J.; Moreno-Vicente, A.; Kopcha, W.P.; Sun, Y.; Mansoor, I.F.; Lipke, M.C.; Hall, G.S.; Poblet, J.M.; Rodríguez-Fortea, A.; et al. Unexpected Formation of Metallofulleroids from Multicomponent Reactions, with Crystallographic and Computational Studies of the Cluster Motion. Angew. Chem. 2021, 133, 25473–25477. [Google Scholar] [CrossRef]
- Su, Y.-L.; Liu, G.-X.; De Angelis, L.; He, R.; Al-Sayyed, A.; Schanze, K.S.; Hu, W.-H.; Qiu, H.; Doyle, M.P. Radical Cascade Multicomponent Minisci Reactions with Diazo Compounds. ACS Catal. 2022, 12, 1357–1363. [Google Scholar] [CrossRef]
- Barcellos, A.M.; Mangiavacchi, F.; Abenante, L.; Dias, Í.F.C.; Sacramento, M. Chapter 1—Multicomponent Reactions in the Synthesis of Organochalcogen Compounds. In Organochalcogen Compounds; Lenardão, E.J., Santi, C., Perin, G., Alves, D., Eds.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–30. ISBN 978-0-12-819449-2. [Google Scholar]
- Zoll, A.J.; Molas, J.C.; Mercado, B.Q.; Ellman, J.A. Imine Directed Cp*RhIII-Catalyzed N−H Functionalization and Annulation with Amino Amides, Aldehydes, and Diazo Compounds. Angew. Chem. 2023, 135, e202210822. [Google Scholar] [CrossRef]
- Russo, C.; Brunelli, F.; Tron, G.C.; Giustiniano, M. Isocyanide-Based Multicomponent Reactions Promoted by Visible Light Photoredox Catalysis. Chem. Eur. J. 2023, 29, e202203150. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Graus, S.; Herrera, R.P.; Gimeno, M.C. Gold Catalyzed Multicomponent Reactions beyond A3 Coupling. Molecules 2018, 23, 2255. [Google Scholar] [CrossRef]
- Rossa, T.A.; Fantinel, M.; Bortoluzzi, A.J.; Sá, M.M. Multicomponent Synthesis of Structurally Diverse Imidazoles Featuring Azirines, Amines and Aldehydes. Eur. J. Org. Chem. 2018, 2018, 4171–4177. [Google Scholar] [CrossRef]
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent Advances in Multi-Component Reactions and Their Mechanistic Insights: A Triennium Review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Müller, T.J.J. Multi-Component Syntheses of Heterocycles by Transition-Metal Catalysis. Chem. Soc. Rev. 2007, 36, 1095–1108. [Google Scholar] [CrossRef]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.X.; Liang, Y.; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. [Google Scholar] [CrossRef]
- Strübing, D.; Neumann, H.; Hübner, S.; Klaus, S.; Beller, M. Straightforward Synthesis of Di-, Tri- and Tetracyclic Lactams via Catalytic Pauson–Khand and Alder–Ene Reactions of MCR Products. Tetrahedron 2005, 61, 11345–11354. [Google Scholar] [CrossRef]
- Sharma, U.K.; Ranjan, P.; Van der Eycken, E.V.; You, S.L. Sequential and Direct Multicomponent Reaction (MCR)-Based Dearomatization Strategies. Chem. Soc. Rev. 2020, 49, 8721–8748. [Google Scholar] [CrossRef]
- Pathare, R.S.; Ansari, A.J.; Verma, S.; Maurya, A.; Maurya, A.K.; Agnihotri, V.K.; Sharon, A.; Pardasani, R.T.; Sawant, D.M. Sequential Pd(0)/Fe(III) Catalyzed Azide–Isocyanide Coupling/Cyclization Reaction: One-Pot Synthesis of Aminotetrazoles. J. Org. Chem. 2018, 83, 9530–9537. [Google Scholar] [CrossRef]
- Ojeda, G.M.; Ranjan, P.; Fedoseev, P.; Amable, L.; Sharma, U.K.; Rivera, D.G.; Eycken, E.V.V.D. Combining the Ugi-Azide Multicomponent Reaction and Rhodium(III)-Catalyzed Annulation for the Synthesis of Tetrazole-Isoquinolone/Pyridone Hybrids. Beilstein J. Org. Chem. 2019, 15, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mgimpatsang, K.C.; Li, X.; Dömling, A. Isoquinolone-4-Carboxylic Acids by Ammonia-Ugi-4CR and Copper-Catalyzed Domino Reaction. J. Org. Chem. 2021, 86, 9771–9780. [Google Scholar] [CrossRef] [PubMed]
- Godineau, E.; Landais, Y. Radical and Radical–Ionic Multicomponent Processes. Chem. Eur. J. 2009, 15, 3044–3055. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Ravelli, D.; Protti, S.; Basso, A. Photoinduced Multicomponent Reactions. Angew. Chem. Int. Ed. 2016, 55, 15476–15484. [Google Scholar] [CrossRef] [PubMed]
- Bakulina, O.; Inyutina, A.; Dar’in, D.; Krasavin, M. Multicomponent Reactions Involving Diazo Reagents: A 5-Year Update. Molecules 2021, 26, 6563. [Google Scholar] [CrossRef] [PubMed]
- Vachan, B.S.; Karuppasamy, M.; Vinoth, P.; Vivek Kumar, S.; Perumal, S.; Sridharan, V.; Menéndez, J.C. Proline and Its Derivatives as Organocatalysts for Multi- Component Reactions in Aqueous Media: Synergic Pathways to the Green Synthesis of Heterocycles. Adv. Synth. Catal. 2020, 362, 87–110. [Google Scholar] [CrossRef]
- Guillena, G.; Ramón, D.J.; Yus, M. Organocatalytic Enantioselective Multicomponent Reactions (OEMCRs). Tetrahedron Asymmetry 2007, 18, 693–700. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Barbas, C.F., III. Towards Organo-Click Chemistry: Development of Organocatalytic Multicomponent Reactions Through Combinations of Aldol, Wittig, Knoevenagel, Michael, Diels–Alder and Huisgen Cycloaddition Reactions. Chem. Eur. J. 2004, 10, 5323–5331. [Google Scholar] [CrossRef]
- Wu, M.-Y.; He, W.-W.; Liu, X.-Y.; Tan, B. Asymmetric Construction of Spirooxindoles by Organocatalytic Multicomponent Reactions Using Diazooxindoles. Angew. Chem. Int. Ed. 2015, 54, 9409–9413. [Google Scholar] [CrossRef]
- Nasiriani, T.; Javanbakht, S.; Nazeri, M.T.; Farhid, H.; Khodkari, V.; Shaabani, A. Isocyanide-Based Multicomponent Reactions in Water: Advanced Green Tools for the Synthesis of Heterocyclic Compounds. Top Curr. Chem. (Z) 2022, 380, 50. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Lygin, A.V.; de Meijere, A. Isocyanides in the Synthesis of Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2010, 49, 9094–9124. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Mohammadkhani, L. Synthesis of Various N-Heterocycles Using the Four-Component Ugi Reaction. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 131, pp. 351–403. ISBN 0065-2725. [Google Scholar]
- Dömling, A. Innovations and Inventions: Why Was the Ugi Reaction Discovered Only 37 Years after the Passerini Reaction? J. Org. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Passerini, M.; Isonitriles, I.I. Compounds with Aldehydes or with Ketones and Monobasic Organic Acids. Gazz. Chim. Ital. 1921, 51, 181–189. [Google Scholar]
- Passerini, M.; Simone, L. Sopra Gli Isonitrili (I). Composto Del p-Isonitril-Azobenzolo Con Acetone Ed Acido Acetico. Gazz. Chim. Ital 1921, 51, 126–129. [Google Scholar]
- Ugi, I.; Dömling, A.; Hörl, W. Multicomponent Reactions in Organic Chemistry. Endeavour 1994, 18, 115–122. [Google Scholar] [CrossRef]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Shaaban, S.; Abdel-Wahab, B.F. Groebke–Blackburn–Bienaymé Multicomponent Reaction: Emerging Chemistry for Drug Discovery. Mol. Div. 2016, 20, 233–254. [Google Scholar] [CrossRef]
- Parenty, A.D.; Song, Y.-F.; Richmond, C.J.; Cronin, L. A General and Efficient Five-Step One-Pot Procedure Leading to Nitrogen-Bridgehead Heterocycles Containing an Imidazole Ring. Org. Lett. 2007, 9, 2253–2256. [Google Scholar] [CrossRef]
- Sisko, J.; Kassick, A.J.; Mellinger, M.; Filan, J.J.; Allen, A.; Olsen, M.A. An Investigation of Imidazole and Oxazole Syntheses Using Aryl-Substituted TosMIC Reagents1. J. Org. Chem. 2000, 65, 1516–1524. [Google Scholar] [CrossRef]
- Bon, R.S.; Hong, C.; Bouma, M.J.; Schmitz, R.F.; de Kanter, F.J.; Lutz, M.; Spek, A.L.; Orru, R.V. Novel Multicomponent Reaction for the Combinatorial Synthesis of 2-Imidazolines. Org. Lett. 2003, 5, 3759–3762. [Google Scholar] [CrossRef]
- Gulevich, A.V.; Zhdanko, A.G.; Orru, R.V.; Nenajdenko, V.G. Isocyanoacetate Derivatives: Synthesis, Reactivity, and Application. Chem. Rev. 2010, 110, 5235–5331. [Google Scholar] [CrossRef] [PubMed]
- Elders, N.; Ruijter, E.; de Kanter, F.J.J.; Groen, M.B.; Orru, R.V.A. Selective Formation of 2-Imidazolines and 2-Substituted Oxazoles by Using a Three-Component Reaction. Chem. Eur. J. 2008, 14, 4961–4973. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.K.Z.; Takada, S.C.S.; Suarez, P.A.Z.; Alves, M.B. Revisiting the Passerini Reaction under Eco-Friendly Reaction Conditions. Synlett 2006, 2006, 1539–1542. [Google Scholar] [CrossRef]
- Ugi, I. From Isocyanides via Four-component Condensations to Antibiotic Syntheses. Angew. Chem. Int. Ed. Eng. 1982, 21, 810–819. [Google Scholar] [CrossRef]
- Bock, H.; Ugi, I. Multicomponent Reactions. II. Stereoselective Synthesis of 1 (S)-camphor-2-cis-methylidene-isocyanide and Its Application in Passerini-and Ugi-reaction. J. Prakt. Chem. 1997, 339, 385–389. [Google Scholar] [CrossRef]
- Van Leusen, A.M.; Wildeman, J.; Oldenziel, O.H. Chemistry of Sulfonylmethyl Isocyanides. 12. Base-Induced Cycloaddition of Sulfonylmethyl Isocyanides to Carbon, Nitrogen Double Bonds. Synthesis of 1, 5-Disubstituted and 1, 4, 5-Trisubstituted Imidazoles from Aldimines and Imidoyl Chlorides. J. Org. Chem. 1977, 42, 1153–1159. [Google Scholar] [CrossRef]
- Groebke, K.; Weber, L.; Mehlin, F. Synthesis of Imidazo [1,2-a] Annulated Pyridines, Pyrazines and Pyrimidines by a Novel Three-Component Condensation. Synlett 1998, 1998, 661–663. [Google Scholar] [CrossRef]
- Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Parallel Synthesis of 3-Aminoimidazo [1, 2-a] Pyridines and Pyrazines by a New Three-Component Condensation. Tetrahedron Lett. 1998, 39, 3635–3638. [Google Scholar] [CrossRef]
- Bienayme, H.; Bouzid, K. A New Heterocyclic Multicomponent Reaction for the Combinatorial Synthesis of Fused 3-aminoimidazoles. Angew. Chem. Int. Ed. 1998, 37, 2234–2237. [Google Scholar] [CrossRef]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019, 7007–7049. [Google Scholar] [CrossRef]
- Rudick, J.G.; Shaabani, S.; Dömling, A. Isocyanide-Based Multicomponent Reactions; Frontiers Media SA: Lausanne, Switzerland, 2020; Volume 7, p. 918. ISBN 2296-2646. [Google Scholar]
- Sadjadi, S.; Heravi, M.M. Recent Application of Isocyanides in Synthesis of Heterocycles. Tetrahedron 2011, 67, 2707–2752. [Google Scholar] [CrossRef]
- Heravi, M.M.; Nazari, N. Isocyanide-Based Cascade Reactions in the Synthesis of Aza-Heterocycles: A Marriage of Convenience. Curr. Org. Chem. 2017, 21, 1440–1529. [Google Scholar] [CrossRef]
- Mohammadkhani, L.; Heravi, M.M. Synthesis of N-Heterocycles Containing 1,5-Disubstituted-1H-Tetrazole via Post-Ugi-Azide Reaction. Mol. Div. 2020, 24, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.; Heravi, M.M.; Nazari, N. Isocyanide-Based Multicomponent Reactions in the Synthesis of Heterocycles. RSC Adv. 2016, 6, 53203–53272. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Dehghani, M.; Ahmadi, T. Towards Click Chemistry: Multicomponent Reactions via Combinations of Name Reactions. Tetrahedron 2018, 74, 3391–3457. [Google Scholar] [CrossRef]
- Li, X.; Zarganes-Tzitzikas, T.; Kurpiewska, K.; Dömling, A. Amenamevir by Ugi-4CR. Green Chem. 2023, 25, 1322–1325. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Zheng, Q.; Patil, P.; Dömling, A. A Bifurcated Multicomponent Synthesis Approach to Polycyclic Quinazolinones. J. Org. Chem. 2022, 87, 13023–13033. [Google Scholar] [CrossRef]
- Zhang, B.; Kurpiewska, K.; Dömling, A. Highly Stereoselective Ugi/Pictet–Spengler Sequence. J. Org. Chem. 2022, 87, 7085–7096. [Google Scholar] [CrossRef]
- Sutanto, F.; Shaabani, S.; Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Patil, P.; Ghonchepour, E.; Dömling, A. Multicomponent Reaction–Derived Covalent Inhibitor Space. Sci. Adv. 2021, 7, eabd9307. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.; Boiarska, Z.; Butera, R.; Neochoritis, C.G.; Kurpiewska, K.; Kalinowska-Tłuscik, J.; Dömling, A. Diaminoimidazopyrimidines: Access via the Groebke–Blackburn–Bienaymé Reaction and Structural Data Mining. Eur. J. Org. Chem. 2020, 2020, 5601–5605. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Katsampoxaki-Hodgetts, K.; Dömling, A. Multicomponent Reactions: “Kinderleicht”. J. Chem. Educ. 2020, 97, 3739–3745. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, M.G.; Ali, A.M.; Plewka, J.; Surmiak, E.; Labuzek, B.; Neochoritis, C.G.; Atmaj, J.; Skalniak, L.; Zhang, R.; Holak, T.A.; et al. Multicomponent Peptide Stapling as a Diversity-Driven Tool for the Development of Inhibitors of Protein–Protein Interactions. Angew. Chem. Int. Ed. 2020, 59, 5235–5241. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Novotná, M.; Mitríková, T.; Wang, Z.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Dömling, A. Isocyanide-Based Multicomponent Reactions of Free Phenylboronic Acids. Eur. J. Org. Chem. 2019, 2019, 6132–6137. [Google Scholar] [CrossRef]
- Kunig, V.B.K.; Ehrt, C.; Dömling, A.; Brunschweiger, A. Isocyanide Multicomponent Reactions on Solid-Phase-Coupled DNA Oligonucleotides for Encoded Library Synthesis. Org. Lett. 2019, 21, 7238–7243. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Shaabani, S.; Dömling, A. Macrocycles: MCR Synthesis and Applications in Drug Discovery. Drug Disc. Today Technol. 2018, 29, 11–17. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Madhavachary, R.; Rossetti, A.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Ugi Multicomponent Reaction Based Synthesis of Medium-Sized Rings. Org. Lett. 2017, 19, 6176–6179. [Google Scholar] [CrossRef]
- Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G.C.; Zhu, J. To Each His Own: Isonitriles for All Flavors. Functionalized Isocyanides as Valuable Tools in Organic Synthesis. Chem. Soc. Rev. 2017, 46, 1295–1357. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-Mediated and Metal-Catalyzed Reactions of Isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.T.; Farhid, H.; Mohammadian, R.; Shaabani, A. Cyclic Imines in Ugi and Ugi-Type Reactions. ACS Comb. Sci. 2020, 22, 361–400. [Google Scholar] [CrossRef]
- Wang, H.; Xu, B. Recent Advances in Inert Bonds Activation with Isocyanides. Chin. J. Org. Chem. 2015, 35, 588–602. [Google Scholar] [CrossRef]
- Mazzoni, R.; Marchetti, F.; Cingolani, A.; Zanotti, V. Bond Forming Reactions Involving Isocyanides at Diiron Complexes. Inorganics 2019, 7, 25. [Google Scholar] [CrossRef]
- Altundas, B.; Marrazzo, J.-P.R.; Fleming, F.F. Metalated Isocyanides: Formation, Structure, and Reactivity. Org. Bio. Chem. 2020, 18, 6467–6482. [Google Scholar] [CrossRef] [PubMed]
- Bosica, G.; Abdilla, R. Recent Advances in Multicomponent Reactions Catalysed under Operationally Heterogeneous Conditions. Catalysts 2022, 12, 725. [Google Scholar] [CrossRef]
- Wang, Z.; Domling, A. Multicomponent Reactions in Medicinal Chemistry. In Multicomponent Reactions towards Heterocycles; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 91–137. ISBN 978-3-527-83243-9. [Google Scholar]
- Nazeri, M.T.; Nasiriani, T.; Farhid, H.; Javanbakht, S.; Bahri, F.; Shadi, M.; Shaabani, A. Sustainable Synthesis of Pseudopeptides via Isocyanide-Based Multicomponent Reactions in Water. ACS Sustain. Chem. Eng. 2022, 10, 8115–8134. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Van der Eycken, E.; Sharma, U.K. Multicomponent Reactions towards Heterocycles: Concepts and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; ISBN 978-3-527-34908-1. [Google Scholar]
- Nazeri, M.T.; Shaabani, A. Synthesis of Polysubstituted Pyrroles via Isocyanide-Based Multicomponent Reactions as an Efficient Synthesis Tool. New J. Chem. 2021, 45, 21967–22011. [Google Scholar] [CrossRef]
- Cankařová, N.; Krchňák, V. Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application. Int. J. Mol. Sci. 2020, 21, 9160. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Novikov, A.S.; Boyarskiy, V.P.; Kukushkin, V.Y. The Halogen Bond with Isocyano Carbon Reduces Isocyanide Odor. Nat. Commun. 2020, 11, 2921. [Google Scholar] [CrossRef]
- Azuaje, J.; Coelho, A.; Maatougui, A.E.; Blanco, J.M.; Sotelo, E. Supported P-Toluenesulfonic Acid as a Highly Robust and Eco-Friendly Isocyanide Scavenger. ACS Comb. Sci. 2011, 13, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.I.; Kigga, M.; Heravi, M.M. Multicomponent Reactions Based on In Situ Generated Isocyanides for the Construction of Heterocycles. Chem. Heterocycl. Compd. 2021, 57, 709–719. [Google Scholar] [CrossRef]
- Azuaje, J.; El Maatougui, A.; Pérez-Rubio, J.M.; Coelho, A.; Fernández, F.; Sotelo, E. Multicomponent Assembly of Diverse Pyrazin-2(1 H)-One Chemotypes. J. Org. Chem. 2013, 78, 4402–4409. [Google Scholar] [CrossRef]
- Azuaje, J.; Pérez-Rubio, J.M.; Yaziji, V.; El Maatougui, A.; González-Gomez, J.C.; Sánchez-Pedregal, V.M.; Navarro-Vázquez, A.; Masaguer, C.F.; Teijeira, M.; Sotelo, E. Integrated Ugi-Based Assembly of Functionally, Skeletally, and Stereochemically Diverse 1,4-Benzodiazepin-2-Ones. J. Org. Chem. 2015, 80, 1533–1549. [Google Scholar] [CrossRef]
- Hantzsch, A. Condensationsprodukte aus Aldehydammoniak und ketonartigen Verbindungen. Ber. Dtsch. Chem. Ges. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Biginelli, P. Ueber Aldehyduramide des Acetessigäthers. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319. [Google Scholar] [CrossRef]
- Azuaje, J.; Tubío, C.R.; Escalante, L.; Gómez, M.; Guitián, F.; Coelho, A.; Caamaño, O.; Gil, A.; Sotelo, E. An Efficient and Recyclable 3D Printed α-Al2O3 Catalyst for the Multicomponent Assembly of Bioactive Heterocycles. Appl. Catal. 2017, 530, 203–210. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Rocha, R.O.; Lapis, A.A.M. What Do We Know about the Ionic Liquid Effect in Catalyzed Multicomponent Reactions?: A Critical Review. Curr. Opin. Green Sustain. Chem. 2022, 35, 100608. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Eberlin, M.N.; Sherwood, J. Solvent Screening Is Not Solvent Effect: A Review on the Most Neglected Aspect of Multicomponent Reactions. Eur. J. Org. Chem. 2022, 2022, e202200172. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Construction and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation: A Green Approach. ChemistrySelect 2022, 7, e202104032. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, B.; Tao, L. Stepping Further from Coupling Tools: Development of Functional Polymers via the Biginelli Reaction. Molecules 2022, 27, 7886. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Murgia, I.; Giustiniano, M.; Pirali, T.; Tron, G.C. The 115 Years Old Multicomponent Bargellini Reaction: Perspectives and New Applications. Molecules 2021, 26, 558. [Google Scholar] [CrossRef] [PubMed]
- Grazia Martina, M.; Giannessi, L.; Radi, M. Multicomponent Synthesis of Purines and Pyrimidines: From the Origin of Life to New Sustainable Approaches for Drug-Discovery Applications. Eur. J. Org. Chem. 2023, 26, e202201288. [Google Scholar] [CrossRef]
- Gibson, S.E.; Mainolfi, N. The Intermolecular Pauson–Khand Reaction. Angew. Chem. Int. Ed. 2005, 44, 3022–3037. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, C.; Zheng, N.; Yang, Z.; Shi, L. Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020). Catalysts 2020, 10, 1199. [Google Scholar] [CrossRef]
- Román, R.; Mateu, N.; López, I.; Medio-Simon, M.; Fustero, S.; Barrio, P. Vinyl Fluorides: Competent Olefinic Counterparts in the Intramolecular Pauson–Khand Reaction. Org. Lett. 2019, 21, 2569–2573. [Google Scholar] [CrossRef]
- Jang, Y.; Lindsay, V.N.G. Synthesis of Cyclopentenones with Reverse Pauson–Khand Regiocontrol via Ni-Catalyzed C–C Activation of Cyclopropanone. Org. Lett. 2020, 22, 8872–8876. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sikora, K.; Timbs, O. Cancer 2025: Introduction. Expert Rev. Anticancer Ther. 2004, 4, S11–S12. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Griglio, A.; Torre, E.; Serafini, M.; Bianchi, A.; Schmid, R.; Coda Zabetta, G.; Massarotti, A.; Sorba, G.; Pirali, T.; Fallarini, S. A Multicomponent Approach in the Discovery of Indoleamine 2,3-Dioxygenase 1 Inhibitors: Synthesis, Biological Investigation and Docking Studies. Bioorg. Med. Chem. Lett. 2018, 28, 651–657. [Google Scholar] [CrossRef]
- Tojo, S.; Kohno, T.; Tanaka, T.; Kamioka, S.; Ota, Y.; Ishii, T.; Kamimoto, K.; Asano, S.; Isobe, Y. Crystal Structures and Structure–Activity Relationships of Imidazothiazole Derivatives as IDO1 Inhibitors. ACS Med. Chem. Lett. 2014, 5, 1119–1123. [Google Scholar] [CrossRef]
- Salah Ayoup, M.; Wahby, Y.; Abdel-Hamid, H.; Ramadan, E.S.; Teleb, M.; Abu-Serie, M.M.; Noby, A. Design, Synthesis and Biological Evaluation of Novel α-Acyloxy Carboxamides via Passerini Reaction as Caspase 3/7 Activators. Eur. J. Org. Chem. 2019, 168, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.; Mostoufi, A.; Kalantar, H.; Fereidoonnezhad, M. Synthesis and Biological Evaluations of Novel Pyrazinoic Acid Derivatives as Anticancer Agents. Med. Chem. Res. 2022, 31, 580–593. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Y. Teaching an Old Dog New Tricks: Drug Discovery by Repositioning Natural Products and Their Derivatives. Drug Discov. Today 2022, 27, 1936–1944. [Google Scholar] [CrossRef]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Braga, F.C.; Buenz, E.J.; Campana, P.R.V.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; et al. Future Directions for the Discovery of Natural Product-Derived Immunomodulating Drugs: An IUPHAR Positional Review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Wiemann, J.; Heller, L.; Csuk, R. An Access to a Library of Novel Triterpene Derivatives with a Promising Pharmacological Potential by Ugi and Passerini Multicomponent Reactions. Eur. J. Org. Chem. 2018, 150, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Ingold, M.; Colella, L.; Hernández, P.; Batthyány, C.; Tejedor, D.; Puerta, A.; García-Tellado, F.; Padrón, J.M.; Porcal, W.; López, G.V. A Focused Library of NO-Donor Compounds with Potent Antiproliferative Activity Based on Green Multicomponent Reactions. Chem. Med. Chem. 2019, 14, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Nazifi, M.; Mansourian, M.; Hosseinzadeh, L.; Shokoohinia, Y. Ugi Efficient Synthesis, Biological Evaluation and Molecular Docking of Coumarin-Quinoline Hybrids as Apoptotic Agents through Mitochondria-Related Pathways. Bioorg. Chem. 2019, 91, 103147. [Google Scholar] [CrossRef] [PubMed]
- Butera, R.; Ważyńska, M.; Magiera-Mularz, K.; Plewka, J.; Musielak, B.; Surmiak, E.; Sala, D.; Kitel, R.; de Bruyn, M.; Nijman, H.W.; et al. Design, Synthesis, and Biological Evaluation of Imidazopyridines as PD-1/PD-L1 Antagonists. ACS Med. Chem. Lett. 2021, 12, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, S.; Amato, J.; Capasso, D.; Di Gaetano, S.; Massarotti, A.; Piccolo, M.; Irace, C.; Tron, G.C.; Pagano, B.; Randazzo, A.; et al. Bio-Inspired Dual-Selective BCL-2/c-MYC G-Quadruplex Binders: Design, Synthesis, and Anticancer Activity of Drug-like Imidazo[2,1-i]Purine Derivatives. J. Med. Chem. 2020, 63, 2035–2050. [Google Scholar] [CrossRef]
- Xiong, J.; He, H.-T.; Yang, H.-Y.; Zeng, Z.-G.; Zhong, C.-R.; Shi, H.; Ouyang, M.-L.; Tao, Y.-Y.; Pang, Y.-L.; Zhang, Y.-H.; et al. Synthesis of 4-Tetrazolyl-Substituted 3,4-Dihydroquinazoline Derivatives with Anticancer Activity via a One-Pot Sequential Ugi-Azide/Palladium-Catalyzed Azide-Isocyanide Cross-Coupling/Cyclization Reaction. J. Org. Chem. 2022, 87, 9488–9496. [Google Scholar] [CrossRef]
- Nichugovskiy, A.; Maksimova, V.; Trapeznikova, E.; Eshtukova-Shcheglova, E.; Ivanov, I.; Yakubovskaya, M.; Kirsanov, K.; Cheshkov, D.; Tron, G.C.; Maslov, M. Synthesis of Novel Lipophilic Polyamines via Ugi Reaction and Evaluation of Their Anticancer Activity. Molecules 2022, 27, 6218. [Google Scholar] [CrossRef]
- El Maatougui, A.; Azuaje, J.; González-Gómez, M.; Miguez, G.; Crespo, A.; Carbajales, C.; Escalante, L.; García-Mera, X.; Gutiérrez-De-Terán, H.; Sotelo, E. Discovery of Potent and Highly Selective A2B Adenosine Receptor Antagonist Chemotypes. J. Med. Chem. 2016, 59, 1967–1983. [Google Scholar] [CrossRef]
- Crespo, A.; El Maatougui, A.; Biagini, P.; Azuaje, J.; Coelho, A.; Brea, J.; Loza, M.I.; Cadavid, M.I.; García-Mera, X.; Gutiérrez-de-Terán, H.; et al. Discovery of 3,4-Dihydropyrimidin-2(1H)-Ones As a Novel Class of Potent and Selective A2B Adenosine Receptor Antagonists. ACS Med. Chem. Lett. 2013, 4, 1031–1036. [Google Scholar] [CrossRef]
- Carbajales, C.; Azuaje, J.; Oliveira, A.; Loza, M.I.; Brea, J.; Cadavid, M.I.; Masaguer, C.F.; García-Mera, X.; Gutiérrez-de-Terán, H.; Sotelo, E. Enantiospecific Recognition at the A2B Adenosine Receptor by Alkyl 2-Cyanoimino-4-Substituted-6-Methyl-1,2,3,4-Tetrahydropyrimidine-5-Carboxylates. J. Med. Chem. 2017, 60, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.H.M.; Prieto-Díaz, R.; Neo, S.; Tong, L.; Chen, X.; Carannante, V.; Önfelt, B.; Hartman, J.; Haglund, F.; Majellaro, M.; et al. A2B Adenosine Receptor Antagonists Rescue Lymphocyte Activity in Adenosine-Producing Patient-Derived Cancer Models. J. Immunother. Cancer 2022, 10, e004592. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Díaz, R.; González-Gómez, M.; Fojo-Carballo, H.; Azuaje, J.; El Maatougui, A.; Majellaro, M.; Loza, M.I.; Brea, J.; Fernández-Dueñas, V.; Paleo, M.R.; et al. Exploring the Effect of Halogenation in a Series of Potent and Selective A2B Adenosine Receptor Antagonists. J. Med. Chem. 2023, 66, 890–912. [Google Scholar] [CrossRef] [PubMed]
- El-Malah, A.; Mahmoud, Z.; Hamed Salem, H.; Abdou, A.M.; Soliman, M.M.H.; Hassan, R.A. Design, Ecofriendly Synthesis, Anticancer and Antimicrobial Screening of Innovative Biginelli Dihydropyrimidines Using β-Aroylpyruvates as Synthons. Green Chem. Lett. Rev. 2021, 14, 221–233. [Google Scholar] [CrossRef]
- Li, Y.; Tan, T.; Zhao, Y.; Wei, Y.; Wang, D.; Chen, R.; Tao, L. Anticancer Polymers via the Biginelli Reaction. ACS Macro Lett. 2020, 9, 1249–1254. [Google Scholar] [CrossRef]
- Çağlar Yavuz, S.; Akkoç, S.; Türkmenoğlu, B.; Sarıpınar, E. Synthesis of Novel Heterocyclic Compounds Containing Pyrimidine Nucleus Using the Biginelli Reaction: Antiproliferative Activity and Docking Studies. J. Heterocycl. Chem. 2020, 57, 2615–2627. [Google Scholar] [CrossRef]
- Ashma, A.; Yahya, S.; Subramani, A.; Tamilarasan, R.; Sasikumar, G.; Askar Ali, S.J.; Al-Lohedan, H.A.; Karnan, M. Synthesis of New Nicotinic Acid Hydrazide Metal Complexes: Potential Anti-Cancer Drug, Supramolecular Architecture, Antibacterial Studies and Catalytic Properties. J. Mol. Struct. 2022, 1250, 131860. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Al-Mutabagani, L.A.; Gomha, S.M.; Ahmed, H.A. Three-Component Synthesis of Some New Coumarin Derivatives as Anticancer Agents. Front. Chem. 2022, 9, 1191. [Google Scholar] [CrossRef]
- Mirza, S.; Asma Naqvi, S.; Mohammed Khan, K.; Salar, U.; Choudhary, M.I. Facile Synthesis of Novel Substituted Aryl-Thiazole (SAT) Analogs via One-Pot Multi-Component Reaction as Potent Cytotoxic Agents against Cancer Cell Lines. Bioorg. Chem. 2017, 70, 133–143. [Google Scholar] [CrossRef]
- Yakaiah, S.; Sagar Vijay Kumar, P.; Baby Rani, P.; Durga Prasad, K.; Aparna, P. Design, Synthesis and Biological Evaluation of Novel Pyrazolo-Oxothiazolidine Derivatives as Antiproliferative Agents against Human Lung Cancer Cell Line A549. Bioorg. Med. Chem. Lett. 2018, 28, 630–636. [Google Scholar] [CrossRef]
- Foroutan, A.; Corazzari, M.; Grolla, A.A.; Colombo, G.; Travelli, C.; Genazzani, A.A.; Theeramunkong, S.; Galli, U.; Tron, G.C. Identification of Novel Aza-Analogs of TN-16 as Disrupters of Microtubule Dynamics through a Multicomponent Reaction. Eur. J. Med. Chem. 2023, 245, 114895. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).