Lupin, a Unique Legume That Is Nodulated by Multiple Microsymbionts: The Role of Horizontal Gene Transfer
Abstract
1. Introduction
2. Lupin, a Unique Legume in So Many Ways
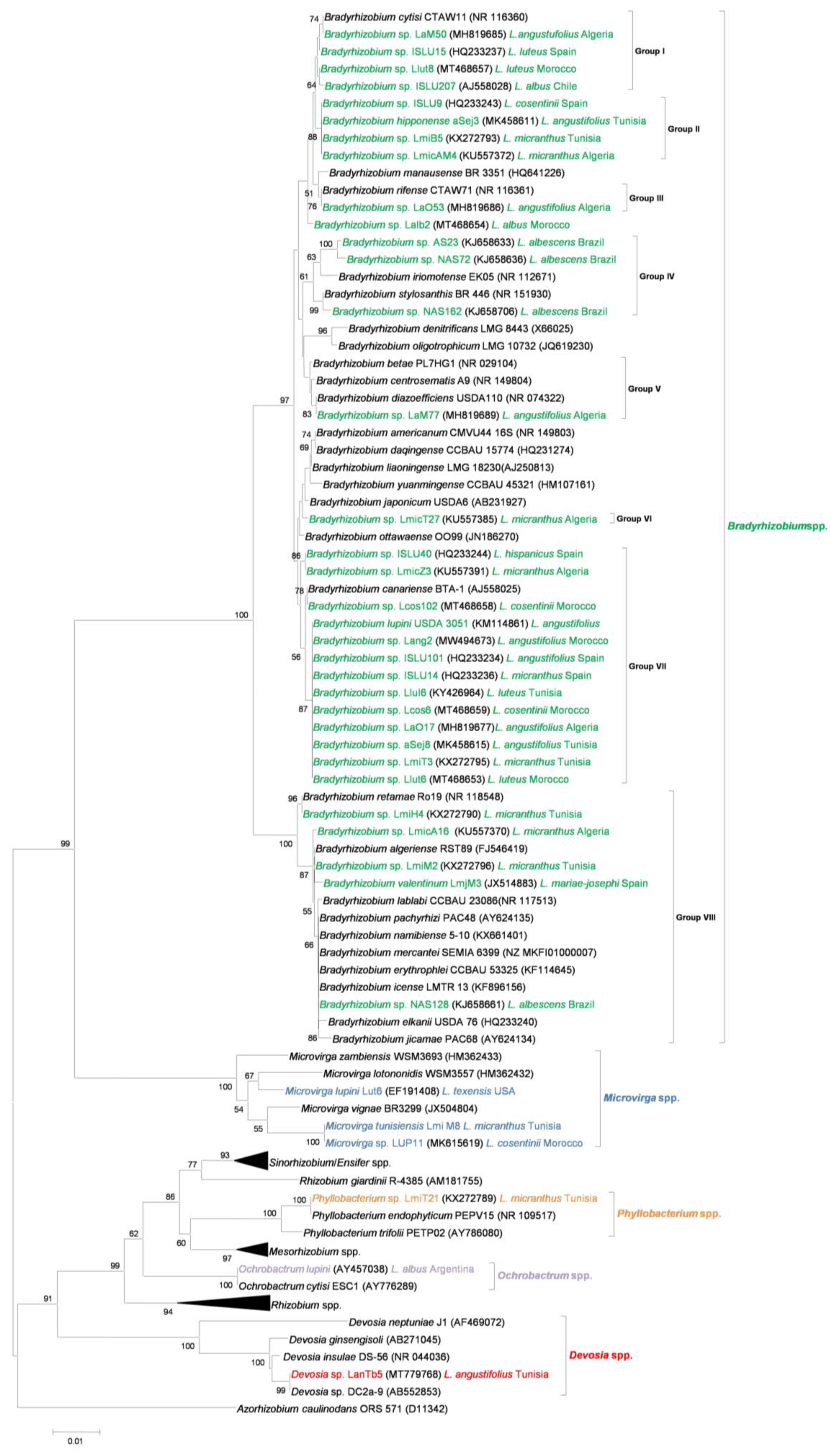
3. Rhizobia That Nodulate Lupins, Many More Than Initially Expected
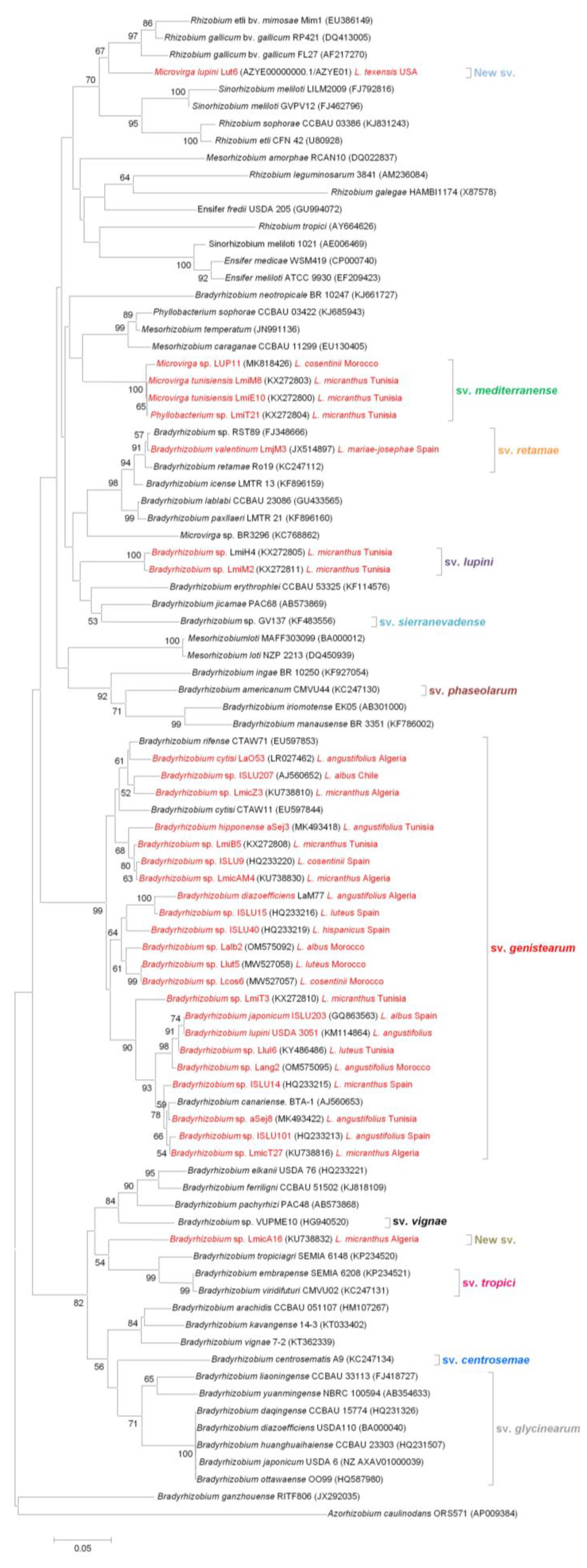
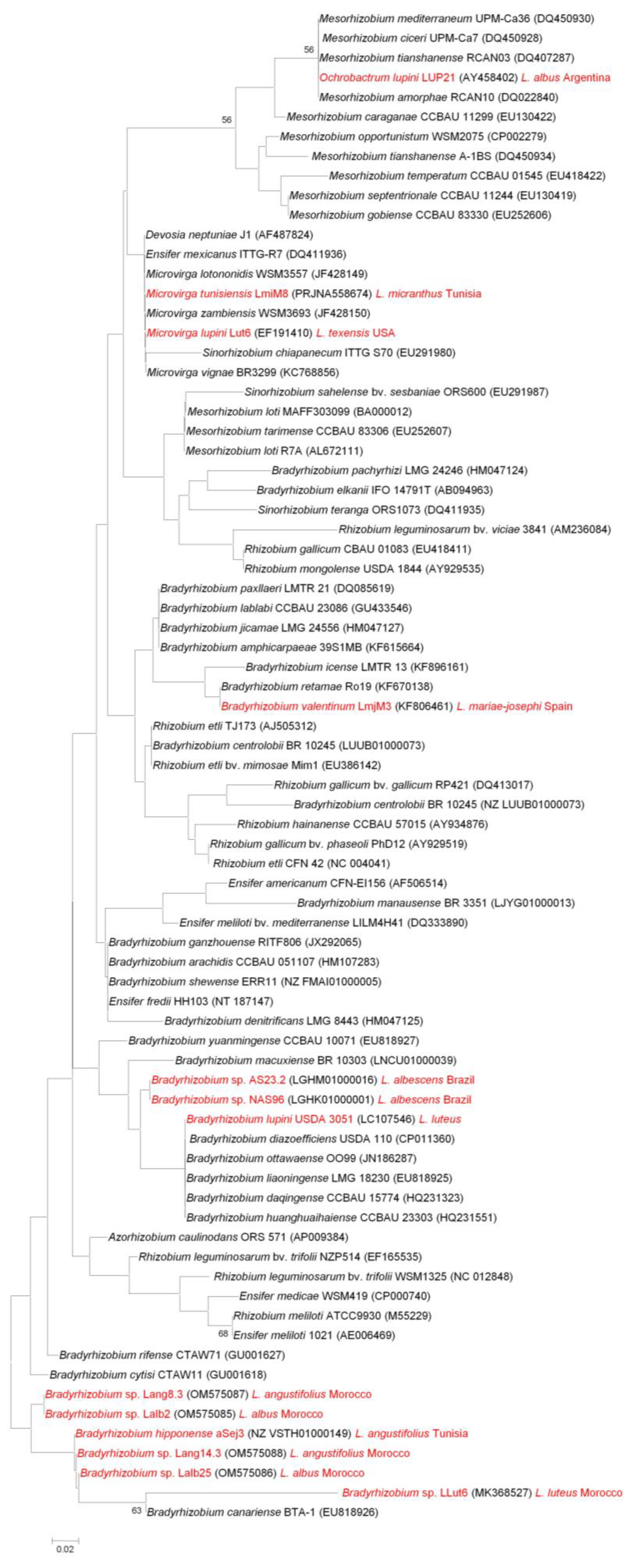
4. Horizontal Transfer of nod and nif Genes: On How to Become a Lupin Microsymbiont
4.1. nodC, an Essential Gene for Nodulation and Host Specificity
4.2. nifH, a Gene Required for Effective Nitrogen Fixation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sprent, J.I.; Ardley, J.K.; James, E.K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 2017, 215, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial associations with legumes. CRC Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Acinas, S.G.; Marcelino, L.A.; Klepac-Ceraj, V.; Polz, M.F. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J. Bacteriol. 2004, 186, 2629–2635. [Google Scholar] [CrossRef]
- Gornung, E. Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: A review of research. Cytogenet. Genome Res. 2013, 141, 90–102. [Google Scholar] [CrossRef]
- Idris, A.B.; Hassan, H.G.; Salaheldin Ali, M.A.; Eltaher, S.M.; Idris, L.B.; Altayb, H.N.; Abass, A.M.; Ibrahim, M.M.A.; Ibrahim, E.A.M.; Hassan, M.A. Molecular phylogenetic analysis of 16S rRNA sequences identified two lineages of Helicobacter pylori strains detected from different regions in Sudan suggestive of differential evolution. Int. J. Microbiol. 2020, 2020, 8825718. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: Pluses, perils, and pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Caputo, A.; Fournier, P.E.; Raoult, D. Genome and pan-genome analysis to classify emerging bacteria. Biol. Direct 2019, 14, 5. [Google Scholar] [CrossRef]
- Msaddak, A.; Rejili, M.; Durán, D.; Rey, L.; Palacios, J.M.; Imperial, J.; Ruiz-Argüeso, T.; Mars, M. Definition of two new symbiovars, sv. lupini and sv. mediterranense, within the genera Bradyrhizobium and Phyllobacterium efficiently nodulating Lupinus micranthus in Tunisia. Syst. Appl. Microbiol. 2018, 41, 487–493. [Google Scholar] [CrossRef]
- Rincón, A.; Arenal, F.; González, I.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Diversity of rhizobial bacteria isolated from nodules of the gypsophyte Ononis tridentata L. growing in Spanish soils. Microb. Ecol. 2008, 56, 223–233. [Google Scholar] [CrossRef]
- Fuks, G.; Elgart, M.; Amir, A.; Zeisel, A.; Turnbaugh, P.J.; Soen, Y.; Shental, N. Combining 16S rRNA gene variable regions enables high resolution microbial community profiling. Microbiome 2018, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Msaddak, A.; Rey, L.; Imperial, J.; Palacios, J.M.; Mars, M.; Pueyo, J.J. Phylogenetic analyses of rhizobia isolated from nodules of Lupinus angustifolius in Northern Tunisia reveal Devosia sp. as a new microsymbiont of lupin species. Agronomy 2021, 11, 1510. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tse, H.; Yuen, K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, A.; Abdelkhalek, A.; Sadowsky, M.J. Recent changes to the classification of symbiotic, nitrogen-fixing, legume-associating bacteria: A review. Symbiosis 2017, 71, 91–109. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W.M.; Elliott, G.N.; Bontemps, C.; De Los Santos, P.E.; Gross, E.; Dos Reis, F.B.; Janet, I.S.; et al. Legume-nodulating betaproteobacteria: Diversity, Host range, and future prospects. Mol. Plant-Microbe Interact. 2011, 24, 1276–1288. [Google Scholar] [CrossRef]
- Benhizia, Y.; Benhizia, H.; Benguedouar, A.; Muresu, R.; Giacomini, A.; Squartini, A. Gamma proteobacteria can nodulate legumes of the genus Hedysarum. Syst. Appl. Microbiol. 2004, 27, 462–468. [Google Scholar] [CrossRef]
- Shiraishi, A.; Matsushita, N.; Hougetsu, T. Nodulation in black locust by the gammaproteobacteria Pseudomonas sp. and the betaproteobacteria Burkholderia sp. Syst. Appl. Microbiol. 2010, 33, 269–274. [Google Scholar] [CrossRef]
- Esma, T.; Ahlem, R.; Boutheina, T.; Soumia, S.; Razika, G.; Hayet, B.; Yacine, B.; Ammar, B. Contribution to the study of the relationship between gammaproteobacteria and rhizobia in legume species of the genus Hedysarum. Legum. Res. 2020, 43, 872–877. [Google Scholar] [CrossRef]
- Schultze, M.; Kondorosi, A. Regulation of symbiotic root nodule development. Annu. Rev. Genet. 1998, 32, 33–57. [Google Scholar] [CrossRef]
- Andrews, M.; Andrews, M.E. Specificity in legume-rhizobia symbioses. Int. J. Mol. Sci. 2017, 18, 705. [Google Scholar] [CrossRef] [PubMed]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Laguerre, G.; Nour, S.M.; Macheret, V.; Sanjuan, J.; Drouin, P.; Amarger, N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 2001, 147, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Remigi, P.; Zhu, J.; Young, J.P.W.; Masson-Boivin, C. Symbiosis within symbiosis: Evolving nitrogen-fixing legume symbionts. Trends Microbiol. 2016, 24, 63–75. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Patrick, H.N.; Lowther, W.L.; Scott, D.B.; Ronson, C.W.; Sullivan, J.T.; Patrickt, H.N.; Lowthert, W.L.; Scottt, D.B.; Ronson, C.W. Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene transfer in the environment. Proc. Natl. Acad. Sci. USA 1995, 92, 8985–8989. [Google Scholar] [CrossRef]
- Rivas, R.; Velázquez, E.; Willems, A.; Vizcaíno, N.; Subba-Rao, N.S.; Mateos, P.F.; Gillis, M.; Dazzo, F.B.; Martínez-Molina, E. A new species of Devosia that forms a unique nitrogen-fixing root-nodule symbiosis with the aquatic legume Neptunia natans (L.f.) Druce. Appl. Environ. Microbiol. 2003, 68, 5217–5222. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Willems, A.; Abril, A.; Planchuelo, A.M.; Rivas, R.; Ludeña, D.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, E. Nodulation of Lupinus albus by strains of Ochrobactrum lupini sp. nov. Appl. Environ. Microbiol. 2005, 71, 1318–1327. [Google Scholar] [CrossRef]
- Lee, I.P.A.; Eldakar, O.T.; Gogarten, J.P.; Andam, C.P. Bacterial cooperation through horizontal gene transfer. Trends Ecol. Evol. 2022, 37, 223–232. [Google Scholar] [CrossRef]
- Andrews, M.; De Meyer, S.; James, E.K.; Stępkowski, T.; Hodge, S.; Simon, M.F.; Young, J.P.W. Horizontal Transfer of symbiosis genes within and between rhizobial genera: Occurrence and importance. Genes 2018, 9, 321. [Google Scholar] [CrossRef]
- Diao, X.; Freeling, M.; Lisch, D. Horizontal transfer of a plant transposon. PLoS Biol. 2006, 4, e5. [Google Scholar] [CrossRef]
- Ivancevic, A.M.; Kortschak, R.D.; Bertozzi, T.; Adelson, D.L. Horizontal transfer of BovB and L1 retrotransposons in eukaryotes. Genome Biol. 2018, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Feschotte, C. Horizontal acquisition of transposable elements and viral sequences: Patterns and consequences. Curr. Opin. Genet. Dev. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Caneschi, W.L.; Sanchez, A.B.; Felestrino, É.B.; de Carvalho Lemes, C.G.; Cordeiro, I.F.; Fonseca, N.P.; Villa, M.M.; Vieira, I.T.; Moraes, L.Â.G.; de Almeida Barbosa Assis, R.; et al. Serratia liquefaciens FG3 isolated from a metallophyte plant sheds light on the evolution and mechanisms of adaptive traits in extreme environments. Sci. Rep. 2019, 9, 18006. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, S.; Kuzmanović, N.; Patz, S.; Lohwasser, U.; Bunk, B.; Spröer, C.; Lorenz, M.; Elhady, A.; Frühling, A.; Neumann-Schaal, M.; et al. Two new Rhizobiales species isolated from root nodules of common sainfoin (Onobrychis viciifolia) show different plant colonisation strategies. Microbiol. Spec. 2022, 10, e0109922. [Google Scholar] [CrossRef] [PubMed]
- Burmølle, M.; Hansen, L.H.; Sørensen, S.J. Establishment and early succession of a multispecies biofilm composed of soil bacteria. Microb. Ecol. 2007, 54, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.C. Family III. Rhizobiaceae Conn. In Bergey’s Manual of Systematic Bacteriology; Krieg, N.R., Holt, J.G., Eds.; Williams & Wilkins: Baltimore, ND, USA, 1938; Volume 1, pp. 234–254. [Google Scholar]
- Rogel, M.A.; Ormeño-Orrillo, E.; Martinez Romero, E. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 2011, 34, 96–104. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Durán, D.; Boulila, F.; Ahnia, H.; Boulila, A.; Temprano, F.; Palacios, J.M.; Imperial, J.; Ruiz-Argüeso, T.; Rey, L. Diversity of Bradyrhizobium strains nodulating Lupinus micranthus on both sides of the Western Mediterranean: Algeria and Spain. Syst. Appl. Microbiol. 2016, 39, 266–274. [Google Scholar] [CrossRef]
- Sánchez-Cañizares, C.; Rey, L.; Durán, D.; Temprano, F.; Sánchez-Jiménez, P.; Navarro, A.; Polajnar, M.; Imperial, J.; Ruiz-Argüeso, T. Endosymbiotic bacteria nodulating a new endemic lupine Lupinus mariae-josephi from alkaline soils in Eastern Spain represent a new lineage within the Bradyrhizobium genus. Syst. Appl. Microbiol. 2011, 34, 207–215. [Google Scholar] [CrossRef]
- Stępkowski, T.; Banasiewicz, J.; Granada, C.E.; Andrews, M.; Passaglia, L.M.P. Phylogeny and phylogeography of rhizobial symbionts nodulating legumes of the tribe Genisteae. Genes 2018, 9, 163. [Google Scholar] [CrossRef]
- Valverde, A.; Velázquez, E.; Fernández-Santos, F.; Vizcaíno, N.; Mateos, P.F.; Martínez-Molina, E.; Igual, J.M.; Willems, A. Phyllobacterium trifolii sp. nov., nodulating Trifolium and Lupinus in Spanish soils. Int. J. Syst. Evol. Microbiol. 2005, 55, 1985–1989. [Google Scholar] [CrossRef]
- Msaddak, A.; Rejili, M.; Durán, D.; Rey, L.; Imperial, J.; Palacios, J.M.; Ruiz-Argüeso, T.; Mars, M. Members of Microvirga and Bradyrhizobium genera are native endosymbiotic bacteria nodulating Lupinus luteus in Northern Tunisian soils. FEMS Microbiol. Ecol. 2017, 93, fix068. [Google Scholar] [CrossRef] [PubMed]
- Tounsi-Hammami, S.; Le Roux, C.; Dhane-Fitouri, S.; De Lajudie, P.; Duponnois, R.; Ben Jeddi, F. Genetic diversity of rhizobia associated with root nodules of white lupin (Lupinus albus L.) in Tunisian calcareous soils. Syst. Appl. Microbiol. 2019, 42, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Missbah El Idrissi, M.; Lamin, H.; ElFaik, S.; Tortosa, G.; Peix, A.; Bedmar, E.J.; Abdelmoumen, H. Microvirga sp. symbiovar mediterranense nodulates Lupinus cosentinii grown wild in Morocco. J. Appl. Microbiol. 2020, 128, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Eastwood, R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proc. Natl. Acad. Sci. USA 2006, 103, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Aïnouche, A.K.; Bayer, R.J. Phylogenetic relationships in Lupinus (Fabaceae: Papilionoideae) based on internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. Am. J. Bot. 1999, 86, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Ohadoma, S.C.; Akah, P.A.; Okolo, C.E. Isolation and characterization of flavonol glycosides from leaves extract of Lupinus arboreus Sims. Pharm. Biosci. J. 2016, 4, 6–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, W.; Wang, P.; Grant, G.; Li, S. Flavonoids from Lupinus texensis and their free radical scavenging activity. Nat. Prod. Res. 2011, 25, 1641–1649. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 2009, 5, 31. [Google Scholar] [CrossRef]
- Ishaq, A.R.; El-Nashar, H.A.S.; Younis, T.; Mangat, M.A.; Shahzadi, M.; Ul Haq, A.S.; El-Shazly, M. Genus Lupinus (Fabaceae): A review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol. 2022, 74, 1700–1717. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Sachdev, D.O.; Gosavi, D.D.; Salwe, K.J. Evaluation of antidiabetic, antioxidant effect and safety profile of gomutra ark in Wistar albino rats. Anc. Sci. Life 2012, 31, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Confortin, T.C.; Todero, I.; Soares, J.F.; Luft, L.; Brun, T.; Rabuske, J.E.; Nogueira, C.U.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extracts from Lupinus albescens: Antioxidant power and antifungal activity in vitro against phytopathogenic fungi. Environ. Technol. 2019, 40, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Hanania, M.; Radwan, S.; Odeh, S.A.; Qumber, A. Determination of minerals, total phenolic content, flavonoids, antioxidants and antimicrobial activities of ethanolic extract of sweet Lupinus angustifolius of Palestine. Eur. J. Med. Plants 2019, 28, 1–6. [Google Scholar] [CrossRef]
- Fernández-Pascual, M.; Pueyo, J.J.; Felipe, M.R.; Golvano, M.P.; Lucas, M.M. Singular features of the Bradyrhizobium-Lupinus symbiosis. Dyn. Soil Dyn. Plant 2007, 1, 1–16. [Google Scholar]
- Coba de la Peña, T.; Pueyo, J.J. Legumes in the reclamation of marginal soils, from cultivar and inoculant selection to transgenic approaches. Agron. Sustain. Dev. 2012, 32, 65–91. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Fajardo, S.; Fernández-Pascual, M.; Lucas, M.M.; Pueyo, J.J. Nodulated white lupin plants growing in contaminated soils accumulate unusually high mercury concentrations in their nodules, roots and especially cluster roots. Horticulturae 2021, 7, 302. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Ruiz-Díez, B.; Fajardo, S.; López-Berdonces, M.A.; Higueras, P.L.; Fernández-Pascual, M. Lupinus albus plants acquire mercury tolerance when inoculated with an Hg-resistant Bradyrhizobium strain. Plant Physiol. Biochem. 2013, 73, 168–175. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Lucas, M.M.; Pueyo, J.J. Adaptive mechanisms make lupin a choice crop for acidic soils affected by aluminum toxicity. Front. Plant Sci. 2022, 12, 810692. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Quiñones, M.A.; Coba de la Peña, T.; Fedorova, E.E.; Lucas, M.M. Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Front. Plant Sci. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Gladstones, J.S. Distribution, origin, taxonomy, history and importance. In Lupinus as Crop Plants: Biology, Production and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Cambridge, UK, 1998; pp. 1–37. [Google Scholar]
- Sprent, J.I.; Ardley, J.K.; James, E.K. From North to South: A latitudinal look at legume nodulation processes. S. Afr. J. Bot. 2013, 89, 31–41. [Google Scholar] [CrossRef]
- Corby, H.D.L. Types of rhizobial nodules and their distribution among the Leguminosae. Kirkia 1988, 13, 53–123. [Google Scholar]
- Ardley, J.K.; Reeve, W.G.; O’Hara, G.W.; Yates, R.J.; Dilworth, M.J.; Howieson, J.G. Nodule morphology, symbiotic specificity and association with unusual rhizobia are distinguishing features of the genus Listia within the Southern African crotalarioid clade Lotononis s.l. Ann. Bot. 2013, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- González-Sama, A.; Lucas, M.M.; De Felipe, M.R.; Pueyo, J.J. An unusual infection mechanism and nodule morphogenesis in white lupin (Lupinus albus). New Phytol. 2004, 163, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, E.E.; De Felipe, M.R.; Pueyo, J.J.; Lucas, M.M. Conformation of cytoskeletal elements during the division of infected Lupinus albus L. nodule cells. J. Exp. Bot. 2007, 58, 2225–2236. [Google Scholar] [CrossRef]
- Scholla, M.H.; Moorefield, J.A.; Elkan, G.H. DNA homology between species of the rhizobia. Syst. Appl. Microbiol. 1990, 13, 288–294. [Google Scholar] [CrossRef]
- Auling, G.; Busse, J.; Hahn, M.; Hennecke, H.; Kroppenstedt, R.-M.; Probst, A.; Stackebrandt, E. Phylogenetic heterogeneity and chemotaxonomic properties of certain gram-negative aerobic carboxydobacteria. Syst. Appl. Microbiol. 1988, 10, 264–272. [Google Scholar] [CrossRef]
- Mellal, H.; Yacine, B.; Boukaous, L.; Khouni, S.; Benguedouar, A.; Castellano-Hinojosa, A.; Bedmar, E.J. Phylogenetic diversity of Bradyrhizobium strains isolated from root nodules of Lupinus angustifolius grown wild in the North East of Algeria. Syst. Appl. Microbiol. 2019, 42, 397–402. [Google Scholar] [CrossRef]
- Msaddak, A.; Durán, D.; Reijili, M.; Mars, M.; Ruiz-Argüeso, T.; Imperial, J.; Palacios, J.M.; Rey, L. Diverse bacteria affiliated with the genera Microvirga, Phyllobacterium, and Bradyrhizobium nodulate Lupinus micranthus growing in soils of Northern Tunisia. Appl. Environ. Microbiol. 2017, 83, e02820-16. [Google Scholar] [CrossRef]
- Jarabo-Lorenzo, A.; Pérez-Galdona, R.; Donate-Correa, J.; Rivas, R.; Velázquez, E.; Hernández, M.; Temprano, F.; Martínez-Molina, E.; Ruiz-Argüeso, T.; León-Barrios, M. Genetic diversity of bradyrhizobial populations from diverse geographic origins that nodulate Lupinus spp. and Ornithopus spp. Syst. Appl. Microbiol. 2003, 26, 611–623. [Google Scholar] [CrossRef]
- Vinuesa, P.; León-Barrios, M.; Silva, C.; Willems, A.; Jarabo-Lorenzo, A.; Pérez-Galdona, R.; Werner, D.; Martínez-Romero, E. Bradyrhizobium canariense sp. nov., an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int. J. Syst. Evol. Microbiol. 2005, 55, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Stȩpkowski, T.; Hughes, C.E.; Law, I.J.; Markiewicz, Ł.; Gurda, D.; Chlebicka, A.; Moulin, L. Diversification of lupine Bradyrhizobium strains: Evidence from nodulation gene trees. Appl. Environ. Microbiol. 2007, 73, 3254–3264. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, E.; Valverde, A.; Rivas, R.; Gomis, V.; Peix, A.; Gantois, I.; Igual, J.M.; León-Barrios, M.; Willems, A.; Mateos, P.F.; et al. Strains nodulating Lupinus albus on different continents belong to several new chromosomal and symbiotic lineages within Bradyrhizobium. Antonie van Leeuwenhoek 2010, 97, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Durán, D.; Rey, L.; Sánchez-Cañizares, C.; Navarro, A.; Imperial, J.; Ruiz-Argueso, T. Genetic diversity of indigenous rhizobial symbionts of the Lupinus mariae-josephae endemism from alkaline-limed soils within its area of distribution in Eastern Spain. Syst. Appl. Microbiol. 2013, 36, 128–136. [Google Scholar] [CrossRef]
- Granada, C.E.; Beneduzi, A.; Lisboa, B.B.; Turchetto-Zolet, A.C.; Vargas, L.K.; Passaglia, L.M.P. Multilocus sequence analysis reveals taxonomic differences among Bradyrhizobium sp. symbionts of Lupinus albescens Plants growing in arenized and non-arenized areas. Syst. Appl. Microbiol. 2015, 38, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rejili, M.; Off, K.; Brachmann, A.; Marín, M. Bradyrhizobium hipponense sp. nov., isolated from Lupinus angustifolius growing in the Northern region of Tunisia. Int. J. Syst. Evol. Microbiol. 2020, 70, 5539–5550. [Google Scholar] [CrossRef] [PubMed]
- Lamrabet, M.; ElFaik, S.; Laadraoui, C.; Bouhnik, O.; Lamin, H.; Alami, S.; Abdelmoumen, H.; Bedmar, E.J.; El Idrissi, M.M. Phylogenetic and symbiotic diversity of Lupinus albus and L. angustifolius microsymbionts in the Maamora Forest, Morocco. Syst. Appl. Microbiol. 2022, 45, 126338. [Google Scholar] [CrossRef]
- Missbah El Idrissi, M.; Bouhnik, O.; ElFaik, S.; Alami, S.; Lamin, H.; Bedmar, E.J.; Abdelmoumen, H. Characterization of Bradyrhizobium spp. nodulating Lupinus cosentinii and L. luteus microsymbionts in Morocco. Front. Agron. 2021, 3, 661295. [Google Scholar] [CrossRef]
- Pascual, H. Lupinus mariae-josephi (Fabaceae), nueva y sorprendente especie descubierta en España. An. Jard. Bot. Madr. 2004, 61, 69–72. [Google Scholar] [CrossRef]
- Navarro Peris, A.; Fos Martín, S.; Ferrando Pardo, I.; Laguna Lumbreras, E. Localización del endemismo aparentemente extinto Lupinus mariae-josephi. Flora Montiberica 2006, 33, 59–63. [Google Scholar]
- Durán, D.; Rey, L.; Navarro, A.; Busquets, A.; Imperial, J.; Ruiz-Argüeso, T. Bradyrhizobium valentinum sp. nov., isolated from effective nodules of Lupinus mariae-josephae, a lupine endemic of basic-lime soils in Eastern Spain. Syst. Appl. Microbiol. 2014, 37, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ardley, J.K.; Parker, M.A.; De Meyer, S.E.; Trengove, R.D.; O’Hara, G.W.; Reeve, W.G.; Yates, R.J.; Dilworth, M.J.; Willems, A.; Howieson, J.G. Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int. J. Syst. Evol. Microbiol. 2012, 62, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Beligala, D.H.; Michaels, H.J.; Devries, M.; Phuntumart, V. Multilocus sequence analysis of root nodule bacteria associated with Lupinus spp. and Glycine max. Adv. Microbiol. 2017, 07, 790–812. [Google Scholar] [CrossRef]
- Ferchichi, N.; Toukabri, W.; Vrhovsek, U.; Angeli, A.; Masuero, D.; Mhamdi, R.; Trabelsi, D. Inoculation of Lupinus albus with the nodule-endophyte Paenibacillus glycanilyticus LJ121 improves grain nutritional quality. Arch. Microbiol. 2020, 202, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Carro, L.; Flores-Félix, J.D.; Ramírez-Bahena, M.H.; García-Fraile, P.; Martínez-Hidalgo, P.; Igual, J.M.; Tejedor, C.; Peix, A.; Velázquez, E. Paenibacillus lupini sp. nov., isolated from nodules of Lupinus albus. Int. J. Syst. Evol. Microbiol. 2014, 64, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Kępczyńska, E.; Karczyński, P. Medicago truncatula root developmental changes by growth-promoting microbes isolated from Fabaceae, growing on organic farms, involve cell cycle changes and WOX5 gene expression. Planta 2020, 251, 25. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, L.H.; Moreno Díaz, P.; Jiménez Dávalos, J. Characterization and evaluation of the PGPR potential of the microflora associated with tarwi (Lupinus mutabilis Sweet). Ecol. Appl. 2020, 19, 65–76. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Alonso-Vega, P.; Rodríguez, R.; Carro, L.; Cerda, E.; Alonso, P.; Martínez-Molina, E. The genus Micromonospora is widespread in legume root nodules: The example of Lupinus angustifolius. ISME J. 2010, 4, 1265–1281. [Google Scholar] [CrossRef]
- Carro, L.; Pujic, P.; Trujillo, M.E.; Normand, P. Micromonospora is a normal occupant of actinorhizal nodules. J. Biosci. 2013, 38, 685–693. [Google Scholar] [CrossRef]
- Muresu, R.; Polone, E.; Sulas, L.; Baldan, B.; Tondello, A.; Delogu, G.; Cappuccinelli, P.; Alberghini, S.; Benhizia, Y.; Benhizia, H.; et al. Coexistence of predominantly nonculturable rhizobia with diverse, endophytic bacterial taxa within nodules of wild legumes. FEMS Microbiol. Ecol. 2008, 63, 383–400. [Google Scholar] [CrossRef]
- Zakhia, F.; Jeder, H.; Willems, A.; Gillis, M.; Dreyfus, B.; De Lajudie, P. Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microb. Ecol. 2006, 51, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.I. Legume Nodulation. A Global Perspective; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar] [CrossRef]
- Wernegreen, J.J.; Riley, M.A. Comparison of the evolutionary dynamics of symbiotic and housekeeping loci: A case for the genetic coherence of rhizobial lineages. Mol. Biol. Evol. 1999, 16, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, O.M.; Collavino, M.M.; Mancini, U. Nodulation competitiveness and diversification of symbiosis genes in common beans from the American centers of domestication. Sci. Rep. 2022, 12, 4591. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Distribution and correlation between phylogeny and functional traits of cowpea (Vigna unguiculata L. Walp.)-nodulating microsymbionts from Ghana and South Africa. Sci. Rep. 2018, 8, 18006. [Google Scholar] [CrossRef]
- Drew, G.C.; Stevens, E.J.; King, K.C. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 2021, 19, 623–638. [Google Scholar] [CrossRef]
- Watanabe, T.; Horiike, T. The evolution of molybdenum dependent nitrogenase in cyanobacteria. Biology 2021, 10, 329. [Google Scholar] [CrossRef]
- Lau, M.C.Y.; Cameron, C.; Magnabosco, C.; Brown, C.T.; Schilkey, F.; Grim, S.; Hendrickson, S.; Pullin, M.; Lollar, B.S.; van Heerden, E.; et al. Phylogeny and phylogeography of functional genes shared among seven terrestrial subsurface metagenomes reveal N-cycling and microbial evolutionary relationships. Front. Microbiol. 2014, 5, 531. [Google Scholar] [CrossRef]
- Barny, M.A.; Schoonejans, E.; Economou, A.; Johnston, A.W.B.; Downie, J.A. The C-terminal domain of the Rhizobium leguminosarum chitin synthase NodC is important for function and determines the orientation of the N-terminal region in the inner membrane. Mol. Microbiol. 1996, 19, 443–453. [Google Scholar] [CrossRef]
- Guerrouj, K.; Ruíz-Díez, B.; Chahboune, R.; Ramírez-Bahena, M.-H.; Abdelmoumen, H.; Quiñones, M.A.; El Idrissi, M.M.; Velázquez, E.; Fernández-Pascual, M.; Bedmar, E.J.; et al. Definition of a novel symbiovar (sv. retamae) within Bradyrhizobium retamae sp. nov., nodulating Retama sphaerocarpa and Retama monosperma. Syst. Appl. Microbiol. 2013, 36, 218–223. [Google Scholar] [CrossRef]
- Bejarano, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Peix, A. Vigna unguiculata is nodulated in Spain by endosymbionts of Genisteae legumes and by a new symbiovar (vignae) of the genus Bradyrhizobium. Syst. Appl. Microbiol. 2014, 37, 533–540. [Google Scholar] [CrossRef]
- Cobo-Díaz, J.F.; Martínez-Hidalgo, P.; Fernández-González, A.J.; Martínez-Molina, E.; Toro, N.; Velázquez, E.; Fernández-López, M. The endemic Genista versicolor from Sierra Nevada National Park in Spain is nodulated by putative new Bradyrhizobium species and a novel symbiovar (sierranevadense). Syst. Appl. Microbiol. 2014, 37, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bahena, M.H.; Flores-Félix, J.D.; Chahboune, R.; Toro, M.; Velázquez, E.; Peix, A. Bradyrhizobium centrosemae (symbiovar centrosemae) sp. nov., Bradyrhizobium americanum (symbiovar phaseolarum) sp. nov. and a new symbiovar (tropici) of Bradyrhizobium viridifuturi establish symbiosis with Centrosema species native to America. Syst. Appl. Microbiol. 2016, 39, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.S.; Peters, J.W. New insights into the evolutionary history of biological nitrogen fixation. Front. Microbiol. 2013, 4, 201. [Google Scholar] [CrossRef] [PubMed]
- Banasiewicz, J.; Granada, C.E.; Lisboa, B.B.; Grzesiuk, M.; Matuśkiewicz, W.; Bałka, M.; Schlindwein, G.; Vargas, L.K.; Passaglia, L.M.P.; Stępkowski, T. Diversity and phylogenetic affinities of Bradyrhizobium isolates from Pampa and Atlantic forest biomes. Syst. Appl. Microbiol. 2021, 44, 126203. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, F.G.; Menna, P.; Batista, J.S.D.S.; Hungria, M. Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inoculant strain to indigenous diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian savannah soil. Appl. Environ. Microbiol. 2007, 73, 2635–2643. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, E.T.; Chen, W.F.; Chen, W.X. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang Province of China. Soil Biol. Biochem. 2008, 40, 238–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msaddak, A.; Mars, M.; Quiñones, M.A.; Lucas, M.M.; Pueyo, J.J. Lupin, a Unique Legume That Is Nodulated by Multiple Microsymbionts: The Role of Horizontal Gene Transfer. Int. J. Mol. Sci. 2023, 24, 6496. https://doi.org/10.3390/ijms24076496
Msaddak A, Mars M, Quiñones MA, Lucas MM, Pueyo JJ. Lupin, a Unique Legume That Is Nodulated by Multiple Microsymbionts: The Role of Horizontal Gene Transfer. International Journal of Molecular Sciences. 2023; 24(7):6496. https://doi.org/10.3390/ijms24076496
Chicago/Turabian StyleMsaddak, Abdelhakim, Mohamed Mars, Miguel A. Quiñones, M. Mercedes Lucas, and José J. Pueyo. 2023. "Lupin, a Unique Legume That Is Nodulated by Multiple Microsymbionts: The Role of Horizontal Gene Transfer" International Journal of Molecular Sciences 24, no. 7: 6496. https://doi.org/10.3390/ijms24076496
APA StyleMsaddak, A., Mars, M., Quiñones, M. A., Lucas, M. M., & Pueyo, J. J. (2023). Lupin, a Unique Legume That Is Nodulated by Multiple Microsymbionts: The Role of Horizontal Gene Transfer. International Journal of Molecular Sciences, 24(7), 6496. https://doi.org/10.3390/ijms24076496







