Roles of TGF-β1 in Viral Infection during Pregnancy: Research Update and Perspectives
Abstract
1. Introduction
- Method:
2. Trophoblasts and the Placenta Villi in Pregnancy
3. Viral Infections in Pregnancy
3.1. RuV
3.2. Human Cytomegalovirus
3.3. HIV
3.4. HBV
3.5. HSV
3.6. IAV
3.7. ZIKV
3.8. SARS-CoV-2
4. TGF-β1 and Its Essential Roles in Human Body Development and Reproductive Tract
5. Roles of TGF β in Viral Infection at the Non-Maternal–Fetal Interface
5.1. RuV
5.2. HCMV
5.3. HIV
5.4. HBV
5.5. HSV
5.6. IAV
5.7. ZIKV
5.8. SARS-CoV-2
6. TGF-β1 and Viral Infection at the Maternal–Fetal Interface
6.1. HCMV
6.2. HIV
6.3. HBV
6.4. ZIKV
7. The Smad Pathway and Promising Future Approaches
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TGF-β1 | Transforming growth factor-beta 1 |
| CRS | Congenital rubella syndrome |
| CZS | Congenital Zika syndrome |
| MTCT | Mother-to-child transmission |
| RuV | Rubella virus |
| HCMV | Human cytomegalovirus |
| HIV | Human immunodeficiency virus |
| HBV | Hepatitis B virus |
| HSV | Herpes simplex virus |
| IAV | Influenza A virus |
| ZIKV | Zika virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SIV | Simian immunodeficiency virus |
| STB | Syncytiotrophoblasts |
| CTB | Cytotrophoblasts |
| EVT | Extravillous trophoblasts |
| MOI | Multiplicity of infection |
| HBx | Hepatitis B virus X protein |
| HSP | Heat-shock proteins |
| GRP | Glucose-regulated protein |
| COVID-19 | Coronavirus disease of 2019 |
| ToRCH | Toxoplasma, others, rubella, cytomegalovirus, herpes simplex virus |
References
- Mor, G. Introduction to the immunology of pregnancy. Immunol. Rev. 2022, 308, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Boss, A.L.; Chamley, L.W.; James, J.L. Placental formation in early pregnancy: How is the centre of the placenta made? Hum. Reprod. Update 2018, 24, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Cornish, E.F.; Filipovic, I.; Asenius, F.; Williams, D.J.; McDonnell, T. Innate immune responses to acute viral infection during pregnancy. Front. Immunol. 2020, 11, 572567. [Google Scholar] [CrossRef] [PubMed]
- Alberca, R.W.; Pereira, N.Z.; Oliveira, L.; Gozzi-Silva, S.C.; Sato, M.N. Pregnancy, viral infection, and COVID-19. Front. Immunol. 2020, 11, 1672. [Google Scholar] [CrossRef]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Investig. 2017, 127, 1591–1599. [Google Scholar] [CrossRef]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef]
- Takada, K.; Shimodai-Yamada, S.; Suzuki, M.; Trinh, Q.D.; Takano, C.; Kawakami, K.; Asai-Sato, M.; Komatsu, A.; Okahashi, A.; Nagano, N.; et al. Restriction of SARS-CoV-2 replication in the human placenta. Placenta 2022, 127, 73–76. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Takada, K.; Hayakawa, S. Placental barrier against COVID-19. Placenta 2020, 99, 45–49. [Google Scholar] [CrossRef]
- Hobman, T.C. Rubella virus. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 687–711. [Google Scholar]
- Rasmussen, S.A.; Jamieson, D.J. Teratogen update: Zika virus and pregnancy. Birth Defects Res. 2020, 112, 1139–1149. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of primitive human placental trophoblast to zika virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1587–E1596. [Google Scholar] [CrossRef]
- Bayer, A.; Delorme-Axford, E.; Sleigher, C.; Frey, T.K.; Trobaugh, D.W.; Klimstra, W.B.; Emert-Sedlak, L.A.; Smithgall, T.E.; Kinchington, P.R.; Vadia, S.; et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am. J. Obstet. Gynecol. 2015, 212, 71.e1–71.e8. [Google Scholar] [CrossRef]
- Pham, N.T.K.; Trinh, Q.D.; Takada, K.; Komine-Aizawa, S.; Hayakawa, S. Low susceptibility of rubella virus in first-trimester trophoblast cell lines. Viruses 2022, 14, 1169. [Google Scholar] [CrossRef]
- Du, M.R.; Guo, P.F.; Piao, H.L.; Wang, S.C.; Sun, C.; Jin, L.P.; Tao, Y.; Li, Y.H.; Zhang, D.; Zhu, R.; et al. Embryonic trophoblasts induce decidual regulatory t cell differentiation and maternal–fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J. Immunol. 2014, 192, 1502–1511. [Google Scholar] [CrossRef]
- Ramhorst, R.; Fraccaroli, L.; Aldo, P.; Alvero, A.B.; Cardenas, I.; Leiros, C.P.; Mor, G. Modulation and recruitment of inducible regulatory t cells by first trimester trophoblast cells. Am. J. Reprod. Immunol. 2012, 67, 17–27. [Google Scholar] [CrossRef]
- Yang, D.; Dai, F.; Yuan, M.; Zheng, Y.; Liu, S.; Deng, Z.; Tan, W.; Chen, L.; Zhang, Q.; Zhao, X.; et al. Role of transforming growth factor-beta1 in regulating fetal-maternal immune tolerance in normal and pathological pregnancy. Front. Immunol. 2021, 12, 689181. [Google Scholar] [CrossRef]
- Graham, C.H.; Lysiak, J.J.; McCrae, K.R.; Lala, P.K. Localization of transforming growth factor-beta at the human fetal-maternal interface: Role in trophoblast growth and differentiation. Biol. Reprod. 1992, 46, 561–572. [Google Scholar] [CrossRef]
- Ingman, W.V.; Robertson, S.A. The essential roles of tgfb1 in reproduction. Cytokine Growth Factor Rev. 2009, 20, 233–239. [Google Scholar] [CrossRef]
- Zhao, M.R.; Qiu, W.; Li, Y.X.; Zhang, Z.B.; Li, D.; Wang, Y.L. Dual effect of transforming growth factor beta1 on cell adhesion and invasion in human placenta trophoblast cells. Reproduction 2006, 132, 333–341. [Google Scholar] [CrossRef]
- Pham, N.T.K.; Trinh, Q.D.; Takada, K.; Takano, C.; Sasano, M.; Okitsu, S.; Ushijima, H.; Komine-Aizawa, S.; Hayakawa, S. The epithelial-to-mesenchymal transition-like process induced by tgf-beta1 enhances rubella virus binding and infection in a549 cells via the smad pathway. Microorganisms 2021, 9, 662. [Google Scholar] [CrossRef]
- Denney, L.; Branchett, W.; Gregory, L.G.; Oliver, R.A.; Lloyd, C.M. Epithelial-derived tgf-beta1 acts as a pro-viral factor in the lung during influenza a infection. Mucosal. Immunol. 2018, 11, 523–535. [Google Scholar] [CrossRef]
- Allen, S.J.; Mott, K.R.; Wechsler, S.L.; Flavell, R.A.; Town, T.; Ghiasi, H. Adaptive and innate transforming growth factor beta signaling impact herpes simplex virus 1 latency and reactivation. J. Virol. 2011, 85, 11448–11456. [Google Scholar] [CrossRef] [PubMed]
- Esaki, S.; Nigim, F.; Moon, E.; Luk, S.; Kiyokawa, J.; Curry, W., Jr.; Cahill, D.P.; Chi, A.S.; Iafrate, A.J.; Martuza, R.L.; et al. Blockade of transforming growth factor-beta signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int. J. Cancer 2017, 141, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tuttle, D.L.; Oshier, J.T.; Knot, H.J.; Streit, W.J.; Goodenow, M.M.; Harrison, J.K. Transforming growth factor-beta1 increases cxcr4 expression, stromal-derived factor-1alpha-stimulated signalling and human immunodeficiency virus-1 entry in human monocyte-derived macrophages. Immunology 2005, 114, 565–574. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Dutta, R.K.; Nair, M.; Chand, H.S.; Rahman, I.; Unwalla, H.J. Tgf-beta1 increases viral burden and promotes HIV-1 latency in primary differentiated human bronchial epithelial cells. Sci. Rep. 2019, 9, 12552. [Google Scholar] [CrossRef] [PubMed]
- Bacsi, A.; Aranyosi, J.; Beck, Z.; Ebbesen, P.; Andirko, I.; Szabo, J.; Lampe, L.; Kiss, J.; Gergely, L.; Toth, F.D. Placental macrophage contact potentiates the complete replicative cycle of human cytomegalovirus in syncytiotrophoblast cells: Role of interleukin-8 and transforming growth factor-beta1. J. Interf. Cytokine Res. 1999, 19, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Prokopowicz, D.; Jaroszewicz, J.; Flisiak, I. Plasma transforming growth factor-beta(1) in acute viral hepatitis. Med. Sci. Monit. 2005, 11, CR304–CR308. [Google Scholar]
- Chou, Y.C.; Chen, M.L.; Hu, C.P.; Chen, Y.L.; Chong, C.L.; Tsai, Y.L.; Liu, T.L.; Jeng, K.S.; Chang, C. Transforming growth factor-beta1 suppresses hepatitis b virus replication primarily through transcriptional inhibition of pregenomic rna. Hepatology 2007, 46, 672–681. [Google Scholar] [CrossRef]
- Li, W.; Yu, X.; Chen, X.; Wang, Z.; Yin, M.; Zhao, Z.; Zhu, C. Hbv induces liver fibrosis via the tgf-beta1/mir-21-5p pathway. Exp. Ther. Med. 2021, 21, 169. [Google Scholar] [CrossRef]
- Wang, Y. The inhibition of microrna-15a suppresses hepatitis b virus-associated liver cancer cell growth through the smad/tgf-beta pathway. Oncol. Rep. 2017, 37, 3520–3526. [Google Scholar] [CrossRef]
- Huntington, K.E.; Carlsen, L.; So, E.Y.; Piesche, M.; Liang, O.; El-Deiry, W.S. Integrin/tgf-beta1 inhibitor glpg-0187 blocks SARS-CoV-2 delta and omicron pseudovirus infection of airway epithelial cells in vitro, which could attenuate disease severity. Pharmaceuticals 2022, 15, 618. [Google Scholar] [CrossRef]
- Wang, E.Y.; Chen, H.; Sun, B.Q.; Wang, H.; Qu, H.Q.; Liu, Y.; Sun, X.Z.; Qu, J.; Fang, Z.F.; Tian, L.; et al. Serum levels of the iga isotype switch factor tgf-beta1 are elevated in patients with COVID-19. FEBS Lett. 2021, 595, 1819–1824. [Google Scholar] [CrossRef]
- Montalvo Villalba, M.C.; Valdes Ramirez, O.; Mune Jimenez, M.; Arencibia Garcia, A.; Martinez Alfonso, J.; Gonzalez Baez, G.; Roque Arrieta, R.; Rosell Simon, D.; Alvarez Gainza, D.; Sierra Vazquez, B.; et al. Interferon gamma, tgf-beta1 and rantes expression in upper airway samples from SARS-CoV-2 infected patients. Clin. Immunol. 2020, 220, 108576. [Google Scholar] [CrossRef]
- Trinh, Q.D.; Pham, N.T.K.; Takada, K.; Takano, C.; Komine-Aizawa, S.; Hayakawa, S. Tgf-beta1 promotes zika virus infection in immortalized human first-trimester trophoblasts via the smad pathway. Cells 2022, 11, 3026. [Google Scholar] [CrossRef]
- Kovacs, A.A.Z. Zika, the newest torch infectious disease in the americas. Clin. Infect. Dis. 2020, 70, 2673–2674. [Google Scholar] [CrossRef]
- Morand, A.; Zandotti, C.; Charrel, R.; Minodier, P.; Fabre, A.; Chabrol, B.; De Lamballerie, X. From torch to torchz: Zika virus infection highlights infectious fetopathies. Arch. Pediatr. 2017, 24, 911–913. [Google Scholar] [CrossRef]
- Warnecke, J.M.; Pollmann, M.; Borchardt-Loholter, V.; Moreira-Soto, A.; Kaya, S.; Sener, A.G.; Gomez-Guzman, E.; Figueroa-Hernandez, L.; Li, W.; Li, F.; et al. Seroprevalences of antibodies against torch infectious pathogens in women of childbearing age residing in brazil, mexico, germany, poland, turkey and china. Epidemiol. Infect. 2020, 148, e271. [Google Scholar] [CrossRef]
- Yu, W.; Hu, X.; Cao, B. Viral infections during pregnancy: The big challenge threatening maternal and fetal health. Matern.-Fetal Med. 2022, 4, 72–86. [Google Scholar] [CrossRef]
- Semmes, E.C.; Coyne, C.B. Innate immune defenses at the maternal-fetal interface. Curr. Opin. Immunol. 2022, 74, 60–67. [Google Scholar] [CrossRef]
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and pregnancy: Effects on maternal and child health. Front. Cell. Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.J.; Jeyarajah, M.J. How trophoblasts fuse: An in-depth look into placental syncytiotrophoblast formation. Cell. Mol. Life Sci. 2022, 79, 433. [Google Scholar] [CrossRef] [PubMed]
- Maudhoo, A.; Khalil, A. Viral pulmonary infection in pregnancy—Including COVID-19, sars, influenza a, and varicella. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 85, 17–25. [Google Scholar] [CrossRef]
- Hayakawa, S.; Komine-Aizawa, S.; Mor, G.G. COVID-19 pandemic and pregnancy. J. Obstet. Gynaecol. Res. 2020, 46, 1958–1966. [Google Scholar] [CrossRef]
- Alouini, S.; Guinard, J.; Belin, O.; Mesnard, L.; Werner, E.; Prazuck, T.; Pichon, C. Maternal-fetal implications of SARS-CoV-2 infection during pregnancy, viral, serological analyses of placenta and cord blood. Int. J. Environ. Res. Public Health 2022, 19, 2105. [Google Scholar] [CrossRef]
- Zimmerman, L.A.; Knapp, J.K.; Antoni, S.; Grant, G.B.; Reef, S.E. Progress toward rubella and congenital rubella syndrome control and elimination—Worldwide, 2012–2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 196–201. [Google Scholar] [CrossRef]
- Namiki, T.; Takano, C.; Aoki, R.; Trinh, Q.D.; Morioka, I.; Hayakawa, S. Parenchymal calcification is associated with the neurological prognosis in patients with congenital rubella syndrome. Congenit. Anom. 2022, 62, 38–41. [Google Scholar] [CrossRef]
- Ekuma, U.O.; Ogbu, O.; Oli, A.N.; Okolo, M.O.; Edeh, P.A.; Al-Dahmoshi, H.O.M.; Akrami, S.; Saki, M. The burden of likely rubella infection among healthy pregnant women in abakaliki, ebonyi state, nigeria. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 5743106. [Google Scholar] [CrossRef]
- Shukla, S.; Maraqa, N.F. Congenital rubella. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gudeloglu, E.; Akillioglu, M.; Demirdag, T.B.; Unal, N.A.; Tapisiz, A.A. Congenital rubella syndrome: A short report and literature review. Trop. Dr. 2023, 53, 171–175. [Google Scholar] [CrossRef]
- Singh, G.; Gaidhane, A. A review of sensorineural hearing loss in congenital cytomegalovirus infection. Cureus 2022, 14, e30703. [Google Scholar] [CrossRef]
- Xia, W.; Yan, H.; Zhang, Y.; Wang, C.; Gao, W.; Lv, C.; Wang, W.; Liu, Z. Congenital human cytomegalovirus infection inducing sensorineural hearing loss. Front. Microbiol. 2021, 12, 649690. [Google Scholar] [CrossRef]
- Davis, N.L.; King, C.C.; Kourtis, A.P. Cytomegalovirus infection in pregnancy. Birth Defects Res. 2017, 109, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Naing, Z.W.; Scott, G.M.; Shand, A.; Hamilton, S.T.; van Zuylen, W.J.; Basha, J.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Congenital cytomegalovirus infection in pregnancy: A review of prevalence, clinical features, diagnosis and prevention. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 9–18. [Google Scholar] [CrossRef]
- Jose Antonio, M.M.; Monica Grisel, R.M.; Alberto, C.S.; Carla Ileana, A.A.; Luis Antonio, U.N.; Maria de Los Angeles, B.S.; Norma Angelica, M.J.; Mara Soraya, R.E.; Victor, R.P.; Jesus Enrique, G.M. Maternal and neonatal risk factors associated with increased mother-to-child transmission of HIV-1 in mexico: Results of a case–control study. Int. J. STD AIDS 2022, 33, 1111–1118. [Google Scholar] [CrossRef]
- WHO. HIV/Aids: Mother-to-Child Transmission; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of cd4+ t-cell depletion in HIV-1 and HIV-2 infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Grignolo, S.; Agnello, R.; Gerbaldo, D.; Gotta, C.; Alicino, C.; Del Puente, F.; Taramasso, L.; Bruzzone, B.; Gustavino, C.; Trasino, S.; et al. Pregnancy and neonatal outcomes among a cohort of HIV-infected women in a large italian teaching hospital: A 30-year retrospective study. Epidemiol. Infect. 2017, 145, 1658–1669. [Google Scholar] [CrossRef]
- Spinillo, A.; Iasci, A.; Dal Maso, J.; Di Lenardo, L.; Grella, P.; Guaschino, S. The effect of fetal infection with human immunodeficiency virus type 1 on birthweight and length of gestation. Sigo study group of HIV infection in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 57, 13–17. [Google Scholar] [CrossRef]
- Kumar, M.; Abbas, Z.; Azami, M.; Belopolskaya, M.; Dokmeci, A.K.; Ghazinyan, H.; Jia, J.; Jindal, A.; Lee, H.C.; Lei, W.; et al. Asian pacific association for the study of liver (apasl) guidelines: Hepatitis b virus in pregnancy. Hepatol. Int. 2022, 16, 211–253. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, C.; Huang, S.; Wang, J.; Qi, Y.; Yao, G.; Sun, W.; Qian, Y.; Ye, L.; Liu, H.; et al. Impact of maternal infection with hepatitis b virus on pregnancy complications and neonatal outcomes for women undergoing assisted reproductive technology treatment: A population-based study. J. Viral Hepat. 2021, 28, 613–620. [Google Scholar] [CrossRef]
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bajema, K.L.; Stankiewicz Karita, H.C.; Tenforde, M.W.; Hawes, S.E.; Heffron, R. Maternal hepatitis b infection and pregnancy outcomes in the united states: A population-based cohort study. Open Forum Infect. Dis. 2018, 5, ofy134. [Google Scholar] [CrossRef]
- Deftereou, T.E.; Trypidi, A.; Alexiadi, C.A.; Theotokis, P.; Manthou, M.E.; Meditskou, S.; Simopoulou, M.; Lambropoulou, M. Congenital herpes simplex virus: A histopathological view of the placenta. Cureus 2022, 14, e29101. [Google Scholar] [CrossRef]
- Krenn, V.; Bosone, C.; Burkard, T.R.; Spanier, J.; Kalinke, U.; Calistri, A.; Salata, C.; Rilo Christoff, R.; Pestana Garcez, P.; Mirazimi, A.; et al. Organoid modeling of zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 2021, 28, 1362–1379.e7. [Google Scholar] [CrossRef] [PubMed]
- Hammad, W.A.B.; Konje, J.C. Herpes simplex virus infection in pregnancy—An update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Heggarty, E.; Sibiude, J.; Mandelbrot, L.; Vauloup-Fellous, C.; Picone, O. Genital herpes and pregnancy: Evaluating practices and knowledge of french health care providers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 249, 84–91. [Google Scholar] [CrossRef]
- Vazquez-Pagan, A.; Schultz-Cherry, S. Serological responses to influenza vaccination during pregnancy. Microorganisms 2021, 9, 2305. [Google Scholar] [CrossRef]
- Fuentes-Zacarias, P.; Murrieta-Coxca, J.M.; Gutierrez-Samudio, R.N.; Schmidt, A.; Schmidt, A.; Markert, U.R.; Morales-Prieto, D.M. Pregnancy and pandemics: Interaction of viral surface proteins and placenta cells. Biochim. Biophys. Acta Mol. (BBA)-Basis Dis. 2021, 1867, 166218. [Google Scholar] [CrossRef]
- Littauer, E.Q.; Esser, E.S.; Antao, O.Q.; Vassilieva, E.V.; Compans, R.W.; Skountzou, I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog. 2017, 13, e1006757. [Google Scholar] [CrossRef]
- Hewagama, S.; Walker, S.P.; Stuart, R.L.; Gordon, C.; Johnson, P.D.; Friedman, N.D.; O’Reilly, M.; Cheng, A.C.; Giles, M.L. 2009 H1N1 influenza a and pregnancy outcomes in victoria, australia. Clin. Infect. Dis. 2010, 50, 686–690. [Google Scholar] [CrossRef]
- Teixeira, F.M.E.; Pietrobon, A.J.; Oliveira, L.M.; Oliveira, L.; Sato, M.N. Maternal-fetal interplay in zika virus infection and adverse perinatal outcomes. Front. Immunol. 2020, 11, 175. [Google Scholar] [CrossRef]
- Espino, A.; Gouilly, J.; Chen, Q.; Colin, P.; Guerby, P.; Izopet, J.; Amara, A.; Tabiasco, J.; Al-Daccak, R.; El Costa, H.; et al. The mechanisms underlying the immune control of zika virus infection at the maternal-fetal interface. Front. Immunol. 2022, 13, 1000861. [Google Scholar] [CrossRef] [PubMed]
- Ades, A.E.; Soriano-Arandes, A.; Alarcon, A.; Bonfante, F.; Thorne, C.; Peckham, C.S.; Giaquinto, C. Vertical transmission of zika virus and its outcomes: A bayesian synthesis of prospective studies. Lancet Infect. Dis. 2021, 21, 537–545. [Google Scholar] [CrossRef] [PubMed]
- de Noronha, L.; Zanluca, C.; Burger, M.; Suzukawa, A.A.; Azevedo, M.; Rebutini, P.Z.; Novadzki, I.M.; Tanabe, L.S.; Presibella, M.M.; Duarte Dos Santos, C.N. Zika virus infection at different pregnancy stages: Anatomopathological findings, target cells and viral persistence in placental tissues. Front. Microbiol. 2018, 9, 2266. [Google Scholar] [CrossRef]
- Mourosi, J.T.; Awe, A.; Jain, S.; Batra, H. Nucleic acid vaccine platform for dengue and zika flaviviruses. Vaccines 2022, 10, 834. [Google Scholar] [CrossRef]
- Masmejan, S.; Pomar, L.; Favre, G.; Panchaud, A.; Giannoni, E.; Greub, G.; Baud, D. Vertical transmission and materno-fetal outcomes in 13 patients with coronavirus disease 2019. Clin. Microbiol. Infect. 2020, 26, 1585–1587. [Google Scholar] [CrossRef]
- Shook, L.L.; Sullivan, E.L.; Lo, J.O.; Perlis, R.H.; Edlow, A.G. COVID-19 in pregnancy: Implications for fetal brain development. Trends Mol. Med. 2022, 28, 319–330. [Google Scholar] [CrossRef]
- Shook, L.L.; Fourman, L.T.; Edlow, A.G. Immune responses to SARS-CoV-2 in pregnancy: Implications for the health of the next generation. J. Immunol. 2022, 209, 1465–1473. [Google Scholar] [CrossRef]
- Parcial, A.L.N.; Salomao, N.G.; Portari, E.A.; Arruda, L.V.; de Carvalho, J.J.; de Matos Guedes, H.L.; Conde, T.C.; Moreira, M.E.; Batista, M.M.; Paes, M.V.; et al. SARS-CoV-2 is persistent in placenta and causes macroscopic, histopathological, and ultrastructural changes. Viruses 2022, 14, 1885. [Google Scholar] [CrossRef]
- Li, P.; Xie, M.; Zhang, W. Clinical characteristics and intrauterine vertical transmission potential of coronavirus disease 2019 infection in 9 pregnant women: A retrospective review of medical records. Am. J. Obstet. Gynecol. 2020, 223, 955–956. [Google Scholar] [CrossRef]
- Patane, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 rna on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100145. [Google Scholar] [CrossRef]
- AlQahtani, M.A.; AlDajani, S.M. A systemic review of vertical transmission possibility in pregnant women with coronavirus disease 2019-positive status. J. Fam. Med. Prim. Care 2020, 9, 4521–4525. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Kovachev, E.; Todorov, N. Cytomegalovirus infection during pregnancy and its impact on the intrauterine fetal development—Case report. Open Access Maced. J. Med. Sci. 2016, 4, 449–452. [Google Scholar] [CrossRef]
- Avgil, M.; Ornoy, A. Herpes simplex virus and epstein-barr virus infections in pregnancy: Consequences of neonatal or intrauterine infection. Reprod. Toxicol. 2006, 21, 436–445. [Google Scholar] [CrossRef]
- Goncalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef]
- Guan, M.; Johannesen, E.; Tang, C.Y.; Hsu, A.L.; Barnes, C.L.; Burnam, M.; McElroy, J.A.; Wan, X.F. Intrauterine fetal demise in the third trimester of pregnancy associated with mild infection with the SARS-CoV-2 delta variant without protection from vaccination. J. Infect. Dis. 2022, 225, 748–753. [Google Scholar] [CrossRef]
- Fuwa, K.; Hayakawa, S. Mechanisms and possible controls of the in utero zika virus infection: Where is the holy grail? Am. J. Reprod. Immunol. 2017, 77, e12605. [Google Scholar] [CrossRef]
- Centeno-Tablante, E.; Medina-Rivera, M.; Finkelstein, J.L.; Herman, H.S.; Rayco-Solon, P.; Garcia-Casal, M.N.; Rogers, L.; Ghezzi-Kopel, K.; Zambrano Leal, M.P.; Andrade Velasquez, J.K.; et al. Update on the transmission of zika virus through breast milk and breastfeeding: A systematic review of the evidence. Viruses 2021, 13, 123. [Google Scholar] [CrossRef]
- Trinh, Q.D.; Takada, K.; Pham, N.T.K.; Takano, C.; Namiki, T.; Ikuta, R.; Hayashida, S.; Okitsu, S.; Ushijima, H.; Komine-Aizawa, S.; et al. Enhancement of rubella virus infection in immortalized human first-trimester trophoblasts under low-glucose stress conditions. Front. Microbiol. 2022, 13, 904189. [Google Scholar] [CrossRef]
- Pereira, L. Congenital viral infection: Traversing the uterine-placental interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef]
- Kirschen, G.W.; Burd, I. Modeling of vertical transmission and pathogenesis of cytomegalovirus in pregnancy: Opportunities and challenges. Front. Virol. 2023, 3, 1106634. [Google Scholar] [CrossRef]
- Weisblum, Y.; Panet, A.; Haimov-Kochman, R.; Wolf, D.G. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin. Immunopathol. 2014, 36, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Sibiude, J.; Le Chenadec, J.; Mandelbrot, L.; Hoctin, A.; Dollfus, C.; Faye, A.; Bui, E.; Pannier, E.; Ghosn, J.; Garrait, V.; et al. Update of perinatal human immunodeficiency virus type 1 transmission in france: Zero transmission for 5482 mothers on continuous antiretroviral therapy from conception and with undetectable viral load at delivery. Clin. Infect. Dis. 2023, 76, e590–e598. [Google Scholar] [CrossRef] [PubMed]
- Ter Schiphorst, E.; Hansen, K.C.; Holm, M.; Honge, B.L. Mother-to-child HIV-2 transmission: Comparison with HIV-1 and evaluation of factors influencing the rate of transmission. A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 399–408. [Google Scholar] [CrossRef]
- Al-Husaini, A.M. Role of placenta in the vertical transmission of human immunodeficiency virus. J. Perinatol. 2009, 29, 331–336. [Google Scholar] [CrossRef]
- WHO. Operationalizing elimination of mother-to-child transmission of hepatitis b virus in the western pacific region. In World Health Organization Regional Office for the Western Pacific, Manila, Licence: CC BY-NC-SA 3.0 IGO; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Lv, N.; Chu, X.D.; Sun, Y.H.; Zhao, S.Y.; Li, P.L.; Chen, X. Analysis on the outcomes of hepatitis b virus perinatal vertical transmission: Nested case-control study. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1286–1291. [Google Scholar] [CrossRef]
- Xu, D.Z.; Yan, Y.P.; Choi, B.C.; Xu, J.Q.; Men, K.; Zhang, J.X.; Liu, Z.H.; Wang, F.S. Risk factors and mechanism of transplacental transmission of hepatitis b virus: A case-control study. J. Med. Virol. 2002, 67, 20–26. [Google Scholar] [CrossRef]
- Belanger, B.G.; Lui, F. Embryology, teratology torch. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Felker, A.M.; Nguyen, P.; Kaushic, C. Primary HSV-2 infection in early pregnancy results in transplacental viral transmission and dose-dependent adverse pregnancy outcomes in a novel mouse model. Viruses 2021, 13, 1929. [Google Scholar] [CrossRef]
- Westhoff, G.L.; Little, S.E.; Caughey, A.B. Herpes simplex virus and pregnancy: A review of the management of antenatal and peripartum herpes infections. Obstet. Gynecol. Surv. 2011, 66, 629–638. [Google Scholar] [CrossRef]
- Kriebs, J.M. Understanding herpes simplex virus: Transmission, diagnosis, and considerations in pregnancy management. J. Midwifery Women’s Health 2008, 53, 202–208. [Google Scholar] [CrossRef]
- Nunes, M.C.; Madhi, S.A. Prevention of influenza-related illness in young infants by maternal vaccination during pregnancy. F1000Research 2018, 7, 122. [Google Scholar] [CrossRef]
- Irving, W.L.; James, D.K.; Stephenson, T.; Laing, P.; Jameson, C.; Oxford, J.S.; Chakraverty, P.; Brown, D.W.; Boon, A.C.; Zambon, M.C. Influenza virus infection in the second and third trimesters of pregnancy: A clinical and seroepidemiological study. BJOG 2000, 107, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Jabrane-Ferrat, N.; Veas, F. Zika virus targets multiple tissues and cell types during the first trimester of pregnancy. Methods Mol. Biol. 2020, 2142, 235–249. [Google Scholar] [PubMed]
- Schwartz, D.A.; Morotti, D.; Beigi, B.; Moshfegh, F.; Zafaranloo, N.; Patane, L. Confirming vertical fetal infection with coronavirus disease 2019: Neonatal and pathology criteria for early onset and transplacental transmission of severe acute respiratory syndrome coronavirus 2 from infected pregnant mothers. Arch. Pathol. Lab. Med. 2020, 144, 1451–1456. [Google Scholar] [CrossRef]
- Gupta, A.; Malhotra, Y.; Patil, U.; Muradas, A.R.; Lee, W.T.; Krammer, F.; Amanat, F.; Clare, C.A.; Vinod, S.; Ghaly, E. In utero vertical transmission of coronavirus disease 2019 in a severely ill 29-week preterm infant. Am. J. Perinatol. Rep. 2020, 10, e270–e274. [Google Scholar] [CrossRef]
- Mankertz, A.; Chen, M.-H.; Goldberg, T.L.; Hübschen, J.M.; Pfaff, F.; Ulrich, R.G.; ICTV Report Consortium. ICTV virus taxonomy profile: Matonaviridae 2022. J. Gen. Virol. 2022, 103, 001817. [Google Scholar] [CrossRef]
- Fonseca, I.C.; Wong, J.; Mireskandari, K.; Chitayat, D. Newborn with bilateral congenital cataracts: Never forget congenital rubella syndrome. Paediatr. Child Health 2020, 25, 72–76. [Google Scholar] [CrossRef]
- Chauhan, N.; Sen, M.S.; Jhanda, S.; Grover, S. Psychiatric manifestations of congenital rubella syndrome: A case report and review of literature. J. Pediatr. Neurosci. 2016, 11, 137–139. [Google Scholar]
- Centers for Disease Control and Prevention. Nationwide rubella epidemic—Japan, 2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 457–462. [Google Scholar]
- Kanai, M.; Kamiya, H.; Okuno, H.; Sunagawa, T.; Tanaka-Taya, K.; Matsui, T.; Oishi, K.; Kitajima, H.; Takeda, M.; Mori, Y. Epidemiology of congenital rubella syndrome related to the 2012–2013 rubella epidemic in Japan. J. Pediatric. Infect. Dis. Soc. 2022, 11, 400–403. [Google Scholar] [CrossRef]
- Ujiie, M. Rubella resurgence in Japan 2018–2019. J. Travel Med. 2019, 26, taz047. [Google Scholar] [CrossRef]
- Tilahun, G.T.; Tolasa, T.M.; Wole, G.A. Modeling the dynamics of rubella disease with vertical transmission. Heliyon 2022, 8, e11797. [Google Scholar] [CrossRef] [PubMed]
- Bate, S.L.; Dollard, S.C.; Cannon, M.J. Cytomegalovirus seroprevalence in the united states: The national health and nutrition examination surveys, 1988–2004. Clin. Infect. Dis. 2010, 50, 1439–1447. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Syggelou, A.; Iacovidou, N.; Kloudas, S.; Christoni, Z.; Papaevangelou, V. Congenital cytomegalovirus infection. Ann. N. Y. Acad. Sci. 2010, 1205, 144–147. [Google Scholar] [CrossRef]
- Fowler, K.B.; Stagno, S.; Pass, R.F. Maternal age and congenital cytomegalovirus infection: Screening of two diverse newborn populations, 1980-1990. J. Infect. Dis. 1993, 168, 552–556. [Google Scholar] [CrossRef]
- Reynolds, D.W.; Stagno, S.; Hosty, T.S.; Tiller, M.; Alford, C.A., Jr. Maternal cytomegalovirus excretion and perinatal infection. N. Engl. J. Med. 1973, 289, 1–5. [Google Scholar] [CrossRef]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: Implications for transmission and pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef]
- Pass, R.F. Epidemiology and transmission of cytomegalovirus. J. Infect. Dis. 1985, 152, 243–248. [Google Scholar] [CrossRef]
- Stagno, S.; Pass, R.F.; Cloud, G.; Britt, W.J.; Henderson, R.E.; Walton, P.D.; Veren, D.A.; Page, F.; Alford, C.A. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 1986, 256, 1904–1908. [Google Scholar] [CrossRef]
- Hemmings, D.G.; Kilani, R.; Nykiforuk, C.; Preiksaitis, J.; Guilbert, L.J. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J. Virol. 1998, 72, 4970–4979. [Google Scholar] [CrossRef]
- Toth, F.D.; Mosborg-Petersen, P.; Kiss, J.; Aboagye-Mathiesen, G.; Hager, H.; Juhl, C.B.; Gergely, L.; Zdravkovic, M.; Aranyosi, J.; Lampe, L. Interactions between human immunodeficiency virus type 1 and human cytomegalovirus in human term syncytiotrophoblast cells coinfected with both viruses. J. Virol. 1995, 69, 2223–2232. [Google Scholar] [CrossRef]
- Vijayan, V.; Naeem, F.; Veesenmeyer, A.F. Management of infants born to mothers with HIV infection. Am. Fam. Physician 2021, 104, 58–62. [Google Scholar] [PubMed]
- Chigwedere, P.; Seage, G.R.; Lee, T.H.; Essex, M. Efficacy of antiretroviral drugs in reducing mother-to-child transmission of HIV in Africa: A meta-analysis of published clinical trials. AIDS Res. Hum. Retrovir. 2008, 24, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Zijenah, L.S.; Bandason, T.; Bara, W.; Chipiti, M.M.; Katzenstein, D.A. Impact of option b(+) combination antiretroviral therapy on mother-to-child transmission of HIV-1, maternal and infant virologic responses to combination antiretroviral therapy, and maternal and infant mortality rates: A 24-month prospective follow-up study at a primary health care clinic, in harare, zimbabwe. AIDS Patient Care STDS 2022, 36, 145–152. [Google Scholar] [PubMed]
- Soriano-Arandes, A.; Noguera-Julian, A.; Lopez-Lacort, M.; Soler-Palacin, P.; Mur, A.; Mendez, M.; Mayol, L.; Vallmanya, T.; Almeda, J.; Carnicer-Pont, D.; et al. Pregnancy as an opportunity to diagnose human-immunodeficiency virus immigrant women in catalonia. Enferm. Infecc. Microbiol. Clin. 2018, 36, 9–15. [Google Scholar] [CrossRef]
- Cooper, E.R.; Charurat, M.; Mofenson, L.; Hanson, I.C.; Pitt, J.; Diaz, C.; Hayani, K.; Handelsman, E.; Smeriglio, V.; Hoff, R.; et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J. Acquir. Immune Defic. Syndr. 2002, 29, 484–494. [Google Scholar] [CrossRef]
- Mano, H.; Chermann, J.C. Replication of human immunodeficiency virus type 1 in primary cultured placental cells. Res. Virol. 1991, 142, 95–104. [Google Scholar] [CrossRef]
- McGann, K.A.; Collman, R.; Kolson, D.L.; Gonzalez-Scarano, F.; Coukos, G.; Coutifaris, C.; Strauss, J.F.; Nathanson, N. Human immunodeficiency virus type 1 causes productive infection of macrophages in primary placental cell cultures. J. Infect. Dis. 1994, 169, 746–753. [Google Scholar] [CrossRef]
- Kilani, R.T.; Chang, L.J.; Garcia-Lloret, M.I.; Hemmings, D.; Winkler-Lowen, B.; Guilbert, L.J. Placental trophoblasts resist infection by multiple human immunodeficiency virus (HIV) type 1 variants even with cytomegalovirus coinfection but support HIV replication after provirus transfection. J. Virol. 1997, 71, 6359–6372. [Google Scholar] [CrossRef]
- Vidricaire, G.; Tardif, M.R.; Tremblay, M.J. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J. Biol. Chem. 2003, 278, 15832–15841. [Google Scholar] [CrossRef]
- Alter, M.J. Epidemiology of hepatitis b in europe and worldwide. J. Hepatol. 2003, 39 (Suppl. S1), S64–S69. [Google Scholar] [CrossRef]
- Eke, A.C.; Eleje, G.U.; Eke, U.A.; Xia, Y.; Liu, J. Hepatitis b immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis b virus. Cochrane Database Syst. Rev. 2017, 2, CD008545. [Google Scholar] [CrossRef]
- Goudeau, A.; Yvonnet, B.; Lesage, G.; Barin, F.; Denis, F.; Coursaget, P.; Chiron, J.P.; Diop Mar, I. Lack of anti-hbc igm in neonates with hbsag carrier mothers argues against transplacental transmission of hepatitis b virus infection. Lancet 1983, 2, 1103–1104. [Google Scholar] [CrossRef]
- Schweitzer, I.L. Vertical transmission of the hepatitis b surface antigen. Am. J. Med. Sci. 1975, 270, 287–291. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yue, Y.F.; Bai, G.Q.; Shi, L.; Jiang, H. Mechanism of intrauterine infection of hepatitis b virus. World J. Gastroenterol. 2004, 10, 437–438. [Google Scholar] [CrossRef]
- Bai, G.; Wang, Y.; Zhang, L.; Tang, Y.; Fu, F. The study on the role of hepatitis b virus x protein and apoptosis in HBV intrauterine infection. Arch. Gynecol. Obstet. 2012, 285, 943–949. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, F.J.; Xu, D.Z.; Yan, Y.P.; Men, K.; Zhang, J.X. Uptake of hepatitis b virus into choriocarcinoma cells in the presence of proinflammatory cytokine tumor necrosis factor-alpha. Am. J. Obstet. Gynecol. 2004, 191, 1971–1978. [Google Scholar] [CrossRef]
- Singhal, P.; Naswa, S.; Marfatia, Y.S. Pregnancy and sexually transmitted viral infections. Indian J. Sex Transm. Dis. AIDS 2009, 30, 71–78. [Google Scholar]
- Brown, Z.A.; Selke, S.; Zeh, J.; Kopelman, J.; Maslow, A.; Ashley, R.L.; Watts, D.H.; Berry, S.; Herd, M.; Corey, L. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 1997, 337, 509–515. [Google Scholar] [CrossRef]
- Baker, D.A. Consequences of herpes simplex virus in pregnancy and their prevention. Curr. Opin. Infect. Dis. 2007, 20, 73–76. [Google Scholar] [CrossRef]
- Mertz, G.J.; Rosenthal, S.L.; Stanberry, L.R. Is herpes simplex virus type 1 (HSV-1) now more common than HSV-2 in first episodes of genital herpes? Sex. Transm. Dis. 2003, 30, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.A.; Wald, A.; Morrow, R.A.; Selke, S.; Zeh, J.; Corey, L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003, 289, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pinninti, S.G.; Kimberlin, D.W. Preventing herpes simplex virus in the newborn. Clin. Perinatol. 2014, 41, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Florman, A.L.; Gershon, A.A.; Blackett, P.R.; Nahmias, A.J. Intrauterine infection with herpes simplex virus: Resultant congenital malformations. JAMA 1973, 225, 129–132. [Google Scholar] [CrossRef]
- Hutto, C.; Arvin, A.; Jacobs, R.; Steele, R.; Stagno, S.; Lyrene, R.; Willett, L.; Powell, D.; Andersen, R.; Werthammer, J.; et al. Intrauterine herpes simplex virus infections. J. Pediatr. 1987, 110, 97–101. [Google Scholar] [CrossRef]
- Koi, H.; Zhang, J.; Makrigiannakis, A.; Getsios, S.; MacCalman, C.D.; Strauss, J.F., 3rd; Parry, S. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol. Reprod. 2002, 67, 1572–1579. [Google Scholar] [CrossRef]
- Norskov-Lauritsen, N.; Aboagye-Mathisen, G.; Juhl, C.B.; Petersen, P.M.; Zachar, V.; Ebbesen, P. Herpes simplex virus infection of cultured human term trophoblast. J. Med. Virol. 1992, 36, 162–166. [Google Scholar] [CrossRef]
- Oliveira, L.H.; Fonseca, M.E.; de-Bonis, M. Echovirus type 19 and herpes simplex type 2 infection in human placenta tissue explants. Braz. J. Med. Biol. Res. 1993, 26, 703–717. [Google Scholar]
- Coffey, V.P.; Jessop, W.J. Maternal influenza and congenital deformities: A prospective study. Lancet 1959, 2, 935–938. [Google Scholar] [CrossRef]
- Kwit, K.; Pomorska-Mol, M.; Markowska-Daniel, I. Pregnancy outcome and clinical status of gilts following experimental infection by H1N2, H3N2 and H1N1pdm09 influenza a viruses during the last month of gestation. Arch. Virol. 2015, 160, 2415–2425. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef]
- Harris, J.W. Influenza occurring in pregnant women: A statistical study of thirteen hundred and fifty cases. J. Am. Med. Assoc. 1919, 72, 978–980. [Google Scholar] [CrossRef]
- Nuzum, J.W.; Pilot, I.; Stangl, F.H.; Bonar, B.E. Pandemic influenza and pneumonia in a large civilian hospital. J. Am. Med. Assoc. 1918, 71, 1562–1567. [Google Scholar] [CrossRef]
- Siston, A.M.; Rasmussen, S.A.; Honein, M.A.; Fry, A.M.; Seib, K.; Callaghan, W.M.; Louie, J.; Doyle, T.J.; Crockett, M.; Lynfield, R.; et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the united states. JAMA 2010, 303, 1517–1525. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Honein, M.A.; Rasmussen, S.A.; Williams, J.L.; Swerdlow, D.L.; Biggerstaff, M.S.; Lindstrom, S.; Louie, J.K.; Christ, C.M.; Bohm, S.R.; et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009, 374, 451–458. [Google Scholar] [CrossRef]
- Wilson, M.G.; Stein, A.M. Teratogenic effects of asian influenza. An extended study. JAMA 1969, 210, 336–337. [Google Scholar] [CrossRef]
- Zou, S. Potential impact of pandemic influenza on blood safety and availability. Transfus. Med. Rev. 2006, 20, 181–189. [Google Scholar] [CrossRef]
- Likos, A.M.; Kelvin, D.J.; Cameron, C.M.; Rowe, T.; Kuehnert, M.J.; Norris, P.J.; National Heart, Lung, Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II). Influenza viremia and the potential for blood-borne transmission. Transfusion 2007, 47, 1080–1088. [Google Scholar] [CrossRef]
- Tsuruoka, H.; Xu, H.; Kuroda, K.; Hayashi, K.; Yasui, O.; Yamada, A.; Ishizaki, T.; Yamada, Y.; Watanabe, T.; Hosaka, Y. Detection of influenza virus rna in peripheral blood mononuclear cells of influenza patients. Jpn. J. Med. Sci. Biol. 1997, 50, 27–34. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Suzaki, A.; Trinh, Q.D.; Izumi, Y.; Shibata, T.; Kuroda, K.; Hayakawa, S. H1N1/09 influenza a virus infection of immortalized first trimester human trophoblast cell lines. Am. J. Reprod. Immunol. 2012, 68, 226–232. [Google Scholar] [CrossRef]
- Trinh, Q.D.; Izumi, Y.; Komine-Aizawa, S.; Shibata, T.; Shimotai, Y.; Kuroda, K.; Mizuguchi, M.; Ushijima, H.; Mor, G.; Hayakawa, S. H3N2 influenza a virus replicates in immortalized human first trimester trophoblast cell lines and induces their rapid apoptosis. Am. J. Reprod. Immunol. 2009, 62, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Besnard, M.; Lastere, S.; Teissier, A.; Cao-Lormeau, V.; Musso, D. Evidence of perinatal transmission of zika virus, french polynesia, december 2013 and february 2014. Eurosurveillance 2014, 19, 20751. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, french polynesia, south pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on yap island, federated states of micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Calvet, G.A.; Siqueira, A.M.; Wakimoto, M.; De Sequeira, P.C.; Nobre, A.; Quintana, M.D.S.B.; De Mendonça, M.C.L.; Lupi, O.; De Souza, R.V.; et al. Zika virus outbreak in rio de janeiro, brazil: Clinical characterization, epidemiological and virological aspects. PLoS Negl. Trop. Dis. 2016, 10, e0004636. [Google Scholar] [CrossRef]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika virus evolution and spread in the americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef]
- Hamanaka, T.; Ribeiro, C.T.M.; Pone, S.; Gomes, S.C.; Nielsen-Saines, K.; Brickley, E.B.; Moreira, M.E.; Pone, M. Longitudinal follow-up of gross motor function in children with congenital zika virus syndrome from a cohort in rio de janeiro, brazil. Viruses 2022, 14, 1173. [Google Scholar] [CrossRef]
- Lazear, H.M.; Diamond, M.S. Zika virus: New clinical syndromes and its emergence in the western hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef]
- Miao, J.; Yuan, H.; Rao, J.; Zou, J.; Yang, K.; Peng, G.; Cao, S.; Chen, H.; Song, Y. Identification of a small compound that specifically inhibits zika virus in vitro and in vivo by targeting the ns2b-ns3 protease. Antivir. Res. 2022, 199, 105255. [Google Scholar] [CrossRef]
- Luria-Perez, R.; Sanchez-Vargas, L.A.; Munoz-Lopez, P.; Mellado-Sanchez, G. Mucosal vaccination: A promising alternative against flaviviruses. Front. Cell. Infect. Microbiol. 2022, 12, 887729. [Google Scholar] [CrossRef]
- Kim, I.J.; Lanthier, P.A.; Clark, M.J.; De La Barrera, R.A.; Tighe, M.P.; Szaba, F.M.; Travis, K.L.; Low-Beer, T.C.; Cookenham, T.S.; Lanzer, K.G.; et al. Efficacy of an inactivated zika vaccine against virus infection during pregnancy in mice and marmosets. NPJ Vaccines 2022, 7, 9. [Google Scholar] [CrossRef]
- Kale, A.; Joshi, D.; Menon, I.; Bagwe, P.; Patil, S.; Vijayanand, S.; Braz Gomes, K.; D’Souza, M. Novel microparticulate zika vaccine induces a significant immune response in a preclinical murine model after intramuscular administration. Int. J. Pharm. 2022, 624, 121975. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Weisblum, Y.; Oiknine-Djian, E.; Vorontsov, O.M.; Haimov-Kochman, R.; Zakay-Rones, Z.; Meir, K.; Shveiky, D.; Elgavish, S.; Nevo, Y.; Roseman, M.; et al. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J. Virol. 2017, 91, e01905-16. [Google Scholar] [CrossRef]
- El Costa, H.; Gouilly, J.; Mansuy, J.M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. Zika virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 2016, 6, 35296. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Keshavarz, P.; Hosseinpour, P.; Erfani, A.; Roshanshad, A.; Pourdast, A.; Nowrouzi-Sohrabi, P.; Chaichian, S.; Poordast, T. Coronavirus disease 2019 (COVID-19): A systematic review of pregnancy and the possibility of vertical transmission. J. Reprod. Infertil. 2020, 21, 157–168. [Google Scholar]
- Tossetta, G.; Fantone, S.; Muti, N.D.; Balercia, G.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: A systematic review. J. Hypertens. 2022, 40, 1629–1638. [Google Scholar] [CrossRef]
- He, Z.; Fang, Y.; Zuo, Q.; Huang, X.; Lei, Y.; Ren, X.; Liu, D. Vertical transmission and kidney damage in newborns whose mothers had coronavirus disease 2019 during pregnancy. Int. J. Antimicrob. Agents 2021, 57, 106260. [Google Scholar] [CrossRef]
- Kuhrt, K.; McMicking, J.; Nanda, S.; Nelson-Piercy, C.; Shennan, A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am. J. Obstet. Gynecol. MFM 2020, 2, 100135. [Google Scholar] [CrossRef]
- Malhotra, Y.; Rossberg, M.C.; Bajaj, K.; Shtern, A.; Moore, R.M. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia. Obstet. Gynecol. 2020, 136, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Marzollo, R.; Aversa, S.; Prefumo, F.; Saccani, B.; Perez, C.R.; Sartori, E.; Motta, M. Possible coronavirus disease 2019 pandemic and pregnancy: Vertical transmission is not excluded. Pediatr. Infect. Dis. J. 2020, 39, e261–e262. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 224, 35–53.e3. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, A.M.; Tal, O.; Tal, R. Vertical transmission of coronavirus disease 2019, a response. Am. J. Obstet. Gynecol. 2021, 224, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ace2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Hanna, N.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 953–954. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278. [Google Scholar] [CrossRef]
- Latifi, Z.; Nejabati, H.R.; Abroon, S.; Mihanfar, A.; Farzadi, L.; Hakimi, P.; Hajipour, H.; Nouri, M.; Fattahi, A. Dual role of tgf-beta in early pregnancy: Clues from tumor progression. Biol. Reprod. 2019, 100, 1417–1430. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. Tgf-beta family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. Tgf-beta signaling from receptors to smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef]
- Marinelli Busilacchi, E.; Costantini, A.; Mancini, G.; Tossetta, G.; Olivieri, J.; Poloni, A.; Viola, N.; Butini, L.; Campanati, A.; Goteri, G.; et al. Nilotinib treatment of patients affected by chronic graft-versus-host disease reduces collagen production and skin fibrosis by downmodulating the tgf-beta and p-smad pathway. Biol. Blood Marrow Transplant. 2020, 26, 823–834. [Google Scholar] [CrossRef]
- Monsivais, D.; Matzuk, M.M.; Pangas, S.A. The tgf-beta family in the reproductive tract. Cold Spring Harb. Perspect. Biol. 2017, 9, a022251. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hill, C.S. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell 2009, 16, 329–343. [Google Scholar] [CrossRef]
- Jones, R.L.; Stoikos, C.; Findlay, J.K.; Salamonsen, L.A. Tgf-beta superfamily expression and actions in the endometrium and placenta. Reproduction 2006, 132, 217–232. [Google Scholar] [CrossRef]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Slabuszewska-Jozwiak, A.; Jakiel, G. Role of transforming growth factor beta in uterine fibroid biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Licini, C.; Tossetta, G.; Avellini, C.; Ciarmela, P.; Lorenzi, T.; Toti, P.; Gesuita, R.; Voltolini, C.; Petraglia, F.; Castellucci, M.; et al. Analysis of cell-cell junctions in human amnion and chorionic plate affected by chorioamnionitis. Histol. Histopathol. 2016, 31, 759–767. [Google Scholar]
- Tossetta, G.; Paolinelli, F.; Avellini, C.; Salvolini, E.; Ciarmela, P.; Lorenzi, T.; Emanuelli, M.; Toti, P.; Giuliante, R.; Gesuita, R.; et al. Il-1beta and tgf-beta weaken the placental barrier through destruction of tight junctions: An in vivo and in vitro study. Placenta 2014, 35, 509–516. [Google Scholar] [CrossRef]
- Ma, W.; Qin, Y.; Chapuy, B.; Lu, C. Lrrc33 is a novel binding and potential regulating protein of tgf-beta1 function in human acute myeloid leukemia cells. PLoS ONE 2019, 14, e0213482. [Google Scholar] [CrossRef]
- Lash, G.E.; Naruse, K.; Innes, B.A.; Robson, S.C.; Searle, R.F.; Bulmer, J.N. Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy. Placenta 2010, 31, 545–548. [Google Scholar] [CrossRef]
- Simpson, H.; Robson, S.C.; Bulmer, J.N.; Barber, A.; Lyall, F. Transforming growth factor beta expression in human placenta and placental bed during early pregnancy. Placenta 2002, 23, 44–58. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Mehta, R.B.; Lindau, R.; Mirrasekhian, E.; Rodriguez-Martinez, H.; Berg, G.; Lash, G.E.; Jenmalm, M.C.; Ernerudh, J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory t cells and homeostatic m2 macrophages. J. Immunol. 2015, 194, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Takano, C.; Horie, M.; Taiko, I.; Trinh, Q.D.; Kanemaru, K.; Komine-Aizawa, S.; Hayakawa, S.; Miki, T. Inhibition of epithelial-mesenchymal transition maintains stemness in human amniotic epithelial cells. Stem Cell Rev. Rep. 2022, 18, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Schliefsteiner, C.; Ibesich, S.; Wadsack, C. Placental hofbauer cell polarization resists inflammatory cues in vitro. Int. J. Mol. Sci. 2020, 21, 736. [Google Scholar] [CrossRef]
- Del Gobbo, V.; Giganti, M.G.; Zenobi, R.; Villani, V.; Premrov, M.G. The immunosuppressive cytokines influence the fetal survival in patients with pregnancy-induced hypertension. Am. J. Reprod. Immunol. 2000, 44, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chi, H.; Qiao, J. Role of regulatory t cells in regulating fetal-maternal immune tolerance in healthy pregnancies and reproductive diseases. Front. Immunol. 2020, 11, 1023. [Google Scholar] [CrossRef]
- Jorgensen, N.; Persson, G.; Hviid, T.V.F. The tolerogenic function of regulatory t cells in pregnancy and cancer. Front. Immunol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Benian, A.; Madazli, R.; Aksu, F.; Uzun, H.; Aydin, S. Plasma and placental levels of interleukin-10, transforming growth factor-beta1, and epithelial-cadherin in preeclampsia. Obstet. Gynecol. 2002, 100, 327–331. [Google Scholar]
- Djurovic, S.; Schjetlein, R.; Wisloff, F.; Haugen, G.; Husby, H.; Berg, K. Plasma concentrations of lp(a) lipoprotein and tgf-beta1 are altered in preeclampsia. Clin. Genet. 1997, 52, 371–376. [Google Scholar] [CrossRef]
- Abudukeyoumu, A.; Li, M.Q.; Xie, F. Transforming growth factor-beta1 in intrauterine adhesion. Am. J. Reprod. Immunol. 2020, 84, e13262. [Google Scholar] [CrossRef]
- Goteri, G.; Altobelli, E.; Tossetta, G.; Zizzi, A.; Avellini, C.; Licini, C.; Lorenzi, T.; Castellucci, M.; Ciavattini, A.; Marzioni, D. High temperature requirement a1, transforming growth factor beta1, phosphosmad2 and ki67 in eutopic and ectopic endometrium of women with endometriosis. Eur. J. Histochem. 2015, 59, 2570. [Google Scholar] [CrossRef]
- Rallon, N.I.; Barreiro, P.; Soriano, V.; Garcia-Samaniego, J.; Lopez, M.; Benito, J.M. Elevated tgf-beta1 levels might protect HCV/HIV-coinfected patients from liver fibrosis. Eur. J. Clin. Investig. 2011, 41, 70–76. [Google Scholar] [CrossRef]
- Vadaq, N.; van de Wijer, L.; van Eekeren, L.E.; Koenen, H.; de Mast, Q.; Joosten, L.A.B.; Netea, M.G.; Matzaraki, V.; van der Ven, A. Targeted plasma proteomics reveals upregulation of distinct inflammatory pathways in people living with HIV. iScience 2022, 25, 105089. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Calvanese, M.; Gentile, A.; Musto, R.; Gaudiello, G.; Scamardella, G.; Terracciano, D.; Frisso, G.; Pero, R.; et al. Diagnostic and therapeutic potential for hnp-1, hbd-1 and hbd-4 in pregnant women with COVID-19. Int. J. Mol. Sci. 2022, 23, 3450. [Google Scholar] [CrossRef]
- Gwon, Y.D.; Mahani, S.A.N.; Nagaev, I.; Mincheva-Nilsson, L.; Evander, M. Rift valley fever virus propagates in human villous trophoblast cell lines and induces cytokine mrna responses known to provoke miscarriage. Viruses 2021, 13, 2265. [Google Scholar] [CrossRef]
- Kumar, A.; Devi, S.G.; Kar, P.; Agarwal, S.; Husain, S.A.; Gupta, R.K.; Sharma, S. Association of cytokines in hepatitis e with pregnancy outcome. Cytokine 2014, 65, 95–104. [Google Scholar] [CrossRef]
- Periolo, N.; Avaro, M.; Czech, A.; Russo, M.; Benedetti, E.; Pontoriero, A.; Campos, A.; Peralta, L.M.; Baumeister, E. Pregnant women infected with pandemic influenza A(H1N1)pdm09 virus showed differential immune response correlated with disease severity. J. Clin. Virol. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Salomao, N.; Rabelo, K.; Avvad-Portari, E.; Basilio-de-Oliveira, C.; Basilio-de-Oliveira, R.; Ferreira, F.; Ferreira, L.; de Souza, T.M.; Nunes, P.; Lima, M.; et al. Histopathological and immunological characteristics of placentas infected with chikungunya virus. Front. Microbiol. 2022, 13, 1055536. [Google Scholar] [CrossRef]
- Helantera, I.; Loginov, R.; Koskinen, P.; Tornroth, T.; Gronhagen-Riska, C.; Lautenschlager, I. Persistent cytomegalovirus infection is associated with increased expression of tgf-beta1, pdgf-aa and icam-1 and arterial intimal thickening in kidney allografts. Nephrol. Dial. Transplant. 2005, 20, 790–796. [Google Scholar] [CrossRef]
- Shimamura, M.; Murphy-Ullrich, J.E.; Britt, W.J. Human cytomegalovirus induces tgf-beta1 activation in renal tubular epithelial cells after epithelial-to-mesenchymal transition. PLoS Pathog. 2010, 6, e1001170. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Wang, B.; Cheng, Z.; Zhao, R. Human cytomegalovirus promotes the activation of tgf-beta1 in human umbilical vein endothelial cells by mmp-2 after endothelial mesenchymal transition. Adv. Clin. Exp. Med. 2019, 28, 1441–1450. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, B.; Hu, M.; Qian, D.; Wang, B. Human cytomegalovirus infection enhances invasiveness and migration of glioblastoma cells by epithelial-to-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2020, 13, 2637–2647. [Google Scholar] [PubMed]
- Reinhold, D.; Wrenger, S.; Kahne, T.; Ansorge, S. HIV-1 tat: Immunosuppression via tgf-beta1 induction. Immunol. Today 1999, 20, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Weinberg, E.M.; Tai, A.W.; Peng, L.F.; Brockman, M.A.; Kim, K.A.; Kim, S.S.; Borges, C.B.; Shao, R.X.; Chung, R.T. HIV increases HCV replication in a tgf-beta1-dependent manner. Gastroenterology 2008, 134, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Kafka, J.K.; Osborne, B.J.; Sheth, P.M.; Nazli, A.; Dizzell, S.; Huibner, S.; Kovacs, C.; Verschoor, C.P.; Bowdish, D.M.; Kaul, R.; et al. Latent tgf-beta1 is compartmentalized between blood and seminal plasma of HIV-positive men and its activation in semen is negatively correlated with viral load and immune activation. Am. J. Reprod. Immunol. 2015, 73, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.; Kliszczak, A.E.; Giannoulatou, E.; Peppa, D.; Pellegrino, P.; Williams, I.; Drakesmith, H.; Borrow, P. Dynamics of transforming growth factor (tgf)-beta superfamily cytokine induction during HIV-1 infection are distinct from other innate cytokines. Front. Immunol. 2020, 11, 596841. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Parira, T.; Dutta, R.; Agudelo, M.; Morris, A.; Nair, M.; Unwalla, H.J. HIV infects bronchial epithelium and suppresses components of the mucociliary clearance apparatus. PLoS ONE 2017, 12, e0169161. [Google Scholar] [CrossRef]
- Lien, K.; Mayer, W.; Herrera, R.; Padilla, N.T.; Cai, X.; Lin, V.; Pholcharoenchit, R.; Palefsky, J.; Tugizov, S.M. HIV-1 proteins gp120 and tat promote epithelial-mesenchymal transition and invasiveness of HPV-positive and HPV-negative neoplastic genital and oral epithelial cells. Microbiol. Spectr. 2022, 10, e0362222. [Google Scholar] [CrossRef]
- Pan, J.; Clayton, M.; Feitelson, M.A. Hepatitis b virus x antigen promotes transforming growth factor-beta1 (tgf-beta1) activity by up-regulation of tgf-beta1 and down-regulation of alpha2-macroglobulin. J. Gen. Virol. 2004, 85, 275–282. [Google Scholar] [CrossRef]
- Flisiak, R.; Jaroszewicz, J.; Lapinski, T.W.; Flisiak, I.; Rogalska, M.; Prokopowicz, D. Plasma transforming growth factor beta1, metalloproteinase-1 and tissue inhibitor of metalloproteinases-1 in acute viral hepatitis type b. Regul. Pept. 2005, 131, 54–58. [Google Scholar] [CrossRef]
- Guo, G.H.; Tan, D.M.; Zhu, P.A.; Liu, F. Hepatitis b virus x protein promotes proliferation and upregulates tgf-beta1 and ctgf in human hepatic stellate cell line, lx-2. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 59–64. [Google Scholar]
- Ming, D.; Yu, X.; Guo, R.; Deng, Y.; Li, J.; Lin, C.; Su, M.; Lin, Z.; Su, Z. Elevated tgf-beta1/il-31 pathway is associated with the disease severity of hepatitis b virus-related liver cirrhosis. Viral Immunol. 2015, 28, 209–216. [Google Scholar] [CrossRef]
- Li, M.H.; Chen, Q.Q.; Zhang, L.; Lu, H.H.; Sun, F.F.; Zeng, Z.; Lu, Y.; Yi, W.; Xie, Y. Association of cytokines with hepatitis b virus and its antigen. J. Med. Virol. 2020, 92, 3426–3435. [Google Scholar] [CrossRef]
- Li, M.H.; Lu, Y.; Sun, F.F.; Chen, Q.Q.; Zhang, L.; Lu, H.H.; Zeng, Z.; Yi, W.; Xie, Y. Transforming growth factor beta as a possible independent factor in chronic hepatitis b. Arch. Virol. 2021, 166, 1853–1858. [Google Scholar] [CrossRef]
- Yu, X.; Guo, R.; Ming, D.; Deng, Y.; Su, M.; Lin, C.; Li, J.; Lin, Z.; Su, Z. The transforming growth factor beta1/interleukin-31 pathway is upregulated in patients with hepatitis b virus-related acute-on-chronic liver failure and is associated with disease severity and survival. Clin. Vaccine Immunol. 2015, 22, 484–492. [Google Scholar] [CrossRef]
- Li, W.; Duan, X.; Zhu, C.; Liu, X.; Jeyarajan, A.J.; Xu, M.; Tu, Z.; Sheng, Q.; Chen, D.; Zhu, C.; et al. Hepatitis b and hepatitis c virus infection promote liver fibrogenesis through a tgf-beta1-induced oct4/nanog pathway. J. Immunol. 2022, 208, 672–684. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.F.; Wang, R.R.; Zhi, S.W. [effect of transforming growth factor-beta1 on HBV replication and antigen synthesis in hepg2.2.15 cells with steatosis]. Zhonghua Gan Zang Bing Za Zhi 2017, 25, 732–737. [Google Scholar]
- Mendez-Samperio, P.; Hernandez, M.; Ayala, H.E. Induction of transforming growth factor-beta 1 production in human cells by herpes simplex virus. J. Interf. Cytokine Res. 2000, 20, 273–280. [Google Scholar] [CrossRef]
- Kaygusuz, I.; Godekmerdan, A.; Keles, E.; Karlidag, T.; Yalcin, S.; Yildiz, M.; Tazegul, A. The role of viruses in idiopathic peripheral facial palsy and cellular immune response. Am. J. Otolaryngol. 2004, 25, 401–406. [Google Scholar] [CrossRef]
- Nie, Y.; Cui, D.; Pan, Z.; Deng, J.; Huang, Q.; Wu, K. HSV-1 infection suppresses tgf-beta1 and smad3 expression in human corneal epithelial cells. Mol. Vis. 2008, 14, 1631–1638. [Google Scholar]
- Choi, J.A.; Ju, H.H.; Kim, J.E.; Lee, J.; Jee, D.; Park, C.K.; Paik, S.Y. Cytokine profile and cytoskeletal changes after herpes simplex virus type 1 infection in human trabecular meshwork cells. J. Cell. Mol. Med. 2021, 25, 9295–9305. [Google Scholar] [CrossRef]
- Drevets, P.; Chucair-Elliott, A.; Shrestha, P.; Jinkins, J.; Karamichos, D.; Carr, D.J. The use of human cornea organotypic cultures to study herpes simplex virus type 1 (HSV-1)-induced inflammation. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Deng, B.C.; Zhou, Y.; Wang, Y.; Cui, W.; Wang, W.; Liu, P. Immunological features in patients with pneumonitis due to influenza a H1N1 infection. J. Investig. Allergol. Clin. Immunol. 2011, 21, 44–50. [Google Scholar] [PubMed]
- Li, C.; Jiao, S.; Wang, G.; Gao, Y.; Liu, C.; He, X.; Zhang, C.; Xiao, J.; Li, W.; Zhang, G.; et al. The immune adaptor adap regulates reciprocal tgf-beta1-integrin crosstalk to protect from influenza virus infection. PLoS Pathog. 2015, 11, e1004824. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.L.; Folsgaard, N.V.; Vissing, N.H.; Birch, S.; Brix, S.; Bisgaard, H. Airway mucosal immune-suppression in neonates of mothers receiving A(H1N1)pnd09 vaccination during pregnancy. Pediatr. Infect. Dis. J. 2015, 34, 84–90. [Google Scholar] [CrossRef]
- BustosRivera-Bahena, G.; Lopez-Guerrero, D.V.; Marquez-Bandala, A.H.; Esquivel-Guadarrama, F.R.; Montiel-Hernandez, J.L. Tgf-beta1 signaling inhibit the in vitro apoptotic, infection and stimulatory cell response induced by influenza H1N1 virus infection on a549 cells. Virus Res. 2021, 297, 198337. [Google Scholar] [CrossRef]
- de Sousa, J.R.; Azevedo, R.S.S.; Martins Filho, A.J.; Araujo, M.T.F.; Moutinho, E.R.C.; Baldez Vasconcelos, B.C.; Cruz, A.C.R.; Oliveira, C.S.; Martins, L.C.; Baldez Vasconcelos, B.H.; et al. Correlation between apoptosis and in situ immune response in fatal cases of microcephaly caused by zika virus. Am. J. Pathol. 2018, 188, 2644–2652. [Google Scholar] [CrossRef]
- Jiyarom, B.; Giannakopoulos, S.; Strange, D.P.; Panova, N.; Gale, M., Jr.; Verma, S. Rig-i and mda5 are modulated by bone morphogenetic protein (bmp6) and are essential for restricting zika virus infection in human sertoli cells. Front. Microbiol. 2022, 13, 1062499. [Google Scholar] [CrossRef]
- Karadeniz, H.; Avanoglu Guler, A.; Ozger, H.S.; Yildiz, P.A.; Erbas, G.; Bozdayi, G.; Deveci Bulut, T.; Gulbahar, O.; Yapar, D.; Kucuk, H.; et al. The prognostic value of lung injury and fibrosis markers, kl-6, tgf-beta1, fgf-2 in COVID-19 patients. Biomark. Insights 2022, 17, 11772719221135443. [Google Scholar] [CrossRef]
- Laloglu, E.; Alay, H. Role of transforming growth factor-beta 1 and connective tissue growth factor levels in coronavirus disease-2019-related lung injury: A prospective, observational, cohort study. Rev. Soc. Bras. Med. Trop. 2022, 55, e06152021. [Google Scholar] [CrossRef]
- Mezger, M.C.; Conzelmann, C.; Weil, T.; von Maltitz, P.; Albers, D.P.J.; Munch, J.; Stamminger, T.; Schilling, E.M. Inhibitors of activin receptor-like kinase 5 interfere with SARS-CoV-2 s-protein processing and spike-mediated cell fusion via attenuation of furin expression. Viruses 2022, 14, 1308. [Google Scholar] [CrossRef]
- Zachar, V.; Fink, T.; Koppelhus, U.; Ebbesen, P. Role of placental cytokines in transcriptional modulation of HIV type 1 in the isolated villous trophoblast. AIDS Res. Hum. Retroviruses 2002, 18, 839–847. [Google Scholar] [CrossRef]
- Cui, H.; Li, Q.L.; Chen, J.; Na, Q.; Liu, C.X. Hepatitis b virus x protein modifies invasion, proliferation and the inflammatory response in an htr-8/svneo cell model. Oncol. Rep. 2015, 34, 2090–2098. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, X.; Li, Q.; Chen, J.; Yin, Z.; Xiao, J.; Zhang, D.; Li, W.; Qiao, Y.; Chen, S. Role of human cytomegalovirus in the proliferation and invasion of extravillous cytotrophoblasts isolated from early placentae. Int. J. Clin. Exp. Med. 2015, 8, 17248–17260. [Google Scholar]
- Busnadiego, O.; Gonzalez-Santamaria, J.; Lagares, D.; Guinea-Viniegra, J.; Pichol-Thievend, C.; Muller, L.; Rodriguez-Pascual, F. LOXL4 is induced by transforming growth factor β1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling. Mol. Cell. Biol. 2013, 33, 2388–2401. [Google Scholar] [CrossRef]
- Booth, L.; Roberts, J.L.; Cash, D.R.; Tavallai, S.; Jean, S.; Fidanza, A.; Cruz-Luna, T.; Siembiba, P.; Cycon, K.A.; Cornelissen, C.N.; et al. GRP78/BiP/HSPA5/Dna K is a universal therapeutic target for human disease. J. Cell Physiol. 2015, 230, 1661–1676. [Google Scholar] [CrossRef]
- Elfiky, A.A.; Ibrahim, I.M.; Elgohary, A.M. SARS-CoV-2 delta variant is recognized through GRP78 host-cell surface receptor, in silico perspective. Int. J. Pept. Res. Ther. 2022, 28, 146. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Gopal, U.; Austin, R.C.; Pizzo, S.V. Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders. IUBMB Life 2021, 73, 843–854. [Google Scholar] [CrossRef]
- Khongwichit, S.; Sornjai, W.; Jitobaom, K.; Greenwood, M.; Greenwood, M.P.; Hitakarun, A.; Wikan, N.; Murphy, D.; Smith, D.R. A functional interaction between GRP78 and zika virus e protein. Sci. Rep. 2021, 11, 393. [Google Scholar] [CrossRef]
- Nain, M.; Mukherjee, S.; Karmakar, S.P.; Paton, A.W.; Paton, J.C.; Abdin, M.Z.; Basu, A.; Kalia, M.; Vrati, S. GRP78 is an important host factor for Japanese encephalitis virus entry and replication in mammalian cells. J. Virol. 2017, 91, e02274-16. [Google Scholar] [CrossRef]
- Prusty, B.K.; Siegl, C.; Gulve, N.; Mori, Y.; Rudel, T. Gp96 interacts with hhv-6 during viral entry and directs it for cellular degradation. PLoS ONE 2014, 9, e113962. [Google Scholar] [CrossRef]
- Pujhari, S.; Brustolin, M.; Macias, V.M.; Nissly, R.H.; Nomura, M.; Kuchipudi, S.V.; Rasgon, J.L. Heat shock protein 70 (hsp70) mediates zika virus entry, replication, and egress from host cells. Emerg. Microbes Infect. 2019, 8, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jia, J.; Jiang, X.; Xu, M.; Guo, J.; Tang, T.; Xu, X.; Wu, Z.; Hu, B.; Yao, K.; et al. Gp96 is critical for both human herpesvirus 6a (hhv-6a) and hhv-6b infections. J. Virol. 2020, 94, e00311-20. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Skeate, J.G.; Kast, W.M. Annexin a2 in virus infection. Front. Microbiol. 2018, 9, 2954. [Google Scholar] [CrossRef] [PubMed]
- Dawar, F.U.; Tu, J.; Khattak, M.N.; Mei, J.; Lin, L. Cyclophilin a: A key factor in virus replication and potential target for anti-viral therapy. Curr. Issues Mol. Biol. 2017, 21, 1–20. [Google Scholar]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin ii binds to capsid protein vp1 of enterovirus 71 and enhances viral infectivity. J. Virol. 2011, 85, 11809–11820. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, S.; Garcia-Manso, A.; Del Barrio, G.; Dalton, K.P.; Gonzalez-Molleda, L.; Arrojo-Fernandez, J.; Nicieza, I.; Parra, F. Role of annexin a2 in cellular entry of rabbit vesivirus. J. Gen. Virol. 2009, 90, 2724–2730. [Google Scholar] [CrossRef]
- Braaten, D.; Franke, E.K.; Luban, J. Cyclophilin a is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 1996, 70, 3551–3560. [Google Scholar] [CrossRef]
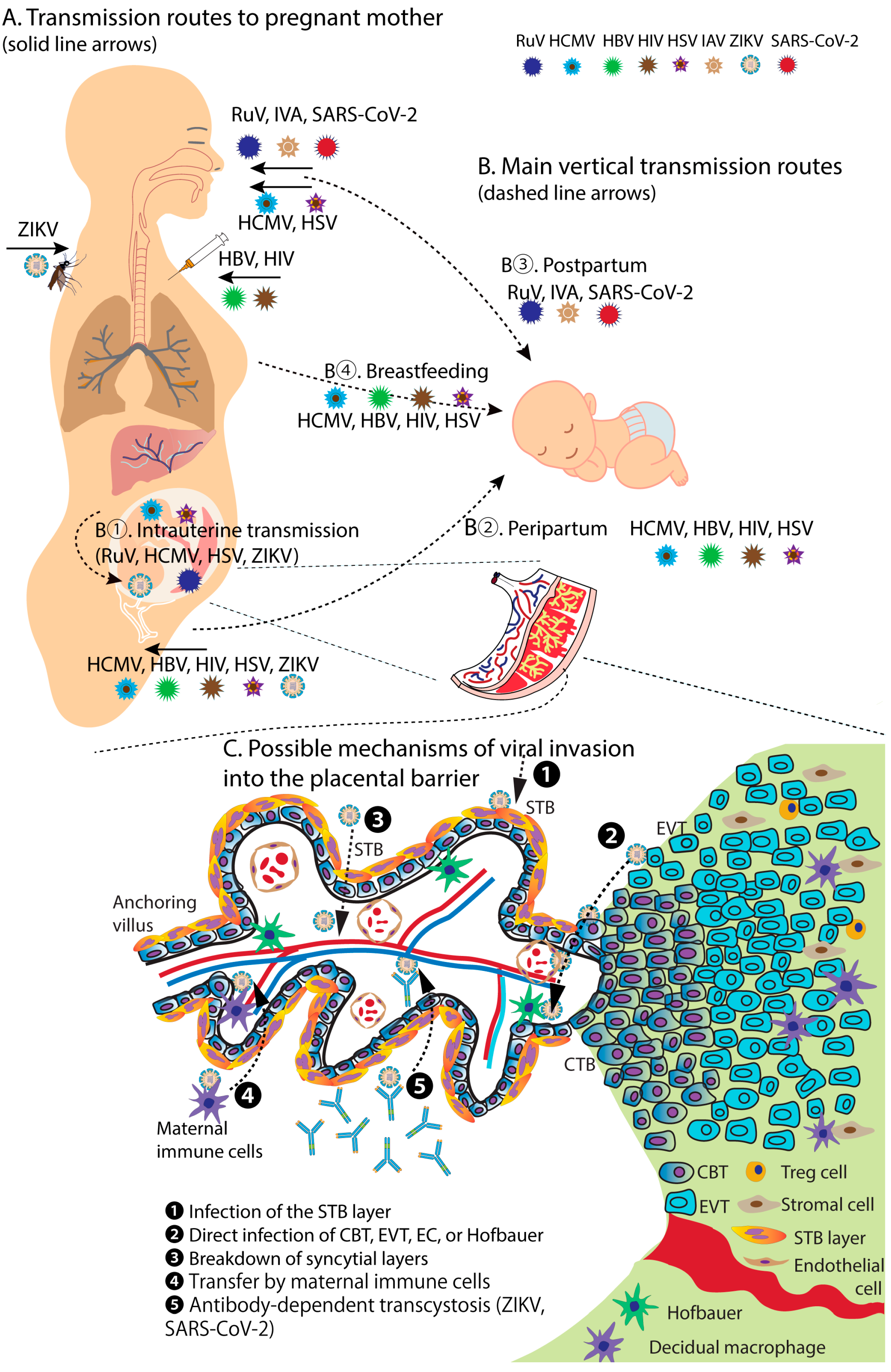
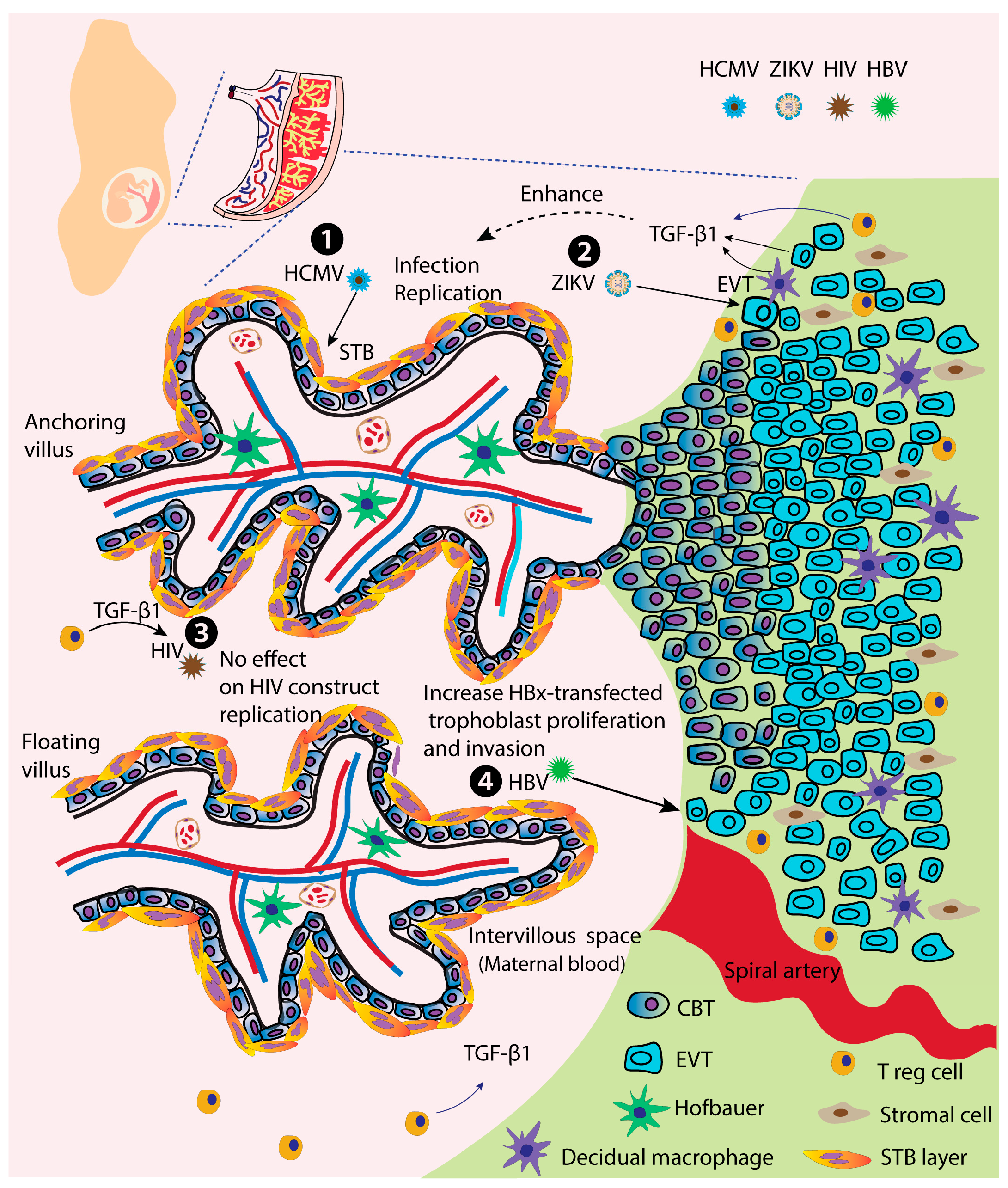
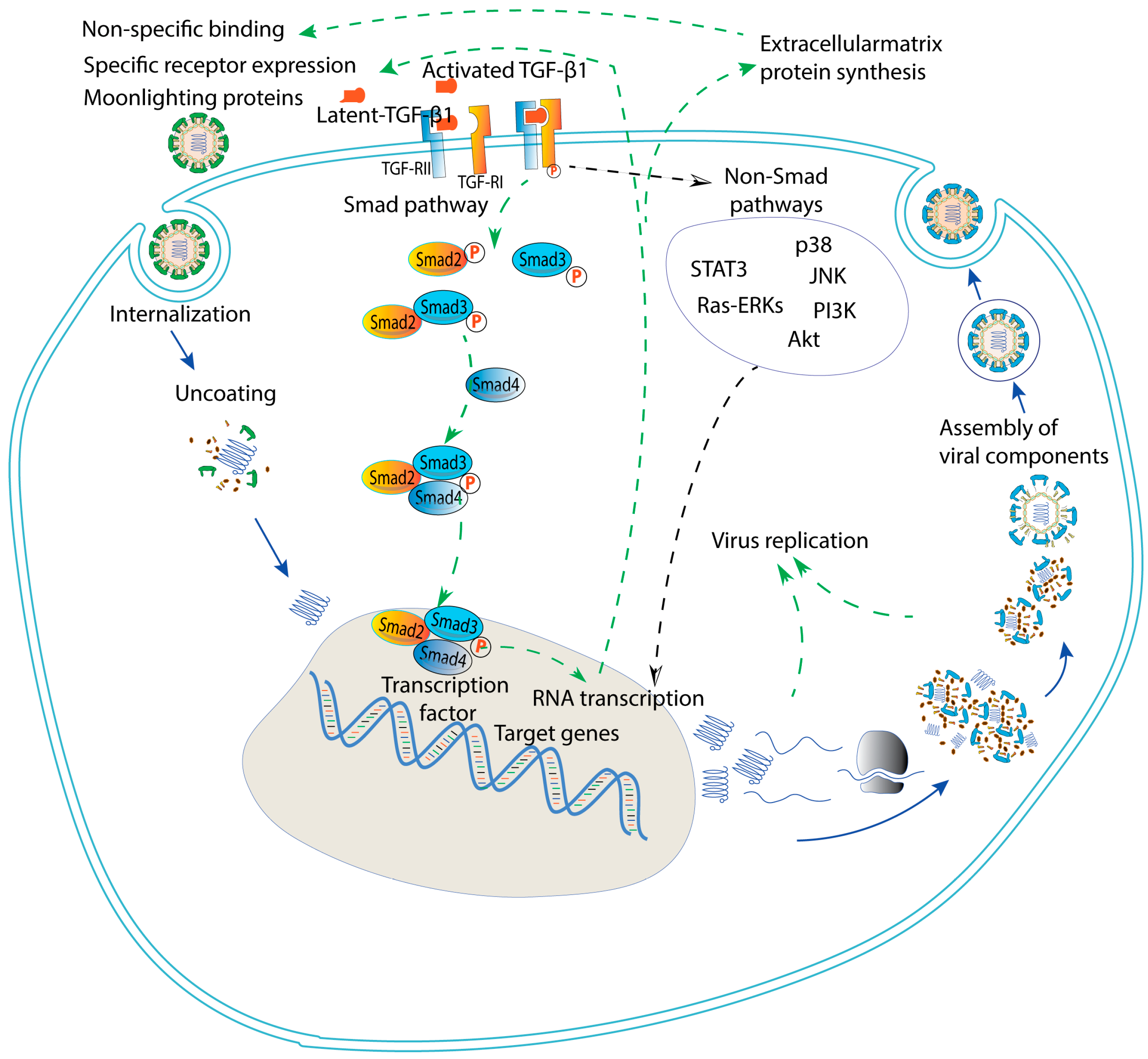
| Virus | Transmission Routes | Major Infected Cells/Organs | Pregnant and Fetal Outcomes | Vaccine Availability | Representative References |
|---|---|---|---|---|---|
| RuV | Respiratory tract. Direct or droplet contact | Respiratory mucosa and cervical lymph nodes. Others: skin, eye, brain | CRS | Yes | [48,49,50,51,52] |
| HCMV | Through bodily fluids: saliva, urine, blood, breast milk | Epithelial cells, fibroblasts, endothelial cells, and immune cells | Congenital CMV infection | No | [53,54,55,56] |
| HIV | Sexual contact, sharing injecting equipment | Immune system, primarily targeting CD4+ T cells | Increase miscarriage, stillbirth, or premature delivery. Congenital HIV infection | No | [57,58,59,60,61] |
| HBV | Sexual contact, sharing injecting equipment | Liver cells | Premature delivery or low birth weight, chronic HBV | Yes | [62,63,64,65] |
| HSV | Sexual contact, infected skin or mucous membranes | Skin and mucous membranes, nerve cells | Congenital HSV infection, lead to neurological damage, blindness, and death | No | [66,67,68,69] |
| IAV | Respiratory tract, through respiratory droplets | Primarily infects respiratory tract cells | Increased risk of pneumonia, premature delivery, or stillbirth. | Yes | [6,45,70,71,72,73] |
| ZIKV | Aedes mosquito bite, sexual contact, blood transfusion | Infects skin, lymph nodes, and other tissues including placenta | Fetal loss, stillbirth, miscarriage, CZS with brain abnormalities | No | [74,75,76,77,78] |
| SARS-CoV-2 | Respiratory droplets | Primarily infects cells in the respiratory tract | Preterm delivery, fetal distress, and stillbirth | Yes | [46,79,80,81,82,83,84,85] |
| Virus | Main Routes of Vertical Transmission | Representative References |
|---|---|---|
| RuV | The virus can cross the placenta. Transplacental infection can occur at any stage of pregnancy, highest incidence during the first trimester (organogenesis period) | [9,92,93] |
| HCMV | Placental and perinatal transmissions, especially if the mother has a primary infection during pregnancy or at the time of delivery; through breastfeeding | [55,93,94,95] |
| HIV | The majority of MTCT of HIV occurs during delivery or through breastfeeding | [57,58,96,97,98] |
| HBV | Perinatal transmission during delivery is the primary route | [62,99,100,101] |
| HSV | Any stage of pregnancy, highest during delivery when the fetus passes through the infected birth canal | [68,102,103,104,105] |
| IAV | Through respiratory secretions. The risk of vertical transmission is low compared to other viruses | [3,45,106,107] |
| ZIKV | Can cross the placenta. Vertically transplacental infection is highest during the first and second trimesters of pregnancy | [75,76,90,108] |
| SARS-CoV-2 | Risk of vertical transmission is generally low. Higher in certain situations: severe maternal COVID-19, infected close to the time of delivery | [79,83,109,110] |
| Virus | Type of Studies, Involved Organs/Cell Types | Effects/Roles | Reference |
|---|---|---|---|
| RuV | In vitro, lung epithelial cells | Increase the virus binding and infection in A549 cells | [20] |
| HCMV | In vitro, renal tubular epithelial cells and umbilical vein endothelial cells | Increased expression and activation of TGF-β1 by HCMV infection | [225,226] |
| HIV | Ex vivo, bronchial epithelial cells. In vitro, macrophages | Increase CXCR4 expression in macrophages, increase the viral burden in bronchial epithelial cells | [24,25,232] |
| HBV | In vitro, hepatocellular carcinoma cells | Inhibit the expression of HBsAg and HBeAg, and suppress HBV replication in HepG2 cells | [28,30] |
| HSV | Ex vivo, human cornea organotypic culture | Enhance HSV-1 replication in 3-dimensional human corneal keratocytes | [247] |
| IAV | In vitro, lung epithelial cells | Inhibit apoptosis induced by IAV infection on A549 cells | [251] |
| ZIKV | In vitro, Sertoli cells | Not affect ZIKV replication in human Sertoli cells | [253] |
| SARS-CoV-2 | In vitro, airway epithelial cells | Increase furin expression leading to enhanced susceptibility to SARS-CoV-2 | [31,256] |
| Virus | Type of Studies, Organs/Cells Involved, Pregnancy Period | Effects/Roles | Reference |
|---|---|---|---|
| RuV | NR | NR | |
| HCMV | In vitro, STB | TGF-β1 and IL-8 promote HCMV replication in STB | [26] |
| HIV | In vitro, STB | Not increase HIV construct replication | [257] |
| HBV | In vitro, first-trimester trophoblast HTR-8/SVneo cells | Increases HBx-transfected HTR-8/SVneo cell proliferation and invasion | [258] |
| HSV | NR | NR | |
| IAV | NR | NR | |
| ZIKV | In vitro, first-trimester tropho-blast cells | Increase the virus binding and replication in trophoblasts | [34] |
| SARS-CoV-2 | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, Q.D.; Pham, N.T.K.; Takada, K.; Ushijima, H.; Komine-Aizawa, S.; Hayakawa, S. Roles of TGF-β1 in Viral Infection during Pregnancy: Research Update and Perspectives. Int. J. Mol. Sci. 2023, 24, 6489. https://doi.org/10.3390/ijms24076489
Trinh QD, Pham NTK, Takada K, Ushijima H, Komine-Aizawa S, Hayakawa S. Roles of TGF-β1 in Viral Infection during Pregnancy: Research Update and Perspectives. International Journal of Molecular Sciences. 2023; 24(7):6489. https://doi.org/10.3390/ijms24076489
Chicago/Turabian StyleTrinh, Quang Duy, Ngan Thi Kim Pham, Kazuhide Takada, Hiroshi Ushijima, Shihoko Komine-Aizawa, and Satoshi Hayakawa. 2023. "Roles of TGF-β1 in Viral Infection during Pregnancy: Research Update and Perspectives" International Journal of Molecular Sciences 24, no. 7: 6489. https://doi.org/10.3390/ijms24076489
APA StyleTrinh, Q. D., Pham, N. T. K., Takada, K., Ushijima, H., Komine-Aizawa, S., & Hayakawa, S. (2023). Roles of TGF-β1 in Viral Infection during Pregnancy: Research Update and Perspectives. International Journal of Molecular Sciences, 24(7), 6489. https://doi.org/10.3390/ijms24076489





