Comparison between Three Different Techniques for the Detection of EGFR Mutations in Liquid Biopsies of Patients with Advanced Stage Lung Adenocarcinoma
Abstract
1. Introduction
2. Results
2.1. Patient Cohort
2.2. Mutation Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Mutation Testing
4.2.1. Tissue DNA Isolation and Screening
4.2.2. ctDNA Isolation from Plasma
4.2.3. ctDNA Screening: Therascreen®
4.2.4. ctDNA Screening: Idylla™
4.2.5. ctDNA Screening: Next-Generation Sequencing (NGS)
4.2.6. Limits of Detection of the Three Mutation Assays
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 15 December 2022).
- Wu, J.; Lin, Z. Non-small cell lung cancer targeted therapy: Drugs and mechanisms of drug resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiong, Y.; Zeng, Y.; Wang, Y.; Zeng, J.; Liu, J.; Xu, S.; Li, L.S. Current status of immunotherapy for non-small cell lung cancer. Front. Pharmacol. 2022, 13, 989461. [Google Scholar] [CrossRef] [PubMed]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular epidemiology of the main druggable genetic alterations in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Baumdick, M.; Gelléri, M.; Uttamapinant, C.; Beránek, V.; Chin, J.W.; Bastiaens, P.I.H. A conformational sensor based on genetic code expansion reveals an autocatalytic component in EGFR activation. Nat. Commun. 2018, 9, 3847. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in cancer: Signaling mechanisms, drugs, and acquired resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Attili, I.; Passaro, A.; Pisapia, P.; Malapelle, U.; de Marinis, F. Uncommon EGFR compound mutations in non-small cell lung cancer (NSCLC): A systematic review of available evidence. Curr. Oncol. 2022, 29, 255–266. [Google Scholar] [CrossRef]
- Hayashi, H.; Nadal, E.; Gray, J.E.; Ardizzoni, A.; Caria, N.; Puri, T.; Grohe, C. Overall treatment strategy for patients with metastatic NSCLC with activating EGFR mutations. Clin. Lung Cancer 2022, 23, e69–e82. [Google Scholar] [CrossRef]
- Kitadai, R.; Okuma, Y. Treatment strategies for non-small cell lung cancer harboring common and uncommon EGFR mutations: Drug sensitivity based on exon classification, and ctdructure-function analysis. Cancers 2022, 14, 2519. [Google Scholar] [CrossRef]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Paliogiannis, P.; Attene, F.; Cossu, A.; Defraia, E.; Porcu, G.; Carta, A.; Sotgiu, M.I.; Pazzola, A.; Cordero, L.; Capelli, F.; et al. Impact of tissue type and content of neoplastic cells of samples on the quality of epidermal growth factor receptor mutation analysis among patients with lung adenocarcinoma. Mol. Med. Rep. 2015, 12, 187–191. [Google Scholar] [CrossRef]
- Nagano, T.; Tachihara, M.; Nishimura, Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells 2018, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Hou, Z.; Zhang, Y.; Jiang, B. Drug resistance mechanisms and progress in the treatment of EGFR-mutated lung adenocarcinoma. Oncol. Lett. 2022, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, E.; De Carlo, E.; Del Conte, A.; Stanzione, B.; Revelant, A.; Fassetta, K.; Spina, M.; Bearz, A. Acquired resistance to Osimertinib in EGFR-mutated non-small cell lung cancer: How do we overcome it? Int. J. Mol. Sci. 2022, 23, 6936. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.G.; Vallée, A.; Théoleyre, S. EGFR T790M resistance mutation in non small-cell lung carcinoma. Clin. Chim. Acta 2015, 444, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.A.; Han, J.Y.; Ahn, M.J.; Ramalingam, S.S.; Delmonte, A.; Hsia, T.C.; Laskin, J.; Kim, S.W.; He, Y.; Tsai, C.M.; et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: Osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer 2020, 126, 373–380. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, L.; Wang, Z.; Duan, J.; Bai, H.; Wang, J. The Status of the EGFR T790M Mutation is associated with the Clinical Benefits of Osimertinib Treatment in Non-small Cell Lung Cancer Patients: A Meta-Analysis. J. Cancer 2020, 11, 3106–3113. [Google Scholar] [CrossRef]
- Santoni-Rugiu, E.; Melchior, L.C.; Urbanska, E.M.; Jakobsen, J.N.; Stricker, K.; Grauslund, M. Intrinsic resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant non small cell lung cancer: Differences and similarities with acquired resistance. Cancers 2019, 11, 92. [Google Scholar] [CrossRef]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef]
- Wang, S.; Tsui, S.T.; Liu, C.; Song, Y.; Liu, D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 59. [Google Scholar] [CrossRef]
- Ma, L.; Chen, R.; Wang, F.; Ma, L.L.; Yuan, M.M.; Chen, R.R.; Liu, J. EGFR L718Q mutation occurs without T790M mutation in a lung adenocarcinoma patient with acquired resistance to osimertinib. Ann. Transl. Med. 2019, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Noor, Z.S.; Remon, J. Are liquid biopsies a surrogate for tissue EGFR testing? Ann. Oncol. 2018, 29 (Suppl. 1), i38–i46. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Ramos, I.; Trigo, J.M.; Palka, M.; Gomez-Rueda, A.; Jantus-Lewintre, E.; Camps, C.; Isla, D.; Iranzo, P.; Ponce-Aix, S.; et al. Clinical utility of plasma-based digital next-generation sequencing in patients with advance-stage lung adenocarcinomas with insufficient tumor samples for tissue genotyping. Ann. Oncol. 2019, 30, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Swalduz, A.; Jovelet, C. Clinical relevance of an amplicon-based liquid biopsy for detecting ALK and ROS1 fusion and resistance mutations in patients with non-small-cell lung cancer. JCO Precis. Oncol. 2020, 4, 272–282. [Google Scholar] [CrossRef]
- Passiglia, F.; Rizzo, S.; Di Maio, M.; Galvano, A.; Badalamenti, G.; Listì, A.; Gulotta, L.; Castiglia, M.; Fulfaro, F.; Bazan, V.; et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 13379. [Google Scholar] [CrossRef]
- Pesta, M.; Shetti, D.; Kulda, V.; Knizkova, T.; Houfkova, K.; Bagheri, M.S.; Svaton, M.; Polivka, J. Applications of liquid biopsies in non-small-cell lung cancer. Diagnostics 2022, 12, 1799. [Google Scholar] [CrossRef]
- Syed, Y.Y. Therascreen® EGFR RGQ PCR kit: A Companion diagnostic for Afatinib and Gefitinib in non-small cell lung cancer. Mol Diagn Ther. 2016, 20, 191–198. [Google Scholar] [CrossRef]
- Gao, G.; Ren, S.; Li, A.; Xu, J.; Xu, Q.; Su, C. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int. J. Cancer 2012, 131, E822–E829. [Google Scholar] [CrossRef]
- Colombino, M.; Paliogiannis, P.; Cossu, A.; Santeufemia, D.A.; Sardinian Lung Cancer (SLC) Study Group; Sini, M.C.; Casula, M.; Palomba, G.; Manca, A.; Pisano, M.; et al. EGFR, KRAS, BRAF, ALK, and cMET genetic alterations in 1440 Sardinian patients with lung adenocarcinoma. BMC Pulm. Med. 2019, 19, 209. [Google Scholar] [CrossRef]
- Soria, J.C.; Wu, Y.L.; Nakagawa, K.; Kim, S.W.; Yang, J.J.; Ahn, M.J. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): A phase 3 randomised trial. Lancet Oncol. 2015, 16, 990–998. [Google Scholar] [CrossRef]
- Lu, S.; Shih, J.Y.; Jang, T.W.; Liam, C.K.; Yu, Y. Afatinib as first-line treatment in Asian patients with EGFR mutation-positive NSCLC: A narrative review of real-world evidence. Adv. Ther. 2021, 38, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.L.; Heideman, D.A.; Thunnissen, E.; Paul, M.A.; van Wijk, A.W.; Postmus, P.E.; Smit, E.F. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer 2014, 85, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Gaspar, C.; Pina, M.; Azevedo, I.; Rodrigues, A. Real-World T790M Mutation Frequency and Impact of Rebiopsy in Patients with EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Cureus 2020, 12, e12128. [Google Scholar] [CrossRef]
- Del Re, M.; Petrini, I.; Mazzoni, F.; Valleggi, S.; Gianfilippo, G.; Pozzessere, D.; Chella, A.; Crucitta, S.; Rofi, E.; Restante, G.; et al. Incidence of T790M in Patients with NSCLC Progressed to Gefitinib, Erlotinib, and Afatinib: A Study on Circulating Cell-free DNA. Clin. Lung Cancer 2020, 21, 232–237. [Google Scholar] [CrossRef]
- Ikushima, H.; Sakatani, T.; Usui, K. Clinical Features of Patients with an Epidermal Growth Factor Receptor T790M Mutation Detected in Circulating Tumor DNA. Oncology 2020, 98, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Tsai, T.H.; Wu, S.G.; Liu, Y.N.; Yu, C.J.; Shih, J.Y. Complex EGFR mutations with secondary T790M mutation confer shorter osimertinib progression-free survival and overall survival in advanced non-small cell lung cancer. Lung Cancer 2020, 145, 1–9. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Colombino, M.; Sini, M.C.; Manca, A.; Casula, M.; Palomba, G.; Pisano, M.; Doneddu, V.; Zinellu, A.; Santeufemia, D.; et al. Global prognostic impact of driver genetic alterations in patients with lung adenocarcinoma: A real-life study. BMC Pulm. Med. 2022, 22, 32. [Google Scholar] [CrossRef]
- Pinto, C.; Biffoni, M.; Popoli, P.; Marchetti, A.; Marchetti, P.; Martini, N.; Normanno, N. Molecular tests and target therapies in oncology: Recommendations from the Italian workshop. Future Oncol. 2021, 17, 3529–3539. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Otake, S.; Ohyama, H.; Amemiya, K.; Higuchi, R.; Oyama, T.; Mochizuki, H.; Goto, T.; Omata, M. Dual-molecular barcode sequencing detects rare variants in tumor and cell free DNA in plasma. Sci. Rep. 2020, 10, 3391. [Google Scholar] [CrossRef]
- Heeke, S.; Hofman, V.; Benzaquen, J.; Otto, J.; Tanga, V.; Zahaf, K.; Allegra, M.; Long-Mira, E.; Lassalle, S.; Marquette, C.H.; et al. Detection of EGFR mutations from plasma of NSCLC patients using an automatic cartridge-based PCR system. Front. Pharmacol. 2021, 12, 657743. [Google Scholar] [CrossRef]
- Ahmad, E.; Ali, A.; Nimisha; Kumar Sharma, A.; Ahmed, F.; Mehdi Dar, G.; Mohan Singh, A.; Apurva; Kumar, A.; Athar, A.; et al. Molecular approaches in cancer. Clin. Chim. Acta 2022, 537, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Colombino, M.; Casula, M.; Manca, A.; Mandalà, M.; Cossu, A.; Italian Melanoma Intergroup (IMI). Molecular pathways in melanomagenesis: What we learned from next-generation sequencing approaches. Curr. Oncol. Rep. 2018, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, M.; Singh, R.R.; Chen, W.; Huang, R.S.P.; Almohammedsalim, A.A.; Barkoh, B.A.; Simien, C.M.; Hernandez, M.; Behrens, C.; Patel, K.P.; et al. Study of preanalytic and analytic variables for clinical next-generation sequencing of circulating cell-free nucleic acid. J. Mol. Diagn. 2017, 19, 514–524. [Google Scholar] [CrossRef] [PubMed]
- MacConaill, L.E.; Burns, R.T.; Nag, A.; Coleman, H.A.; Slevin, M.K.; Giorda, K.; Light, M.; Lai, K.; Jarosz, M.; McNeill, M.S.; et al. Unique, dual-indexed sequencing adapters with UMIs effectively eliminate index cross-talk and significantly improve sensitivity of massively parallel sequencing. BMC Genomics 2018, 19, 30. [Google Scholar] [CrossRef]
- Kivioja, T.; Vaharautio, A.; Karlsson, K.; Bonke, M.; Enge, M.; Linnarsson, S.; Taipale, J. Counting absolute numbers of molecules using unique molecular identifiers. Nat. Methods 2011, 9, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Jantus-Lewintre, E.; García-Peláez, B.; Royuela, A.; Insa, A.; Cruz, P. Comprehensive cross-platform comparison of methods for noninvasive EGFR mutation testing: Results of the RING observational trial. Mol. Oncol. 2021, 15, 43–56. [Google Scholar] [CrossRef]
- Caputo, A.; D’Ardia, A.; Sabbatino, F.; Picariello, C.; Ciaparrone, C.; Zeppa, P.; D’Antonio, A. Testing EGFR with Idylla on cytological specimens of lung cancer: A Review. Int. J. Mol. Sci. 2021, 22, 4852. [Google Scholar] [CrossRef] [PubMed]
- Simarro, J.; Pérez-Simó, G.; Mancheño, N.; Ansotegui, E.; Muñoz-Núñez, C.F.; Gómez-Codina, J.; Juan, Ó.; Palanca, S. Technical validation and clinical implications of ultrasensitive PCR approaches for EGFR-Thr790Met mutation detection in pretreatment FFPE samples and in liquid biopsies from non-small cell lung cancer patients. Int. J. Mol. Sci. 2022, 23, 8526. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Hughes, J.; van den Hout, A.; Burns, C.; Woodhouse, R.; Dennis, L.; Hegde, P.; Oxnard, G.R.; Vietz, C. Driver mutation variant allele frequency in circulating tumor DNA and association with clinical outcome in patients with non-small cell lung cancer and EGFR- and KRAS-mutated tumors. J. Mol. Diagn. 2022, 24, 543–553. [Google Scholar] [CrossRef]
- Fadda, G.M.; Lobrano, R.; Casula, M.; Pisano, M.; Pazzola, A.; Cossu, A.; Palmieri, G.; Paliogiannis, P. Liquid biopsy in the oncological management of a histologically undiagnosed lung carcinoma: A case report. J. Pers. Med. 2022, 12, 1874. [Google Scholar] [CrossRef]
- Tsongalis, G.J.; Al Turkmani, M.R.; Suriawinata, M.; Babcock, M.J.; Mitchell, K.; Ding, Y.; Scicchitano, L.; Tira, A.; Buckingham, L.; Atkinson, S.; et al. Comparison of tissue molecular biomarker testing turnaround times and concordance between standard of care and the Biocartis Idylla platform in patients with colorectal cancer. Am. J. Clin. Pathol. 2020, 154, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Mokany, E.; Tan, Y.L.; Bone, S.M.; Fuery, C.J.; Todd A, V. MNAzyme qPCR with superior multiplexing capacity. Clin. Chem. 2013, 59, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.Y.; Walker, S.M.; Lonergan, T.; Lima, N.E.; Todd, A.V.; Mokany, E. Superior multiplexing capacity of PlexPrimers enables sensitive and specific detection of SNPs and clustered mutations in qPCR. PLoS ONE 2017, 12, e0170087. [Google Scholar] [CrossRef] [PubMed]
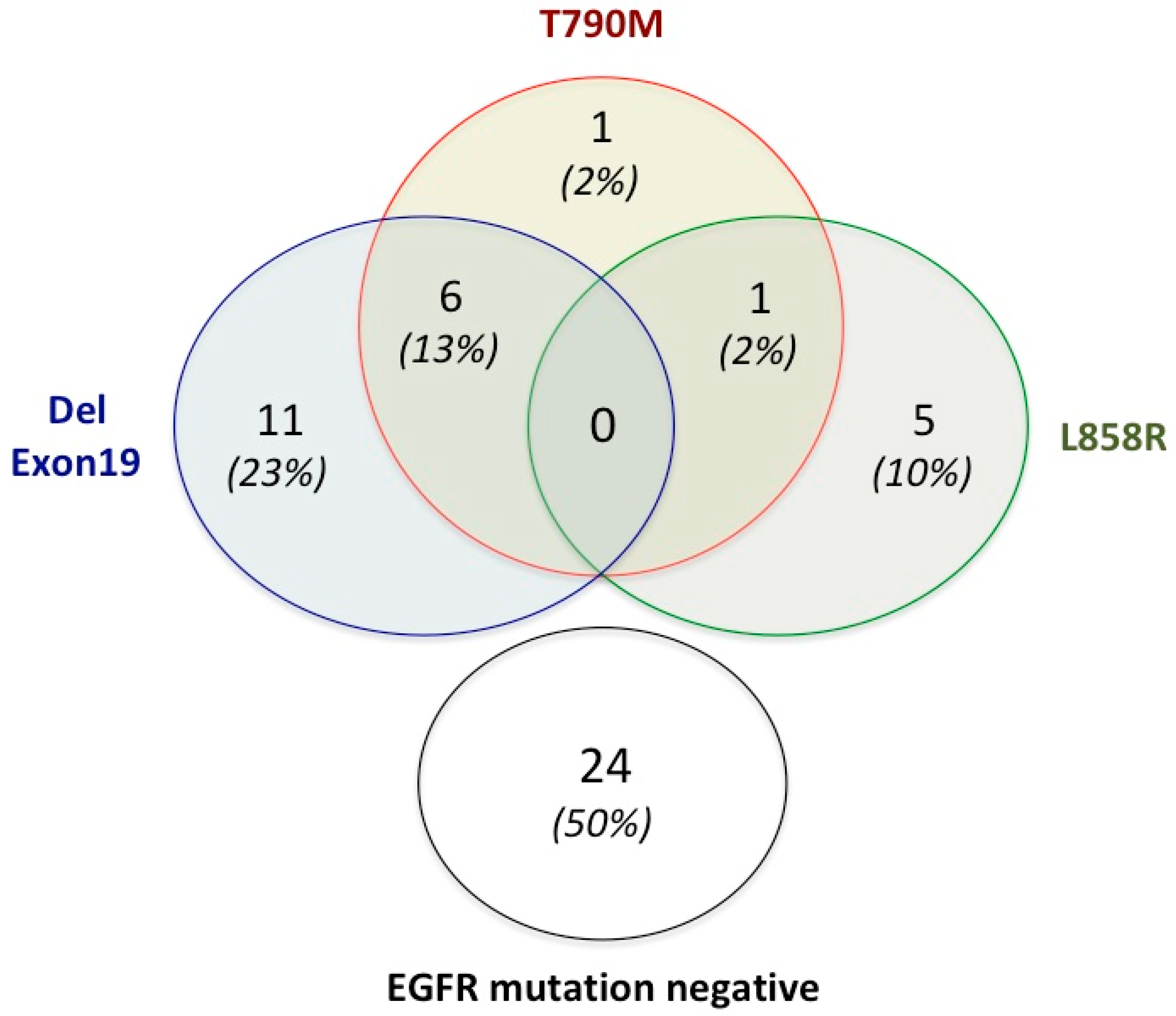

| Characteristics | Total of Patients (%) |
|---|---|
| Age at diagnosis, years | Median 70 (range 41–85) |
| Gender | |
| Male Female | 13 (27.1) 35 (72.9) |
| Smoking status | |
| Never Smokers Former Smokers Smokers | 34 (70.8) 5 (10.4) 9 (18.8) |
| Disease Stage | |
| IIIB IV | 20 (41.7) 28 (58.3) |
| Disease sites | |
| Single location Multiple locations With CNS involvement Without CNS involvement | 29 (60.4) 19 (39.6) 4 (8.3) 15 (31.3) |
| Type of treatment | |
| First-generation EGFR TKI (Gefitinib) Second-generation EGFR TKI (Afatinib) | 41 (85.4) 7 (14.6) |
| EGFR Mutation | FFPE (n. %) | ctDNA+ (n. %) | ctDNA− (n. %) |
|---|---|---|---|
| Exon 18 G719A/S | 3 (6.2%) | ND | ND |
| Exon 19 Deletions | 31 (64.6%) | 20 (64.5%) | 11 (35.5%) |
| Exon 21 L858R | 12 (25.0%) | 7 (58.3%) | 5 (41.7%) |
| Exon 21 L861Q | 2 (4.2%) | ND | ND |
| Exon 19 Deletions | L858R | ||||||
|---|---|---|---|---|---|---|---|
| Metastasis | Patients | FFPE | ctDNA+ | p | FFPE | ctDNA+ | p |
| 1 site | 29 (60.4%) | 18 (62) | 10/18 (56) | 0.402 | 8 (28) | 4/8 (50) | 0.394 |
| ≥2 sites | 19 (39.6%) | 13 (68) | 10/13 (77) | 0.418 | 4 (21) | 3/4 (75) | 0.270 |
| Stage | Patients | FFPE | ctDNA+ | FFPE | ctDNA+ | ||
| IIIB | 20 (41.7%) | 11 (55) | 7/11 (64) | 0.426 | 8 (40) | 4/8 (50) | 0.690 |
| IV | 28 (58.3%) | 20 (71) | 13/20 (65) | 0.385 | 4 (14) | 3/4 (75) | 0.150 |
| Sample | Thera Screen | Idylla | % Agreement Idylla with Therascreen | p | NGS | % Agreement NGS with Therascreen | p |
|---|---|---|---|---|---|---|---|
| Total ctDNA | 54 | 49 | 90.7 | 0.057 | 40 | 74.1 | <0.001 |
| ctDNA+ | 27 | 23 | 85.2 | 0.111 | 13 | 48.1 | <0.001 |
| ctDNA− | 27 | 26 | 96.3 | 1.000 | 27 | 100 | 1.000 |
| EGFR Alteration | Therascreen | Idylla | Agreement % | p |
|---|---|---|---|---|
| T790M mutation | 8/54 | 8/54 | 100 | 1.000 |
| Exon19 deletion | 27/54 | 23/54 | 85.2 | 0.562 |
| L858R mutation | 17/54 | 18/54 | 94.4 | 1.000 |
| EGFR alteration | Therascreen | NGS | ||
| T790M mutation | 8/54 | 3/54 | 37.5 | 0.201 |
| Exon19 deletion | 27/54 | 20/54 | 74.1 | 0.244 |
| L858R mutation | 17/54 | 15/54 | 88.2 | 0.833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casula, M.; Pisano, M.; Paliogiannis, P.; Colombino, M.; Sini, M.C.; Zinellu, A.; Santeufemia, D.; Manca, A.; Casula, S.; Tore, S.; et al. Comparison between Three Different Techniques for the Detection of EGFR Mutations in Liquid Biopsies of Patients with Advanced Stage Lung Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 6410. https://doi.org/10.3390/ijms24076410
Casula M, Pisano M, Paliogiannis P, Colombino M, Sini MC, Zinellu A, Santeufemia D, Manca A, Casula S, Tore S, et al. Comparison between Three Different Techniques for the Detection of EGFR Mutations in Liquid Biopsies of Patients with Advanced Stage Lung Adenocarcinoma. International Journal of Molecular Sciences. 2023; 24(7):6410. https://doi.org/10.3390/ijms24076410
Chicago/Turabian StyleCasula, Milena, Marina Pisano, Panagiotis Paliogiannis, Maria Colombino, Maria Cristina Sini, Angelo Zinellu, Davide Santeufemia, Antonella Manca, Stefania Casula, Silvia Tore, and et al. 2023. "Comparison between Three Different Techniques for the Detection of EGFR Mutations in Liquid Biopsies of Patients with Advanced Stage Lung Adenocarcinoma" International Journal of Molecular Sciences 24, no. 7: 6410. https://doi.org/10.3390/ijms24076410
APA StyleCasula, M., Pisano, M., Paliogiannis, P., Colombino, M., Sini, M. C., Zinellu, A., Santeufemia, D., Manca, A., Casula, S., Tore, S., Lobrano, R., Sardinian Lung Cancer Study Group, Cossu, A., & Palmieri, G. (2023). Comparison between Three Different Techniques for the Detection of EGFR Mutations in Liquid Biopsies of Patients with Advanced Stage Lung Adenocarcinoma. International Journal of Molecular Sciences, 24(7), 6410. https://doi.org/10.3390/ijms24076410









