miR-33a and Its Association with Lipid Profile in Patients with Carotid Atherosclerosis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Validated 20X primers for hsa-miR: miR-33a-5p, miR-33a-3p (ThermoFischerScientific, Waltham, MA, USA)
- Leukocyte RNA Purification Plus Kit (NORGEN Biotec Corp., Thorold, ON, Canada)
- TaqMan™ Advanced miRNA cDNA Synthesis Kit (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA)
- Real-time CFX96 Touch amplifier (BioRaD, Hercules, CA, USA)
Statistics
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Ono, K. Functions of microRNA-33a/b and microRNA therapeutics. J. Cardiol. 2015, 67, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.; Sheedy, F.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; van Gils, J.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Raskurazhev, A.A.; Kuznetsova, P.I.; Shabalina, A.A.; Tanashyan, M.M. MicroRNA and Hemostasis Profile of Carotid Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 10974. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Béjot, Y.; Cabrejo, L.; Cha, J.-K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2020, 382, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Chung, J.-W.; Jang, H.-S.; Lee, J.; Hong, K.-S.; Bang, O.Y.; Kim, G.-M.; Seo, W.-K. Achieved low-density lipoprotein cholesterol level and stroke risk: A meta-analysis of 23 randomised trials. Eur. J. Prev. Cardiol. 2019, 28, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibanez, B.; Fernández-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991, Erratum in J. Am. Coll. Cardiol. 2018, 71, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Torres-Paz, Y.E.; Huesca-Gómez, C.; Sánchez-Muñoz, F.; Martínez-Alvarado, R.; Soto, M.E.; Torres-Tamayo, M.; Fuentevilla-Álvarez, G.; Gamboa, R. Increased expression of miR-33a in monocytes from Mexican hypertensive patients in elevated carotid intima-media thickness. J. Hum. Hypertens. 2018, 32, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Qin, S.; Chen, Y.; Liu, H.-Y.; Yuan, E.; Deng, H.; Liu, S.-M. B-RCA revealed circulating miR-33a/b associates with serum cholesterol in type 2 diabetes patients at high risk of ASCVD. Diabetes Res. Clin. Pract. 2018, 140, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Rotllan, N.; Canfrán-Duque, A.; Zhang, X.; Pati, P.; Arias, N.; Moen, J.; Mayr, M.; Ford, D.A.; Baldán, Á.; et al. Genetic Dissection of the Impact of miR-33a and miR-33b during the Progression of Atherosclerosis. Cell Rep. 2017, 21, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Lei, H.; Liu, Q.; Chen, Y.; Zhao, L.; Luo, S.; Zuo, Z.; He, Q.; Huang, W.; Zhang, N.; et al. Effects of miR-33a-5P on ABCA1/G1-Mediated Cholesterol Efflux under Inflammatory Stress in THP-1 Macrophages. PLoS ONE 2014, 9, e109722. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, M. Is microRNA-33 an Appropriate Target in the Treatment of Atherosclerosis? Nutrients 2023, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Raskurazhev, A.A.; Tanashyan, M.M.; Shabalina, A.A.; Kuznetsova, P.I.; Kornilova, A.A.; Burmak, A.G. Micro-RNA in Patients with Carotid Atherosclerosis. Hum. Physiol. 2020, 46, 880–885. [Google Scholar] [CrossRef]

| Study Population (n = 61) | LDL < 1.8 mmol/L (n = 26) | LDL ≥ 1.8 mmol/L (n = 35) | p * | |
|---|---|---|---|---|
| Male, n (%) | 34 (55.7) | 12 (46.2) | 22 (62.9) | 0.30 |
| Age, years (median [Q1; Q3]) | 66.0 [61.0; 71.0] | 66.0 [62.0; 73.3] | 65 [61.0; 70.0] | 0.46 |
| BMI, kg/m2 (median [Q1; Q3]) | 27.2 [25.5; 29.4] | 26.4 [24.6; 27.6] | 27.7 [26.3; 29.8] | 0.03 |
| Carotid stenosis degree, % | 50.0 [35.0; 70.0] | 50.0 [36.3; 70.0] | 55.0 [37.5; 70.0] | 0.60 |
| Smokers, n (%) | 21 (34.4) | 9 (34.6) | 12 (34.3) | >0.99 |
| Stroke, n (%) | 20 (32.8) | 10 (38.5) | 10 (28.6) | 0.59 |
| AH, n (%) | 56 (91.8) | 25 (96.2) | 31 (88.6) | 0.55 |
| CHD, n (%) | 24 (39.3) | 11 (42.3) | 13 (37.1) | 0.89 |
| MI, n (%) | 10 (16.4) | 8 (30.8) | 2 (5.7) | 0.02 |
| DM, n (%) | 26 (42.6) | 14 (53.8) | 12 (34.3) | 0.21 |
| AF, n (%) | 7 (11.5) | 6 (23.1) | 1 (2.9) | 0.04 |
| ASA, n (%) | 55 (90.2) | 22 (84.6) | 33 (94.3) | 0.41 |
| Anticoagulants, n (%) | 13 (21.3) | 7 (26.9) | 6 (17.1) | 0.54 |
| Statins, n (%) | 50 (82.0) | 21 (80.8) | 29 (82.9) | >0.99 |
| Study Population (n = 61) | LDL < 1.8 mmol/L (n = 26) | LDL ≥ 1.8 mmol/L (n = 35) | p * | |
|---|---|---|---|---|
| LDL, mmol/L | 2.0 [1.4; 2.7] | 1.0 [0.96; 1.5] | 2.6 [2.2; 2.9] | N/A |
| HDL, mmol/L | 1.7 [1.3; 2.1] | 1.5 [1.3; 2.2] | 1.7 [1.5; 2.1] | 0.31 |
| Total cholesterol, mmol/L | 4.8 [4.2; 6.0] | 4.4 [4.1; 5.0] | 5.2 [4.6; 6.9] | 0.006 |
| TG, mmol/L | 1.4 [1.0; 2.0] | 1.3 [0.9; 1.8] | 1.8 [1.1;2.1] | 0.06 |
| Study Population (n = 61) | LDL < 1.8 mmol/L (n = 26) | LDL ≥ 1.8 mmol/L (n = 35) | p * | |
|---|---|---|---|---|
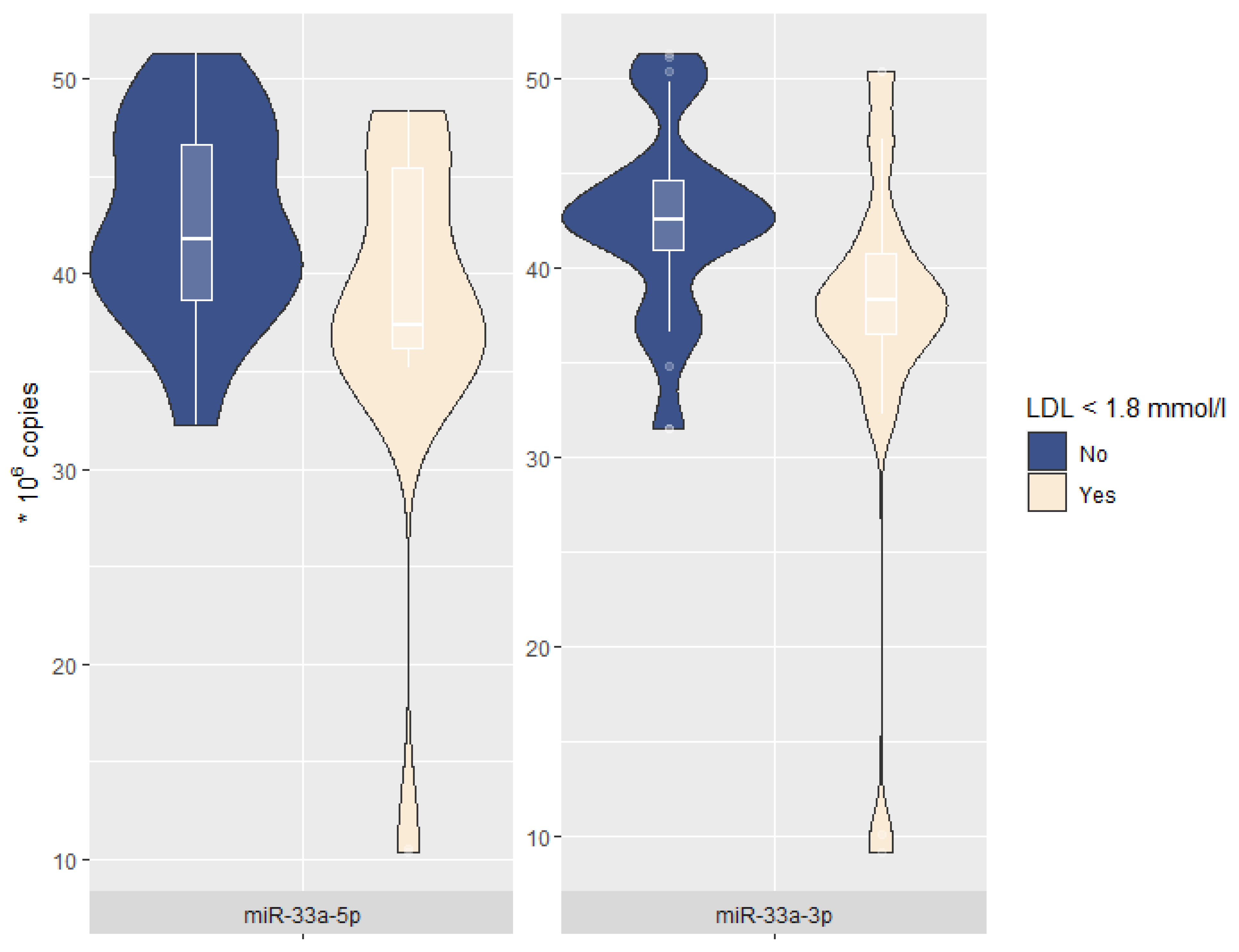
| miR-33a-5p, * 106 copies | 41.3 [36.8; 46.3] | 37.4 [36.2; 45.4] | 41.8 [38.7; 46.6] | 0.008 |
| miR-33a-3p, * 106 copies | 41.3 [36.9; 43.6] | 38.3 [36.5; 40.8] | 42.5 [40.9; 44.6] | 0.002 |
| miR-33a-5p | p | miR-33a-3p | p | |
|---|---|---|---|---|
| LDL | 0.03 [0.005–0.059] | 0.02 | 0.03 [0.007–0.061] | 0.02 |
| HDL | 0.006 [−0.010–0.022] | 0.47 | 0.004 [−0.012–0.020] | 0.60 |
| Total cholesterol | 0.010 [−0.040–0.060] | 0.69 | 0.014 [−0.035–0.064] | 0.58 |
| TG | 0.021 [−0.002–0.044] | 0.08 | 0.021 [−0.002–0.043] | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanashyan, M.M.; Shabalina, A.A.; Kuznetsova, P.I.; Raskurazhev, A.A. miR-33a and Its Association with Lipid Profile in Patients with Carotid Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 6376. https://doi.org/10.3390/ijms24076376
Tanashyan MM, Shabalina AA, Kuznetsova PI, Raskurazhev AA. miR-33a and Its Association with Lipid Profile in Patients with Carotid Atherosclerosis. International Journal of Molecular Sciences. 2023; 24(7):6376. https://doi.org/10.3390/ijms24076376
Chicago/Turabian StyleTanashyan, Marine M., Alla A. Shabalina, Polina I. Kuznetsova, and Anton A. Raskurazhev. 2023. "miR-33a and Its Association with Lipid Profile in Patients with Carotid Atherosclerosis" International Journal of Molecular Sciences 24, no. 7: 6376. https://doi.org/10.3390/ijms24076376
APA StyleTanashyan, M. M., Shabalina, A. A., Kuznetsova, P. I., & Raskurazhev, A. A. (2023). miR-33a and Its Association with Lipid Profile in Patients with Carotid Atherosclerosis. International Journal of Molecular Sciences, 24(7), 6376. https://doi.org/10.3390/ijms24076376








